Abstract
Objectives:
To investigate the effects of 6 nights of sleep extension on sustained attention and sleep pressure before and during total sleep deprivation and after a subsequent recovery sleep.
Design:
Subjects participated in two experimental conditions (randomized cross-over design): extended sleep (EXT, 9.8 ± 0.1 h (mean ± SE) time in bed) and habitual sleep (HAB, 8.2 ± 0.1 h time in bed). In each condition, subjects performed two consecutive phases: (1) 6 nights of either EXT or HAB (2) three days in-laboratory: baseline, total sleep deprivation and after 10 h of recovery sleep.
Setting:
Residential sleep extension and sleep performance laboratory (continuous polysomnographic recording).
Participants:
14 healthy men (age range: 26–37 years).
Interventions:
EXT vs. HAB sleep durations prior to total sleep deprivation.
Measurements and Results:
Total sleep time and duration of all sleep stages during the 6 nights were significantly higher in EXT than HAB. EXT improved psychomotor vigilance task performance (PVT, both fewer lapses and faster speed) and reduced sleep pressure as evidenced by longer multiple sleep latencies (MSLT) at baseline compared to HAB. EXT limited PVT lapses and the number of involuntary microsleeps during total sleep deprivation. Differences in PVT lapses and speed and MSLT at baseline were maintained after one night of recovery sleep.
Conclusion:
Six nights of extended sleep improve sustained attention and reduce sleep pressure. Sleep extension also protects against psychomotor vigilance task lapses and microsleep degradation during total sleep deprivation. These beneficial effects persist after one night of recovery sleep.
Citation:
Arnal PJ, Sauvet F, Leger D, van Beers P, Bayon V, Bougard C, Rabat A, Millet GY, Chennaoui M. Benefits of sleep extension on sustained attention and sleep pressure before and during total sleep deprivation and recovery. SLEEP 2015;38(12):1935–1943.
Keywords: sleep deprivation, sleep extension, cognitive performance, recovery sleep, involuntary microsleep
INTRODUCTION
A clear trend toward a reduction in sleep duration at night leading to growing sleep debt has been reported in western countries, particularly in professionally active populations.1 Many human endeavors such as those required for military operations, health care or aviation require high levels of cognitive performance throughout a 24-hour period. It has long been established that both acute total sleep deprivation (TSD) and chronic sleep restriction impair the ability to maintain wakefulness, increase subjective sleepiness and sleep propensity, and, most critically, reduce various aspects of cognitive performance.2,3 In studies conducted in both the laboratory and different professional situations inducing insufficient sleep, the most consistently and dramatically affected cognitive capacities were sustained attention and occurrence of involuntary microsleeps.4–7 This impairment is due to a state of instability caused by competing sleep initiating and endogenous wake promoting factors identified as a “flip-flop switch.”8 This impairment of cognitive performance constitutes a trait-like characteristic, i.e., individuals show stability in their response with repeated testing.3,9 Van Dongen et al. reported that the amount of sleep prior to exposure to TSD (called sleep-history) has an effect on many neurobehavioral functions.9 However, psycho-motor vigilance task (PVT) performance impairments during TSD were not dependent on sleep amount prior to TSD in this study. Similarly, in previous studies of chronic sleep restriction (≥ 7 consecutive nights), it has been shown that performance and alertness were degraded in a dose-dependent manner.10,11
Recently, Rupp et al. reported that 7 days of sleep extension before one week of sleep restriction (3 h/night) influenced the rate of degradation in cognitive performance and alertness both during the sleep restriction and subsequent recovery periods. In other words, they proposed that “banking” sleep before a period of sleep loss helps sustaining performance and alertness.12 Others studies have shown that sleep extension can improve the baseline levels of physical performance, sustained attention, and mood.13–15 From a practical standpoint, increasing total sleep time over a relatively short period represents an attractive non-pharmacological countermeasure to limit the deleterious effects that sleep deprivation has on performance. The only study, to our knowledge, that has investigated the effects of sleep extension on PVT performance during TSD found no effect.9 Neither have we been able to find any study on the effect of sleep extension on subsequent recovery day after TSD. Furthermore, involuntary microsleeps that reflect the instability of the waking state are an important consequence of sleep deprivation and have been found to be associated with the risk of accident.16,17 The effects of sleep extension on the occurrence of involuntary microsleeps during TSD are also unknown.
Therefore, the aim of this study was to assess the effects of 6 nights of sleep extension on sustained attention and sleep pressure before and during TSD and on the subsequent recovery day. We hypothesized that sleep extension would: (1) improve sustained attention and reduce sleep pressure at baseline (2) limit the degradation of sustained attention and sleep pressure during total sleep deprivation, and (3) have persistent effects after one night of recovery sleep.
METHODS
Subjects
Fourteen healthy men, aged 31.4 ± 3.9 years (mean ± SD), with a body mass index (BMI) of 24.0 ± 2.0 kg/m2, range 21.5–27.3 were included in the study after giving their informed written consent. Eighteen persons were selected and seen at a preliminary visit. Based on our exclusion criteria (see below), 4 persons were not included. The ethics committee of the Hotel Dieu – Ile de France 1 (Paris) and the French National Agency for the Safety of Medicines and Health Products (ANSM) approved the protocol (N°ID RCB: 2013-A01403-42), which was conducted according to the principles expressed in the Declaration of Helsinki of 1975 as revised in 2001. All subjects underwent a detailed medical history and examination including an electrocardiogram at rest. Subjects were excluded if they reported any of the following during the preceding month: (1) an average > 9 h and < 6 h sleep per night Sunday through Thursday, (2) a difference > 45 min between weeknight and weekend night sleep (subjects with sleep debt), (3) an average lights-out time earlier than 21:00 Sunday through Thursday, (4) an average wake-up time later than 09:00 Monday through Friday. To confirm these subjective data, actigraphy was used during 3 consecutive weeks to measure accurately subjects' habitual sleep time during week nights (Monday through Thursday). Data were scored manually for total sleep time (min) defined by the time of sleep within the identified sleep period (elapsed time from the start of sleep to sleep end time) and time in bed. The reported difference between week and weekend nights was around 30 minutes and the habitual week night sleep time was measured to be between 7.5 and 8 h per night. Additional exclusion criteria were: shift-workers, smokers, daily consumption of alcoholic beverages and those consuming > 400 mg of caffeine per day (i.e., about 8 caffeinated sodas or 3–4 cups of coffee), subjects with a BMI > 28 kg/m2, and those taking medication. Subjects with excessive daytime somnolence (Epworth Sleepiness Scales > 11),18 sleep complaints (Pittsburgh Sleep Quality Index > 5),19 who could not be considered as an intermediate chronotype or moderately morning type on the Horne and Ostberg questionnaire (< 42 and > 69),20 or who scored ≥ 13 on the Beck Depression Inventory21 were also excluded.
Protocol
Subjects participated in 2 experimental counterbalanced conditions (crossover design): extended sleep (EXT, 9.8 ± 0.1 h (mean ± SE) time in bed) and habitual sleep (HAB, 8.2 ± 0.1 h time in bed]). In each condition, subjects performed 2 consecutives phases: (1) 6 nights of either EXT or HAB then (2) 3 days in-laboratory. Two weeks before the first phase, a familiarization night was spent in the laboratory to avoid any first-night laboratory effect. Moreover, a control week with 8 h in bed was realized before first phase to avoid starting the experiment with the subjects in sleep debt. Time in bed during the control week was checked with actigraphy (Actiwatch TM, Cambridge Neurotechnology, Cambridgeshire, UK).
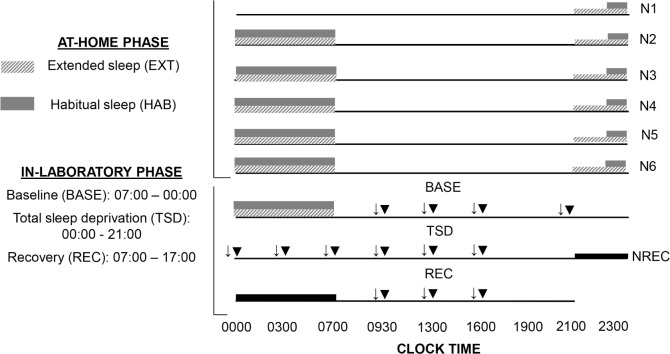
The washout period between the 2 experimental conditions was 6 weeks (Figure 1). The first phase consisted in 5 nights at home (N1 to N5) with sleep recorded by polysomnography (PSG). In HAB, subjects were instructed to maintain their habitual sleep time and spend ≥ 8 h in bed (bedtime between 22:30 and 23:00 and wake at 07:00). In EXT, they spent 10 h in bed between 21:00 and 07:00. In both conditions, volunteers maintained a wake time of 07:00 to accustom them to the waking time of 07:00 used during the second laboratory phase. Volunteers were allowed to maintain their usual lifestyles but needed to return the PSG equipment to the laboratory every morning.
Figure 1.
Experimental protocol. N, night; ↓, psychomotor vigilance task; ▼, sleep latency test.
The second phase was conducted in the laboratory and started at 17:00 after the 5th night at home. It consisted in one night of either extended or habitual sleep, a baseline (07:00– 00:00, BASE), a period of total sleep deprivation (00:00–21:00, TSD) a night of recovery sleep (21:00–07:00 in both conditions, NREC), and one subsequent recovery day (07:00–17:00, REC). Day of week entry into the second phase was standardized to Saturday.
Testing Facilities in the Laboratory Phase
During the testing and sleep periods, subjects were in individual temperature-controlled (22 ± 1°C), 3 × 4 m rooms that included a bed, restroom facilities, and a computer workstation. Laboratory illumination was maintained at 150–200 lux during the entire period of sleep deprivation (with lights off during sleep periods). Subjects were prohibited exercise, caffeine, tobacco, alcohol, or other psychoactive substances 48 hours before and during the study. Meals and caloric intake were standardized for all subjects (2600 kcal/day). Water was allowed ad libitum. When not engaged in any specific testing or meals, subjects followed a standardized activity program (reading, watching videos, and playing video games). Six investigators were systematically present in the laboratory with at least 2 of them with the subjects. Two teams of 12-h shifts were organized to maintain a good level of investigator alertness. When the subjects were about to fall asleep (eyes closed, head down), they were gently and immediately woken up (i.e., no period of sleep > 30 seconds).
MEASUREMENTS
Nighttime Sleep Assessment
During N1 to N6 and NREC, an ultra-miniaturized PSG was used to limit patient discomfort (Actiwave, CamNtech LtD, Cambridge, UK) and provide continuous monitoring for 6 EEG (F3, C3, O1 and F4, C4, O2), 2 electrocardiograms and 2 electroculograms (outer canthus of each eye), and 2 electro-myograms (chin). Contralateral mastoid leads served as references for all unipolar measurements (electroencepha lograms and electroculograms). PSG data were scored by two trained research technicians in accordance with AASM criteria,22 using Somnologica software (TM, Medcare, Reykjavik, Iceland). Sleep period time, wakefulness after sleep onset, total sleep time (TST), sleep efficiency (TST / sleep period time), sleep onset latency (first epoch − 30 s of any sleep stage), and the time spend in various sleep stages (sleep stages 1, 2, 3, and REM) were determined. The PSG remained in place for further testing throughout the protocol.
Psychomotor Vigilance Task
During the in-laboratory phase, a previously validated,23 10-min version of the psychomotor vigilance task (PVT) was given on a personal computer. PVT was analyzed for speed (1 / reaction time × 1000), and number of lapses (reaction times > 500 ms). Subjects performed PVT as indicated in Figure 1.
Multiple Sleep Latency Assessment
During the in-laboratory phase, the multiple sleep latency test (MSLT)24 was used to assess physiological sleep pressure using the PSG to determine whether the subjects fell asleep. Onset of sleep was defined as attaining 3 subsequent 30-s epochs of stage 1 or one epoch of stage 2/stage 3/REM. The MSLT was terminated 20 min after lights-out if there had been no sleep or after sleep onset. As indicated in Figure 1, MSLT testing was carried out immediately after the PVT.
Involuntary Microsleep Assessment
Throughout the in-laboratory phase, microsleeps (defined as 3 to 14 s epoch in stage 1, 2 or 3) were monitored.25 Moreover, each microsleep had to be preceded by a period of wakefulness ≥ 15 s to be scored as a microsleep. Three time periods during the day (08:00–11:00, 11:00–14:00, 14:00–17:00) and night (23:00–02:00, 02:00–05:00, and 05:00–08:00) were analyzed. Cumulative sleep duration over a given time period was calculated as the sum of sleep and microsleep durations in that period.25
Subjective Levels of Sleepiness
The subjective level of sleepiness was assessed with the Karolinska Sleepiness Scale (KSS).26 This scale consists of 9 scores from 1: extremely alert to 9: extremely sleepy, falls asleep all the time. Subjects performed KSS every morning at 08:30 during the at-home phase. During the in-laboratory phase, subjects filled out visual analogic scales on a personal computer during 3 min (data not shown), then they performed KSS and PVT in standardized conditions. A few other measurements (saliva, blood samples, and knee extensors test) were also performed during this protocol and will be reported elsewhere.
Statistics
All data in text, tables and figures are presented as mean ± standard error of the mean, unless otherwise stated. Statistical analyses were performed using SigmaPlot 12.0 (Systat Software Inc, San Jose, USA). All data were assessed for normality using the Shapiro-Wilk test before statistical analysis was performed. Two-way repeated-measures ANOVAs (condition × night) were conducted on PSG parameters and KSS from N1 to NREC. NREC was compared to N6 to assess the effect of TSD on sleep. Two-way repeated-measures ANOVAs (condition × time since awakening) were also conducted on PVT parameters, sleep latencies, number of involuntary microsleeps and cumulative duration and KSS to evaluate differences between EXT and HAB in BASE, TSD and REC. When the ANOVA revealed significant interactions or main effect, a Tukey post hoc test was used to identify differences between HAB and EXT. For time since awakening effect, all points were compared to a single control value (09:30 at BASE). A paired t-test was performed to compare total sleep duration between HAB and EXT over the control week. Statistical significance was set at P < 0.05 for all statistical analyses.
RESULTS
Nighttime Sleep Assessment
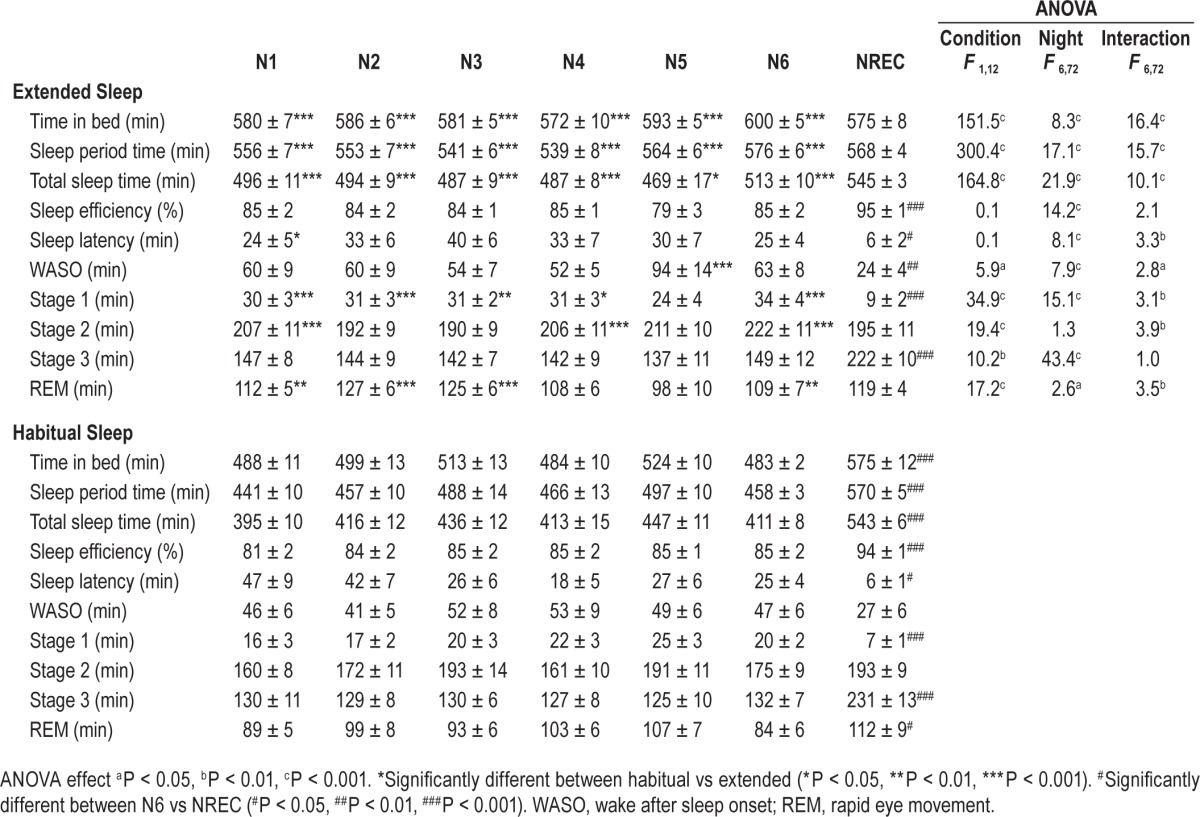
Average time in bed during the control week was 8.0 ± 0.3 and 8.0 ± 0.2 h for HAB and EXT, respectively (P = 0.97). Average time in bed and total sleep time over the 6 nights was 8.2 ± 0.1 and 7.0 ± 0.1 h for HAB and 9.8 ± 0.1 and 8.2 ± 0.1 h for EXT, respectively (significant condition main effect for both). Time in bed, sleep period time, total sleep time, wake after sleep onset, and stages 1, 2, 3 and REM sleep over the 6 nights of EXT were significantly longer compared to HAB (significant condition and night main effects, Table 1). No significant differences between the 2 conditions were observed for sleep efficiency and sleep latency (Table 1). Likewise, no significant differences were observed between HAB and EXT for any of the PSG parameters during the recovery night (Table 1).
Table 1.
Sleep parameters measured in extended and habitual sleep.
Psychomotor Vigilance Task
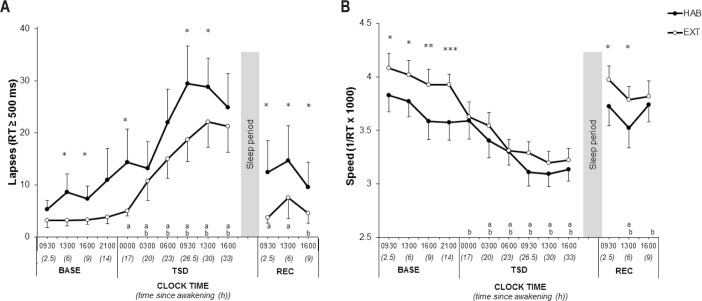
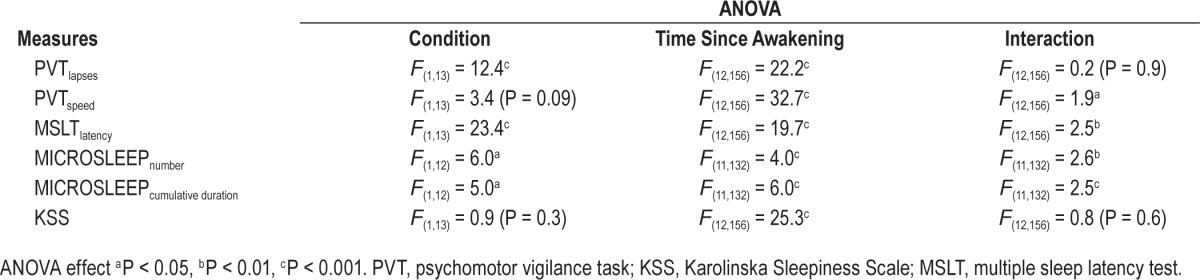
There were significantly fewer PVT lapses in EXT than HAB (significant condition and time since awakening main effects, Figure 2A and Table 2). There was no significant interaction for PVT lapses. Compared to 09:30 in BASE, PVT lapses increased significantly during TSD from 00:00 to 16:00 in HAB and from 03:00 to 16:00 in EXT. PVT lapses remained significantly more important in REC at 09:30 and 13:00 in HAB and at 16:00 in EXT (Figure 2A and Table 2). There was no significant condition main effect for PVT speed. However, there were significant interaction and time since awakening main effects (Figure 2B and Table 2). PVT speed decreased significantly during TSD from 03:00 to 16:00 in HAB and from 00:00 to 16:00 in EXT. PVT speed remained significantly lower in REC at 13:00 in HAB and from 13:00 to 16:00 in EXT (Figure 2B and Table 2).
Figure 2.
Number of lapses (A) and response speed (B) in the psychomotor vigilance test for extended (filled circle) and habitual (open circles) sleep conditions. *Significantly different between habitual (HAB) and extended (EXT) (*P < 0.05 **P < 0.01 ***P < 0.001). aSignificantly different from BASE at 09:30 in habitual (P < 0.05). bSignificantly different from BASE at 09:30 in extended (P < 0.05). BASE, baseline; TSD, total sleep deprivation; REC, recovery; RT, reaction time.
Table 2.
ANOVA table for the PVT lapses, PVT speed, MSLT latency, number, and cumulative duration of involuntary MICROSLEEP and KSS during in-laboratory phase (BASE, TSD, and REC).
Multiple Sleep Latency Assessment
Sleep latencies were longer in EXT than HAB (significant interaction, condition and time since awakening main effects, Figure 3 and Table 2). Compared to 09:30 in BASE, sleep latencies decreased significantly during TSD from 03:00 to 16:00 in HAB and from 00:00 to 16:00 in EXT. Sleep latencies remained lower in REC at 09:30 and 13:00 only in EXT (Figure 3 and Table 2).
Figure 3.
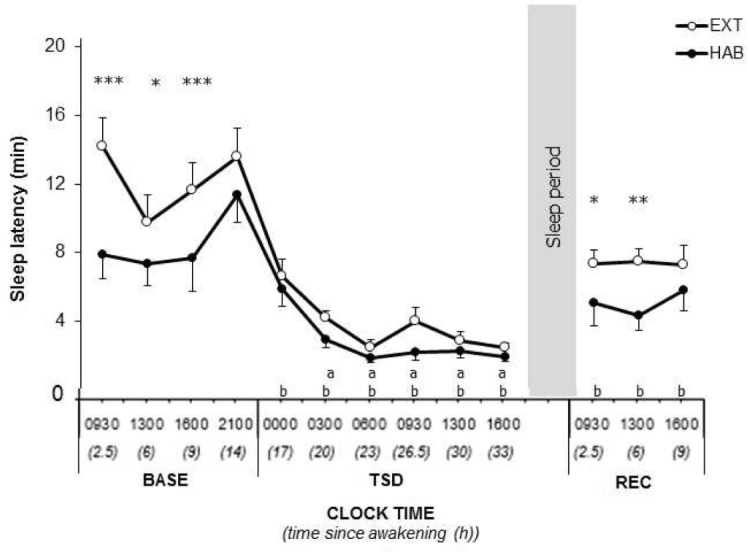
Multiple sleep latency test in extended sleep condition (filled circle) and habitual (open circles) sleep conditions. *Significantly different between habitual (HAB) and extended (EXT) (*P < 0.05 **P < 0.01 ***P < 0.001). aSignificantly different from BASE at 09:30 in habitual (P < 0.05). bSignificantly different from BASE at 09:30 in extended (P < 0.05). BASE, baseline; TSD, total sleep deprivation; REC, recovery.
Involuntary Microsleeps
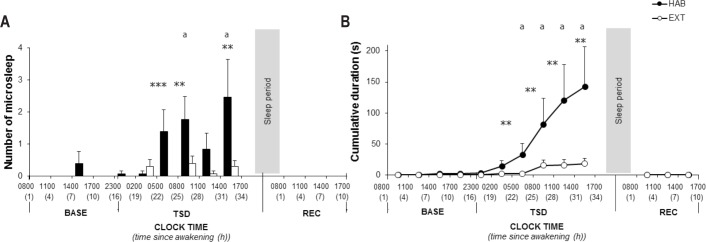
The number of involuntary microsleeps was lower in EXT than HAB at 05:00–08:00, 08:00–11:00, and 14:00–17:00 in TSD (significant interaction, condition and time since awakening main effects, Figure 4A and 4B and Table 2). There were no involuntary microsleeps in REC in either condition. The number of involuntary microsleeps and cumulative duration increased significantly during TSD in HAB from 05:00–08:00 to 14:00–17:00 time intervals (Figure 4A and 4B and Table 2). There was no significant change in the number of involuntary microsleeps and cumulative duration in EXT.
Figure 4.
Number of involuntary microsleep episodes of continuous EEG monitoring during in-laboratory phase (A) in habitual (black bars) and in extended (white bars) sleep condition and cumulative duration of microsleep episodes (B) in habitual (filled circle) and in extended (open circle) sleep conditions. *Significantly different between habitual (HAB) and extended (EXT) (*P < 0.05 **P < 0.01 ***P < 0.001). aSignificantly different from BASE at 09:30 in habitual (P < 0.05). BASE, baseline; TSD, total sleep deprivation; REC, recovery.
Subjective Levels of Sleepiness
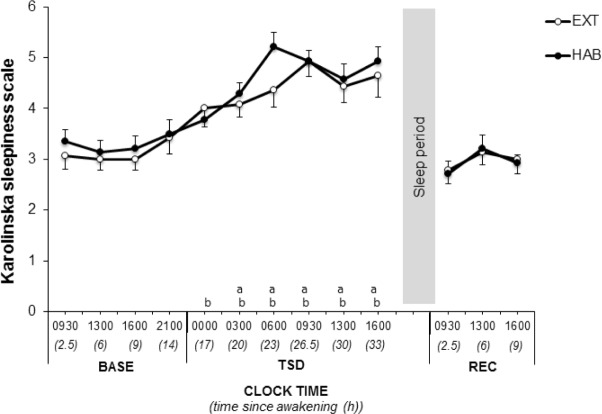
There were no changes in subjective sleepiness measured by KSS across N1 to N6 (P > 0.05). KSS increased significantly during TSD between 03:00–16:00 in HAB and 00:00–16:00 in EXT and recovered to baseline levels in REC (time since awakening main effect, Figure 5 and Table 2). No difference was observed in KSS at any time in BASE, TSD and REC between HAB and EXT (Figure 5 and Table 2).
Figure 5.
Karolinska Sleepiness Scale in extended (filled circle) and habitual (open circles) sleep conditions. aSignificantly different from BASE at 09:30 in habitual (P < 0.05). bSignificantly different from BASE at 09:30 in extended (P < 0.05). BASE, baseline; TSD, total sleep deprivation; REC, recovery; RT, reaction time.
DISCUSSION
The principal findings of this randomized, crossover study are that 6 nights of extended sleep in healthy subjects: (1) improve sustained attention (both fewer lapses and faster speed) and reduced sleep pressure at baseline, (2) limit the increase of PVT lapses and microsleeps during total sleep deprivation without changing PVT speed and MSLT, and (3) have persistent effects after one night of recovery sleep.
The present study shows that it is possible to increase sleep duration in subjects with a very mild sleep deficit before the extension period. The increase in TST after 6 nights in EXT (8.2 vs 7 h per night) was due to a significant extension of stages 1, 2, 3 and REM. Contrary to other studies, we observed an increase of stage 3 during sleep extension. This result was unexpected since slow wave sleep is known to be mainly under homeostatic regulation.27,28 Using a similar experimental protocol, Rupp et al. found that 7 nights of sleep extension (8.7 vs. 6.8 h) increased TST and stage 1, 2, and REM sleep duration.12 Wehr et al. reported that changes due to sleep extension persisted after 4 weeks of an extended time in bed of 14 h per night (between 18:00–08:00), with more TST (8.2 vs 7.2 h) and stage 1, 2, and REM sleep.29 Other authors have studied the effect of 9 days of a 16-h dark/8-h light cycle, and they also found a total sleep time of around 8.7 hours.30 Indirectly, these studies confirm epidemiological studies showing chronic sleep debt in the general population (with an average sleep duration below their sleep needs). The duration of sleep need, when the opportunity of sleeping more is given (at least 10 h in bed) seems to be slightly above 8 h in healthy, young people without sleep disturbances. This duration corroborates the recently updated recommendations of the National Sleep Foundation for sleep requirements in adults.31
In our study, the increase in total sleep time over the 6 nights induced an improvement in sustained attention (both fewer lapses and faster speed) and reduced sleep pressure (longer MSLT) at baseline. These results are in line with previous studies showing that reaction times14,15 and sleep latencies14 were improved after one week of sleep extension (10 h in bed). This improvement could be beneficial regardless of the level of sleep debt and initial sleep pressure.15,32 Indeed, Roehrs et al.14 have shown that reaction time and sleep latencies of MSLT were improved after 6 nights of EXT in both subjects with MSLT > 16 min (called “alert”) and others with MSLT < 6 min (called “sleepy”). As expected, the sleepy subjects showed an immediate and uniform increase in alertness, while alert subjects did not show improvements until late in the extension period. In our study, sleep latency values and sustained attention (mainly PVT lapses) at baseline were rather low in HAB, suggesting that some subjects were in very mild sleep debt. However one has to consider that subjects performed soporific, standardized activities immediately before MSLT, i.e. KSS and PVT. Moreover MSLT also assesses “sleep ability,” i.e., the ability to fall asleep rapidly without other signs of sleepiness.33 Contrary to sustained attention and sleep pressure, we observed no difference in the number of involuntary microsleeps measured throughout the day at baseline. This difference between results in PVT lapses and microsleeps, which are often associated, could be explained by the fact that a large part of these lapses (specifically minor lapses) can be realized with eyes open.34 This measure was probably not sensitive enough for very mild sleep debt.
The present study showed that 6 nights of sleep extension limited the number of PVT lapses and involuntary microsleeps but not PVT speed and sleep pressure during TSD. As expected, sustained attention, sleep pressure and stability of the waking state were heavily impaired by TSD. These results confirm the abundant literature reporting an overall slowing of reaction times, an increase in the number of omissions and a decrease of sleep latencies in both acute total sleep deprivation35–37 and chronic partial sleep restriction.10–12 Our results on sustained attention are in line with those of Rupp et al. showing that 7 days of sleep extension before one week of sleep restriction (3 h/night) influence the rate of degradation of PVT performance (only lapses) and modifies the maintenance of wakefulness test during the sleep restriction period.
Yet our results are contrary to those of Van Dongen et al. who reported no effect of sleep amounts prior to TSD on PVT lapse impairments during TSD.9 The authors explained that the variance associated with order effects probably due to a short wash-out period may have contributed to the statistical non-significance of the sleep-history effect for PVT. In our study, the sleep pressure (i.e. MSLT) during TSD was similar between EXT and HAB and was reinforced by an absence of SWS differences between the conditions during the night of recovery sleep. Sleep extension seems to have a protective effect on the stability of the waking state as shown by the reduction of PVT lapses and involuntary microsleeps during TSD. Our results on involuntary microsleeps during TSD confirm the few studies that have actually measured involuntary microsleeps using EEG during a period of TSD.25,38 Indeed, Beaumont et al.25 have shown that, in their control group, the number of involuntary microsleeps became significant after 22 h of continuous wakefulness. In our study, we also observed a significant increase in the number of involuntary microsleeps after 22 h of continuous wakefulness in HAB. In addition, we report no significant increase in the number of involuntary microsleeps or cumulative duration during TSD in EXT. Finally as already shown,26 subjective sleepiness was significantly increased by TSD. However, KSS was not influenced by EXT in any phase. Rupp et al. have shown similar results using the Stanford Sleepiness Scale during sleep restriction.12 This result supports the notion that subjective and objective measures represent distinct entities and both need to be assessed.
The positive effects of sleep extension observed at baseline were still present after one night of recovery sleep. Indeed, improvements in sustained attention (both fewer lapses and faster speed) and reduced sleep pressure induced by 6 nights of extended sleep persist after the night of recovery sleep, suggesting that sleep extension has long lasting effects. The long-term effect of sleep extension has already been evidenced after one week of sleep restriction.12 However, in our study, neither sustained attention nor sleep pressure were completely restored after one night of recovery sleep. Despite that, there were no involuntary microsleeps during recovery showing the low sensitivity of this measurement for low levels of sleep pressure. Several hypotheses could be formulated about the possible mechanisms behind the effects of extended sleep on subsequent periods of sleep loss. There is ample agreement that the cognitive role of sleep is explained by sleep-dependent brain changes.39 Previous studies have shown modulation of cerebral protein synthesis and the expression of genes involved in neural plasticity during sleep.40–42 We can speculate that changes in total sleep time may induce a modulation of neurotrophic factors such as brain derived neurotrophic factor and insulin growth factor-1 known to be involved in cognitive performance.43 It is also known that glucose metabolism in the brain, often used as an indication of neuronal activity in several cortical and subcortical structures,43 is impaired by sleep deprivation as demonstrated by positron emission tomography studies in sleep-deprived subjects.44 It is thus possible that sleep extension improves brain glucose metabolism.45 Finally, adenosine has been proposed as an endogenous marker of sleep drive. It has been shown that TSD increases adenosine concentrations in the extracellular adenosine levels (hypnogogic substance) in the basal forebrain.46 In line with Rupp et al., it can be hypothesized that sleep extension down regulates adenosine A1 receptors in the anatomical structures47 controlling attentional capacities,44 allowing attenuation of the deleterious effects of sleep deprivation.
However, some limitations in the present study must be taken into consideration. We were careful to recruit subjects without sleep debt. However, sleep latency data, PVT lapses, and speed at baseline in the habitual sleep condition suggest that some subjects were in very mild sleep debt. The large number of tests performed during our protocol is another limitation of our study and, it would be hazardous to generalize our results to the general population because all our subjects were young, healthy males.
CONCLUSION
Six nights of extended sleep improve sustained attention and alertness, limit the degradation of these two parameters during total sleep deprivation and improve their recovery speed. The results of the present randomized, crossover study confirm those of Rupp et al. that addressed the question of the effects of sleep banking on chronic sleep restriction and extend them to total sleep deprivation. From a practical standpoint, our work provides a potential countermeasure to manage the sleep/ wake cycle in various professional areas. Indeed, the present findings suggest that “banking” sleep prior to total sleep deprivation may help to sustain performance and alertness in various environments such as those encountered by the military, shift workers, long-distance drivers, health workers or those working in aviation. Moreover, the present study shows that these effects are still present after one night of recovery sleep. It would be interesting to integrate sleep extension into fatigue management software systems. Further studies are needed to (1) investigate a possible differential effect of sleep extension in people with polymorphisms known to be implicated in sleep deprivation resilience, and (2) examine whether extending sleep over short periods with pharmacological aids has the same beneficial effects.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was carried out in the French armed forces biomedical research institute (IRBA), Brétigny sur Orge, France. Pierrick J. Arnal was supported by a doctoral research grant from the General Directorate for Armament (DGA, ministry of defence). Research support was provided by the DGA, contracts 14Ca703 and PDH-1-SMO-2-508. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Bruno Gourby, Mathias Guillard, Raafat Fares, Juliette Regnaud at IRBA and the Hotel-Dieu hospital (VIFASOM) and Nadia Ghazani, Michèle Bazin, Didier Clément and Goran Stankovic from Public Assistance Hospitals of Paris Cochin-Brocca-Hôtel-Dieu, Virginie Morel and Michelle Lhote-richard for their technical and logistic contributions to this work. We also thank Wanda Lipski for his proofreading of the manuscript.
ABBREVIATIONS
- BASE
baseline
- BMI
body mass index
- EEG
electroencephalography
- EXT
extended sleep
- HAB
habitual sleep
- KSS
Karolinska Sleepiness Scale
- MSLT
multiple sleep latency test
- PSG
polysomnography
- PVT
psychomotor vigilance task
- REC
recovery
- REM
rapid eye movement
- TSD
total sleep deprivation
- TST
total sleep time
Footnotes
A commentary on this article appears in this issue on page 1843.
REFERENCES
- 1.National Sleep Foundation. Sleep in America Poll. 2008. Available at https://sleepfoundation.org/sleep-polls-data/sleep-in-america-poll/2008-sleep-performance-and-the-workplace.
- 2.Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29:320–39. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leproult R, Colecchia EF, Berardi AM, Stickgold R, Kosslyn SM, Van Cauter E. Individual differences in subjective and objective alertness during sleep deprivation are stable and unrelated. Am J Physiol Regul Integr Comp Physiol. 2003;284:R280–90. doi: 10.1152/ajpregu.00197.2002. [DOI] [PubMed] [Google Scholar]
- 4.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–22. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 5.Van Dongen HP, Caldwell JA, Jr., Caldwell JL. Individual differences in cognitive vulnerability to fatigue in the laboratory and in the workplace. Prog Brain Res. 2011;190:145–53. doi: 10.1016/B978-0-444-53817-8.00009-8. [DOI] [PubMed] [Google Scholar]
- 6.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–29. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 7.Sauvet F, Bougard C, Coroenne M, et al. In-flight automatic detection of vigilance states using a single EEG channel. IEEE Trans Biomed Eng. 2014;61:2840–7. doi: 10.1109/TBME.2014.2331189. [DOI] [PubMed] [Google Scholar]
- 8.Saper CB, Cano G, Scammell TE. Homeostatic, circadian, and emotional regulation of sleep. J Comp Neurol. 2005;493:92–8. doi: 10.1002/cne.20770. [DOI] [PubMed] [Google Scholar]
- 9.Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–33. [PubMed] [Google Scholar]
- 10.Belenky G, Wesensten N, Thorne D, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 11.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 12.Rupp TL, Wesensten NJ, Bliese PD, Balkin TJ. Banking sleep: realization of benefits during subsequent sleep restriction and recovery. Sleep. 2009;32:311–21. doi: 10.1093/sleep/32.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mah CD, Mah KE, Kezirian EJ, Dement WC. The effects of sleep extension on the athletic performance of collegiate basketball players. Sleep. 2011;34:943–50. doi: 10.5665/SLEEP.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roehrs T, Timms V, Zwyghuizen-Doorenbos A, Roth T. Sleep extension in sleepy and alert normals. Sleep. 1989;12:449–57. doi: 10.1093/sleep/12.5.449. [DOI] [PubMed] [Google Scholar]
- 15.Kamdar BB, Kaplan KA, Kezirian EJ, Dement WC. The impact of extended sleep on daytime alertness, vigilance, and mood. Sleep Med. 2004;5:441–8. doi: 10.1016/j.sleep.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Horne J, Reyner L. Vehicle accidents related to sleep: a review. Occup Environ Med. 1999;56:289–94. doi: 10.1136/oem.56.5.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright N, McGown A. Vigilance on the civil flight deck: incidence of sleepiness and sleep during long-haul flights and associated changes in physiological parameters. Ergonomics. 2001;44:82–106. doi: 10.1080/00140130150203893. [DOI] [PubMed] [Google Scholar]
- 18.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 19.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 20.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 21.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 22.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events : rules, terminology, and technical specifications. [Google Scholar]
- 23.Khitrov MY, Laxminarayan S, Thorsley D, et al. PC-PVT: a platform for psychomotor vigilance task testing, analysis, and prediction. Behav Res Methods. 2014;46:140–7. doi: 10.3758/s13428-013-0339-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519–24. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 25.Beaumont M, Batejat D, Pierard C, et al. Slow release caffeine and prolonged (64-h) continuous wakefulness: effects on vigilance and cognitive performance. J Sleep Res. 2001;10:265–76. doi: 10.1046/j.1365-2869.2001.00266.x. [DOI] [PubMed] [Google Scholar]
- 26.Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 27.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–38. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dijk DJ. Regulation and functional correlates of slow wave sleep. J Clin Sleep Med. 2009;5:S6–15. [PMC free article] [PubMed] [Google Scholar]
- 29.Wehr TA, Moul DE, Barbato G, et al. Conservation of photoperiod-responsive mechanisms in humans. Am J Physiol. 1993;265:R846–57. doi: 10.1152/ajpregu.1993.265.4.R846. [DOI] [PubMed] [Google Scholar]
- 30.Rajaratnam SM, Middleton B, Stone BM, Arendt J, Dijk DJ. Melatonin advances the circadian timing of EEG sleep and directly facilitates sleep without altering its duration in extended sleep opportunities in humans. J Physiol. 2004;561:339–51. doi: 10.1113/jphysiol.2004.073742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation's sleep time duration recommendations: methodology and results summary. Sleep Health. 1:40–3. doi: 10.1016/j.sleh.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Dement WC. Sleep extension: getting as much extra sleep as possible. Clin Sport Med. 2005;24:251–68, viii. doi: 10.1016/j.csm.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Harrison Y, Horne JA. “High sleepability without sleepiness”. The ability to fall asleep rapidly without other signs of sleepiness. Neurophysiol Clin. 1996;26:15–20. doi: 10.1016/0987-7053(96)81530-9. [DOI] [PubMed] [Google Scholar]
- 34.Anderson C, Wales AW, Horne JA. PVT lapses differ according to eyes open, closed, or looking away. Sleep. 2010;33:197–204. doi: 10.1093/sleep/33.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doran SM, Van Dongen HP, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol. 2001;139:253–67. [PubMed] [Google Scholar]
- 36.Chua EC, Yeo SC, Lee IT, et al. Sustained attention performance during sleep deprivation associates with instability in behavior and physiologic measures at baseline. Sleep. 2014;37:27–39. doi: 10.5665/sleep.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basner M, Dinges DF. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep. 2011;34:581–91. doi: 10.1093/sleep/34.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lagarde D, Batejat D, Van Beers P, Sarafian D, Pradella S. Interest of modafinil, a new psychostimulant, during a sixty-hour sleep deprivation experiment. Fundam Clin Pharmacol. 1995;9:271–9. doi: 10.1111/j.1472-8206.1995.tb00295.x. [DOI] [PubMed] [Google Scholar]
- 39.Abel T, Havekes R, Saletin JM, Walker MP. Sleep, plasticity and memory from molecules to whole-brain networks. Curr Biol. 2013;23:R774–88. doi: 10.1016/j.cub.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;41:35–43. doi: 10.1016/s0896-6273(03)00814-6. [DOI] [PubMed] [Google Scholar]
- 41.Mackiewicz M, Shockley KR, Romer MA, et al. Macromolecule biosynthesis: a key function of sleep. Physiol Genomics. 2007;31:441–57. doi: 10.1152/physiolgenomics.00275.2006. [DOI] [PubMed] [Google Scholar]
- 42.Cirelli C. Cellular consequences of sleep deprivation in the brain. Sleep Med Rev. 2006;10:307–21. doi: 10.1016/j.smrv.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Sonntag WE, Bennett C, Ingram R, et al. Growth hormone and IGF-I modulate local cerebral glucose utilization and ATP levels in a model of adult-onset growth hormone deficiency. Am J Physiol Endocrinol Metab. 2006;291:E604–10.P. doi: 10.1152/ajpendo.00012.2006. [DOI] [PubMed] [Google Scholar]
- 44.Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–52. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 45.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nature reviews. Endocrinology. 2009;5:253–61. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science (New York, N.Y. 1997;276:1265–8. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henny P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neurosci. 2008;27:654–70. doi: 10.1111/j.1460-9568.2008.06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]