Abstract
Objectives:
The significance of hippocampal sleep spindles and their relation to epileptic activity is still a matter of controversy. Hippocampal spindles have been considered a physiological phenomenon, an evoked response to afferent epileptic discharges, or even the expression of an epileptic manifestation. To address this question, we investigated the presence and rate of hippocampal spindles in focal pharmacoresistant epilepsy patients undergoing scalp-intracerebral electroencephalography (EEG).
Design:
Sleep recording with scalp-intracerebral EEG.
Setting:
Tertiary referral epilepsy center.
Patients:
Twenty-five epilepsy patients (extratemporal: n = 6, temporal: n = 15, and multifocal including the temporal lobe: n = 4).
Interventions:
N/A.
Measurements and Results:
We analyzed associations between hippocampal spindles and hippocampal electrophysiological findings (interictal spiking, seizure onset zone) and magnetic resonance imaging volumetry. Sixteen of 25 patients (64%) had hippocampal spindles (extratemporal epilepsy: 6/6; temporal epilepsy: 10/15; and multifocal epilepsy: 0/4; P = 0.005). Median spindle rate was 0.6 (range, 0.1–8.6)/min in nonrapid eye movement sleep. Highest spindle rates were found in hippocampi of patients with extratemporal epilepsy (P < 0.001). A negative association was found between hippocampal spiking activity and spindle rate (P = 0.003). We found no association between the presence (n = 21) or absence (n = 17) of hippocampal seizure onset zone and hippocampal spindle rate (P = 0.114), and between a normal (n = 30) or atrophic (n = 8) hippocampus and hippocampal spindle rate (P = 0.195).
Conclusions:
Hippocampal spindles represent a physiological phenomenon, with an expression that is diminished in epilepsy affecting the temporal lobe. Hippocampal spiking lowered the rate of hippocampal spindles, suggesting that epileptic discharges may at least in part be a transformation of these physiological events, similar to the hypothesis considering generalized spike-and-waves a transformation of frontal spindles.
Citation:
Frauscher B, Bernasconi N, Caldairou B, von Ellenrieder N, Bernasconi A, Gotman J, Debeau F. Interictal hippocampal spiking influences the occurrence of hippocampal sleep spindles. SLEEP 2015;38(12):1927–1933.
Keywords: epilepsy, hippocampus, intracerebral EEG, sleep physiology, MRI volumetry
INTRODUCTION
Sleep spindles are distinct electroencephalographic (EEG) events and a hallmark of nonrapid eye movement (NREM) sleep stage 2. They are characterized by waxing and waning oscillations with a frequency between 10–16 Hz and duration between 0.5 and 2 sec, and are predominantly found in frontocentroparietal regions.1–3
The significance of sleep spindles in the hippocampus and their possible relation to epileptic activity is still unclear.4–11 They, however, seem to play an important role in learning and memory consolidation during sleep.7,12
The first description of spindles in the hippocampus dates back to the 1970s,13 and so far three studies using intracranial EEG recordings specifically looked at the relationship between sleep spindles and epileptic activity.4–6 Montplaisir and colleagues4 found that spindle activity was more prominent in epileptic patients in whom the hippocampal region was not the site of the primary focus, and concluded that hippocampal spindles are either a physiological phenomenon or an evoked response of the hippocampus to an epileptic “afferent” discharge. Malow and colleagues5 confirmed these findings in a small group of patients with temporal lobe epilepsy (TLE), and also concluded that hippocampal spindles are physiological for most cases. In one of their subjects, however, atypical hippocampal spindles were recorded during both wakefulness and sleep and, in this case, thought to be epileptic.5 Finally, Nakabayashi and colleagues6 studied six patients with subdural electrodes, of whom five had a diagnosis of TLE. The authors identified events with a 10–16 Hz frequency in epileptic temporal lobes in three of these patients during both wakefulness and sleep, and concluded that temporal spindle-like oscillatory bursts represent epileptic activity.6
We recently demonstrated that sleep spindles are widespread and occur in different intracerebral structures at the time of scalp spindles. In contrast to these extratemporal widespread findings, hippocampal spindles, although identified in the majority of nonepileptic hippocampi, occurred rarely simultaneously with scalp spindles. Moreover, hippocampal spindles were more spatially restricted and their duration was shorter compared to that of scalp spindles.11
Because of apparent contradictory findings, we aimed to study the morphology, timing, and rate of hippocampal sleep spindles in a series of patients with temporal and extratemporal lobe epilepsy (extraTLE) who underwent combined scalp and intracerebral EEG recordings for refractory epilepsy at our center. We hypothesized that sleep spindles represent a physiological phenomenon of the hippocampus, and that hippocampal spindle rates should be higher in “healthy” hippocampi compared to structurally abnormal and epileptic hippocampi, where “healthy” was defined from intracerebral EEG and from hippocampal anatomy. We examined if their presence and rates were correlated with electrophysiological epileptic findings [interictal spiking activity and localization of the seizure onset zone (SOZ)] and with hippocampal structural abnormalities as measured by magnetic resonance imaging (MRI) volumetry.
METHODS
Patient Selection
Fifty-seven consecutive patients with pharmacoresistant epilepsy underwent continuous intracerebral EEG recording combined with scalp EEG at the Montreal Neurological Institute and Hospital between January 2010 and July 2014.
Patients were included in this study if: (1) they had at least one depth electrode in one hippocampus; (2) a 3 T MRI acquisition for hippocampal volumetry; (3) at least five well-identifiable sleep spindles during the first sleep cycle in the surface EEG recording; and (4) a localizable epileptic generator. Exclusion criteria were the presence of a secondarily generalized seizure during the 12 h, or presence of a partial EEG seizure (clinical or asymptomatic) during the 6 h prior to the evaluation period. Twenty-five subjects fulfilled these criteria.
This study was approved by the Research Ethics Board of the Montreal Neurological Institute and Hospital. All patients signed a Research Ethics Board approved written informed consent.
Intracerebral EEG Recording and Postimplantation Verification of Electrode Localization
Depth electrodes were implanted stereotactically using an image-guided system (ROSA robotic neuronavigation system, Medtech SAS, Montpellier, France; Stealth neuronavigation system, Medtronic, Dublin, Ireland). In 13 patients commercially available electrodes (5–18 contacts, 2 mm in length, separated by 1.5 mm) were used (DIXI Medical, Besancon, France), and in 12 patients, implantations consisted of electrodes manufactured on site (nine contacts, 0.5–1 mm in length and separated by 5 mm). Intracerebral electrode positions were tailored for each patient and depended on the clinical hypothesis made regarding the location of the seizure generator and propagation of ictal discharge.
Hippocampal electrodes were inserted using an orthogonal approach, with at least two contacts recording hippocampal EEG activity. In case of two hippocampal electrodes we used the electrode recording from the anterior hippocampus, as this position was available for all investigated subjects. Electrode contact locations were determined by either postimplantation MRI (n = 13), postimplantation computed tomography (CT) coregistered with a preimplantation MRI (n = 8) using Statistical Parametric Mapping 8 software (UCL, London, United Kingdom), or the information from the reconstructed planned position of the electrodes from the ROSA robotic neuronavigation system (n = 4).14
Subdermal thin wire electrodes15 were placed at the time of implantation at positions F3, F4, Fz, C3, C4, Cz, and P3, P4, Pz for the majority of the patients or at positions Fz, Cz, C3, C4, and Pz for a minority allowing for identification of sleep stages. EEGs were recorded using the Harmonie EEG system (Stellate, Montreal, QC, Canada).
Scoring of Wake and Sleep, and Identification of Hippocampal Spindles
For scoring of wake and sleep and identification of hippocampal spindles we selected the first suitable continuous recording between 20:00 and 08.00, which was at least 72 h postelectrode implantation in order to avoid a potential influence of anesthesia on sleep as well as the acute effect of electrode implantation. Wakefulness and sleep were scored manually during 30-sec epochs using a bipolar scalp EEG montage.16 Because of the proximity of some depth electrodes to the mastoid region, and the risk of infection, we did not use a traditional mastoid referential montage for the scoring of sleep. The identification of hippocampal spindles was done visually by a sleep expert, as previously proposed.17 Automated methods were not used because none of the existing spindle detectors are validated for their use during intracerebral EEG recording and in the presence of epileptic activity.
Hippocampal spindles were marked in a bipolar montage during a 10-min period of wakefulness, which was selected at least 30 min prior to sleep onset (first 30-sec epoch of sleep stage N1); it was demonstrated previously that hippocampal spindles can precede scalp spindles for a mean of 11 min.10 Hippocampal spindles were also marked during the complete N2 and N3 sleep of the first sleep cycle. Spindles were defined according to their typical waxing and waning morphology, their frequency between 10 and 16 Hz, and duration of at least 0.5 sec. We selected a time scale of 30 mm/sec using a split screen showing in the left panel the unfiltered signal and on the right panel the filtered signal (high pass, 10 Hz; low pass, 16 Hz). The filtered signal in the right panel was checked for rhythmic regular events with spindle-shape morphology in the 10–16 Hz spectrum. All identified events in the filtered EEG were then checked for plausibility in the unfiltered signal. Representative examples of hippocampal spindles are given in Figure 1, and examples of events that were not considered as hippocampal spindles are given in Figure 2. Spindle rates were calculated per minute, and the duration of hippocampal spindles provided in all cases with at least five hippocampal spindles during the first sleep cycle.
Figure 1.
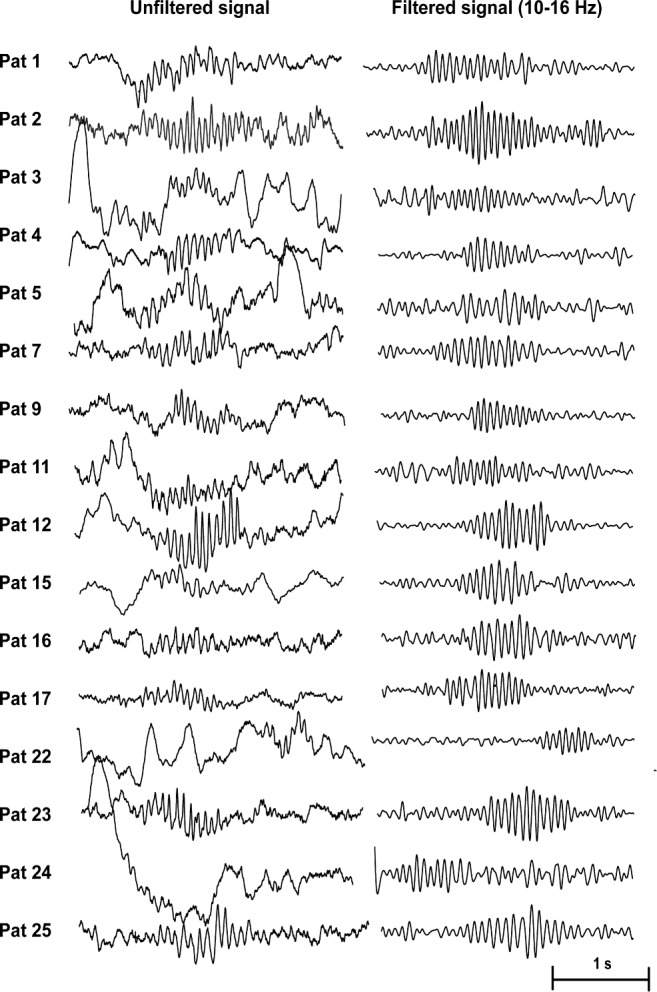
Representative examples of hippocampal spindles as observed in the individual patients (n = 16).
Figure 2.
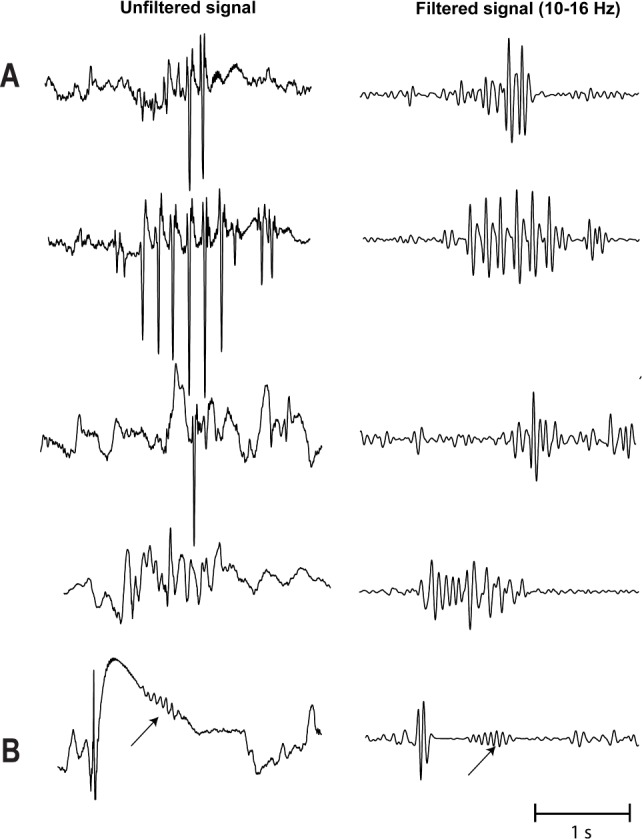
Examples of hippocampal events that were not considered as sleep spindles. (A) The morphology in the filtered signal might suggest abortive, irregular spindle-like morphology. This morphology, however, varies significantly in both frequency and amplitude. Unfiltered signal confirmed epileptic spike activity. (B) One event has morphology compatible with a sleep spindle in both unfiltered and filtered signal (see arrow). It was not considered a sleep spindle due to a duration < 0.5 sec.
Identification of Interictal Hippocampal Spikes and Seizure Onset Zone
The rate of hippocampal interictal spiking was defined on 15 sec pages independently by two electrophysiologists during the same night of the sleep recording. Spike rates were defined semi-quantitatively with ratings from 0 to 3: “0” indicates no spikes during the whole recording; “1” indicates rare epileptic spikes, with most 15-sec pages being free of epileptic discharge; “2” indicates moderate spiking defined as a mixture of pages with no spikes and pages with one or more spikes; and “3” indicates frequent spiking with ≥ 1 epileptic spike visible in almost all 15-sec pages. The SOZ was defined as the depth electrode contacts showing the first unequivocal ictal intracranial EEG change at seizure onset.18
Magnetic Resonance Acquisition and Hippocampal Volumetry
All patients underwent T1-weighted MRI on a 3 T scanner using a three-dimensional magnetization-prepared rapid-acquisition gradient echo, providing isotropic voxels of 1 × 1 × 1 mm3. Images underwent automated correction for intensity nonuniformity and intensity standardization and were linearly registered to the Montreal Neurological Institute template. The hippocampus was automatically segmented using a surface-based multitemplate algorithm.19 Hippocampal volumes were z-scored with respect to the distribution in 34 age- and sex-matched healthy controls.
Statistical Analysis
Statistical analysis was performed using IBM SPSS 22 for Windows. The normality distributions were tested with the Shapiro-Wilks test. As data were not normally distributed, differences in hippocampal spindle rate and hippocampal spindle duration were calculated using either the Mann-Whitney U test in case of two variables or the Kruskal-Wallis test in case of more than two variables. Correlations were calculated using the Spearman rho coefficient. A value of P < 0.05 was considered statistically significant. In case of multiple comparisons, Bonferroni correction was applied, and the significance level was set accordingly.
RESULTS
Demographic and Electroclinical Findings
Twenty-five patients (13 men; mean age, 33.0 ± 6.4 y; mean epilepsy duration, 14.3 ± 8.5 y) were studied and divided in three groups as defined by the main SOZ. Group 1: six patients with extraTLE; with a frontal lobe generator (n = 5), or a parasagittal fronto-parietal generator (n = 1); Group 2: 15 patients with TLE (n = 12) or with temporal lobe plus epilepsy (n = 3); with a mesiotemporal epileptic focus (n = 7), a mesial and neocortical temporal focus (n = 5), or with a neocortical temporal focus (n = 3). Group 3: four patients with multifocal epilepsy including the temporal lobe. Electroclinical findings of the study sample are provided in Table S1 (supplemental material). Thirteen patients underwent bilateral implantation in the hippocampus, and 12 patients had a unilateral hippo-campus implantation, with a total of 38 hippocampal recordings available for analysis.
Presence and Rate of Hippocampal Spindles and Type of Epilepsy
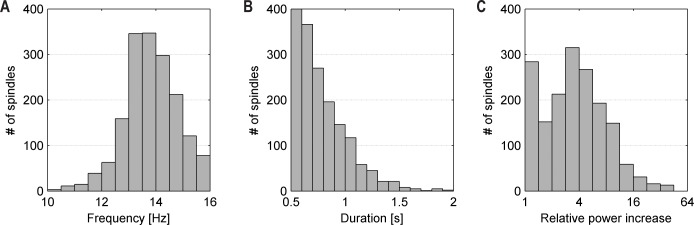
Hippocampal spindles were found in 16 patients (64%) during N2 and N3 sleep (extraTLE: 6/6; TLE: 10/15; and multifocal epilepsy: 0/4; P = 0.005). Median spindle rate was 0.6 (range, 0.1–8.6)/min. Examples of representative hippocampal sleep spindles for the individual patients are given in Figure 1, and Table S1 provides the individual spindle rates. The mean spindle duration was 0.8 ± 0.2 sec with a mean frequency of 13.9 ± 1.0 Hz. The relative power increase was 4.9 ± 5.1 compared to the prespindle baseline. Histograms of the morphological characterization of hippocampal spindles are provided in Figure 3. During wakefulness (defined as > 30 min prior to N1 sleep onset), no spindles according to our definition were identified. In one patient with TLE, two events resembling classic sleep spindles were found; however, as their duration did not reach 0.5 sec, they were not counted as spindle events (see definition of sleep spindles in the Methods section).
Figure 3.
Histograms of spindle characteristics. (A) Frequency, computed as the maximum magnitude of the discrete Fourier transform of the spindle intervals interpolated to 0.5 Hz resolution. (B) Duration. (C) Power increase in the sigma band (10–16 Hz) during the spindle relative to the background (0.5-sec interval immediately before the spindle).
Hippocampal spindle rates differed significantly across the different groups. The highest spindle rates were found in patients with extraTLE, followed by patients with TLE, and no spindles were recorded in patients with multifocal epilepsy (P < 0.001). This is illustrated in Figure 4. The duration of spindles, however, did not differ between patients with extraTLE (median, 0.8; range, 0.7–0.9 sec) compared to those with TLE (median, 0.7; range, 0.6–0.8 sec; P = 0.255).
Figure 4.

Boxplot presentation of the difference in spindle rates across the different types of epilepsy. Hippocampal spindle rates were significantly higher in hippocampi of patients with extratemporal lobe epilepsy (7 hippocampi), followed by temporal lobe epilepsy (25 hippocampi), whereas no spindles were found in hippocampi of patients with multifocal epilepsy involving the temporal lobe (6 hippocampi). Outliers are indicated by asterisks.
Within the TLE group, the spindle rates were not significantly different between hippocampi of patients with mesial TLE and those with combined mesial and neocortical TLE [14 hippocampi of patients with mesiotemporal epilepsy versus 9 hippocampi of patients with combined mesial and neocortical TLE: 0.2 (range, 0–2.2) versus 0.1 (range, 0–1.7); P = 1.000].
We identified five patients with a unilateral epileptic focus who had a bilateral hippocampus implantation. The overall group comparison between the hippocampal spindle rates ipsilateral to the focus (median, 0.4; range, 0.1–1.7) and the hippocampal spindle rates contralateral to the focus (median, 0.1; range, 0.1–3.8) was not significant (P = 0.465). Interestingly, the two patients who had relatively high hippocampal spindle rates (> 0.5/min) showed considerably higher spindle rates contralateral to the epileptic focus (patient 12: 3.8/min contralateral versus 1.7/min ipsilateral; patient 23: 2.2/min contralateral versus 0.4/min ipsilateral).
Influence of Interictal Spiking, SOZ, and Hippocampal Atrophy on Hippocampal Spindle Rates
We investigated the effect of epileptic spike occurrence, presence of SOZ, and hippocampal atrophy on spindle rates in the hippocampus. The presence of interictal hippocampal spiking, but not the presence of the SOZ in the hippocampus or hippo-campal atrophy was associated with reduced spindle rates: (1) spiking hippocampi (combined moderate or severe spiking category) versus nonspiking or mildly spiking hippocampi [n = 30: 0.1/min (range, 0–1.7) versus n = 8: 1.7/min (range, 0–8.6); P = 0.003]; hippocampus with SOZ versus without SOZ [n = 21: 0.1 (range, 0–1.7) versus n = 17: 0.5 (range, 0–8.6); P = 0.114]; and atrophic versus nonatrophic hippocampus [n = 8: 0 (range, 0–1.7) versus n = 30: 0.3 (range, 0–8.6/min); P = 0.195]. The association between spindle rate and the degree of interictal hippocampal spiking is illustrated in Figure 5. A higher rate of spiking was accompanied by a lower spindle rate; there is a negative correlation between spindle rates and hippocampal spiking (rho = −0.567, P < 0.001). There was no significant correlation between hippocampal volume and spindle rate (rho = 0.201, P = 0.227). This is illustrated in Figure 6.
Figure 5.
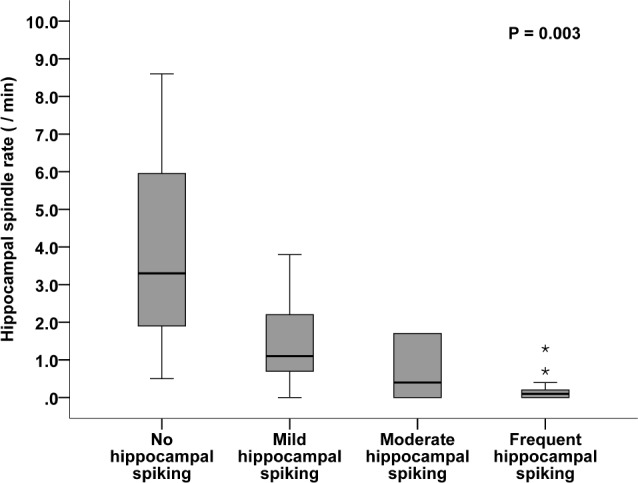
Hippocampal spindle rate is dependent on hippocampal interictal spiking. The spindle rates are higher in hippocampi with no or mild interictal spiking (n = 8) compared to hippocampi with moderate or severe spiking (n = 30). Outliers are indicated by asterisks.
Figure 6.
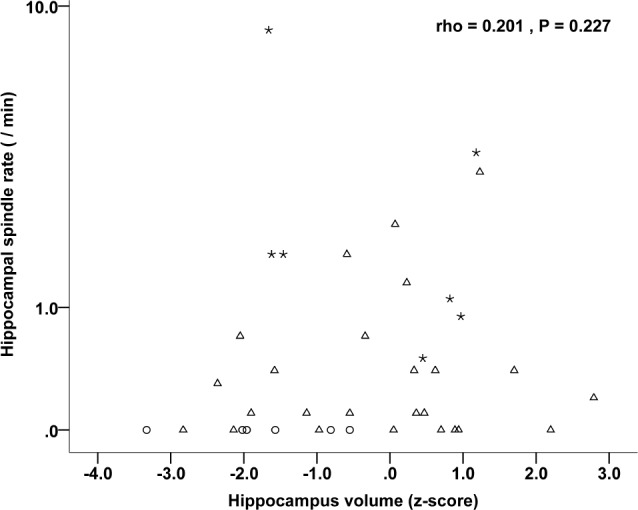
Correlation between the individual hippocampus volumes and the hippocampal spindle rate. Stars represent hippocampi of patients with extraTLE (n = 7), triangles represent hippocampi of patients with TLE (n = 25), and circles represent hippocampi of multifocal patients (n = 6).
DISCUSSION
This study, using intracerebral EEG electrodes, investigated quantitatively the significance of sleep spindles in the hippocampus. Controversies persist on whether hippocampal spindles are a physiological phenomenon, or represent a response to “afferent” epileptic discharges or are even epileptic in nature. This study clearly demonstrated that hippocampal spindles are physiological; they are recorded only during sleep and not during clear wakefulness, and are found in the majority of investigated hippocampi. Their expression, however, is diminished in patients with epilepsy affecting the temporal lobe. Moreover, we showed that interictal hippocampal spiking influences the presence and rates of hippocampal spindles, whereas there are no associations between spindle rates and presence of a hippocampal SOZ or hippocampal volumetric findings.
Hippocampal Spindles are Physiological and their Presence Depends on the Site of the Epileptic Generator
We found that hippocampal spindles were present in the majority of our subjects, during NREM sleep and absent during wakefulness. Their presence and rates were, however, higher in the extraTLE group and intermediate in the TLE group, whereas no spindles were observed in the group of patients with multifocal epilepsy. Moreover, in those patients with spindle rates > 0.5/min, the spindle rate was considerably higher contralateral to the epileptic focus. These findings strongly favor that hippocampal spindles are indeed a physiological sleep phenomenon that can be disturbed in case of epilepsy affecting the temporal lobe.
The results of the current work corroborate qualitatively and quantitatively the studies of Montplaisir and colleagues and Malow and colleagues, who suggested that hippocampal spindles are physiological in nature.4,5 Malow and colleagues, however, described one patient with “atypical” hippocampal spindles which were higher in amplitude and present during both wakefulness and sleep. The authors concluded that these spindles probably reflect epileptic activity and are different from the physiological typical hippocampal spindles also observed in this case.5 In the current study, we decided a priori to only mark typical sleep spindles in the hippocampus based on not only frequency and duration but also on the regular spindle-like morphology, and excluded spindle-like events that did not fit this morphology, and looked more like epileptic discharges (see Figure 2).
In contrast to these two studies, Nakabayashi and colleagues concluded that temporal spindle-like oscillatory bursts represent epileptic activity and that there are no physiological spindles in the temporal lobe.6 They studied six patients with invasive EEG, of whom five had TLE. In three of these patients, they identified events during wakefulness and sleep with a 10–16 Hz frequency that resembled spindles.6 In the current study, one third of our patients with TLE (5/15) also had no spindles. Both studies therefore corroborate the notion that there are no or reduced physiological spindle rates in the epileptic temporal lobe. Furthermore, our study suggests that, apart from the morphological criterion, the absence of spindle-like events during clear wakefulness is important for the definition of physiological hippocampal spindles.
Recent studies focusing on physiological aspects of sleep spindles further support our findings, showing sleep spindles in the hippocampus, although at a lower rate compared to the frontal or parietal cortex.8,9,11 In the current work, we also found hippocampal spindle rates that were lower than rates of scalp spindles, and a mean spindle frequency fitting that of centroparietal fast spindles. It is likely that hippo-campal spindle rates and frequencies may show some degree of variation across the different sleep cycles, as it is known that both spindle density and frequency are influenced by the change in slow wave activity occurring across the night.8 Further evidence for the physiological occurrence of sleep spindles in the hippocampus comes from experimental studies showing connections between the dorsal thalamic nuclei and the mesiotemporal structures. The fact that these connections are more sparse and not reciprocally connected with the reticular nucleus as shown for the frontocentroparietal cortex20,21 might explain the lower spindle rate in the hippocampus compared to that of the frontal and parietal lobe structures.
Hippocampal Spikes, but not SOZ or Atrophy, Affect Hippocampal Spindle Rates
Another interesting finding of the current study is that hippocampal spikes, but not hippocampal SOZ, are related to reduced hippocampal spindle rates. This finding is at first glance surprising, as one would have expected a negative association with the SOZ as a sign of a more severely damaged hippo-campus rather than with hippocampal spiking. One possible explanation can be found in a hypothesis of Pierre Gloor, who proposed that spike-wave discharges of generalized absence seizures are generated by the same thalamocortical circuits as physiological spindles but occur in the presence of hyperexcitable and hyperresponsive cortical neurons.22,23 Similarly, hippocampal spikes could be facilitated by thalamohippocampal activity that in a normal hippocampus triggers spindles. In this context, it has to be kept in mind that spikes in the current study mostly occurred as isolated spikes and not classic spike-wave paroxysms. Moreover, in the patients presenting with hippo-campi having continuous spiking and no spindles, a transformation of spindles to spikes cannot be the only explanation, and it might be alternatively possible that spikes, with rates varying across the different sleep stages in mesiotemporal lobe epilepsy,24 have an occluding effect on spindle activity. With respect to this latter possibility, there are also studies showing that epileptic spikes in the hippocampus represent a pathological transformation of the physiological sharp-wave-ripple complexes that are essential for memory consolidation in the hippocampus.25
We also found no association between hippocampal spindle rates and the hippocampal volumetric assessment. Volume reduction is usually correlated with reduced cell numbers but not necessarily with altered function, and our findings emphasizes the importance of an intact neuronal functionality for the occurrence of spindles rather than an apparent anatomical anomaly. The fact that patients with multifocal epilepsy and probably with severe functional brain network alterations was the group exhibiting no spindles in the hippocampus points in this same direction. When interpreting these results, however, it should be kept in mind that the patient sample was heterogeneous, and that the number of patients with hippocampus atrophy was small. Moreover, we assessed hippocampal spindles in the anterior hippocampus, but we performed the correlation analysis with the whole hippocampal volume. In addition, the distribution of hippocampal spindle rates in the TLE group was comparatively narrow.
Do Reduced Hippocampal Spindle Rates Contribute to the Presence of Memory Deficit in Temporal Lobe Epilepsy?
There is increasing evidence of the role of sleep spindles for memory consolidation via a neocortical hippocampal dialogue.7,26–29 One study even showed a coupling between parahippocampal spindles and ripples that fits the idea of a temporally fine-tuned hippocampus-to-neocortex transfer of information taking place during NREM sleep.7 In this context, it is tempting to speculate whether reduced hippocampal sleep spindle rates contribute to memory impairment in patients with TLE.30,31 A recent study showed that hippocampal interictal epileptiform activity disrupts memory function in humans depending on when and where the spikes occurred.32 Whether reduced spindle rates in patients with temporal lobe epilepsy contribute to memory dysfunction in this patient cohort awaits further confirmation.
Strengths and Potential Limitations
Strengths of the current study are the design with manual and quantitative assessments of hippocampal spindles and the large number of subjects. One potential limitation is that we are restricted to recordings of subjects with epilepsy because long-term intracerebral EEG recording is not done outside of the setting of presurgical epilepsy evaluation. In one patient at our center, however, in whom finally a diagnosis of epilepsy was ruled out (she received a diagnosis of psychogenic nonepileptic seizures; she underwent psychiatric therapy and is seizure-free since 2012), hippocampal spindles of similar morphology, frequency, and duration were observed during NREM sleep. Another patient with a final diagnosis of NREM parasomnia, who also had hippocampal sleep spindles, was reported by Sarasso and colleagues.10 Both cases as well as the literature on rodents demonstrating sleep spindles in the healthy hippocampus33,34 further corroborate our findings.
CONCLUSION
This study demonstrated that hippocampal spindles are a physiological phenomenon, with highest rates in extraTLE and fewer or lack of spindles in epilepsies involving the temporal lobe. Interictal hippocampal spiking lowered the rates of hippocampal spindles, but these were not affected by hippocampal SOZ or neuroimaging results. The negative correlation between hippocampal spiking and hippocampal spindle rate might suggest that hippocampal spikes are at least in part a transformation of these physiological events, similar to the hypothesis considering generalized spike-and-waves a transformation of frontal spindles. Alternatively, spikes might have an occluding effect on hippocampal spindles.
DISCLOSURE STATEMENT
This was not an industry supported study. This work is supported by the Austrian Science Fund (Schrödinger fellowship abroad J3485 to BF), the Austrian Sleep Research Association, and the Canadian Institutes of Health Research (grant MOP-10189). Nicolas von Ellenrieder and Jean Gotman have received fees for consultancy from Precisis Inc; all outside of the submitted work. The other authors have indicated no financial conflicts of interest. The work was performed at the Montreal Neurological Institute and Hospital, McGill University, Montreal, Québec, Canada.
ACKNOWLEDGMENTS
The authors are grateful for the assistance of the staff and technicians at the EEG Department at the Montreal Neurological Institute and Hospital, particularly Lorraine Allard and Nicole Drouin. The authors are also grateful to Dr. Jeffery Hall and Dr. André Olivier from the Department of Neurosurgery at the Montreal Neurological Institute and Hospital for electrode implantation during epilepsy surgery evaluation.
REFERENCES
- 1.Loomis AL, Harvey EN, Hobart G. Potential rhythms of the cerebral cortex during sleep. Science. 1935;81:597–8. doi: 10.1126/science.81.2111.597. [DOI] [PubMed] [Google Scholar]
- 2.Gibbs FA, Gibbs EL. Atlas of electroencephalography. Cambridge: Addison-Wesley Press; 1950. [Google Scholar]
- 3.Jankel WR, Niedermeyer E. Sleep spindles. J Clin Neurophysiol. 1985;2:1–35. doi: 10.1097/00004691-198501000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Montplaisir J, Leduc L, Laverdiere M, Walsh J, Saint-Hilaire JM. Sleep spindles in the human hippocampus: normal or epileptic activity. Sleep. 1981;4:423–8. doi: 10.1093/sleep/4.4.423. [DOI] [PubMed] [Google Scholar]
- 5.Malow BA, Carney PR, Kushwaha R, Bowes RJ. Hippocampal sleep spindles revisited: physiologic or epileptic activity. Clin Neurophysiol. 1999;110:687–93. doi: 10.1016/s1388-2457(99)00008-5. [DOI] [PubMed] [Google Scholar]
- 6.Nakabayashi T, Uchida S, Maehara T, et al. Absence of sleep spindles in human medial and basal temporal lobes. Psychiatry Clin Neurosci. 2001;55:57–65. doi: 10.1046/j.1440-1819.2001.00785.x. [DOI] [PubMed] [Google Scholar]
- 7.Clemens Z, Mölle M, Eross L, et al. Fine-tuned coupling between human parahippocampal ripples and sleep spindles. Eur J Neurosci. 2011;33:511–20. doi: 10.1111/j.1460-9568.2010.07505.x. [DOI] [PubMed] [Google Scholar]
- 8.Andrillon T, Nir Y, Staba RJ, et al. Sleep spindles in humans: insights from intracranial EEG and unit recordings. J Neurosci. 2011;31:17821–4. doi: 10.1523/JNEUROSCI.2604-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peter-Derex L, Comte JC, Mauguiere F, Salin PA. Density and frequency caudo-rostral gradients of sleep spindles recorded in the human cortex. Sleep. 2012;35:69–79. doi: 10.5665/sleep.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarasso S, Proserpio P, Pigorini A, et al. Hippocampal sleep spindles preceding neocortical sleep onset in humans. Neuroimage. 2014;86:425–32. doi: 10.1016/j.neuroimage.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 11.Frauscher B, von Ellenrieder N, Dubeau F, Gotman J. Scalp spindles are associated with widespread intracranial activity with unexpectedly low synchrony. Neuroimage. 2015;105:1–12. doi: 10.1016/j.neuroimage.2014.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrara M, Moroni F, De Gennaro L, Nobili L. Hippocampal sleep features: relations to human memory function. Front Neurol. 2012;3:57. doi: 10.3389/fneur.2012.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brazier MA. Interactions of deep structures during seizures in man. In: Petsche U, Brazier MA, editors. Mechanisms of synchronization in epileptic seizures. Vienna: Springer-Verlag; 1972. pp. 185–207. [Google Scholar]
- 14.Olivier A, Germano IM, Cukiert A, Peters T. Frameless stereotaxy for surgery of the epilepsies: preliminary experience. Technical note. J Neurosurg. 1994;81:629–33. doi: 10.3171/jns.1994.81.4.0629. [DOI] [PubMed] [Google Scholar]
- 15.Ives JR. New chronic EEG electrode for critical/intensive care unit monitoring. J Clin Neurophysiol. 2005;22:119–123. doi: 10.1097/01.wnp.0000152659.30753.47. [DOI] [PubMed] [Google Scholar]
- 16.Berry RB, Brooks R, Gamaldo CE, et al. Darien, IL: American Academy of Sleep Medicine; 2012. for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.0. www.aasment.org. [Google Scholar]
- 17.Warby SC, Wendt S, Welinder P, et al. Sleep-spindle detection: crowdsourcing and evaluating performance of experts, non-experts and automated methods. Nat Methods. 2014;11:385–92. doi: 10.1038/nmeth.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spanedda F, Cendes F, Gotman J. Relations between EEG seizure morphology, interhemispheric spread, and mesial temporal atrophy in bitemporal epilepsy. Epilepsia. 1997;38:1300–14. doi: 10.1111/j.1528-1157.1997.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim H, Besson P, Colliot O, Bernasconi A, Bernasconi N. Surface-based vector analysis using heat equation interpolation: a new approach to quantify local hippocampal volume changes. Med Image Comput Comput Assist Interv. 2008;11:1008–15. doi: 10.1007/978-3-540-85988-8_120. [DOI] [PubMed] [Google Scholar]
- 20.Jones EG, editor. The thalamus (ed. 2) Cambridge: University Press; 2007. [Google Scholar]
- 21.Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–1106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 22.Gloor P. Generalized epilepsy with bilateral synchronous spike and wave discharge. New findings concerning its physiological mechanisms. Electroenceph Clin Neurophysiol. 1978;(Suppl 34):245–9. [PubMed] [Google Scholar]
- 23.Kostopoulos GK. Spike-and-wave discharges of absence seizures as a transformation of sleep spindles: the continuing development of a hypothesis. Clin Neurophysiol. 2000;111:S27–38. doi: 10.1016/s1388-2457(00)00399-0. [DOI] [PubMed] [Google Scholar]
- 24.Clemens Z, Janszky J, Szucs A, Békésy M, Clemens B, Halász P. Interictal epileptic spiking during sleep and wakefulness in mesial temporal lobe epilepsy: a comparative study of scalp and foramen ovale electrodes. Epilepsia. 2003;44:186–92. doi: 10.1046/j.1528-1157.2003.27302.x. [DOI] [PubMed] [Google Scholar]
- 25.Gulyas AI, Freund TT. Generation of physiological and pathological high frequency oscillations: the role of perisomatic inhibition in sharp-wave ripple and interictal spike generation. Curr Opin Neurobiol. 2015;31:26–32. doi: 10.1016/j.conb.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 26.Schabus M, Gruber G, Parapatics S, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27:1479–85. doi: 10.1093/sleep/27.7.1479. [DOI] [PubMed] [Google Scholar]
- 27.Morin A, Doyon J, Dostie V, et al. Motor sequence learning increases sleep spindles and fast frequencies in post-training sleep. Sleep. 2008;31:1149–56. [PMC free article] [PubMed] [Google Scholar]
- 28.Fogel SM, Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Behav Rev. 2011;35:1154–65. doi: 10.1016/j.neubiorev.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Fogel SM, Albouy G, Vien C, et al. fMRI and sleep correlates of the age-related impairment in motor memory consolidation. Hum Brain Mapp. 2014;35:3625–45. doi: 10.1002/hbm.22426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hermann BP, Seidenberg M, Schoenfeld J, Davies K. Neuropsychological characteristics of the syndrome of mesial temporal lobe epilepsy. Arch Neurol. 1997;54:369–76. doi: 10.1001/archneur.1997.00550160019010. [DOI] [PubMed] [Google Scholar]
- 31.Banks SJ, Sziklas V, Sodums D, Jones-Gotman M. fMRI of verbal and nonverbal memory processes in healthy and epileptogenic medial temporal lobes. Epilepsy Behav. 2012;25:42–9. doi: 10.1016/j.yebeh.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Kleen JK, Scott RC, Holmes GL, et al. Hippocampal interictal epileptiform activity disrupts cognition in humans. Neurology. 2013;81:18–24. doi: 10.1212/WNL.0b013e318297ee50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sirota A, Csicsvari J, Buhl D, Buzsaki G. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci U S A. 2003;100:2065–9. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sullivan D, Mizuseki K, Sorgi A, Buzaki G. Comparison of sleep spindles and theta oscillations in the hippocampus. J Neurosci. 2014;34:662–74. doi: 10.1523/JNEUROSCI.0552-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.