Abstract
Background
Gastric cancer exhibits various degrees of fluorine F-18 fluorodeoxyglucose (18F-FDG) uptake on positron emission tomography/computed tomography (PET/CT). We evaluated the relationship between 18F-FDG uptake and the presence/absence of metastasis in individual lymph nodes (LN) on a one-to-one basis.
Methods
We analyzed 21 patients with gastric cancer. We injected 18F-FDG intravenously in the morning, and gastrectomy with LN dissection was performed in the afternoon of the same day. Radiation doses were measured at each LN using a well-type counter, and we then compared 18F-FDG uptake, the shortest diameter, and pathological examination results for each LN.
Results
In our study, 906 LNs were analyzed, including 115 metastatic LNs. Metastatic LNs showed significantly higher 18F-FDG uptake (P < 0.0001), and were significantly enlarged (P < 0.0001). The receiver operating characteristics (ROC) curve had a larger area under the curve (0.71) for 18F-FDG uptake than for the shortest LN diameter (0.60). Considering histology, the ROC curve for intestinal type adenocarcinoma had a larger area under the curve than that for diffuse type (0.75 vs 0.61).
Conclusions
F-FDG uptake is potentially a more useful variable than LN diameter for discriminating between LN with and without metastasis, especially in intestinal type gastric cancer cases.
Keywords: 18F-FDG, Gastric cancer, Lymph node metastasis, PET/CT, Navigation surgery
Background
In gastric cancer, lymph node (LN) metastasis is an important prognostic factor [1, 2]. Among patients with R0 resection for gastric cancer, LN status was the most important independent prognostic factor, followed by the pT category, surgical complication, and distant metastasis [1]. Therefore, accurate knowledge of LN status would be helpful for predicting prognosis and planning the extent of lymphadenectomy. Enhanced computed tomography (CT), which is routinely performed to evaluate LN metastasis in gastric cancer, has a sensitivity of 80.0 % and a specificity of 77.8 %, based on the size of LN [3]. However, Monig et al. reported LN size to be an unreliable indicator of LN metastasis in patients with gastric cancer [4]. As compared to CT scans, fluorine F-18 fluorodeoxyglucose positron emission tomography and computed tomography (18F-FDG PET/CT) shows lower sensitivity and higher specificity for evaluating regional LN metastasis [5]. However, low sensitivity may result from low spatial resolution of both PET scanning and PET/CT scan [3, 5–7]. Considering the difficulty to diagnose LN metastasis preoperatively, prophylactic LN dissection is regarded as essential to curative resection for gastric cancer, resulting in the dissection of non-metastatic LNs.
18F-FDG has been used for not only preoperative diagnosis but also intraoperative diagnosis and navigation surgery using intraoperative gamma probe [8–10]. This navigation system during gastric cancer surgery can be planned, if the radiation dose of 18F-FDG shows nodal involvement precisely. To our knowledge, there are no reports comparing pathological findings and 18F-FDG uptake on a one-to-one basis.
The aim of this study was to clarify the diagnostic power of 18F-FDG by investigating the one-to-one relationship between the 18F-FDG uptake of each dissected LN and the corresponding pathological results.
Methods
Patients
Study patients were recruited between July 2012 and September 2013 at the Department of Gastrointestinal Surgery, the University of Tokyo Hospital, Japan, for a prospective pilot study. Criteria for inclusion in this study were (1) adenocarcinoma of the stomach confirmed by pathological examination, (2) diagnosis of advanced gastric cancer based on preoperative CT scan or endoscopic examination results, (3) necessity of gastrectomy for curative or palliative intent, (4) age 85 years or younger, (5) normal renal function, and (6) European clinical oncology group performance status (ECOG-PS) ≦1. Exclusion criteria were (1) diabetes mellitus, (2) any severe ongoing comorbidity, (3) prior malignant diseases, and (4) synchronous malignancies other than gastric cancer. For patients who meet these criteria, we injected 18F-FDG on the day of surgery, took 18F-FDG PET/CT in the morning, and measured radiation dose of each LN after harvesting LNs by surgery in the afternoon. We only included patients with advanced gastric cancer since 18F-FDG PET/CT suffers from low detection rate of LN involvement for early gastric cancer [11].
Ethics statement
The scientific protocol was approved by the local ethics committee (Graduate School of Medicine and Faculty of Medicine, the University of Tokyo, no. 3799). Written informed consent for participating this study and publishing was obtained from all participants. This trial was registered in the UMIN Clinical Trial Registry (UMIN 000013934, http://www.umin.ac.jp/ctr/).
FDG-PET/CT study
In the morning of the day of gastrectomy, 18F-FDG was injected intravenously 3–4 h prior to surgery, and PET/CT scans were obtained. Patients fasted for at least 5 h before undergoing FDG-PET, and a blood sugar level under 150 mg/dL was required. Each patient received 296 MBq of intravenous FDG. Imaging was then performed 50 min later using an Aquiduo PET/CT scanner (Toshiba Medical Systems, Otawara, Japan). This scanner contains 24,336 lutetium oxyorthosilicate (LSO) crystals in 39 detector rings and has an axial field of view of 16.2 cm and 82 transverse slices with a 2.0 mm thickness. The intrinsic full width at half-maximum (FWHM) spatial resolution in the center of the field of view is ~4.3 mm, and the FWHM axial resolution is 4.7 mm. The sinogram was acquired in the three-dimensional mode. The CT scan was performed with a tube current of 50 mA and a tube voltage of 120 kV for attenuation correction, and one 2.5-min emission scan per position was acquired. Images were reconstructed using Fourier rebinning ordered subset expectation maximization iterative reconstruction, with two iterations and eight subsets, and a 4-mm FWHM Gaussian filter was applied. The data were collected in a 128 × 128 × 41 matrix with a voxel size of 2.0 × 2.0 × 4.0 mm.
PET/CT images were visually evaluated by two of the authors, both of whom are experienced nuclear medicine physicians (MT and KK). The maximum activity concentration within the lesions of interest was determined and expressed as the maximal standardized uptake value (SUV max). All SUV measurements were normalized for patient body weight and for the time elapsed from injection until data acquisition. If the PET/CT scan showed distant metastasis, we reviewed the indications for the scheduled gastrectomy. We determined the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of 18F-FDG PET as described in a previous report [7]. We classified regional LNs into three groups: LNs along the lesser curvature, LNs along the greater curvature, and other regional LNs (LNs in suprapancreatic area and hepatoduodenal ligament). LNs were considered positive or negative on the basis of the group as a whole.
Radiation dose measurements for individual LNs
After 18F-FDG PET/CT scan in the morning, gastrectomy with LN dissection was performed in the afternoon. All LNs were harvested from the surgical specimen before formalin fixation. We measured the radiation dose of each LN using a CAPRAC-t well-type counter (Capintec, Inc., Pittsburgh, PA, USA) with the energy window set at 464.7–557.3 keV before formalin fixation and staining. The energy window was determined by the FWHM peak of the 511 keV radiation spectrum, in order to precisely measure the radiation dose of 18F-FDG. Since it took 40–50 s to prepare for the radiation dose measurement in each LN, we set the count time at 30 s to assure that the time between the first LN and the last LN measurement would be within the half-life of 18F-FDG (109.8 min). In addition, we determined the weight and shortest diameter of each LN before fixation. To assess 18F-FDG uptake of each LN by well-type counter, the modified standardized uptake value of each LN (modified SUV) was calculated using the following formula: modified SUV = CCF × Cdc/(di/w), where CCF is the cross-calibration factor, Cdc is the decay-corrected tracer tissue concentration normalized for the time elapsed from 18F-FDG injection until data acquisition (in counts per second per gram), di is the injected dose (in becquerels), and w is the patient’s body weight (in grams). CCF is the ratio of the radioactivity (count per second) measured with the well-type counter to those (in becquerels) obtained with the dose calibrator. In this study, we determined CCF to be 5.8 based on our measurements using test tubes filled with 18F-FDG solution, the dose calibrator, and the well-type counter. We also compared the diagnostic usefulness of LN size and 18F-FDG uptake.
Surgical specimens including the excised stomach and LNs were examined by an experienced pathologists (KM, HA, and TU) with no knowledge of either the 18FDG-PET/CT findings or the radiation dose measurements. We conducted the tumor staging according to the Union for International Cancer Control (UICC) TNM staging system for the stomach [12]. Each LN was examined employing 2 mm-spaced slices using hematoxylin-eosin-stained sections to avoid missing small focal metastases. The gastric cancers were histologically classified into two groups according to the Lauren classification system [13]. Well and moderately differentiated tubular adenocarcinoma, papillary adenocarcinoma, and solid type poorly differentiated adenocarcinoma were classified as intestinal type carcinomas. Non-solid type poorly differentiated adenocarcinoma, signet ring cell carcinoma, and mucinous carcinoma were classified as diffuse-type carcinomas.
Statistical analysis
All statistical analyses were carried out using JMP 10.0.2 software (SAS institute, Cary, NC, USA). Differences in histological type as categorical variables were compared between metastasis-positive and metastasis-negative LNs employing the chi-square test. The Wilcoxon test was applied for continuous variables including modified SUV, the shortest LN diameter, and the time elapsed from 18F-FDG injection until data acquisition. Differences were considered significant at P < 0.05. The receiver operating characteristics (ROC) curves for the shortest LN diameter and modified SUV were used to discriminate LN metastasis from other findings. For this purpose, the area under each curve was used to measure the discriminatory ability of the model.
Results
Patient characteristics and PET/CT findings
In total, 21 patients were recruited for this study, and 906 LNs were harvested. Characteristics of the 21 patients are summarized in Table 1. Intestinal type was the main histopathology, being seen in 15 cases, diffuse type in the other 6. As for primary lesion, median SUV max was 7.1, and these values were higher in the intestinal type group (8.0 vs 6.4) on PET/CT scan. Median blood sugar was 95.0 mg/dL in the intestinal type group and 97.5 mg/dL in the diffuse-type group. Upon primary tumor staging of these patients, five of six (83 %) in the diffuse-type group were diagnosed as having pT4a. D2 lymphadenectomy was performed in 11 patients with intestinal type and two with diffuse-type gastric cancer. Distant LN sampling was performed in four patients because preoperative or intraoperative findings had indicated LN enlargement. Five patients with stage IV disease underwent palliative gastrectomy for anemia or symptoms of obstruction. The median primary tumor size was 6.5 cm (range, 2.3-16.5 cm). The primary lesion was larger in the diffuse-type group (11.6 vs 5.6 cm). Among 16 cases with LN metastases, 5, including 4 with intestinal type and 1 with diffuse type, had PET-positive LNs. The median SUV max of PET-positive LNs was 4.7. The sensitivity, specificity, PPV, and NPV of preoperative PET/CT were 24, 100, 100, and 57 %, respectively.
Table 1.
Characteristics of the 21 patients and result of PET/CT
| Total | Intestinal type | Diffuse type | |
|---|---|---|---|
| Characteristics | n = 21 | n = 15 | n = 6 |
| Sex: male/female | 16/5 | 10/5 | 6/0 |
| Median age, years (range) | 70 (41–81) | 69 (41–81) | 76 (62–81) |
| Operations: TG/DG/PG | 11/8/2 | 8/6/1 | 4/1/1 |
| Dissection: D0/D1/D1+/D2 | 1/2/5/13 | 1/1/2/11 | 0/1/3/2 |
| Locus: upper/middle/lower | 9/9/3 | 4/9/2 | 5/0/1 |
| T status: pT1b/pT2/pT3/pT4a | 3/4/6/8 | 2/4/6/3 | 1/0/0/5 |
| N status: pN0/pN1/pN2/pN3 | 5/7/3/6 | 5/5/2/3 | 0/2/1/3 |
| pStage: I/II/III/IV | 2/9/5/5 | 1/9/2/3 | 1/0/3/2 |
| SUV max of primary lesion on PET/CT | |||
| Median (range) | 7.1 (2.4-24.1) | 8.0 (2.4-24.1) | 6.4 (3.2-11.6) |
| SUV max of LNs on PET/CT | |||
| Median (range) | 4.7 (1.6-5.5) | 3.15 (1.6-4.9) | 5.5 (5.5) |
TG total gastrectomy, DG distal gastrectomy, PG proximal gastrectomy, SUV standard uptake value, LN lymph node, PET/CT positron emission tomography/computed tomography
Result of radiation dose measurement by well-type counter
The 906 harvested LNs included 115 with metastases (Table 2). The median time between 18F-FDG injection and measurement of 18F-FDG uptake using the well-type counter was 444 min (range 343-527 min). Measurement by the well-type counter revealed significantly higher modified SUV among metastatic LNs than non-metastatic LNs (Table 2). The time between 18F-FDG injection and the completion of LN resection was 364 min in the node-positive group and 336 min in the node-negative group. The median blood sugar levels in node-positive and node-negative patients were 96 and 92 mg/dl, respectively. The time elapsed from 18F-FDG injection until data acquisition by well-type counter was 435 min for the metastasis-positive LNs and 446 min for the metastasis-negative LNs (P = 0.0003). Metastatic LNs were also significantly enlarged as compared to non-metastatic LNs (Table 2). Figure 1 shows the ROC curve for modified SUV used to distinguish between LNs with and without metastasis. The area under the curve for modified SUV was 0.71 for metastatic LNs. Using a cutoff of 2.62, the sensitivity, specificity, PPV, and NPV were 77, 60, 24, and 94 %, respectively. On the other hand, the area under the curve for the shortest LN diameter was 0.60. Using a cutoff of 7.0 mm, the sensitivity, specificity, PPV, and NPV were 39, 77, 21, and 89 %, respectively. The area under the curve was 0.75 in cases with intestinal adenocarcinoma (Fig. 2) and 0.61 in those with diffuse adenocarcinoma (Fig. 3). Using a cutoff of 2.65 in the intestinal type group, the sensitivity, specificity, PPV, and NPV were 80, 65, 23, and 96 %, respectively. In the diffuse-type group, the sensitivity, specificity, PPV, and NPV were 89, 41, 26, and 94 % with a cutoff of 1.98.
Table 2.
Characteristics of 906 LNs in the 21 patients and measurement results by well-type counter
| LN with metastasis | LN without metastasis | P value | |
|---|---|---|---|
| Characteristics | n = 115 | n = 791 | |
| Modified SUV | <0.001 | ||
| Median (range) | 3.50 (0–9.52) | 2.06 (0–14.18) | |
| Histology of primary lesion | 0.001 | ||
| Intestinal | 71 | 605 | |
| Diffuse | 44 | 186 | |
| Time from 18F-FDG injection until data acquisition (min) | 0.0003 | ||
| Median (range) | 435 (343–517) | 446 (347–527) | |
| Shortest LN diameter (mm) | 0.0005 | ||
| Median (range) | 6 (2–19) | 5 (1–16) |
LN lymph node, 18 F-FDG fluorine F-18 fluorodeoxyglucose
Fig. 1.
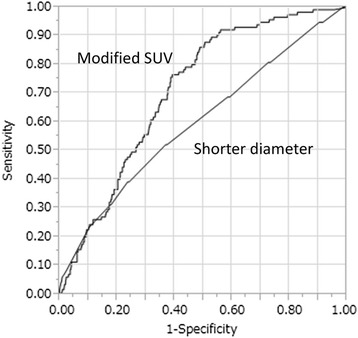
The ROC curve for modified SUV and the shortest LN diameter. The ROC curve for modified SUV had a larger area under the curve (0.71) than that for the shortest LN diameter (0.60)
Fig. 2.
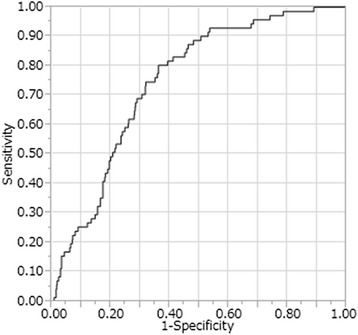
The ROC curves for modified SUV in intestinal type carcinoma. The area under the curve for this parameter is 0.75
Fig. 3.
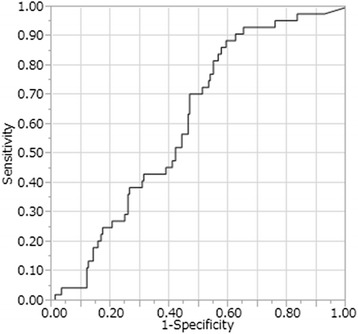
The ROC curves for modified SUV in diffuse-type carcinoma. The area under the curve for this parameter is 0.61
Discussion
To the best of our knowledge, this is the first one-to-one comparison between the 18F-FDG uptake and pathological status of individual LNs. Metastatic LNs showed significantly higher modified SUV than non-metastatic LNs using well-type counter. The area under the ROC curve for modified SUV was larger than that for the shortest LN diameter, indicating 18F-FDG uptake to be a better indicator than CT scan findings for detecting LN metastasis of gastric cancer. Micrometastasis such as isolated cluster-type tumor cells is frequently found in gastric cancer [14], therefore, accurate diagnosis of LN involvement by size is considered to be difficult. Since 18F-FDG uptake reflects the metabolic status of the lesion, this modality has been anticipated to be useful for the assessment of metastasis.
The area under the curve was larger for intestinal type adenocarcinoma than for the diffuse histological type. Intestinal type gastric cancer expresses more GLUT-1 than diffuse type, and this is associated with the fact that intestinal type gastric cancer shows higher SUV max than diffuse type on 18F-FDG PET/CT [15]. Difference of GLUT-1 expression may affect higher detection rate of 18F-FDG uptake for LN metastasis for intestinal type adenocarcinoma by well-type counter.
As for FDG-PET/CT, low sensitivity and high specificity of PET/CT scan for detecting LN metastasis were also demonstrated in current study. There are some reports about the sensitivity and specificity of 18F-FDG PET or PET/CT for detecting LN metastasis of gastric cancer [3, 6, 7, 16]. These studies included limitation of spatial resolution that 18F-FDG uptake in perigastric LN and inflammatory gastric wall or primary lesion could not be distinguished. Additionally, partial volume effect might affect low sensitivity of PET/CT scan. Since our one-to-one comparison was not disturbed by spatial resolution, our study revealed realistic diagnostic power of 18F-FDG for detecting LN metastasis of gastric cancer.
Recently, navigation surgery using a radioactive agent has been considered. Navigation surgeries are beneficial in terms of precise cancer detection, leading to avoidance of unnecessary resection. Sentinel node navigation surgery is focused on lymphatic drainage and is based on the idea that the sentinel node is the first possible site of LN metastasis [17, 18]. Navigation surgery which focuses on the metabolism of cancer cells has also been investigated [19]. Uptake of radioactive agent is detected using gamma probe without harvesting LNs; therefore, this method was expected to prevent unnecessary LN dissection. Navigation surgery using 18F-FDG has been reported for several malignancies [8–10]. In these series, 18F-FDG was injected preoperatively, and focal accumulation of 18F-FDG was detected with an intraoperative gamma probe. We planned this pilot study for navigation surgery using 18F-FDG in case with gastric cancer, but its sensitivity and specificity were not sufficient. Even though sentinel navigation surgery for early gastric cancer has 97.5 % of detection rate and 99 % of accuracy for LN evaluation, its efficacy remains to be clarified [17, 18]. Other tracers have been investigated for diagnostic application in some malignancies [20–23]; therefore, navigation surgery using these new tracers might be feasible.
This study has limitations. The first limitation was the time elapsed from 18F-FDG injection until radiation dose measurement. Considering the half-life of 18F-FDG (109.8 min), the time elapsed from 18F-FDG injection until data acquisition was rather long (444 min). The time elapsed from 18F-FDG injection until data acquisition was significantly longer for metastasis-negative than for metastasis-positive LNs. Extent of LN dissection may influence the length of this time period. Since the D2 lymphadenectomy group had more harvested LNs, it took a longer time to measure the radiation dose of all harvested LNs in this group. 18F-FDG uptake in tumors does not peak until approximately 4–5 h after FDG injection [19], but some of the LNs in this study showed low counts at measurement using the well-type counter. Although we normalized our data for the time factor, it may still have affected our results. We used the modified SUV to represent 18F-FDG uptake. This conversion facilitates correcting for various injected FDG doses and patient body masses, but some of the LNs in this study showed low counts at measurement using the well-type counter. Although we normalized our data for the time factor, our results may still have been affected. CCF was obtained by measuring activity in test tubes filled with 18F-FDG solution using the dose calibrator and the well-type counter, in which the radioactivity ranged from 1094 to 4 cps. The calculated CCF fluctuated more widely at the lower measured activities. Therefore, this CCF fluctuation may be one of the causes of the overlap between the positive LN and negative LN activities observed in this study. We did not investigate the correlation of SUV max on preoperative PET scans and modified SUV in postoperative analysis. Other indexes such as percent injected dose should also be investigated. The second limitation was the limited number of patients. This may affect especially low sensitivity of PET/CT scan.
Conclusions
In conclusion, 18F-FDG uptake is a more useful variable than the shortest LN diameter for detecting LN metastasis of gastric cancer, especially in cases with intestinal type adenocarcinoma. However, its sensitivity and specificity were not sufficient to be applied clinically as navigation yet. Further investigation should be planned for navigation surgery.
Acknowledgements
The authors thank Nobuyuki Kaneko for his excellent technical assistance with the pathological studies, and Seiji Kato and Michiharu Sekimoto for their professional support with the nuclear medicine technology.
Abbreviations
- 18F-FDG
fluorine F-18 fluorodeoxyglucose
- PET/CT
positron emission tomography/computed tomography
- LN
lymph node
- ROC
receiver operating characteristics
- SUV
standardized uptake value
- UICC
Union for International Cancer Control
- ECOG-PS
European clinical oncology group performance status
- FWHM
full width half-maximum
- CCF
cross-calibration factor
- PPV
positive predictive value
- NPV
negative predictive value
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
YO, SA, MT, TM, KM, MF, and YS conceived the study concept and design. YO, HO, KJ, YY, KM, MT, KK, HA, KM, and TU acquired the data. YO, SA, SN, MT, TM, KM, and YS analyzed and interpreted the data. YO, SA, and YS drafted the manuscript. MT and TM critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Contributor Information
Yasuhiro Okumura, Email: okumura-tky@umin.ac.jp.
Susumu Aikou, Email: aikous-tky@umin.ac.jp.
Haruna Onoyama, Email: onoyamah-sur@h.u-tokyo.ac.jp.
Keiichi Jinbo, Email: jinbok-sur@h.u-tokyo.ac.jp.
Yukinori Yamagata, Email: yamagay-tky@umin.ac.jp.
Kazuhiko Mori, Email: morikaz158@gmail.com.
Hiroharu Yamashita, Email: hyamashi@umin.net.
Sachiyo Nomura, Email: nomuras-dis@h.u-tokyo.ac.jp.
Miwako Takahashi, Email: takahashim-rad@h.u-tokyo.ac.jp.
Keitaro Koyama, Email: koyamak-rad@h.u-tokyo.ac.jp.
Toshimitsu Momose, Email: momose-rad@h.u-tokyo.ac.jp.
Hiroyuki Abe, Email: abeh-pat@h.u-tokyo.ac.jp.
Keisuke Matsusaka, Email: ksk.matsusaka@gmail.com.
Tetsuo Ushiku, Email: ushikut-pat@h.u-tokyo.ac.jp.
Masashi Fukayama, Email: fukayamam-pat@h.u-tokyo.ac.jp.
Yasuyuki Seto, Phone: +81-3-3815-5411, Email: seto-tky@umin.ac.jp.
References
- 1.Siewert JR, Bottcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: ten-year results of the German gastric cancer study. Ann Surg. 1998;228:449–61. doi: 10.1097/00000658-199810000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang XF, Huang CM, Lu HS, Wu XY, Wang C, Guang GX, et al. Surgical treatment and prognosis of gastric cancer in 2,613 patients. World J Gastroenterol. 2004;10:3405–8. doi: 10.3748/wjg.v10.i23.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwee RM, Kwee TC. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer. 2009;12:6–22. doi: 10.1007/s10120-008-0492-5. [DOI] [PubMed] [Google Scholar]
- 4.Monig SP, Zirbes TK, Schroder W, Baldus SE, Lindemann DG, Dienes HP, et al. Staging of gastric cancer: correlation of lymph node size and metastatic infiltration. Am J Roentgenol. 1999;173:365–7. doi: 10.2214/ajr.173.2.10430138. [DOI] [PubMed] [Google Scholar]
- 5.Kim EY, Lee WJ, Choi D, Lee SJ, Choi JY, Kim BT, et al. The value of PET/CT for preoperative staging of advanced gastric cancer: comparison with contrast-enhanced CT. Eur J Radiol. 2011;79:183–8. doi: 10.1016/j.ejrad.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Smyth EC, Shah MA. Role of 18F 2-fluoro-2-deoxyglucose positron emission tomography in upper gastrointestinal malignancies. World J Gastroenterol. 2011;17:5059–74. doi: 10.3748/wjg.v17.i46.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yun M, Lim JS, Noh SH, Hyung WJ, Cheong JH, Bong JK, et al. Lymph node staging of gastric cancer using 18F-FDG PET: a comparison study with CT. J Nucl Med. 2005;46:1582–8. [PubMed] [Google Scholar]
- 8.Molina MA, Goodwin WJ, Moffat FL, Serafini AN, Sfakianakis GN, Avisar E. Intra-operative use of PET probe for localization of FDG avid lesions. Cancer Imaging. 2009;9:59–62. doi: 10.1102/1470-7330.2009.9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarikaya I, Povoski SP, Al-Saif OH, Kocak E, Bloomston M, Marsh S, et al. Combined use of preoperative 18F-FDG PET imaging and intraoperative gamma probe detection for accurate assessment of tumor recurrence in patients with colorectal cancer. World J Surg Oncol. 2007;5:80. doi: 10.1186/1477-7819-5-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barranger E, Kerrou K, Petegnief Y, David-Montefiore E, Cortez A, Darai E. Laparoscopic resection of occult metastasis using the combination of FDG-positron emission tomography/computed tomography image fusion with intraoperative probe guidance in a woman with recurrent ovarian cancer. Gynecol Oncol. 2005;96:241–4. doi: 10.1016/j.ygyno.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 11.Mukai K, Ishida Y, Okajima K, Isozaki H, Morimoto T, Nishiyama S. Usefulness of preoperative FDG-PET for detection of gastric cancer. Gastric Cancer. 2006;9:192–6. doi: 10.1007/s10120-006-0374-7. [DOI] [PubMed] [Google Scholar]
- 12.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM classification of malignant tumours. 7. Hoboken, NJ: Wiley; 2009. [Google Scholar]
- 13.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 14.Fukagawa T, Sasako M, Mann GB, Sano T, Katai H, Maruyama K, et al. Immunohistochemically detected micrometastases of the lymph nodes in patients with gastric carcinoma. Cancer. 2001;92:753–60. doi: 10.1002/1097-0142(20010815)92:4<753::AID-CNCR1379>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Stahl A, Ott K, Weber WA, Becker K, Link T, Siewert JR, et al. FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging. 2003;30:288–95. doi: 10.1007/s00259-002-1029-5. [DOI] [PubMed] [Google Scholar]
- 16.Shimada H, Okazumi S, Koyama M, Murakami K. Japanese gastric cancer association task force for research promotion: clinical utility of 18F-fluoro-2-deoxyglucose positron emission tomography in gastric cancer. A systematic review of the literature. Gastric Cancer. 2011;14:13–21. doi: 10.1007/s10120-011-0017-5. [DOI] [PubMed] [Google Scholar]
- 17.Kitagawa Y, Takeuchi H, Takagi Y, Natsugoe S, Terashima M, Murakami N, et al. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol. 2013;31:3704–10. doi: 10.1200/JCO.2013.50.3789. [DOI] [PubMed] [Google Scholar]
- 18.Ryu KW, Eom BW, Nam BH, Lee JH, Kook MC, Choi IJ, et al. Is the sentinel node biopsy clinically applicable for limited lymphadenectomy and modified gastric resection in gastric cancer? A meta-analysis of feasibility studies. J Surg Oncol. 2011;104:578–84. doi: 10.1002/jso.21995. [DOI] [PubMed] [Google Scholar]
- 19.Povoski SP, Hall NC, Murrey DA, Jr, Chow AZ, Gagiani JR, Bahnson EE, et al. Multimodal imaging and detection approach to 18F-FDG-directed surgery for patients with known or suspected malignancies: a comprehensive description of the specific methodology utilized in a single-institution cumulative retrospective experience. World J Surg Oncol. 2011;9:152. doi: 10.1186/1477-7819-9-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janjigian YY, Viola-Villegas N, Holland JP, Divilov V, Carlin SD, Gomes-DaGama EM, et al. Monitoring afatinib treatment in HER2-positive gastric cancer with 18F-FDG and 89Zr-trastuzumab PET. J Nucl Med. 2013;54:936–43. doi: 10.2967/jnumed.112.110239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamura K, Kurihara H, Yonemori K, Tsuda H, Suzuki J, Kono Y, et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J Nucl Med. 2013;54:1869–75. doi: 10.2967/jnumed.112.118612. [DOI] [PubMed] [Google Scholar]
- 22.Herrmann K, Ott K, Buck AK, Lordick F, Wilhelm D, Souvatzoglou M, et al. Imaging gastric cancer with PET and the radiotracers 18F-FLT and 18F-FDG: a comparative analysis. J Nucl Med. 2007;48:1945–50. doi: 10.2967/jnumed.107.044867. [DOI] [PubMed] [Google Scholar]
- 23.McKinley ET, Ayers GD, Smith RA, Saleh SA, Zhao P, Washington MK, et al. Limits of 18F-FLT PET as a biomarker of proliferation in oncology. PLoS One. 2013;8:58938. doi: 10.1371/journal.pone.0058938. [DOI] [PMC free article] [PubMed] [Google Scholar]


