Abstract
Study Objectives:
To summarize evidence about the association between daytime napping and the risk of cardiovascular disease and all-cause mortality, and to quantify the potential dose-response relation.
Design:
Meta-analysis of prospective cohort studies.
Methods and Results:
Electronic databases were searched for articles published up to December 2014 using the terms nap, cardiovascular disease, and all-cause mortality. We selected well-adjusted prospective cohort studies reporting risk estimates for cardiovascular disease and all-cause mortality related to napping. Eleven prospective cohort studies were identified with 151,588 participants (1,625,012 person-years) and a mean follow-up period of 11 years (60% women, 5,276 cardiovascular events, and 18,966 all-cause deaths). Pooled analysis showed that a long daytime nap (≥ 60 min/day) was associated with a higher risk of cardiovascular disease (rate ratio [RR]: 1.82 [1.22–2.71], P = 0.003, I2 = 37%) compared with not napping. All-cause mortality was associated with napping for ≥ 60 min/day (RR: 1.27 [1.11–1.45], P < 0.001, I2 = 0%) compared with not napping. In contrast, napping for < 60 min/day was not associated with cardiovascular disease (P = 0.98) or all-cause mortality (P = 0.08). Meta-analysis demonstrated a significant J-curve dose-response relation between nap time and cardiovascular disease (P for nonlinearity = 0.01). The RR initially decreased from 0 to 30 min/day. Then it increased slightly until about 45 min/day, followed by a sharp increase at longer nap times. There was also a positive linear relation between nap time and all-cause mortality (P for non-linearity = 0.97).
Conclusions:
Nap time and cardiovascular disease may be associated via a J-curve relation. Further studies are needed to confirm the efficacy of a short nap.
Citation:
Yamada T, Hara K, Shojima N, Yamauchi T, Kadowaki T. Daytime napping and the risk of cardiovascular disease and all-cause mortality: a prospective study and dose-response meta-analysis. SLEEP 2015;38(12):1945–1953.
Keywords: napping, siesta, cardiovascular disease, all-cause mortality, meta-analysis
INTRODUCTION
Several recent meta-analyses have shown that a U-shaped curve describes the relationship between sleep duration and cardiovascular disease (CVD) or all-cause mortality, with both short and long sleep durations being significant predictors of worse cardiovascular outcomes and higher all-cause mortality in prospective studies.1,2
A nap is a short sleep, typically taken during daylight hours, and the habit of napping is prevalent worldwide. Daytime naps are usually brief, but can range from several minutes to several hours. The frequency varies from an occasional nap to several planned rest periods daily for habitual nappers. Some people take a nap because of excessive daytime sleepiness resulting from a sleep disorder.
The hypothesis that a midday nap reduces the risk of CVD is based on low CVD mortality rates in countries where napping is widespread (Mediterranean countries and several Latin American countries).3 However, epidemiological studies assessing the relation between daytime napping and CVD or all-cause mortality have yielded conflicting results. A significant positive association was observed in seven cohort studies,4–10 but there was no significant association in another four.11–14 Moreover, the strength of the association has varied among studies that found a positive association.4–10 These studies were heterogeneous with respect to sample size, stratification of nap times, nocturnal sleep duration, and other characteristics that could have influenced outcomes. Therefore, we performed meta-analysis of the dose-response relation across 11 studies to clarify the association between nap time and the risk of CVD or all-cause mortality.
METHODS
Search Strategy
We searched of the Medline, Cochrane Library, Web of Science, and Science Direct databases (until 8 December 2014) for prospective cohort studies examining the association of napping with CVD and/or all-cause mortality. The search terms were cardiovascular disease OR heart disease OR coronary artery disease OR cardiomyopathy OR heart failure OR cerebrovascular disease OR peripheral vascular disease OR mortality OR death AND nap OR siesta OR daytime sleepiness. Details of the search terms (MeSH) are shown in supplemental method 1 in the supplemental material. We also performed a manual search of the references cited by articles thus identified.
Study Selection
We selected prospective cohort studies reporting risk estimates for the influence of daytime napping on CVD and all-cause mortality that excluded patients with self-reported CVD at baseline (or adjusted analyses for a history of CVD), investigated the general population, and provided point estimates of the incidence rate ratio (or hazard ratio) with its 95% confidence interval or standard error (or reported sufficient data for calculation of these parameters). We defined daytime napping on the basis of affirmative answers to questions such as “Do you take a daytime nap?” or “Do you sleep during the day?” We excluded studies on excessive daytime sleepiness (EDS), which was defined as affirmative answers to questions like “Do you have a problem with sleepiness during the daytime?” Each cohort study was approved by a medical ethics committee and was undertaken according to the Declaration of Helsinki. All studies followed the relevant local rules for ethics and data protection.
In all cohorts, follow up was based on linked records (e.g., population registries). Mortality was defined by using death certificates. CVD events were defined as fatal/non-fatal coronary heart disease and stroke.
Data Extraction
We extracted the following information: study characteristics (study title, authors, year of publication, journal, study location, follow-up period, and number of participants and incident cases), participant characteristics (age and sex), exposure to napping (definition of napping, nap time, and prevalence of each napping category), validity of the assessment method, validity of outcome assessment (CVD mortality and all-cause mortality), and validity of the analytical methods (statistical models, covariates included in the models, and risk estimates in each category).
Seven studies4–6,8,10,12,13 reported data separately for men and women, or reported data separately for age groups8 sleep duration,12 or the presence of hypertension.14 We treated each of these cohorts as an independent report and extracted data separately. Person times were estimated as the number of participants multiplied by the average follow-up time when these were not described. Event incidence rates for CVD and all-cause mortality were estimated as the number of events divided by person time (1,000 person-years). If more than one study covered the same cohort, only the report containing the most comprehensive information was analyzed to avoid overlapping populations.
We contacted Dr. Bursztyn directly by Email and confirmed that three studies were performed on the same cohort.9,15,16 Accordingly, we only included data on CVD mortality and all-cause mortality from one study9 and excluded the other two.15,16 Cohen-Mansfield et al. kindly provided the adjusted RR (rate ratio) and 95% confidence interval for napping subjects compared with non-napping subjects in their study (Jiska Cohen-Mansfield, Email communication). Leng et al.10 also kindly provided event data for all-cause mortality and CVD among short nappers (< 60 min/day) and long nappers (≥ 60 min/day) (Yue Leng, Email communication).
Study Validation
The quality of each study was appraised according to the STROBE statement.17 In addition, the Newcastle-Ottawa Scale for assessing the quality of prospective cohort studies in meta-analyses was used to quantify validity.18 Only high-quality prospective observational studies with a Newcastle-Ottawa Scale score ≥ 7 (maximum possible score: 9) and follow-up period ≥ 5 years were included in our meta-analysis. Two authors (TY, KH) independently confirmed the eligibility of each study and then extracted and collated the data. Discrepancies were resolved by discussion.
Statistical Analysis
We performed meta-analysis to determine the pooled incidence RR (rate ratio) of CVD and all-cause mortality by making the following comparisons: (1) napping (irrespective of the nap time) versus not napping, (2) a long nap (≥ 60 min/ day) versus not napping, and (3) a short nap (< 60 min/day) versus not napping. Analyses stratified for sex, age, ethnicity, and follow-up period were also performed. Furthermore, we used the method of Altman et al.19 to evaluate potential interactions between nap time and age or sex, to evaluate whether the pooled RRs differed between groups stratified by age (≥ 65 years vs. < 65 years) or sex (men vs. women).
Moreover, we performed multivariate meta-regression analyses to explore the sources of heterogeneity. Variables such as the age (≥ 65 years vs. < 65 years) and sex (men vs. women) were examined to detect any significant influence on the risk of CVD and all-cause mortality.
In the study of Naska,13 subjects were assumed to take daily naps, but a report of occasional napping does not necessarily imply a daily habit. Therefore, we performed sensitivity analysis excluding this study to determine whether the point estimates changed significantly. The hazard ratio (equivalent to the RR in a cohort study) with its 95% confidence interval (CI) was employed to assess associations in all studies except one,9 which used logistic regression analysis. Because the incidence of events was very low in this study, the odds ratio was considered a relatively accurate estimate of the true RR. When multiple estimates (adjusted for age or obtained by multivariate analysis, etc.) were generated in a study, we used the estimate with the most adjustments. We pooled all RRs by using the DerSimonian-Laird random effects model to compare napping categories and weighted each study as equal to the inverse variance of its effect estimate.20 Forest plots were employed for visual assessment of the multivariate adjusted RRs and corresponding 95% CIs across studies. Cochrane's χ2 test and the I2 test were used to evaluate heterogeneity among the studies.21 Publication bias was evaluated by creating a funnel plot of the effect size for each study versus the standard error. Then asymmetry of the funnel plots was assessed by Begg test22 and Egger test.23
Dose-Response Meta-Analysis
To investigate the dose-response relation between CVD or all-cause mortality and nap time, dose-response meta-analysis was performed taking into account the between-study heterogeneity proposed by Orsini et al.24 to compute the trend from correlated log RR estimates across various nap times. A restricted cubic spline model with three knots (5th, 35th, 65th, and 95th percentiles)25 for the duration of nap time was estimated using generalized least squares regression analysis, taking into account the correlations within each set of published RRs.26 Probability (P) values for curve linearity or nonlinearity were calculated by testing the null hypothesis that the coefficient of the second spline equals zero. A summary risk estimate was calculated for a standardized increment of nap time (10 min/day). This analysis used data on the RRs and 95% CIs, number of cases, person-years, and median or mean nap time (minutes per day) for each group.
The midpoint between the upper and lower borders was set as the median for each nap category if the median or mean exposure was not reported. If the highest category was open-ended, its midpoint was set at 1.25 times the lower border, while we set the median of the lowest nap category at 0.5 times the cutoff point (e.g., if the category was < 30 min, the median was 15 min). These settings were largely consistent with Stang et al.5 For the study of Naska et al.,13 we set the median for “occasional napping” (short midday naps with an average duration < 30 min irrespective of the weekly frequency) at 15 min and the median for “systematic napping” (regular midday naps at least 3 times weekly with an average duration ≥ 30 min) at 30 min. Because estimators were unstable in the random effects cubic spline model due to lack of power, we used a fixed effects model to evaluate the dose-response relation. Statistical analyses were performed with Stata V.12.0 software (Stata-Corp, College Station, Texas, USA) and P < 0.05 was considered significant. All procedures followed the guideline of the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group27 (supplemental method 3, supplemental material) and the PRISMA statement (supplemental method 4, supplemental material).28
RESULTS
Literature Search
Figure 1 summarizes the literature search and study selection process. We identified 2,963 articles from Medline (n = 1,089), the Cochrane library (n = 59), Web of Science (n = 1,078), and Science Direct (n = 938). After excluding duplicate citations (n = 563) and studies that did not fit the inclusion criteria (n = 2,504), 97 articles received full-text evaluation. Of these, we excluded 11 reviews, 57 studies with irrelevant exposures/ outcomes, 15 studies that assessed EDS (supplemental method 1), and 3 studies with overlapping subjects. The remaining 11 studies met our inclusion criteria and were used in the meta-analysis. Among them, 8 studies4–6,8,10,12–14 examined men and women separately.
Figure 1.
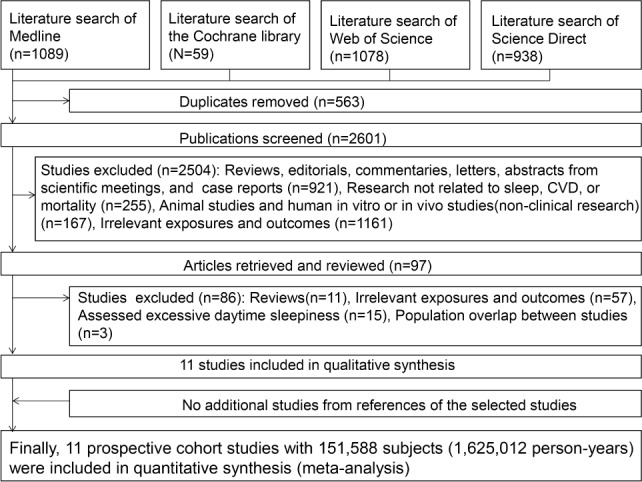
Literature search and study selection.
Qualitative Assessment
Study Characteristics
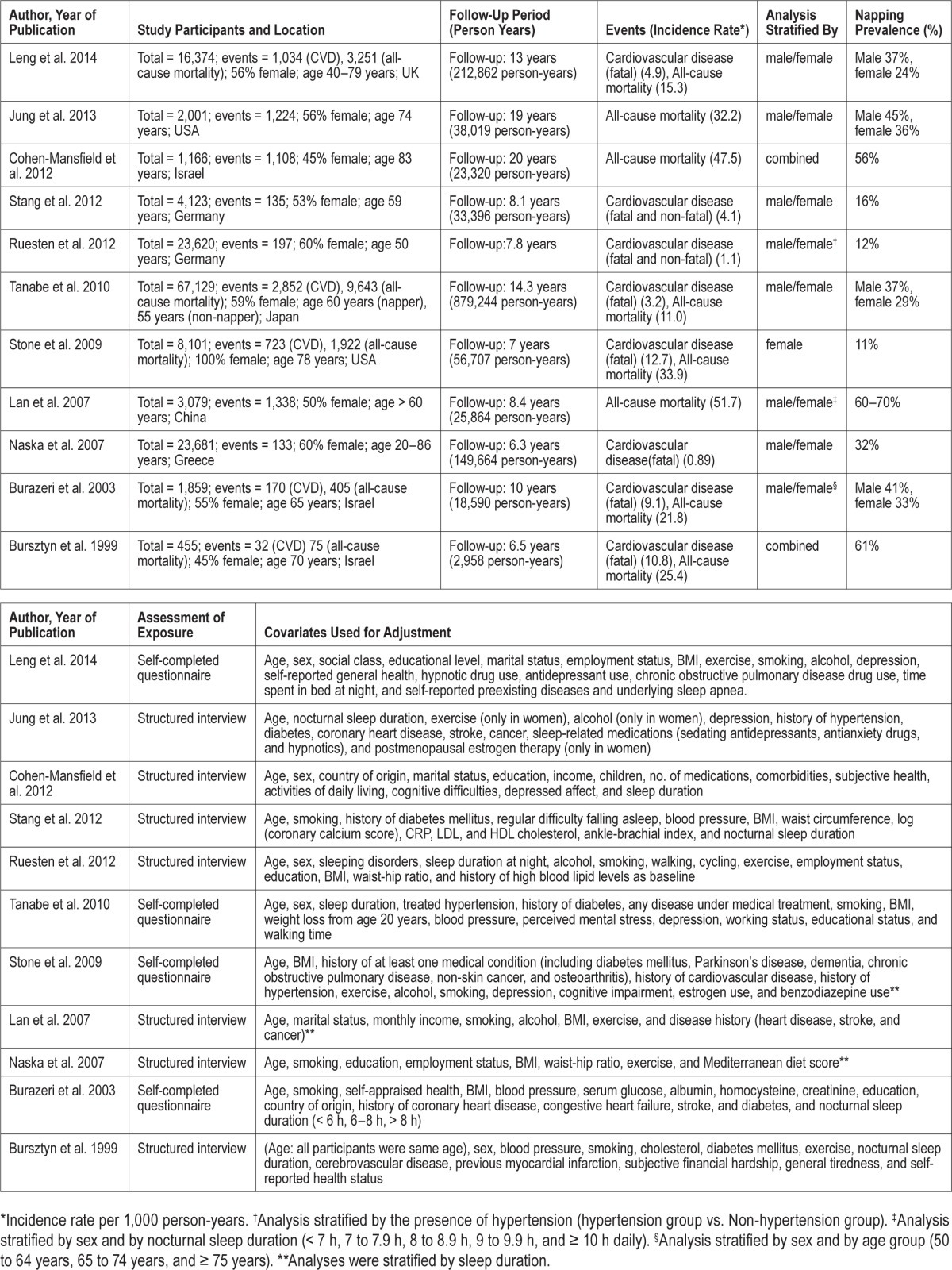
Table 1 lists the characteristics of the 11 studies, which were all prospective cohort studies with a total of 151,588 subjects (1,625,012 person-years, 60% women). There were 5,276 CVD events and 18,966 all-cause deaths during follow-up for 6.3 to 20 years (mean: 11 years). Seven studies were performed in Europe or Israel, 2 studies were done in the USA, and 2 studies were carried out in Asia. Three studies only analyzed CVD, 3 only assessed all-cause mortality, and 5 investigated both endpoints. The definition of napping was similar in all studies (supplemental method 2, supplemental material).
Table 1.
Summary of prospective cohort studies evaluating the association between napping and cardiovascular disease or all-cause mortality.
Study Quality
All studies achieved high scores on the Newcastle-Ottawa Scale, with the maximum score of 9 for 7 studies and a score of 8 for 4 studies (supplemental method 2). Each exposed (napping) cohort was derived from the general cohort and the non-exposed (non-napping) cohort was from the same community as the exposed cohort in all studies. Exposure was assessed by structured interview in 7 studies4,5,9,11–14 and by self-completed questionnaire in 4 studies.6–8,10 Outcomes of interest (CVD and all-cause mortality) were not present at the start of each study. Patients with previous CVD events were excluded from studies assessing CVD5,6,13,14 or adjusted analyses were performed.7–10 Various confounders (age, nocturnal sleep duration, and exercise) were adequately adjusted in all studies. Nocturnal sleep duration was a covariate for multivariate analysis in 8 of 11 studies.4–6,8–11,14 In the other 3 studies,7,12,13 analyses were stratified by sleep duration categories (e.g., < 6 h, 6–8 h, and > 8 h).
Except in the report of Stang et al.,5 CVD outcomes were confirmed from linked records (death certificates and/or medical records), with coding according to the International Statistical Classification of Diseases and Related Health Problems.29 The mean follow-up period ranged from 6 to 20 years (overall mean: 11 years), which was sufficient for outcomes of interest to occur. Loss of participants to follow-up was unlikely to have introduced bias, since the follow-up rate was > 90% in nine studies4,5,7–14 and the lost subjects were described in the remaining report.6
Quantitative Assessment (Meta-Analysis)
Daytime Napping and Cardiovascular Disease
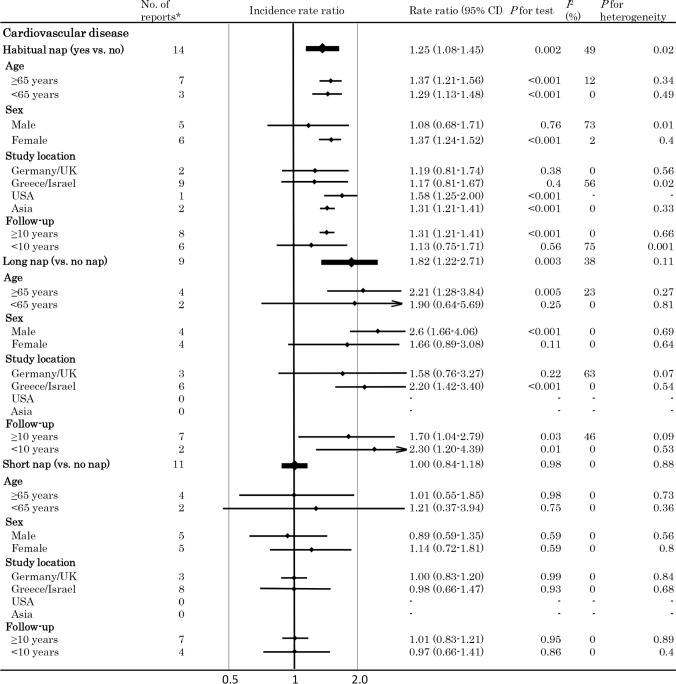
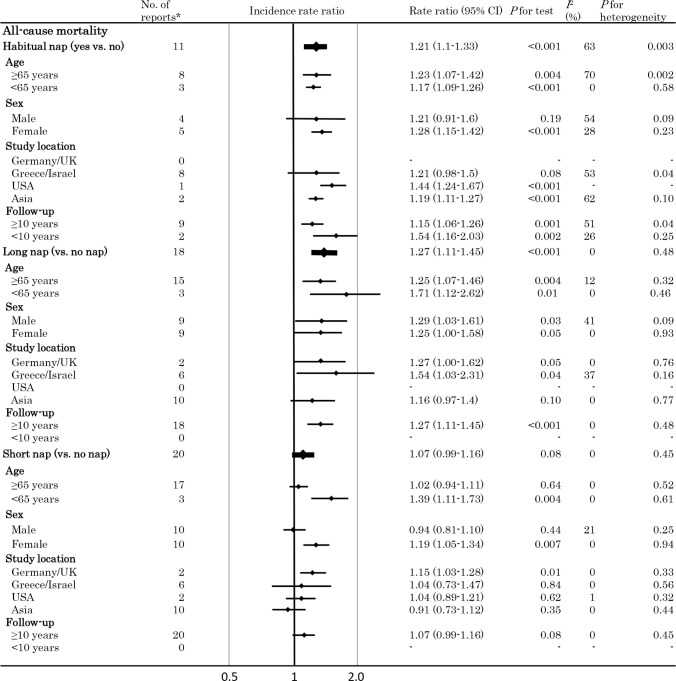
Figures 2 and 3 respectively summarize the pooled random effects RRs (with 95% CIs) for CVD and all-cause mortality, while adjusted hazard ratios originally reported in each study that included meta-analysis are shown in supplemental method 2.
Figure 2.
Meta-analysis of the incidence rate ratio of cardiovascular disease. Plots showing the association between daytime napping and the risk of cardiovascular disease. *Number of available datasets. In seven reports, the results were stratified by sex (male/female), while results were stratified by age group in 1 report (50 to 64 years, 65 to 74 years, and ≥ 75 years), by nocturnal sleep duration in 1 report (< 7 h, 7 to 7.9 h, 8 to 8.9 h, 9 to 9.9 h, and ≥ 10 h), and by the presence of hypertension in 1 report (hypertension group, non-hypertension group). CI, confidence interval.
Figure 3.
Meta-analysis of the incidence rate ratio of all-cause mortality. Plots showing the association between daytime napping and the risk of all-cause mortality. *Number of available datasets. CI, confidence interval.
When the pooled RR of CVD in napping subjects (irrespective of the nap time) versus non-napping subjects was assessed using 14 data sets, a significant increase in risk was found to be associated with napping (RR: 1.25 (1.08–1.45), P = 0.002) and significant heterogeneity was also identified (I2 = 49%, P for heterogeneity = 0.02).
Analysis stratified by the nap time (≥ 60 min/day or < 60 min/day vs. not napping) showed that a long nap (≥ 60 min/ day) was associated with a significantly higher risk of CVD (RR: 1.82 [1.22–2.71]) (n = 9) compared with not napping, whereas a short nap (< 60 min/day) was not (RR: 1.00 (0.84–1.18, P = 0.98) (n = 11). Heterogeneity was not significant in either of these analyses (both I2 = 0%, I2 = 0%). Analysis stratified by sex demonstrated that men taking long naps (≥ 60 min/ day) had a markedly higher risk of CVD (RR: 2.60 [1.66–4.06], P < 0.001, I2 = 0%), and there was no significant interaction for this relation (P for interaction = 0.23).
Analysis of studies performed in Greece and Israel (9 data sets) revealed that the pooled RR of CVD did not differ significantly between subjects taking naps (irrespective of nap time) and non-napping subjects. However, there was a significantly higher risk of CVD associated with a long nap versus not napping, while a short nap was not associated with a significant increase of CVD risk versus not napping, as was also demonstrated by our overall analysis. Sensitivity analysis excluding the study of Naska13 yielded similar results with reduced heterogeneity (RR 1.33 [1.24–1.42], P < 0.001, I2 = 0%, P for heterogeneity = 0.54 for napping; RR 1.03 [0.72–1.48], P = 0.88, I2 = 0%, P for heterogeneity = 0.92 for a short nap). Analyses stratified by age (< 65 vs. ≥ 65 years) revealed that a long nap was more closely associated with CVD in elderly people, without any significant interaction for this relation (P for interaction = 0.81). Finally, multivariate meta-regression analyses demonstrated that the covariates that we investigated were not significantly related to the risk of CVD (age, P = 0.69; sex, P = 0.17).
Daytime Napping and All-Cause Mortality
The pooled RR for all-cause mortality (n = 11) was 1.21 (1.1–1.33) (P = 0.01, I2 = 63%, P for heterogeneity = 0.003) in subjects napping (irrespective of nap time) versus subjects not napping. Analysis stratified by study location (Greece/Israel; 8 data sets) revealed no increase in the risk of all-cause mortality associated with napping (RR: 1.21 (0.98–1.5), P = 0.08, I2 = 53%, P for heterogeneity = 0.04), as was the case for CVD (Figure 3).
However, analysis stratified by nap time (≥ 60 or < 60 min/ day vs. not napping) showed that a long nap (≥ 60 min/day) (18 data sets) was associated with a significant higher risk of all-cause mortality (RR: 1.27 [1.11–1.45], P < 0.001), whereas a short nap (< 60 min/day) (20 data sets) was not (RR: 1.07 [0.99–1.16], P = 0.08)]. Heterogeneity was not significant in both analyses (I2 = 0%, and I2 = 0%). The funnel plot and Egger's test (P > 0.05) did not suggest any publication bias (supplemental result 1, supplemental material).
There was no significant interaction between nap time and age or between nap time and sex. Multivariate meta-regression analysis also indicated that none of the variables assessed had a significant influence (data not shown).
Dose-Response Meta-Analysis
Cardiovascular Disease
The fixed effects cubic spline model included a total of 1,459 events (412,582 person-years). Raw data were available for 11 comparisons with a total of 39 log RRs. We found a significant non-linear relation (J-curve) between nap time and CVD risk (P for non-linearity = 0.01; Figure 4). The RR initially decreased from 0 to 30 min/day. Then the risk began to increase slightly up to about 45 min/day, followed by a sharp increase in risk at longer times.
Figure 4.
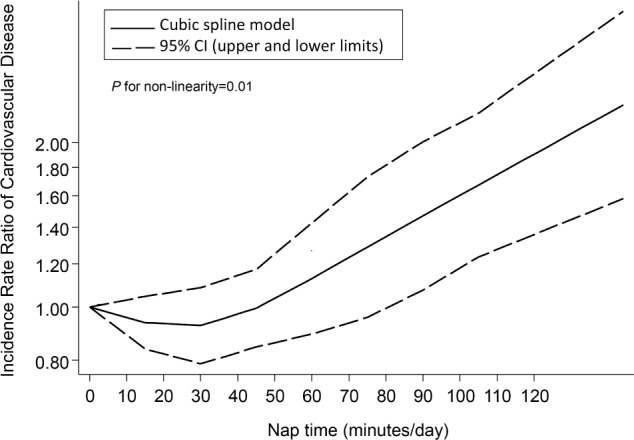
Dose-response relationship between nap time and the risk of cardiovascular diseases. CI, confidence interval.
All-Cause Mortality
The fixed effects cubic spline model included a total of 6,524 events (318,569 person-years). Raw data were available for 10 comparisons with a total of 33 log RRs. We found a non-linear dose-response relation between nap time and all-cause mortality (P for non-linearity = 0.97) (Figure 5), with a sharp increase in risk at longer nap times, although this relation was not significant unlike that for CVD. The linear model revealed that the risk of all-cause mortality increased by 4% for every 10 min/day increment of the total nap time without significant heterogeneity (95% CI: 1.02–1.05, P for trend < 0.001, P for heterogeneity = 0.59).
Figure 5.
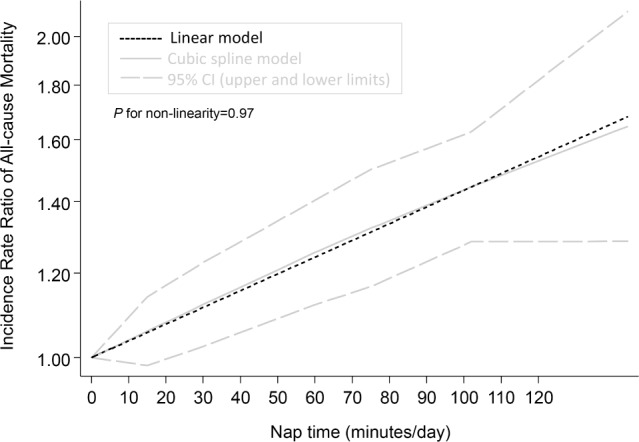
Dose-response relationship between nap time and the risk of all-cause mortality. CI, confidence interval.
DISCUSSION
The present meta-analysis of prospective cohort studies demonstrated that a longer nap was associated with a significantly elevated risk of CVD and all-cause mortality. In addition, our dose-response meta-analysis identified a J-curve dose-response relation between nap time and CVD.
Previous epidemiologic studies have yielded conflicting findings about the relation between daytime napping and CVD or all-cause mortality. Our present results suggested that cultural differences of the nap time might have influenced these previous analyses, since the nap time is disregarded when assessing a simple association between the presence/absence of napping and CVD risk. Significant heterogeneity was not observed in our analysis stratified by nap time (long vs. short), suggesting that heterogeneity was due to differences of nap time among studies.
Our analysis of studies from Greece and Israel showed that the pooled RRs for CVD and all-cause mortality were not significantly higher in napping subjects (irrespective of the nap time) than in non-napping subjects. This finding may be related to differences in the duration of napping among studies, which could influence both the physiological effects and outcomes associated with napping. Our analysis stratified by sex showed that men taking long naps had an increased risk of CVD compared with men not napping, while this did not apply to women. This sex difference may have occurred because the mean nap time was longer for men in most studies.4,6,8
Analyses stratified by age showed that a long nap was more closely associated with CVD in elderly people than younger people. The napping behavior of younger persons aged 20–65 years may differ from that of older persons with respect to the reason for napping (younger persons may take a daytime nap because they work until late at night), the opportunity for napping (younger persons may not have the option of a daytime nap because of work), the length of the nap, and the associated risk of cardiovascular events.
Although it is often stated that napping has a protective effect in younger people and is problematic for older people,8,13,30 Leng et al. found that the positive association between napping and mortality was stronger among persons aged ≤ 65 years than among those > 65 years.10
While our analyses identified a nonlinear relation between nap time and CVD, we found a positive linear relation between nap time and all-cause mortality, but the reason for this difference was unclear. Because CVD is not the only cause of death, other causes might have influenced the relation between nap time and all-cause mortality. For example, a prospective cohort study performed in the UK found that napping was associated with a slightly elevated risk of cancer.31
Daytime napping might be a consequence of night-time sleep disturbance such as obstructive sleep apnea (OSA). Epidemiological studies have shown that obstructive sleep apnea is independently linked to myocardial ischemia, stroke, fatal and nonfatal cardiovascular events, and all-cause mortality.32,33
OSA is characterized by recurrent episodes of apnea and hypopnea associated with repetitive episodes of intermittent hypoxemia, changes of intrathoracic pressure, and arousal. Intermittent hypoxemia promotes oxidative stress by increasing the production of reactive oxygen species and angiogenesis, by sympathetic activation with elevation of the blood pressure, and by promoting systemic and vascular inflammation with endothelial dysfunction that contributes to chronic multiorgan morbidity/mortality related to cardiovascular disease and metabolic dysfunction.34 Moreover, sleep deprivation has been linked to a decrease of leptin, an increase of ghrelin, and increased hunger and appetite35; a harmful impact on carbohydrate metabolism, increased cortisol secretion, and elevated sympathetic nervous activity36; and low-grade inflammation,37 all of which might increase the risk of CVD.
Another point is that major depression is associated with an increased risk of ischemic heart disease,38 but depression was only assessed in 5 of 11 studies, making its influence diffi-cult to ascertain.4,6,7,10,11 Moreover, we have to acknowledge the possibility of “reverse causality,” which means that persons with an increased risk (those who are sicker) are more likely to experience an outcome. The majority of persons who die or develop cardiovascular events are elderly and retired with the opportunity to take a daytime nap. Also, the people taking long daytime naps could be more likely to be ill and have various risk factors for morbidity or mortality.
Our results suggested that a short nap was actually associated with a lower incidence of CVD, so shorter naps may not have the same negative effect as longer naps. Naska et al.13 suggested that cardiovascular stress could be reduced by daytime sleep. Several studies have demonstrated beneficial effects of taking short naps less than 30 minutes in duration, which help to increase alertness and motor skills.39,40 A short nap finishes before the onset of deep slow wave sleep. Entering deep slow wave sleep and then failing to complete the normal sleep cycle can result in a phenomenon known as sleep inertia, in which a person feels groggy, disoriented, and even sleepier than before napping. Although the mechanisms by which a short nap might decrease the risk of CVD are still unclear, such duration-dependent differences in the effects of sleep might partly explain our finding.
A short nap might have the effect of improving an abnormal circadian rhythm and modifying a variety of endocrine abnormalities caused by sleep deprivation. It has been reported that a short nap can reduce stress and blood pressure, with the greatest decline of blood pressure being associated with vasodilation of more than 9% that occurs between the start of resting and actually falling asleep (onset of stage 1 sleep).41 Such blood pressure reduction might be associated with a decreased risk of CVD.
Strengths and Limitations
The strengths of the present meta-analysis were as follows. First, we investigated several large cohorts with a long follow-up period. In addition, the fully adjusted analytical models of these studies contained most of the established risk factors for CVD. Moreover, we investigated the dose-response relation over a wide spectrum of nap times. Our results were significant after adjustment for multiple covariates, including age, sex, nocturnal sleep duration, education, exercise, depression, smoking, alcohol consumption, medical history (hyper-tension, diabetes, coronary heart disease, stroke, and cancer), use of sleep-related medications, and use of estrogen (in women).
This meta-analysis also had some limitations. First, we might have overlooked some relevant articles. However, we searched over 2,000 articles and investigated all references cited in each study selected whenever possible. Also, analysis of publication bias did not suggest that unpublished results were missed. Second, although our stratified analyses (based on nap time and sex) and our sensitivity analysis excluding the study of Naska et al.13 did not show significant within-group heterogeneity, the limited number of studies may have diminished the statistical power for detecting heterogeneity. Third, structured interviews or self-completed questionnaires were employed to assess nap times, so measurement error is unavoidable and recall bias would also have occurred in the four studies that used self-completed questionnaires.6–8,10 Errors in measuring the extent of exposure can lead to the weakening of true associations in prospective studies, especially when exposure is assessed before disease onset. It is even possible that the questions used to identify napping were also answered affirmatively by persons with EDS.
Fourth, although some of the studies excluded subjects with self-reported CVD at baseline, undiagnosed patients may still have been enrolled. Also, the other studies did not exclude baseline CVD and only adjusted for it during analysis. While we analyzed the results from fully adjusted models because the original studies were observational, the findings could still have been influenced by residual confounders or other biases.
Depending on the nature of any such uncontrolled or residual confounders (habits and frailty,13 health worker bias,8 fatal malignancy, funding source, etc.), the associations detected in the individual studies and our meta-analysis could have been biased in either direction.
To explore the dose-response relationship in our meta-analysis, we employed different measures, such as the ‘standardized increment of nap time’ or the midpoint of nap time when modeling the association. However, none of the previous studies reported the exact nap times of the participants, which raises concerns regarding the precision of our analyses. Additional research, including large-scale pooling projects, with accurate and detailed measures of napping will be needed to confirm our conclusions and to determine whether adding a nap time score to a conventional CVD risk model improves estimation of the long-term cardiovascular event risk in the general population, as well as whether a short nap decreases the risk of CVD.
In conclusion, a longer nap was associated with a significantly elevated risk of CVD and all-cause mortality. Dose-response meta-analysis identified a J-curve dose-response relation between nap time and CVD risk, while there was a positive linear relation between nap time and all-cause mortality.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Dr. Jiska Cohen-Mansfield, Dr. Michael Bursztyn, and Dr. Yue Leng for providing information for the meta-analysis.
REFERENCES
- 1.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–92. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 2.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trichopoulos D, Tzonou A, Christopoulos C, Havatzoglou S, Trichopoulou A. Does a siesta protect from coronary heart disease? Lancet. 1987;2:269–70. doi: 10.1016/s0140-6736(87)90848-8. [DOI] [PubMed] [Google Scholar]
- 4.Jung KI, Song CH, Ancoli-Israel S, Barrett-Connor E. Gender differences in nighttime sleep and daytime napping as predictors of mortality in older adults: the Rancho Bernardo study. Sleep Med. 2013;14:12–9. doi: 10.1016/j.sleep.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stang A, Dragano N, Moebus S, et al. Heinz Nixdorf Recall Investigative Group. Midday naps and the risk of coronary artery disease: results of the Heinz Nixdorf Recall Study. Sleep. 2012;35:1705–12. doi: 10.5665/sleep.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanabe N, Iso H, Seki N, et al. JACC Study Group. Daytime napping and mortality, with a special reference to cardiovascular disease: the JACC study. Int J Epidemiol. 2010;39:233–43. doi: 10.1093/ije/dyp327. [DOI] [PubMed] [Google Scholar]
- 7.Stone KL, Ewing SK, Ancoli-Israel S, et al. Self-reported sleep and nap habits and risk of mortality in a large cohort of older women. J Am Geriatr Soc. 2009;57:604–11. doi: 10.1111/j.1532-5415.2008.02171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burazeri G, Gofin J, Kark JD. Siesta and mortality in a Mediterranean population: a community study in Jerusalem. Sleep. 2003;26:578–84. doi: 10.1093/sleep/26.5.578. [DOI] [PubMed] [Google Scholar]
- 9.Bursztyn M, Ginsberg G, Hammerman-Rozenberg R, Stessman J. The siesta in the elderly: risk factor for mortality? Arch Intern Med. 1999;159:1582–6. doi: 10.1001/archinte.159.14.1582. [DOI] [PubMed] [Google Scholar]
- 10.Leng Y, Wainwright NW, Cappuccio FP, et al. Daytime napping and the risk of all-cause and cause-specific mortality: a 13-year follow-up of a British population. Am J Epidemiol. 2014;179:1115–24. doi: 10.1093/aje/kwu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen-Mansfield J, Perach R. Sleep duration, nap habits, and mortality in older persons. Sleep. 35:1003–9. doi: 10.5665/sleep.1970. 20121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lan TY, Lan TH, Wen CP, Lin YH, Chuang YL. Nighttime sleep, Chinese afternoon nap, and mortality in the elderly. Sleep. 2007;30:1105–10. doi: 10.1093/sleep/30.9.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naska A, Oikonomou E, Trichopoulou A, Psaltopoulou T, Trichopoulos D. Siesta in healthy adults and coronary mortality in the general population. Arch Intern Med. 2007;167:296–301. doi: 10.1001/archinte.167.3.296. [DOI] [PubMed] [Google Scholar]
- 14.von Ruesten A, Weikert C, Fietze I, Boeing H. Association of sleep duration with chronic diseases in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. PLoS One. 2012;7:e30972. doi: 10.1371/journal.pone.0030972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bursztyn M, Stessman J. The siesta and mortality: twelve years of prospective observations in 70-year-olds. Sleep. 2005;28:345–7. [PubMed] [Google Scholar]
- 16.Bursztyn M, Ginsberg G, Stessman J. The siesta and mortality in the elderly: effect of rest without sleep and daytime sleep duration. Sleep. 2002;25:187–91. doi: 10.1093/sleep/25.2.187. [DOI] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–9. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Wells G, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. Ottawa Hospital Research Institute. [accessed 8 December 2014]. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 19.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. 15. [DOI] [PubMed] [Google Scholar]
- 22.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 23.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrell FE, Jr, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1988;80:1198–202. doi: 10.1093/jnci/80.15.1198. [DOI] [PubMed] [Google Scholar]
- 26.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006;6:40–57. [Google Scholar]
- 27.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 28.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 29.WHO. International Classification of Diseases (ICD) [Internet] World Health Organization. [accessed 8 December 2014]. http://www.who.int/classifications/icd/en/
- 30.Bursztyn M. Mortality and the siesta, fact and fiction. Sleep Med. 2013;14:3–4. doi: 10.1016/j.sleep.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 31.Cairns BJ, Travis RC, Wang XS, Reeves GK, Green J, Beral V Million Women Study Collaborators. A short-term increase in cancer risk associated with daytime napping is likely to reflect pre-clinical disease: prospective cohort study. Br J Cancer. 2012;107:527–30. doi: 10.1038/bjc.2012.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 33.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 34.Dewan NA, Nieto FJ, Somers VK. Intermittent hypoxemia and OSA: implications for comorbidities. Chest. 2015;147:266–74. doi: 10.1378/chest.14-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 36.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 37.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 38.Gale CR, Batty GD, Osborn DP, Tynelius P, Rasmussen F. Mental disorders across the adult life course and future coronary heart disease: evidence for general susceptibility. Circulation. 2014;129:186–93. doi: 10.1161/CIRCULATIONAHA.113.002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayashi M, Ito S, Hori T. The effects of a 20-min nap at noon on sleepiness, performance and EEG activity. Int J Psychophysiol. 1999;32:173–80. doi: 10.1016/s0167-8760(99)00009-4. [DOI] [PubMed] [Google Scholar]
- 40.Gillberg M, Kecklund G, Axelsson J, Akerstedt T. The effects of a short daytime nap after restricted night sleep. Sleep. 1996;19:570–5. doi: 10.1093/sleep/19.7.570. [DOI] [PubMed] [Google Scholar]
- 41.Zaregarizi M, Edwards B, George K, Harrison Y, Jones H, Atkinson G. Acute changes in cardiovascular function during the onset period of daytime sleep: comparison to lying awake and standing. J Appl Physiol. 2007;103:1332–8. doi: 10.1152/japplphysiol.00474.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.