Abstract
Study Objective:
Serotonin (5-hydroxytryptamine, 5-HT) neurons are now thought to promote wakefulness. Early experiments using the tryptophan hydroxylase inhibitor para-chlorophenylalanine (PCPA) had led to the opposite conclusion, that 5-HT causes sleep, but those studies were subsequently contradicted by electrophysiological and behavioral data. Here we tested the hypothesis that the difference in conclusions was due to failure of early PCPA experiments to control for the recently recognized role of 5-HT in thermoregulation.
Design:
Adult male C57BL/6N mice were treated with PCPA (800 mg/kg intraperitoneally for 5 d; n = 15) or saline (n = 15), and housed at 20°C (normal room temperature) or at 33°C (thermoneutral for mice) for 24 h. In a separate set of experiments, mice were exposed to 4°C for 4 h to characterize their ability to thermoregulate.
Measurements and Results:
PCPA treatment reduced brain 5-HT to less than 12% of that of controls. PCPA-treated mice housed at 20°C spent significantly more time awake than controls. However, core body temperature decreased from 36.5°C to 35.1°C. When housed at 33°C, body temperature remained normal, and total sleep duration, sleep architecture, and time in each vigilance state were the same as controls. When challenged with 4°C, PCPA-treated mice experienced a precipitous drop in body temperature, whereas control mice maintained a normal body temperature.
Conclusions:
These results indicate that early experiments using para-chlorophenylalanine that led to the conclusion that 5-hydroxytryptamine (5-HT) causes sleep were likely confounded by hypothermia. Temperature controls should be considered in experiments using 5-HT depletion.
Citation:
Murray NM, Buchanan GF, Richerson GB. Insomnia caused by serotonin depletion is due to hypothermia. SLEEP 2015;38(12):1985–1993.
Keywords: ascending reticular activating system (ARAS), para-chlorophenylalanine (PCPA), serotonin, sleep, thermoregulation
INTRODUCTION
5-hydroxytryptamine (5-HT) has long been thought to play a key role in sleep-wake regulation. The current consensus is that 5-HT neurons promote wakefulness and are a component of the ascending reticular activating system (ARAS).1–7 However, there was a time when this conclusion was highly controversial.8 Early studies supported the opposite belief, that 5-HT neurons cause sleep, on the basis of the observation that pharmacologically depleting 5-HT with the tryptophan hydroxylase inhibitor para-chlorophenylalanine (PCPA) causes insomnia in cats9–11 and rats.12,13 These classic studies are still frequently referenced6,14,15 as the primary evidence that 5-HT neurons do not cause arousal, because there is no proven explanation for why their conclusion differed from recent studies. There have also been proposals that 5-HT neurons play a role in sleep-wake regulation that is intermediate to the aforementioned polar opposites, such as measuring the duration and intensity of wakefulness and later triggering downstream effects to facilitate sleep when it is needed.8 In this model, 5-HT is released in response to accumulated sleep debt, producing a more relaxed waking state that leads to a transition into sleep.15 One reason why this controversy has never been completely resolved is that there has not been a clear explanation for why early studies found PCPA causes insomnia.
The current general consensus that 5-HT promotes wakefulness is due to evidence from a variety of different experiments. Electrophysiological recordings from 5-HT neurons in the raphe nuclei of cats in vivo across sleep-wake states contradicted the conclusion that 5-HT promotes sleep.16,17 Contrary to what was predicted, 5-HT neurons have their greatest firing rate during wakefulness.16,18 Dorsal raphe 5-HT neurons densely innervate the cortex and thalamus,19,20 and 5-HT converts thalamocortical neurons in brain slices from firing in a burst mode typical of sleep, to a tonically firing mode seen in wakefulness.21 Systemic treatment with agonists for 5-HT receptors 1A,22 1B,23 2A, 2C,24 and 325 increase wakefulness and antagonists for 5-HT receptors 2A and 2C26,27 decrease wakefulness. Rapid eye movement (REM) sleep is reduced by focal application of 5-HT agonists into the dorsal raphe: 5-HT2A/C (DOI)28 and 5-HT7,29 and is increased by 8-OH-DPAT,22 which causes a decrease in firing of 5-HT neurons due to activation of 5-HT1A autoreceptors.30 Optogenetic stimulation of 5-HT neurons in the mouse dorsal raphe doubles the amount of time spent in wakefulness.6 Overall, these and other data have led to the consensus that 5-HT neurons are part of the ARAS, which promotes wakefulness.2,31–33
We have previously suggested that early PCPA studies did not account for the recently recognized role of 5-HT neurons in thermoregulation. A subset of 5-HT neurons increase their firing rate when an animal is exposed to a cold environment.34 5-HT neurons of the raphe pallidus in the medulla increase cutaneous vasoconstriction, shivering, and sympathetic output to brown adipose tissue.35 In Lmx1bf/f/p mice, the transcription factor Lmx1b (LIM homeobox transcription factor 1 β, which is required for development of 5-HT neurons36) is flanked by loxP sites, and is genetically deleted selectively in Pet1-expressing cells (pheochromocytoma 12 ETS factor-1, a transcription factor present specifically in all 5-HT neurons of the central nervous system [CNS]37,38) by expression of cre-recombinase driven by the enhancer region of Pet1.39,40 In these mice, there is near-complete absence of 5-HT neurons and profound failure of thermoregulation.41 There is an increase in wakefulness in Lmx1bf/f/p mice at an ambient temperature (TA) of 23°C, and a drop in core body temperature (TC) when they fall asleep.42 When these animals are housed at a TA of 23°C they increase their locomotor activity, which generates heat. In contrast, at a thermoneutral TA of 33°C, sleep is normal.42 Unlike PCPA treatment, which acutely depletes 5-HT but leaves 5-HT neurons alive, 5-HT neurons are absent beginning during embryonic life in Lmx1bf/f/p mice. Therefore, it is possible that results from Lmx1bf/f/p mice are different than those using PCPA. For example, we previously observed evidence for compensation in Lmx1bf/f/p mice, as acute deletion of 5-HT neurons with diphtheria toxin or acute silencing of 5-HT neurons using designer receptors exclusively activated by designer drugs (DREADDs) causes larger defects in thermoregulation.43,44
Here we found that housing mice treated with PCPA at 20°C, which is cool for a mouse, caused an increase in wakefulness and an associated drop in TC, whereas housing at 33°C, which is in the thermoneutral range for a mouse,45 prevented insomnia or any change in TC. We conclude that 5-HT depletion causes a thermoregulation deficit that can confound studies of sleep, and that 5-HT does not directly cause sleep.
METHODS
Experimental Animals and PCPA
Adult (26–32 g) male C57BL/6N mice (Charles River, Wilmington, MA) and mice homozygous for floxed Lmx1b and hemizygous for ePet1-Cre (Lmx1bf/f; e-Pet1-Cre/+ or Lmx1bf/f/p; generation, breeding, and genotyping previously described40,46) were housed in standard cages in a 12 h light/12 h dark regimen with food and water available ad libitum. Pet1 is a transcription factor expressed selectively in essentially all 5-HT neurons of the CNS.37,38 Lmx1b is a transcription factor that is expressed in developing 5-HT neurons, and is required for their survival during development.36 Expression of Lmx1b also occurs in dopamine neurons, as well as a variety of tissues outside of the CNS.47,48 Genetic deletion of Pet1 does not eliminate all 5-HT neurons, and deletion of Lmx1b causes a variety of abnormalities unrelated to 5-HT neuron loss,47 including loss of dopamine neurons.49 Therefore, selective deletion of Lmx1b in Pet1 expressing neurons of Lmx1bf/f/p mice has been used to induce a highly selective and near-complete elimination of 5-HT neurons during embryonic development.40
In C57BL/6N mice, pharmacological blockade of 5-HT synthesis was achieved with the tryptophan hydroxylase inhibitor PCPA, a potent and selective 5-HT depleter. PCPA (C6506; Sigma, St. Louis, MO) was insoluble in all nontoxic delivery vehicles tested, so we used the methyl ester form (C3635; Sigma). The optimal dose of PCPA was determined by measuring brain 5-HT content using HPLC (HTEC-500; EiCOM USA, San Diego, CA) (Figure 1). All procedures and protocols were approved by the University of Iowa Office of Animal Resources and Institutional Animal Care and Use Committee and are in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Figure 1.
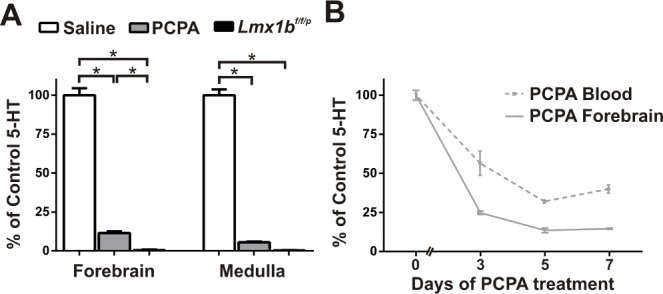
Five consecutive daily treatments with parachlorophenylalanine (PCPA) maximally decreased blood and brain 5-hydroxytryptamine (5-HT). (A) 5-HT levels in forebrain and medulla of PCPA-treated mice (800 mg/kg for 5 days) and Lmx1bf/f/p mice as a percentage of saline treated control mice (n = 7 all groups). *P < 0.001. (B) Blood and forebrain 5-HT reached a minimum following the fifth day of PCPA treatment (blood, n = 5 for each day; cortex day 0, n = 10; day 3, n = 5; day 5, n = 11; day 7, n = 5). For all points compared to day 0: P < 0.001; for blood and forebrain on day 3 compared to day 5: P < 0.05. Data are shown as mean ± standard error of the mean. Unpaired t-test with Welch correction.
High-Performance Liquid Chromatography Verification of 5-HT Depletion
As per our standard laboratory high-performance liquid chromatography (HPLC) protocol,50 animals received a dose of flunixin meglumine (flunixamide; 2.5 mg/kg subcutaneously) for pre-procedural analgesia 2 h prior to whole blood collection and brain removal. Under 1.5–3.0% isoflurane via precision vaporizer (AS-01-0007; Patterson Scientific, Bend, OR), a thoracotomy was performed and whole blood samples were collected via cardiac puncture into a syringe with EDTA for a final concentration of 2 mg/mL. Samples were mixed in a 1:1 ratio with extraction solvent (0.8 M perchloric acid, 0.1 M ascorbic acid and 10 mM EDTA), vortexed for 15 sec, spun in a microfuge at 10,000 g for 10 min, and the supernatant was injected into a Nucleosil C18 column (Phenomenex, Torrance, CA), eluted with a mobile phase (89.5% 0.1 M trichloroacetic acid, 10−2 M sodium acetate, 10−4 M EDTA, and 10.5% methanol; pH 3.8) at 0.7 mL/min using a HPLC pump (515; Waters, Milford, MA) for measurement on an Decade II detector (Antec, Boston, MA).
To eliminate contamination of brain 5-HT with 5-HT in blood (mostly in platelets), animals under flunixamide (2.5 mg/ kg subcutaneously) and 1.0–3.0% isofluorane anesthesia underwent transcardiac cannulation of the aorta and perfusion of the vascular system with 25 mL of chilled phosphate buffered saline (1 M). The brain was exposed, cerebellum was discarded, and a transverse cut was made rostral to the superior colliculus to obtain forebrain tissue. The medulla was then removed, with the caudal limit at the pyramidal decussation and rostral limit just caudal to the basis pontis. The tissues were flash frozen on dry ice, and homogenized with a sonicator (Sonic Dismembrator 60; Fisher Scientific, Houston, TX) at 1W for 45 sec in 100 μL mobile phase solution (80% 0.1 M citrate-acetate buffer, pH 3.5, 20% methanol, with 220 mg/L sodium octane sulfonate and 5 mg/mL EDTA). Homogenates and plasma were spiked with 40 ng 2,3-dihydroxybenzoic acid (2,3-DHBA; internal standard) and extracted by centrifugation at 20,000 g at 4°C for 20 min. The supernatant was filtered with aluminum oxide and a 0.45-μm syringe filter, and 10 μL was transferred to a 96-well plate within a 4°C auto sampler. The supernatant was automatically injected into the precolumn (CA-ODS; EiCOM) and column (SC-30DS; EiCOM) and eluted with the mobile phase at a flow rate of 200 μL/min. 5-HT and 5-hydroxyindoleacetic acid (5-HIAA) standards were used to generate daily calibration curves, using 2,3-DHBA as the internal standard.
PCPA Delivery
Treatment of C57BL/6N mice with PCPA-methyl ester, here referred to as PCPA, at 800 mg/kg intraperitoneally daily for 5 d led to a decrease in brain 5-HT content to nearly 10% of controls. PCPA was delivered in 0.3 mL saline (0.9% NaCl) at 07:00 on days 1 to 5. The fifth injection occurred less than 1 min prior to the recording period for sleep experiments, and 5–6 h prior to HPLC tissue procurement. Volume matched saline injections were used in controls.
Electrophysiological Data Acquisition
Mice were implanted with electroencephalography/electro-myography (EEG/EMG) headmounts (8201; Pinnacle Systems, Inc., Lawrence, KS), connected to a preamplifier that was tethered to a commutator (Pinnacle), and connected to an amplifier and computer as previously described.42 To measure TC, mice were implanted with telemetry temperature probes in the abdomen (Emitter G2; Respironics, Bend, OR) and housed in cages positioned on top of a telemetry receiver (ER-4000 Energizer Receiver; Respironics). TA was concurrently recorded (BAT-12 microprobe thermometer; Physitemp Instruments, Inc., Clifton, NJ).
24-H Sleep and TC Recordings
Mice were housed in modified mouse cages devoid of bedding on top of the telemetry receiver in a temperature-controlled, ventilated box for at least 72 h prior to recording. Animals were housed at 33°C on days 2 – 4 to prevent sleep deprivation that tends to occur at ambient temperatures that are outside of a thermoneutral range for rodents (data not shown). Food and water were provided ad libitum. On day 5, EEG, EMG, TC, TA, locomotor activity, and video recording (AF20 Webcam; Logitech, Newark, CA) were collected for 24 h. Studies began at 07:00 with lights on, and lights off at 19:00 until studies terminated the following morning at 07:00. TA was maintained at 20 ± 2.0°C for one group of mice and 33 ± 2.0°C for the second group with a thermoelectric solid-state heating/cooling air conditioner (AHP-150FFHC; TECA Corp., Chicago, IL). These two temperatures were selected to match those used previously in classic studies with PCPA and with Lmx1bf/f/p mice42 as closely as possible. When TA was set at 20°C there was slightly more variability around the set point than at 33°C due to the on and off cycling of the air conditioner unit, which did not occur with the resistance heater.
Vigilance State Scoring
EEG/EMG data were visualized with custom-written MATLAB software and manually scored in 10-sec epochs as Wake, NREM or REM sleep. These vigilance states were assigned using a standard approach based on EEG/EMG frequency characteristics (Wake: low-amplitude high-frequency (7–13 Hz) EEG with high EMG power; NREM: high-amplitude low-frequency (0.5–4 Hz) EEG with moderate to low EMG power and lack of movement; and REM: moderate-amplitude moderate-frequency (4.5–8 Hz) EEG with minimal EMG power, except for brief bursts and minimal movement correlating with EMG bursts).32 Ambiguous vigilance states were discarded from the analysis; their inclusion did not change significance of sleep-wake parameters as they were a small percentage of epochs (PCPA 5.2 ± 3.1% at TA 20°C versus 4.5 ± 3.4% at TA 33°C; n = 5 each; saline controls 2.3 ± 2.1% at TA 20°C versus 3.0 ± 2.2% at TA 33°C; n = 5 and 6; all comparisons P ≥ 0.8). All statistical tests were unpaired t-tests with Welch correction, and were conducted with GraphPad Prism (GraphPad Software, La Jolla, CA).
Change in Temperature in Response to Motor Activity
To determine the average number of activity units due to locomotor activity in PCPA and saline mice at 20°C that are necessary to increase body temperature by 1°C, we selected the first three epochs of continuous active wake data for each animal that lasted at least 10 min and had a noticeable increase in TC. These epochs were chosen after the first 5 h of recording in each mouse to allow moderate recovery of TC after PCPA injection. The temperature increase was normalized to the average activity count during the 10 min.
Cold Challenges
Mice with implanted telemetry temperature probes were placed in mouse cages devoid of bedding on the telemetry receiver. A 0.5-h baseline was acquired at normal TA (20 ± 2.0°C). The mouse cage and telemetry receiver were transferred into a room with a TA of 4 ± 2.0°C for 4 h. Animal temperature and activity were recorded every 10 sec. If a mouse had a TC that dropped below 30°C, it was moved to normal TA to recover. Following 4 h at 4°C, other animals were also returned to normal TA and reestablished baseline for 0.5 h. All cold challenges took place on the fifth day of PCPA injections, 6–8 h after injection.
RESULTS
PCPA Treatment Significantly Reduced 5-HT in the Brain and Blood
To replicate the pharmacologically induced 5-HT depletion of early sleep studies, we treated mice with PCPA and then measured 5-HT levels with HPLC. PCPA-treated mice had significantly reduced 5-HT in both the forebrain and medulla after 5 consecutive days of treatment as compared to control mice (Figure 1A), similar to previous results.50 Forebrain 5-HT levels were significantly higher in PCPA-treated mice than in Lmx1bf/f/p mice, whereas medullary 5-HT levels were similar. By day 5 of injection, 5-HT levels had reached their lowest level in blood and forebrain (Figure 1B).
PCPA Decreased Sleep at a TA of 20°C But Not 33°C
Next, we studied the role of 5-HT in sleep while housing mice at either a normal TA or a thermoneutral TA. At both TAs, saline and PCPA-treated mice maintained their ability to cycle through wake, NREM, and REM states during 24 h of recording (Figure 2A–2D). At 20°C, PCPA-treated mice spent significantly more time awake and less time in NREM and REM sleep over the course of 24 h compared to controls (wake: 78.2 ± 3.6% versus 59.8 ± 2.7%, t = 4.1, P = 0.004; NREM: 18.0 ± 3.6% versus 29.8 ± 2.2%, t = 2.8, P = 0.03; REM: 3.1 ± 1.0% versus 11.5 ± 1.6%, t = 4.4, P = 0.002; PCPA n = 5, saline n = 6; Figure 2E). These differences were greater during the light period (first 12 h of recording). We then housed separate PCPA-treated and control mice at a thermoneutral TA of 33°C for 24 h. PCPA-treated mice did not have insomnia, and spent significantly less time awake (52.4 ± 2.3%, t = 6.1, P = 0.0005, n = 5), more time in NREM sleep (40.0 ± 3.4%, t = 4.4, P = 0.002) and more time in REM sleep (7.9 ± 1.5%, t = 2.7, P = 0.03; Figure 2F) than PCPA-treated mice at 20°C. At 33°C, PCPA-treated mice had sleep-wake architecture and sleep stage amounts comparable to saline treated mice (wake: t = 0.7, P = 0.53; NREM: t = 1.0, P = 0.35; REM: t = 1.0, P = 0.35; PCPA: n = 5, saline: n = 5; Figure 2G, 2H).
Figure 2.
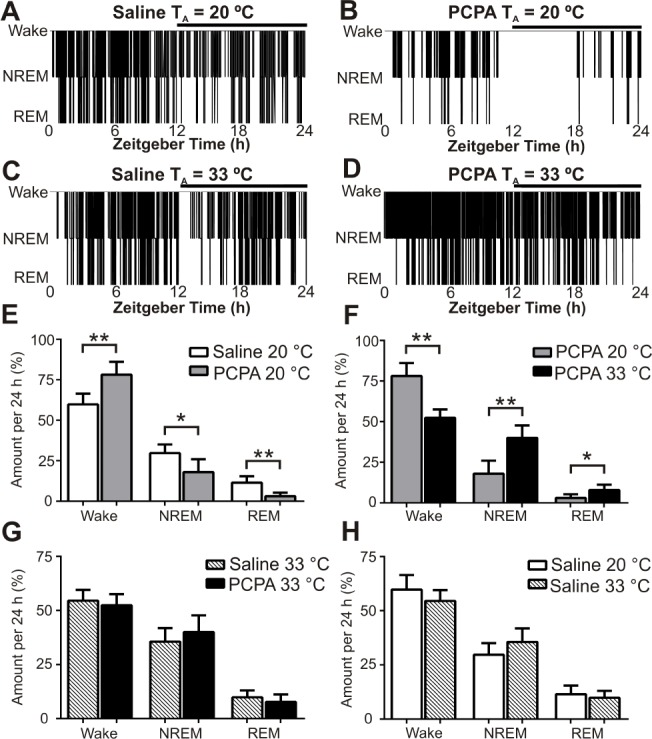
Insomnia occurred in para-chlorophenylalanine (PCPA)-treated mice at an ambient temperature (TA) of 20°C but not at 33°C. All sleep data were obtained after 5 days of treatment with PCPA or saline. Top Panels: Twenty-four hour sleep-wake histograms from a saline-treated and a PCPA-treated mouse at TA 20°C (A,B) and 33°C (C,D). The horizontal black bar indicates lights off. Bottom Panels: Percentage of 24-h recording spent in each vigilance state for saline-treated and PCPA-treated mice at TA 20°C (n = 6 saline, n = 5 PCPA) and 33°C (n = 5 saline, n = 5 PCPA). Percentage of 24-h period in each state for: (E) Saline-treated versus PCPA-treated mice at TA 20°C; (wake: 59.8 ± 2.7% versus 78.2 ± 3.6%, P = 0.004; nonrapid eye movement [NREM]: 29.8 ± 2.2% versus 18.0 ± 3.6%, P = 0.03; rapid eye movement [REM]: 11.5 ± 1.6% versus 3.1 ± 1.0%, P = 0.002); (F) PCPA-treated mice at TA 20°C versus 33°C; (G) Saline-treated versus PCPA-treated mice at TA 33°C; (wake: 54.5 ± 2.2% versus 52.4 ± 2.3%, P = 0.53; NREM: 35.6 ± 2.8% versus 40.0 ± 3.4%, P = 0.35; REM: 9.8 ± 1.4% versus 7.9 ± 1.5%, P = 0.35); (H) Saline-treated mice at TA 20°C versus 33°C. *P < 0.05, **P < 0.005. Data are shown as mean ± standard error of the mean. Unpaired t-test with Welch correction.
5-HT Depletion by PCPA Impaired Thermoregulation and Suppressed Motor Activity
Because sleep was dependent on ambient temperature, we analyzed TC of PCPA-treated mice at each TA to determine if they retained normal thermoregulation ability. PCPA injections at 20°C caused significant hypothermia compared to saline controls (Figures 3A and 3B). The most profound hypothermia at 20°C occurred during the first 5 h after the PCPA injection (33.9 ± 0.3°C versus 36.5 ± 0.2°C, t = 7.1, P = 0.0006, n = 5; Figure 4A). This drop in TC did not occur at a TA of 33°C (Figure 3C and 3D). Average TC of PCPA-treated mice during the first 12 h (lights on) and last 12 h (lights off) of recording at 20°C was lower than control mice (both: P = 0.02, n = 5). Averaged over the total 24 h of recording at 20°C, PCPA led to lower TC than saline (35.1 ± 0.3°C versus 36.5 ± 0.2°C, t = 4.1, P = 0.004, n = 5), whereas at 33°C the TC of PCPA- treated mice was the same as controls (Figure 4B). At 33°C, TC of PCPA-treated mice was significantly greater than at 20°C (Figures 4A and 4B).
Figure 3.
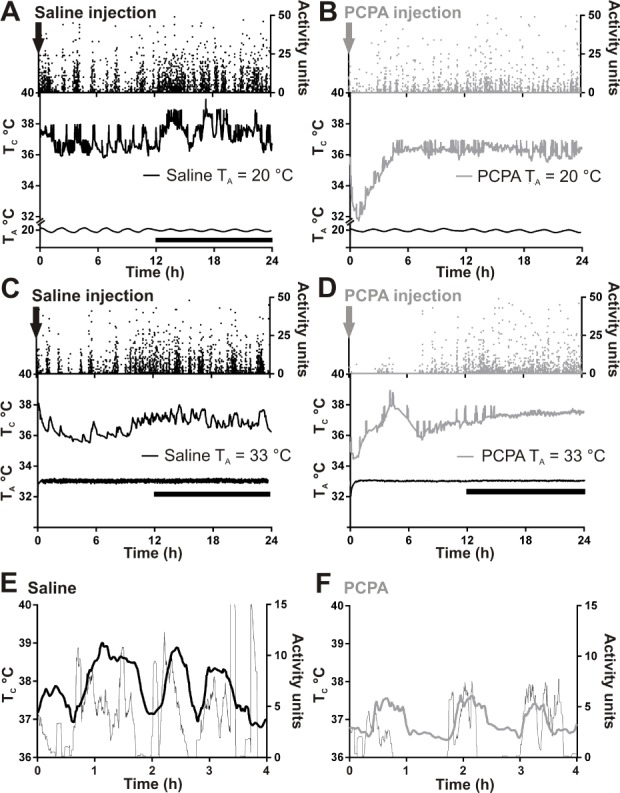
PCPA-treated mice have significantly decreased core body temperature (TC) immediately after injection when ambient temperature (TA) is 20°C but not 33°C. Individual mouse activity (top), TC (middle), and TA (bottom) over 24 h at TA 20°C—(A) Saline-treated mouse and (B) PCPA-treated mouse—versus 33°C—(C) Saline-treated mouse and (D) PCPA-treated mouse. The TC of PCPA-treated mice were at a steady state prior to PCPA injections. Downward arrow at time 0 h marks time of injection. Saline n = 5 for TA 20°C, n = 6 for TA 33°C, PCPA n = 5 for TA 20°C, n = 5 for TA 33°C. Overlay of motor activity (thin line) and TC (thick line) of a saline (E) and PCPA-treated (F) mouse during a 4-h period at TA 20°C after treatment with PCPA. Each trace is smoothed using adjacent averaging. Note that in both cases a burst of motor activity is accompanied by an increase in TC.
Figure 4.
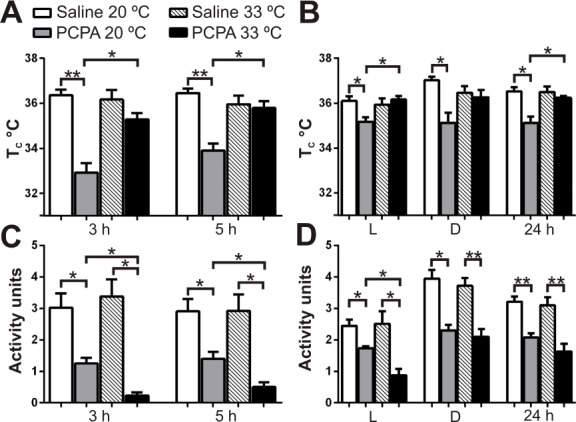
PCPA-treated mice had a significant thermoregulation deficit that led to hypothermia at an ambient temperature (TA) of 20°C. (A) Core body temperature (TC) of PCPA-treated versus saline-treated mice during the first 3 and 5 h after injection at TA 20°C and 33°C. (B) TC of PCPA-treated versus saline-treated mice during the first 12 h light (L), 12 h dark (D), and total 24 h at 20°C and 33°C. At 20°C, all PCPA-treated mice had significantly lower TC than saline- treated mice at 33°C (P < 0.05, significance bars not shown). (C) Motor activity of PCPA-treated versus saline-treated mice during first 3 and 5 h, and (D) first 12 h light, 12 h dark, and total 24 h after injection at 20°C and 33°C. Saline n = 5 for TA 20°C, n = 6 for TA 33°C, PCPA n = 5 for TA 20°C, n = 5 for TA 33°C. *P < 0.05, **P < 0.001. Data are shown as mean ± standard error of the mean. Unpaired t-test with Welch correction.
At 20°C, it was expected that the hypothermic PCPA-treated mice would increase their locomotor activity to generate heat, because that was what occurred with Lmx1bf/f/p mice.42 However, locomotor activity of PCPA-treated mice was significantly less than that of saline-treated mice throughout the entire recording period (Figures 4C and 4D). The same was true at 33°C. Locomotor activity of PCPA mice at 20°C was significantly greater than at 33°C during the first 12 h of recording, but not during the final 12 h of recording or over the total 24 h. The temporal relationship between motor activity and an increase in TC in mice at 20°C is evident in Figures 3E and 3F, as both mice showed elevation in TC soon after an increase in activity units occurred. The average number of activity units necessary to increase body temperature by 1°C was the same in PCPA-treated and control mice at each temperature (average activity units/min for a 1°C increase in TC for PCPA versus saline at a TA of 20°C: 0.42 ± 0.20 versus 0.43 ± 0.16, P = 0.98; at 33°C: 0.32 ± 0.28 vs. 0.33 ± 0.14, P = 0.99). Overall, these data indicate that PCPA-treated mice were able to produce heat by motor activity with the same effectiveness as control mice.
Video recordings showed PCPA-treated mice at 20°C were capable of normal locomotion by walking across their cages. They also shivered throughout the 24-h recording period, which may explain why their TC decreased to 32.9 ± 0.4°C over the first 3 h of recording, but did not become lower despite decreased locomotor activity. The cold adaptive behavior of shivering was not seen in saline-treated mice.
PCPA Induced a Potentially Lethal Thermoregulation Deficit
To further characterize thermoregulation of PCPA-treated mice, we housed them in a cold room at 4°C for 4 h. When presented with this cold challenge, PCPA-treated mice became severely hypothermic after only 1 h, in contrast to saline-treated mice (30.7 ± 1.6°C versus 36.0 ± 0.6°C, t = 3.1, P = 0.03, PCPA n = 4, saline n = 5; Figure 5). In all PCPA-treated mice, TC dropped below 30.0°C after 2 h of exposure to 4°C, requiring three of the four mice to be removed from the cold before the experiment ended. In none of the saline-treated mice did TC drop below 34.3°C.
Figure 5.
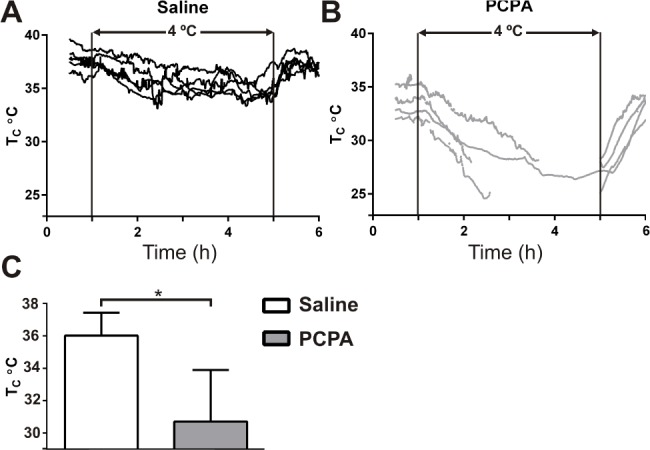
Core body temperature (TC) was not maintained in PCPA-treated mice during a 4°C cold challenge. TC of individual mice before, during, and after exposure to a 4°C cold challenge. Ambient temperature (TA) of 4°C is between the two vertical lines. (A) Saline-treated mice (n = 5). (B) PCPA-treated mice (n = 4). Discontinuous lines represent removal of the animal from 4°C due to hypothermia, the trace returns at the end of the 4-h period to align with the recovery period of other mice. (C) Average TC after 1 h in 4°C. *P < 0.05. Data are shown as mean ± standard error of the mean. Unpaired t-test with Welch correction.
DISCUSSION
When mice were treated with PCPA, brain 5-HT content decreased to 10–12% of normal. At a TA of 20°C, these mice depleted of 5-HT spent significantly more time awake. This excessive wakefulness was similar to that previously seen in PCPA-treated cats and rats9–13,51 and in cats with lesions of the 5-HT system,52–55 results that were pivotal for the early conclusion that 5-HT causes sleep. However, we found that 5-HT depleted mice were hypothermic at a room temperature of 20°C, and only became normothermic when TA was increased to 33°C, which is in the mouse thermoneutral range.45 At a TA of 33°C, 5-HT depletion did not have any effect on wakefulness, total sleep time, sleep architecture, or percentage of time in each vigilance state. We conclude that insomnia following PCPA treatment is due to a confounding effect of hypothermia.
There is now convincing evidence from many different approaches supporting the conclusion that 5-HT neurons cause arousal.1–7,21,42 However, early studies using PCPA had a major and lasting effect on the sleep field, leading to the theory that 5-HT causes sleep.12,13 This theory was later contradicted by in vivo recordings showing increased midbrain 5-HT neuron activity in wakefulness,16,17,56 and yet there remained a dominating influence from the results of the early PCPA studies. Hybrid theories were proposed that preserved the validity of the PCPA data, such as suggesting that 5-HT neurons act to measure the duration and intensity of wakefulness and later trigger downstream effects to facilitate sleep when it is needed,8 or that 5-HT is released in response to accumulated sleep debt, producing a more relaxed waking state that leads a transition into sleep.15 Currently, the older PCPA data have still not been completely dismissed, with some proposing that 5-HT might be able to induce sleep under some situations.2
The lasting influence of the early PCPA studies is likely to be due in part to the fact that there has not been a satisfactory explanation for those data. One explanation has been that insomnia was due to general hyperresponsiveness to stimuli,2 but direct evidence from auditory click-evoked potentials does not show an influence of PCPA on the sensory system that would disrupt sleep.57 Our results also do not support the hypothesis that there is general sensorimotor hyperresponsiveness, as PCPA-treated mice had less locomotor activity than saline-treated mice in a cool environment.
A better explanation is based off 5-HT acting as a critical neurotransmitter for the central mechanisms involved in generating heat in a cool environment. A subset of 5-HT neurons increase their firing rate in vivo when animals are exposed to cold,34 and increased activity of 5-HT neurons is associated with increased heat generation.35,58,59 Lmx1bf/f/p mice, deficient of 5-HT neurons since embryonic life, have profound failure of thermoregulation due to impaired shivering and nonshivering thermogenesis, whereas thermosensory perception and heat conservation are preserved.40,41 Hypothermia is also induced by acute silencing of 5-HT neurons by activation of DREADD receptors44 and acute deletion of 5-HT neurons with diphtheria toxin.43 The importance of 5-HT in thermoregulation was not known at the time of the early PCPA studies. It is likely that the animals depleted of 5-HT in those studies also became cold, and were too uncomfortable to sleep. Inability to sleep is an obvious and intuitive effect of being excessively cold, and is familiar to anyone who has tried to sleep in a cold environment without warm blankets or bedclothes. In fact, it has been shown that when TA is too cool, humans also have decreased NREM and REM sleep.60
A cool TA also induces insomnia in Lmx1bf/f/p mice due to hypothermia.42 However, behavior under cold stress differs between Lmx1bf/f/p mice and PCPA-treated mice. Loco-motor activity is decreased in PCPA-treated mice compared to Lmx1bf/f/p mice, as well as tryptophan hydroxylase 2 knockout mice (Tph2−/−).61,62 Depression of locomotor activity has been seen previously with even a single dose of 300 mg/kg PCPA57,63 and can last for 24 h.64 It is not known why this occurs, but decreased locomotor activity appears to be a primary contributor to reduced heat production and would render mice more susceptible to hypothermia at 20°C than at 33°C. 5-HT neurons are activated with motor challenges,65 so loss of motor facilitation after acute 5-HT depletion by PCPA might contribute to the decrease in motor activity, but it is not clear why this would not also occur with Tph2−/− or Lmx1bf/f/p mice. There is no reason to believe that PCPA-treated mice had a metabolic cause of their decreased locomotion, because any effects of 5-HT depletion such as low hepatic glycogen content,66 muscle atrophy,67 or changes in muscle glucose transport68 would not be expected to occur so rapidly. Finally, it is possible that intraperitoneal injection of PCPA may have induced some kind of discomfort that suppressed locomotion at both ambient temperatures, leading to less heat generation from locomotion. Interestingly, PCPA-treated mice were still capable of normal locomotion, as they were frequently seen on video walking or running normally across their cages.
PCPA-treated, Tph2−/−, and Lmx1bf/f/p mice all had significantly greater locomotor activity at a cool temperature than a warm temperature. In each case, locomotor activity may be a behavioral response for generation of heat. Although PCPA treatment caused less locomotor activity overall, it did not change the effectiveness of motor activity to elevate TC (Figures 3E and 3F). The average number of motor activity units per minute required for an increase in TC of 1°C was the same in PCPA as control mice.
The mechanisms of hypothermia in PCPA-treated mice are not clear. Thermoregulation in rodents can occur via shivering,35,58,59 peripheral vasoconstriction,69 5-HT mediated brown adipose tissue (BAT) activation,70,71 and behavioral responses, such as increased locomotor activity,41 which are all centrally driven. Of note, our protocol does not differentiate between depletion of 5-HT produced by neurons in the raphe pallidus and parapyramidal region, which are associated with activation of sympathetic thermoregulation pathways,71,72 versus that produced by other 5-HT neurons in the midbrain that are implicated in behavioral arousal and locomotor activity, versus 5-HT produced by enterochromaffin cells in the digestive tract that is then released into the bloodstream and acts peripherally. However, there is not good evidence to support a role of peripheral 5-HT in thermoregulation.61 In the current experiments, PCPA-treated mice at 20°C typically formed a ball and raised their hair, and it may have been that this pilo-erection was used to conserve heat. PCPA-treated mice at 20°C also had bouts of shivering, indicating that involuntary motor activity was possible. The various responses to cold also indicate that PCPA-treated mice were capable of thermosensation, as in Lmx1bf/f/p mice.41 It is not known if BAT thermogenesis was normal, but it is impaired in Lmx1bf/f/p mice and mice with 5-HT neurons lesioned by diphtheria toxin.41,73
The most significant insomnia in PCPA mice at 20°C was seen as REM sleep loss. This is the opposite of what would be predicted by evidence that 5-HT inhibits REM sleep.2 Instead, it fits with the well-known loss of thermoregulatory control in REM sleep, possibly due in part to decreased activity of 5-HT neurons during REM.16,74 Loss of vasoconstriction to minimize heat loss in cool environments is severe during REM but not NREM sleep or wake.75–78 We did not measure tail vasoconstriction in the current experiments. However, prior studies on Lmx1bf/f/p mice found normal tail vasoconstriction in response to cold.41
Only one prior study exists on the relationship between PCPA, thermoregulation, and sleep.79 PCPA treatment decreased NREM and REM sleep, but surprisingly there was not a temperature-dependent relationship between PCPA and amount of total time spent in wake and sleep. However, in that study only a single 300 mg/kg PCPA injection was used, which would be unlikely to cause a major decrease in 5-HT levels (Figure 1), and would not have been enough to cause sustained impairment of thermogenesis.
In a different study, Tph2−/− mice were not found to have a defect in thermoregulation or a change in wakefulness over 24 hours, although there were transient increases in wakefulness during transitions from light to dark and dark to light periods.62 As observed in other models of chronic 5-HT disruption,43 it is possible that other neurotransmitter systems compensate for the loss of 5-HT. For example, 5-HT projections from the raphe pallidus terminate in the intermediolateral cell column of the thoracic cord, and these projections contain other co-localized neurotransmitters (e.g., thyrotropin-releasing hormone, and substance P), possibly stimulating heat generation.80–82
A decrease in TC has previously been reported in PCPA-treated rats57,63,79 and mice,83 by 1.2°C at a TA of 23°C63 and by 2.3°C at 20°C.79 In those studies, low TC was most noticeable immediately after PCPA injection, similar to our findings, and then body temperature slowly rises. In our study it is not known why such a rapid and large drop in body temperature occurred following PCPA injection, because the effect on 5-HT levels should not be that rapid. The low levels of 5-HT from the 4 prior days of treatment may have rendered mice more vulnerable to acute stress, causing rapid vasodilation. It is known that 5-HT plays a role in control of feeding57,84 and circadian rhythms,85 which are both affected by PCPA.79,86 Therefore, a change in nutrition status or body weight could leave mice more susceptible to decompensate during acute stress, leading to periods of increased wakefulness. However, if those were the primary problem, insomnia would not have been resolved by an increase in TA. Here, we did not measure food intake or changes in body weight, but we did not observe any changes in body appearance or fur at the end of the treatment. Although these factors may have contributed to the acute drop in body temperature, a defect in central thermoregulation is likely to be the primary cause of hypothermia given the large body of data supporting a role of 5-HT neurons in that function.
A final thermoregulation issue is whether thermoregula-tory deficits caused the sleep disturbance, or instead if chronic sleep disturbance caused thermoregulatory changes. Prior studies that deprived rats of sleep resulted in severe multiorgan pathology and death,87 hypothermia,88 and excessive body heat loss.89 However, in those studies sleep deprivation was much more severe and prolonged than that induced here. Thus, although the current experiments do not define the mechanisms of hypothermia caused by 5-HT depletion from PCPA, they clearly demonstrate that PCPA affects sleep indirectly due to hypothermia.
Multiple studies have shown that 5-HT neurons activate the cerebral cortex via the ARAS.2,3 For example, optogenetic stimulation of 5-HT neurons causes a twofold increase in the amount of wakefulness in mice.6 It may seem confusing, then, that a decrease in 5-HT does not cause an increase in sleep. This can be explained simply by concluding that when the brain is deprived of 5-HT by PCPA, ascending systems that release histamine, noradrenaline, acetylcholine, and other neurotransmitters are able to maintain wakefulness. This is analogous to the observation that optogenetic stimulation of 5-HT neurons in the medulla stimulates breathing,90 and yet deletion of 5-HT neurons does not cause a decrease in baseline breathing.41 This is also analogous to the observation that antihistamines can sometimes cause drowsiness, especially in someone who is already tired, but they usually do not cause sleep and never cause coma, even though histamine neurons are well-known to be a component of the ARAS. Similarly, norepinephrine neurons are also clearly part of the ARAS, and yet complete loss of norepinephrine and epinephrine by genetic deletion of dopa-mine beta-hydroxylase does not cause a significant decrease in wakefulness.91,92 The importance of redundancy among neuromodulatory networks driving wakefulness is powerfully illustrated by the finding that the total amount of daily wakefulness did not change even after simultaneous chemical lesions of the histaminergic tuberomammillary nucleus, noradrenergic locus coeruleus, and cholinergic basal forebrain.93 In that case, wakefulness might have been maintained either by the intact serotonergic and orexinergic networks, or by the small number of neurons remaining in the lesioned populations. Together the aforementioned findings clearly show that arousal is not dependent solely on any one specific component of the ARAS.
It is possible that each component of the ARAS may cause wakefulness for a specific purpose. For example, a subset of 5-HT neurons is important for the arousal response to elevated blood CO2 levels.42 It is possible that other monoamine systems may cause arousal in response to yet unknown interoceptive stimuli, such as stress,94 hypothermia,95 low blood pressure or heart rate,96 or low glucose.97
We conclude that 5-HT does not directly promote sleep. The theory that this is the case was based on experiments that did not control for hypothermia induced by 5-HT depletion. When designing behavioral experiments involving 5-HT depletion with PCPA, it is important to account for serotonin-dependent thermogenesis. Controlling body temperature is a powerful motivator in many animals and humans. In the presence of disease or a biological defect, some ensuing behaviors may be impaired due to a 5-HT defect. They can be easily missed and misinterpreted in relation to a primary outcome and may be more common in both experiments and patients than previously thought.
DISCLOSURE STATEMENT
This was not an industry supported study. Funding was provided by: Dr. Murray – Howard Hughes Medical Institute Fellowship; Dr. Buchanan – NINDS K08NS069667, Citizens United for Research in Epilepsy: Christopher Donalty and Kyle Coggins Memorial SUDEP Research Award, The Beth and Nate Tross Epilepsy Research Fund; Dr. Richerson – NIH P20NS076916, R01HD052772, P01HD36379. The authors have indicated no financial conflicts of interest. Authorship: Dr. Murray – Designed research, performed research, analyzed data, and wrote the paper; Dr. Buchanan – Designed research, analyzed data, and edited the paper; Dr. Richerson – Designed research, analyzed data, and edited the paper.
REFERENCES
- 1.McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- 2.Monti JM. Serotonin control of sleep-wake behavior. Sleep Med Rev. 2011;15:269–81. doi: 10.1016/j.smrv.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–42. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallopin T, Fort P, Eggerman E, et al. Identification of sleep-promoting neurons in vivo. Nature. 2000;404:992–5. doi: 10.1038/35010109. [DOI] [PubMed] [Google Scholar]
- 5.Eriksson KS, Stevens DR, Haas HL. Serotonin excites tuberomammillary neurons by activation of Na(+)/Ca(2+)-exchange. Neuropharmacology. 2001;40:345–51. doi: 10.1016/s0028-3908(00)00175-1. [DOI] [PubMed] [Google Scholar]
- 6.Ito H, Yanase M, Yamashita A, et al. Analysis of sleep disorders under pain using an optogenetic tool: possible involvement of the activation of dorsal raphe nucleus-serotonergic neurons. Mol Brain. 2013;6:59. doi: 10.1186/1756-6606-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–85. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 8.Jouvet M. Sleep and serotonin: an unfinished story. Neuropsychopharmacology. 1999;21:24S–7S. doi: 10.1016/S0893-133X(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 9.Koella WP, Feldstein A, Czicman JS. The effect of parachlorophenylalanine on the sleep of cats. Electroencephalogr Clin Neurophysiol. 1968;25:481–90. doi: 10.1016/0013-4694(68)90158-2. [DOI] [PubMed] [Google Scholar]
- 10.Dement WC, Mitler MM, Henriksen SJ. Sleep changes during chronic administration of parachlorophenylalanine. Revue canadienne de biologie/editee par l'Universite de Montreal. 1972;31:Suppl:239–46. [PubMed] [Google Scholar]
- 11.Denoyer M, Sallanon M, Kitahama K, Aubert C, Jouvet M. Reversibility of para-chlorophenylalanine-induced insomnia by intrahypothalamic microinjection of L-5-hydroxytryptophan. Neuroscience. 1989;28:83–94. doi: 10.1016/0306-4522(89)90234-0. [DOI] [PubMed] [Google Scholar]
- 12.Mouret J, Bobillier P, Jouvet M. Insomnia following parachlorophenylalanine in the rat. Eur J Pharmacol. 1968;5:17–22. doi: 10.1016/0014-2999(68)90151-9. [DOI] [PubMed] [Google Scholar]
- 13.Jouvet M. Biogenic amines and the states of sleep. Science. 1969;163:32–41. doi: 10.1126/science.163.3862.32. [DOI] [PubMed] [Google Scholar]
- 14.Kryger MH, Roth T, Dement WC. Principles and practice of sleep medicine. 5th ed. Philadelphia, PA: Saunders/Elsevier; 2011. [Google Scholar]
- 15.Jones BE. Neurobiology of waking and sleeping. Handb Clin Neurol. 2011;98:131–49. doi: 10.1016/B978-0-444-52006-7.00009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGinty DJ, Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 1976;101:569–75. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- 17.Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 1979;163:135–50. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs BL, Fornal CA. Activity of brain serotonergic neurons in the behaving animal. Pharmacol Rev. 1991;43:563–78. [PubMed] [Google Scholar]
- 19.Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–68. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- 20.Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:641–67. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- 21.Pape HC, McCormick DA. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature. 1989;340:715–8. doi: 10.1038/340715a0. [DOI] [PubMed] [Google Scholar]
- 22.Monti JM, Jantos H. Differential effects of the 5-HT1A receptor agonist flesinoxan given locally or systemically on REM sleep in the rat. Eur J Pharmacol. 2003;478:121–30. doi: 10.1016/j.ejphar.2003.08.039. [DOI] [PubMed] [Google Scholar]
- 23.Monti JM, Monti D, Jantos H, Ponzoni A. Effects of selective activation of the 5-HT1B receptor with CP-94,253 on sleep and wakefulness in the rat. Neuropharmacology. 1995;34:1647–51. doi: 10.1016/0028-3908(95)00112-3. [DOI] [PubMed] [Google Scholar]
- 24.Dugovic C, Wauquier A, Leysen JE, Marrannes R, Janssen PA. Functional role of 5-HT2 receptors in the regulation of sleep and wakefulness in the rat. Psychopharmacology (Berl) 1989;97:436–42. doi: 10.1007/BF00439544. [DOI] [PubMed] [Google Scholar]
- 25.Ponzoni A, Monti JM, Jantos H. The effects of selective activation of the 5-HT3 receptor with m-chlorophenylbiguanide on sleep and wakefulness in the rat. Eur J Pharmacol. 1993;249:259–64. doi: 10.1016/0014-2999(93)90520-r. [DOI] [PubMed] [Google Scholar]
- 26.Dugovic C, Wauqier A. 5-HT2 receptors could be primarily involved in the regulation of slow wave sleep in the rat. Eur J Pharmacol. 1987;137:145–6. doi: 10.1016/0014-2999(87)90196-8. [DOI] [PubMed] [Google Scholar]
- 27.Kirov R, Moyanova S. Age-dependent effect of ketanserin on the sleep-waking phases in rats. Int J Neurosci. 1998;93:257–64. doi: 10.3109/00207459808986431. [DOI] [PubMed] [Google Scholar]
- 28.Monti JM, Jantos H. Effects of the serotonin 5-HT2A/2C receptor agonist DOI and of the selective 5-HT2A or 5-HT2C receptor antagonists EMD 281014 and SB-243213, respectively, on sleep and waking in the rat. Eur J Pharm. 2006;553:163–70. doi: 10.1016/j.ejphar.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 29.Monti JM, Leopoldo M, Jantos H. The serotonin 5-HT7 receptor agonist LP-44 microinjected into the dorsal raphe nucleus suppresses REM sleep in the rat. Behav Brain Res. 2008;191:184–9. doi: 10.1016/j.bbr.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 30.Hjorth S, Magnusson T. The 5-HT 1A receptor agonist, 8-OH-DPAT, preferentially activates cell body 5-HT autoreceptors in rat brain in vivo. Naunyn-Schmiedeberg's Arch Pharmacol. 1988;338:463–71. doi: 10.1007/BF00179315. [DOI] [PubMed] [Google Scholar]
- 31.Thakkar MM, Strecker RE, McCarley RW. Behavioral state control through differential serotonergic inhibition in the mesopontine cholinergic nuclei: a simultaneous unit recording and microdialysis study. J Neurosci. 1998;18:5490–7. doi: 10.1523/JNEUROSCI.18-14-05490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–31. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- 33.Leonard CS, Llinas R. Serotonergic and cholinergic inhibition of mesopontine cholinergic neurons controlling REM sleep: an in vitro electrophysiological study. Neuroscience. 1994;59:309–30. doi: 10.1016/0306-4522(94)90599-1. [DOI] [PubMed] [Google Scholar]
- 34.Martin-Cora FJ, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic medullary and pontine raphe neurons to environmental cooling in freely moving cats. Neuroscience. 2000;98:301–9. doi: 10.1016/s0306-4522(00)00133-0. [DOI] [PubMed] [Google Scholar]
- 35.Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci. 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding YQ, Marklund U, Yuan W, et al. Lmx1b is essential for the development of serotonergic neurons. Nat Neurosci. 2003;6:933–8. doi: 10.1038/nn1104. [DOI] [PubMed] [Google Scholar]
- 37.Fyodorov D, Nelson T, Deneris E. Pet-1, a novel ETS domain factor that can activate neuronal nAchR gene transcription. J Neurobiol. 1998;34:151–63. [PubMed] [Google Scholar]
- 38.Hendricks TJ, Fyodorov DV, Wegman LJ, et al. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–47. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- 39.Lakso M, Sauer B, Mosinger B, Jr., et al. Targeted oncogene activation by site-specific recombination in transgenic mice. Proc Natl Acad Sci U S A. 1992;89:6232–6. doi: 10.1073/pnas.89.14.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao ZQ, Scott M, Chiechio S, et al. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci. 2006;26:12781–8. doi: 10.1523/JNEUROSCI.4143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hodges MR, Tattersall GJ, Harris MB, et al. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci U S A. 2010;107:16354–9. doi: 10.1073/pnas.1004587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cerpa V, Gonzalez A, Richerson GB. Diphtheria toxin treatment of Pet-1-Cre floxed diphtheria toxin receptor mice disrupts thermoregulation without affecting respiratory chemoreception. Neuroscience. 2014;279:65–76. doi: 10.1016/j.neuroscience.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ray RS, Corcoran AE, Brust RD, et al. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333:637–42. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon CJ. Relationship between autonomic and behavioral thermoregulation in the mouse. Physiol Behav. 1985;34:687–90. doi: 10.1016/0031-9384(85)90365-8. [DOI] [PubMed] [Google Scholar]
- 46.Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J. Neurosci. 2009;29:10341–9. doi: 10.1523/JNEUROSCI.1963-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen H, Lun Y, Ovchinnikov D, et al. Limb and kidney defects in Lmx1b mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet. 1998;19:51–5. doi: 10.1038/ng0598-51. [DOI] [PubMed] [Google Scholar]
- 48.Kania A, Johnson RL, Jessell TM. Coordinate roles for LIM homeobox genes in directing the dorsoventral trajectory of motor axons in the vertebrate limb. Cell. 2000;102:161–73. doi: 10.1016/s0092-8674(00)00022-2. [DOI] [PubMed] [Google Scholar]
- 49.Smidt MP, Asbreuk CH, Cox JJ, Chen H, Johnson RL, Burbach JP. A second independent pathway for development of mesencephalic dopaminergic neurons requires Lmx1b. Nat Neurosci. 2000;3:337–41. doi: 10.1038/73902. [DOI] [PubMed] [Google Scholar]
- 50.Buchanan GF, Murray NM, Hajek MA, Richerson GB. Serotonin neurones have anti-convulsant effects and reduce seizure-induced mortality. J Physiol. 2014;592:4395–410. doi: 10.1113/jphysiol.2014.277574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torda C. Effect of brain serotonin depletion on sleep in rats. Brain Res. 1967;6:375–7. doi: 10.1016/0006-8993(67)90204-1. [DOI] [PubMed] [Google Scholar]
- 52.Koella WP. Discussion of insomnia and decrease of cerebral 5-hydroxytryptamine after destruction of the raphe system in the cat. Adv Pharmacol. 1968;6:280–2. doi: 10.1016/s1054-3589(08)60327-0. [DOI] [PubMed] [Google Scholar]
- 53.Pujol JF, Buguet A, Froment JL, Jones B, Jouvet M. The central metabolism of serotonin in the cat during insomnia. A neurophysiological and biochemical study after administration of P-chlorophenylalanine or destruction of the Raphe system. Brain Res. 1971;29:195–212. doi: 10.1016/0006-8993(71)90028-x. [DOI] [PubMed] [Google Scholar]
- 54.Jouvet M. The role of monoamines and acetylcholine-containing neurons in the regulation of the sleep-waking cycle. Ergebnisse der Physiologie, biologischen Chemie und experimentellen Pharmakologie. 1972;64:166–307. doi: 10.1007/3-540-05462-6_2. [DOI] [PubMed] [Google Scholar]
- 55.Jouvet M. Insomnia and decrease of cerebral 5-hydroxytryptamine after destruction of the raphe system in the cat. Adv Pharmacol. 1968;6:265–79. doi: 10.1016/s1054-3589(08)60326-9. [DOI] [PubMed] [Google Scholar]
- 56.Fornal C, Auerbach S, Jacobs BL. Activity of serotonin-containing neurons in nucleus raphe magnus in freely moving cats. Exp Neurol. 1985;88:590–608. doi: 10.1016/0014-4886(85)90074-3. [DOI] [PubMed] [Google Scholar]
- 57.Borbely AA, Huston JP, Waser PG. Physiological and behavioral effects of parachlorophenylalanine in the rat. Psychopharmacologia. 1973;31:131–42. doi: 10.1007/BF00419813. [DOI] [PubMed] [Google Scholar]
- 58.Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nature Neurosci. 2008;11:62–71. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakamura K, Morrison SF. A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci U S A. 2010;107:8848–53. doi: 10.1073/pnas.0913358107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haskell EH, Palca JW, Walker JM, Berger RJ, Heller HC. The effects of high and low ambient temperatures on human sleep stages. Electroencephalogr Cin Neurophysiol. 1981;51:494–501. doi: 10.1016/0013-4694(81)90226-1. [DOI] [PubMed] [Google Scholar]
- 61.Alenina N, Kikic D, Todiras M, et al. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci U S A. 2009;106:10332–7. doi: 10.1073/pnas.0810793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Solarewicz JZ, Angoa-Perez M, Kuhn DM, Mateika JH. The sleep-wake cycle and motor activity, but not temperature, are disrupted over the light-dark cycle in mice genetically depleted of serotonin. Am J Physiol Regul Integr Comp Physiol. 2015;308:R10–7. doi: 10.1152/ajpregu.00400.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Satinoff E, Kent S, Li H, Megirian D, Tomkowiak JM. Circadian rhythms of body temperature and drinking and responses to thermal challenge in rats after PCPA. Pharmacol Biochem Behav. 1991;38:253–7. doi: 10.1016/0091-3057(91)90274-6. [DOI] [PubMed] [Google Scholar]
- 64.Borbely AA, Neuhaus HU, Tobler I. Effect of p-chlorophenylalanine and tryptophan on sleep, EEG and motor activity in the rat. Behav Brain Res. 1981;2:1–22. doi: 10.1016/0166-4328(81)90035-8. [DOI] [PubMed] [Google Scholar]
- 65.Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic dorsal raphe neurons to specific motor challenges in freely moving cats. Neuroscience. 1997;79:161–9. doi: 10.1016/s0306-4522(96)00673-2. [DOI] [PubMed] [Google Scholar]
- 66.Kobayashi B, Ui M, Warashina Y. The role of serotonin in carbohydrate metabolism. II. The effect of serotonin on glycogen content of liver, heart and diaphragm in rats. Endocrinol Jpn. 1960;7:239–48. doi: 10.1507/endocrj1954.7.239. [DOI] [PubMed] [Google Scholar]
- 67.Carre-Pierrat M, Mariol MC, Chambonnier L, et al. Blocking of striated muscle degeneration by serotonin in C. elegans. J Muscle Res Cell Motil. 2006;27:253–8. doi: 10.1007/s10974-006-9070-9. [DOI] [PubMed] [Google Scholar]
- 68.Hajduch E, Rencurel F, Balendran A, Batty IH, Downes CP, Hundal HS. Serotonin (5-Hydroxytryptamine), a novel regulator of glucose transport in rat skeletal muscle. J Biol Chem. 1999;274:13563–8. doi: 10.1074/jbc.274.19.13563. [DOI] [PubMed] [Google Scholar]
- 69.Ootsuka Y, Blessing WW. Activation of 5-HT1A receptors in rostral medullary raphe inhibits cutaneous vasoconstriction elicited by cold exposure in rabbits. Brain Res. 2006;1073-1074:252–61. doi: 10.1016/j.brainres.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 70.Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol. 2003;460:303–26. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- 71.Morrison SF, Madden CJ, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 2014;19:741–56. doi: 10.1016/j.cmet.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiology. 2008;93:773–97. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McGlashon JM, Gorecki MC, Kozlowski AE, et al. Central serotonergic neurons activate and recruit thermogenic brown and beige fat and regulate glucose and lipid homeostasis. Cell Metab. 2015;21:692–705. doi: 10.1016/j.cmet.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jacobs BL, Abercrombie ED, Fornal CA, Levine ES, Morilak DA, Stafford IL. Single-unit and physiological analyses of brain norepinephrine function in behaving animals. Prog Brain Res. 1991;88:159–65. doi: 10.1016/s0079-6123(08)63805-4. [DOI] [PubMed] [Google Scholar]
- 75.Alfoldi P, Rubicsek G, Cserni G, Obal F., Jr Brain and core temperatures and peripheral vasomotion during sleep and wakefulness at various ambient temperatures in the rat. Pflugers Arch. 1990;417:336–41. doi: 10.1007/BF00371001. [DOI] [PubMed] [Google Scholar]
- 76.Franzini C, Cianci T, Lenzi P, Guidalotti PL. Neural control of vasomotion in rabbit ear is impaired during desynchronized sleep. Am J Physiol. 1982;243:R142–6. doi: 10.1152/ajpregu.1982.243.1.R142. [DOI] [PubMed] [Google Scholar]
- 77.Parmeggiani PL, Zamboni G, Cianci T, Calasso M. Absence of thermoregulatory vasomotor responses during fast wave sleep in cats. Electroencephalogr Clin Neurophysiol. 1977;42:372–80. doi: 10.1016/0013-4694(77)90173-0. [DOI] [PubMed] [Google Scholar]
- 78.Walker JM, Walker LE, Harris DV, Berger RJ. Cessation of thermoregulation during REM sleep in the pocket mouse. Am J Physiol. 1983;244:R114–8. doi: 10.1152/ajpregu.1983.244.1.R114. [DOI] [PubMed] [Google Scholar]
- 79.Li H, Satinoff E. Effects of p-chlorophenylalanine on thermoregulation and sleep in rats. Brain Res. 1992;569:46–56. doi: 10.1016/0006-8993(92)90367-i. [DOI] [PubMed] [Google Scholar]
- 80.Appel NM, Wessendorf MW, Elde RP. Coexistence of serotonin- and substance P-like immunoreactivity in nerve fibers apposing identified sympathoadrenal preganglionic neurons in rat intermediolateral cell column. Neurosci Lett. 1986;65:241–6. doi: 10.1016/0304-3940(86)90268-5. [DOI] [PubMed] [Google Scholar]
- 81.Sasek CA, Wessendorf MW, Helke CJ. Evidence for co-existence of thyrotropin-releasing hormone, substance P and serotonin in ventral medullary neurons that project to the intermediolateral cell column in the rat. Neuroscience. 1990;35:105–19. doi: 10.1016/0306-4522(90)90125-n. [DOI] [PubMed] [Google Scholar]
- 82.Thor KB, Helke CJ. Serotonin and substance P colocalization in medullary projections to the nucleus tractus solitarius: dual-colour immunohistochemistry combined with retrograde tracing. J Chem Neuroanat. 1989;2:139–48. [PubMed] [Google Scholar]
- 83.Janoff AS, Rosenberg B. Chemically evoked hypothermia in the mouse: towards a method for investigating thermodynamic parameters of aging and death in mammals. Mech Ageing Dev. 1978;7:335–49. doi: 10.1016/0047-6374(78)90076-3. [DOI] [PubMed] [Google Scholar]
- 84.Voigt JP, Fink H. Serotonin controlling feeding and satiety. Behav Brain Res. 2015;277:14–31. doi: 10.1016/j.bbr.2014.08.065. [DOI] [PubMed] [Google Scholar]
- 85.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 86.MacKenzie RG, Hoebel BG, Ducret RP, Trulson ME. Hyperphagia following intraventricular p-chlorophenylalanine-, leucine- or tryptophan-methyl esters: lack of correlation with whole brain serotonin levels. Pharmacol Biochem Behav. 1979;10:951–5. doi: 10.1016/0091-3057(79)90075-3. [DOI] [PubMed] [Google Scholar]
- 87.Rechtschaffen A, Gilliland MA, Bergmann BM, Winter JB. Physiological correlates of prolonged sleep deprivation in rats. Science. 1983;221:182–4. doi: 10.1126/science.6857280. [DOI] [PubMed] [Google Scholar]
- 88.Everson CA, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: III. Total sleep deprivation. Sleep. 1989;12:13–21. doi: 10.1093/sleep/12.1.13. [DOI] [PubMed] [Google Scholar]
- 89.Prete FR, Bergmann BM, Holtzman P, Obermeyer W, Rechtschaffen A. Sleep deprivation in the rat: XII. Effect on ambient temperature choice. Sleep. 1991;14:109–15. doi: 10.1093/sleep/14.2.109. [DOI] [PubMed] [Google Scholar]
- 90.Depuy SD, Kanbar R, Coates MB, Stornetta RL, Guyenet PG. Control of breathing by raphe obscurus serotonergic neurons in mice. J Neurosci. 2011;31:1981–90. doi: 10.1523/JNEUROSCI.4639-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hunsley MS, Palmiter RD. Norepinephrine-deficient mice exhibit normal sleep-wake states but have shorter sleep latency after mild stress and low doses of amphetamine. Sleep. 2003;26:521–6. [PubMed] [Google Scholar]
- 92.Hunsley MS, Palmiter RD. Altered sleep latency and arousal regulation in mice lacking norepinephrine. Pharmacol Biochem Behav. 2004;78:765–73. doi: 10.1016/j.pbb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 93.Blanco-Centurion C, Gerashchenko D, Shiromani PJ. Effects of saporin-induced lesions of three arousal populations on daily levels of sleep and wake. J Neurosci. 2007;27:14041–8. doi: 10.1523/JNEUROSCI.3217-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cano G, Mochizuki T, Saper CB. Neural circuitry of stress-induced insomnia in rats. J Neurosci. 2008;28:10167–84. doi: 10.1523/JNEUROSCI.1809-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cerri M, Morrison SF. Activation of lateral hypothalamic neurons stimulates brown adipose tissue thermogenesis. Neuroscience. 2005;135:627–38. doi: 10.1016/j.neuroscience.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 96.Stock G, Rupprecht U, Stumpf H, Schlor KH. Cardiovascular changes during arousal elicited by stimulation of amygdala, hypothalamus and locus coeruleus. J Auton Nerv Syst. 1981;3:503–10. doi: 10.1016/0165-1838(81)90083-7. [DOI] [PubMed] [Google Scholar]
- 97.Moriguchi T, Sakurai T, Nambu T, Yanagisawa M, Goto K. Neurons containing orexin in the lateral hypothalamic area of the adult rat brain are activated by insulin-induced acute hypoglycemia. Neurosci Lett. 1999;264:101–4. doi: 10.1016/s0304-3940(99)00177-9. [DOI] [PubMed] [Google Scholar]


