Abstract
Strain UFB2 was isolated from a soybean field soil in Mississippi and identified as a member of Pseudomonas chlororaphis. Strain UFB2 has a broad-spectrum antimicrobial activity against common soil-borne pathogens. Plate assays showed that strain UFB2 was especially efficient in inhibiting the growth of Clavibacter michiganensis 1–07, the causal agent of the devastating bacterial canker of tomato. Here, the complete genome sequence of P. chlororaphis strain UFB2 is reported and described. The strain UFB2 genome consists of a circular chromosome of 6,360,256 bp of which 87.86 % are protein-coding bases. Genome analysis revealed multiple gene islands encoding various secondary metabolites such as 2,4-diacetylphloroglucinol. Further genome analysis will provide more details about strain UFB2 antibacterial activities mechanisms and the use of this strain as a potential biocontrol agent.
Keywords: Pseudomonas chlororaphis strain UFB2, Complete genome, Biocontrol, Bacterial canker of tomato, Secondary metabolites
Introduction
Bacterial strains of Pseudomonas chlororaphis are aerobic Gram-positive bacteria and many of the strains possess a wide-spectrum antifungal activity against soil-borne plant pathogens [1–5]. P. chlororaphis strains have been reported to be efficient plant-growth-promoting bacteria, which can be used as inoculants for biofertilization, phytostimulation and biocontrol [6]. The use of P. chlororaphis strains as biocontrol agents is promising because they are capable of producing a variety of antimicrobial secondary metabolites including phenazine-1-carboxamide, 2-hydroxyphenazine, pyrrolnitrin, hydrogen cyanide, chitinases and proteases [6–8]. Moreover, P. chlororaphis is considered to be nonpathogenic to humans, wildlife, or the environment according to U.S. environmental protection agency (EPA) [9]. Antimicrobial activities and low risks to animals and the environments have made the bacterium P. chlororaphis highly potential biocontrol agents in agriculture [8, 10]. A genome-wide research and analysis could provide useful information about the mechanisms of how P. chlororaphis protects plants against soil-borne phytopathogens. Currently, the whole genomes of a few P. chlororaphis strains that exhibit antifungal activity have been sequenced. These include P. chlororaphis strain PA23 that can protect canola from stem rot disease caused by the fungal pathogen Sclerotinia sclerotiorum [2, 11], and P. chlororaphis PCL1606 that was isolated from avocado rhizosphere and exhibited biocontrol activity against soil-borne phytopathogenic fungi [1]. In addition, another functionally-uncharacterized strain, P. chlororaphis subsp. aurantiaca JD37, was recently sequenced (NCBI reference sequence: NZ_CP009290.1). Genome sequences of P. chlororaphis strains with significant antibacterial activity have not been reported previously.
Strain UFB2 was isolated from a soybean field soil in Mississippi. Preliminary analysis of the 16S rRNA gene indicated that it is a member of P. chlororaphis. Plate assays indicated P. chlororaphis strain UFB2 has a broad spectrum of antimicrobial activities, especially against bacterial canker pathogen of tomato: Clavibacter michiganensis [12, 13]. Greenhouse trials demonstrated both living cells and culture extract of strain UFB2 can be used for disease management of bacterial canker of tomato. In this study, the P. chlororaphis strain UFB2 complete genome sequence and annotation are reported. The gene islands within strain UFB2 genome that encode various secondary metabolites, including antimicrobial compounds, are also described. The detailed description of the strain UFB2 genome will shed light into further studies of biocontrol effectiveness and applications of Pseudomonas species.
Organism information
Classification and features
Strain UFB2 was isolated from rhizosphere soil sample collected from soybean field near Cleveland, Mississippi, USA, where healthy soybean plants were found growing in charcoal rot disease patch. Phylogenetic analyses based on multilocus sequence typing [14] (gyrB, rpoB, rpoD and 16 s rRNA) revealed that strain UFB2 belongs to Pseudomonas chlororaphis (Fig. 2). Strain UFB2 is rod-shaped, motile, non-spore-forming Gram-negative bacterium in the order Pseudomonadales of the class Gammaproteobacteria. UFB2 cells are approximately 3.0 ± 0.8 μm in width and 0.9 ± 0.3 μm in length (Fig. 1). The strain is relatively fast-growing, forming approximately 1 mm opaque yellowish colonies after overnight incubation at 28 °C on nutrient-broth yeast extract agar [15]. Strain UFB2 can also be grown on rich media such as LB [16] and PDA, as well as M9 minimal medium [17]. Phenotypic characterization of strain UFB2 was carried out using the API 50CH system as recommended by manufacturer. According to the result, strain UFB2 could utilize almost all carbon sources in API 50CH tests, including D-glucose, D-galactose, L-rhamnose, D-mannitol, D-raffinose, D-fructose, D-arabinose, D-ribose, L-arabinose, L-xylose and D-xylose, but not potassium gluconate.
Fig. 2.
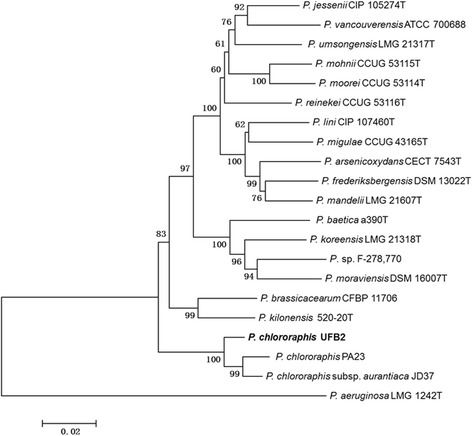
Phylogenetic analysis of concatenated four multilocus sequence typing loci of P. chlororaphis UFB2 and related species. Phylogenetic tree based on the concatenated sequence (3775 bp) of four housekeeping gene fragments [gyrB (729 bp), rpoB (885 bp), rpoD (711 bp) and 16 s rRNA (1450 bp)]. Phylogenetic analyses were performed using MEGA, version 6.06 [51]. The tree was built using the Neighbor-Joining method [52]. Bootstrap analysis with 1000 replicates was performed to assess the support of the clusters
Fig. 1.
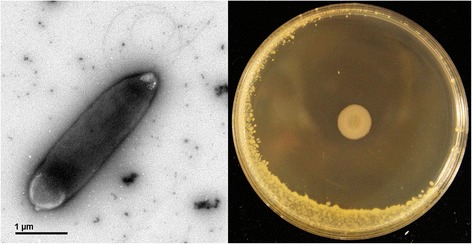
Image of P. chlororaphis UFB2 cells and plate assay of UFB2 antibacterial activity against Clavibacter michiganensis 1–07. The plate bioassay was conducted as described by Scholz-Schroeder and colleagues [44]
Plate bioassays demonstrated that strain UFB2 possesses significant antibacterial activity against a broad array of plant bacterial pathogens. Other than Clavibacter michiganensis 1–07, the tested bacteria sensitive to strain UFB2 also include Erwinia amylovora [18, 19], Burkholderia glumae [20], Ralstonia solanacearum Rso [21, 22] and Erwinia carotovoraWSCH1 [19, 23]. Of the tested plant pathogenic bacteria, the Gram-positive bacterium Clavibacter michiganensis 1–07, the pathogen causing bacterial canker of tomato [24], is most sensitive to strain UFB2 with a radius of 28 ± 1 mm clear inhibitory zone (Fig. 1). In addition, the growth of fungal pathogen Geotrichum candidum Km, which causes sour rot of citrus fruits, tomatoes, carrot and some vegetables [25], can also be inhibited by strain UFB2. To test the field biocontrol efficacy of strain UFB2, greenhouse experiments were set up according to the method described by Lu and Ingram [26]. Preliminary data showed the control efficacies of both strain UFB2 culture extract and living cells on bacterial canker of tomato are equivalent to that of streptomycin at the recommended rate for plant disease management. The genome of strain UFB2 was sequenced with the aim to identify the genes associated with the antimicrobial characters. The information about the genome sequence of strain UFB2 is summarized in Table 1, and its phylogenetic position is shown in Fig. 2.
Table 1.
Classification and general features of Pseudomonas chlororaphis UFB2 according to the MIGS recommendations [55]
| MIGS ID | Property | Term | Evidence codea |
|---|---|---|---|
| Classification | Domain Bacteria | TAS [56] | |
| Phylum Proteobacteria | TAS [57] | ||
| Class Gammaproteobacteria | TAS [58, 59] | ||
| Order Pseudomonadales | TAS [19, 60] | ||
| Family Pseudomonadaceae | TAS [19, 61] | ||
| Genus Pseudomonas | TAS [19, 61–63] | ||
| Species Pseudomonas chlororaphis | TAS [19, 64, 65] | ||
| strain: UFB2 | NAS | ||
| Gram stain | negative | TAS [66] | |
| Cell shape | Rod | TAS [66] | |
| Motility | Motile | TAS [66] | |
| Sporulation | None | NAS | |
| Temperature range | Mesophilic | IDA | |
| Optimum temperature | 33 °C | IDA | |
| pH range; Optimum | not determined | IDA | |
| Carbon source | D-glucose, D-galactose, L-rhamnose, D-mannitol, D-raffinose, D-fructose, D-arabinose, 2D-ribose, L-arabinose, L-xylose, D-xylose. | TAS [66] | |
| MIGS-6 | Habitat | Soil | NAS |
| MIGS-6.3 | Salinity | not determined | IDA |
| MIGS-22 | Oxygen requirement | Aerobic | NAS |
| MIGS-15 | Biotic relationship | free-living/Rhizospheric | NAS |
| MIGS-14 | Pathogenicity | non-pathogen | IDA |
| MIGS-4 | Geographic location | Mississippi, USA | IDA |
| MIGS-5 | Sample collection | 2011 | IDA |
| MIGS-4.1 | Latitude | 34.1 N | IDA |
| MIGS-4.2 | Longitude | 90.6 W | IDA |
| MIGS-4.4 | Altitude | 40 M | IDA |
aEvidence codes - IDA Inferred from Direct Assay, TAS Traceable Author Statement (i.e., a direct report exists in the literature), NAS Non-traceable Author Statement (i.e., not directly observed for the living, isolated sample, but based on a generally accepted property for the species, or anecdotal evidence). These evidence codes are from the Gene Ontology project [67]
Chemotaxonomic data
Fatty acid analysis was performed by gas chromatography (gas chromatograph, model 6890 N, Agilent Technologies) and analyzed by the Microbial Identification System (MIDI, Sherlock Version 6.1; database, TSBA40). The analysis of total cells showed the major fatty acids are C 16:1ω7c (32 %), C 16:0 (28 %), C 18:1ω7c (19 %). Fatty acid 3-hydroxy C 12:0 (5 %), C 12:0 (4 %), 2-hydroxy C 12:0 (4 %) and 3-hydroxy C 10:0 (3 %) were found in minor amount.
Genome sequencing information
Genome project history
P. chlororaphis strain UFB2 was selected for sequencing because of its significant antimicrobial activities and its potential as a biocontrol agent for agricultural use. Genomes of three P chlororaphis strains have been sequenced as of May 2015. Sequencing of the whole genome of strain UFB2 makes more data available for genome comparison and analysis within P. chlororaphis species.
The genome project is deposited in the Genomes OnLine Database [27] and the NCBI BioProject database [28]. The annotated genome is publicly available from the Intergrated Microbial Genomes Database [29] under the accession number Gp0111981 and GenBank under accession number CP011020. A summary of the project information is provided in Table 2.
Table 2.
Project information
| MIGS ID | Property | Term |
|---|---|---|
| MIGS 31 | Finishing quality | Finished |
| MIGS-28 | Libraries used | libraries of 400 bp, mate pair library of 2,000, 5,000 and 8,000 bp |
| MIGS 29 | Sequencing platforms | Illumina |
| MIGS 31.2 | Fold coverage | 600 × |
| MIGS 30 | Assemblers | DNAStar Seqman NGen v12 |
| MIGS 32 | Gene calling method | NCBI Prokaryotic Genome Annotation Pipeline |
| Locus Tag | VM99 | |
| Genbank ID | CP011020 | |
| GenBank Date of Release | Jun 9th, 2015 | |
| GOLD ID | Gp0111981 | |
| BIOPROJECT | PRJNA277727 | |
| MIGS13 | Source Material Identifier | UFB2 |
| Project relevance | Biocontrol |
Growth conditions and genomic DNA preparation
P. chlororaphis strain UFB2 was cultured in liquid NBY medium overnight at 28 °C in a shaker at 220 rpm. The genomic DNA was extracted from 50 mL of the culture using the Wizard Genomic DNA Purification Kit (Promega Corporation, Madison, WI, USA). Totally approximately 900 μg of DNA were obtained with an OD260/280 of 1.9. The DNA sample was used for library construction with Illumina Genomic DNA Sample Preparation Kit (Illumina, CA, USA).
Genome sequencing and assembly
One standard library with an average insert size of 400 bp and three mate pair libraries with an average insert size of 2,000 bp, 5,000 bp and 8,000 bp were prepared and sequenced on the Illumina MiSeq instrument according to the manufacturer’s instructions. The genome was de novo assembled using a method as described by Durfee et al. [30] using DNAStar Seqman NGen (Version 12, DNASTAR, Inc. Madison, WI U.S.). The standard library and 2,000 bp mate pair library were selected for the de novo assembly. A total of 30 million short reads were scanned and extracted from the raw data files as input data. The short reads were preprocessed by Seqman NGen to trim adaptors and filter low-quality reads. Automatic Mer size and a minimum match percentage of 98 % were selected. 29 million short reads were assembled into 29 contigs and SeqMan Pro (Version 12, DNASTAR, Inc. Madison, WI U.S.) was used to order the contigs in one scaffold according to the mate pair data. The first round assembled sequence was then used as a template for a complete reassembly. The 2,000 bp and 8,000 bp mate pair data were incorporated to proofread the first assembly and to maximize coverage and quality. Adjacent contigs, if possible, were merged. Remaining gaps were filled by PCR and Sanger sequencing. No contigs that might correspond to plasmids remained unassembled. IslandViewer [31] was used to predict and identify genomic islands.
Genome annotation
Automatic annotation was performed using the NCBI Prokaryotic Genome Annotation Pipeline [32], which combines gene calling algorithm with similarity-based gene detection approach to predict protein-coding genes, structural RNAs (5S, 16S, 23S), tRNAs and small non-coding RNAs. Additional gene prediction analysis and functional annotation were performed by the Integrated Microbial Genomes platform [29].
Genome properties
The complete genome of P. chlororaphis strain UFB2 consists of one circular chromosome of 6,360,256 bp with a GC content of 62.03 %. 5,556 genes were identified from the genome, of which 5,473 are protein coding genes. 90 of the 5,556 genes were predicted to be pseudogenes or partial genes. The genome encodes 1 noncoding RNA, 5 rRNA operons and 65 tRNAs. Seventy genomic islands ranging from 4 kbp to 43.5 kbp were also identified throughout the strain UFB2 genome, among which majority of the islands encode hypothetical proteins. The genome features of P. chlororaphis strain UFB2 are summarized in Tables 3 and 4, and the circular chromosome of strain UFB2 is shown in Fig. 3.
Table 3.
Genome statistics
| Attribute | Value | % of Total |
|---|---|---|
| Genome size (bp) | 6,360,256 | 100.00 |
| DNA coding (bp) | 5,588,126 | 87.86 |
| DNA G + C (bp) | 3,945,558 | 62.03 |
| DNA scaffolds | 1 | 100.00 |
| Total genes | 5,556 | 100.00 |
| Protein coding genes | 5,473 | 98.51 |
| RNA genes | 83 | 1.49 |
| Pseudo genes | 90 | 1.62 |
| Genes in internal clusters | 5,473 | 98.51 |
| Genes with function prediction | 4,886 | 87.94 |
| Genes assigned to COGs | 4,092 | 73.65 |
| Genes with Pfam domains | 4,748 | 85.46 |
| Genes with signal peptides | 577 | 10.39 |
| Genes with transmembrane helices | 1,228 | 22.10 |
| CRISPR repeats | 0 | 0 |
Table 4.
Number of genes associated with general COG functional categories
| Code | Value | % age | Description |
|---|---|---|---|
| J | 231 | 4.89 | Translation, ribosomal structure and biogenesis |
| A | 1 | 0.02 | RNA processing and modification |
| K | 418 | 8.85 | Transcription |
| L | 123 | 2.60 | Replication, recombination and repair |
| B | 3 | 0.06 | Chromatin structure and dynamics |
| D | 39 | 0.83 | Cell cycle control, Cell division, chromosome partitioning |
| V | 101 | 2.14 | Defense mechanisms |
| T | 316 | 6.69 | Signal transduction mechanisms |
| M | 262 | 5.55 | Cell wall/membrane biogenesis |
| N | 166 | 3.52 | Cell motility |
| W | 44 | 0.93 | Extracellular structures |
| U | 137 | 2.90 | Intracellular trafficking and secretion |
| O | 166 | 3.52 | Posttranslational modification, protein turnover, chaperones |
| C | 304 | 6.44 | Energy production and conversion |
| G | 227 | 4.81 | Carbohydrate transport and metabolism |
| E | 483 | 10.23 | Amino acid transport and metabolism |
| F | 92 | 1.95 | Nucleotide transport and metabolism |
| H | 242 | 5.12 | Coenzyme transport and metabolism |
| I | 234 | 4.96 | Lipid transport and metabolism |
| P | 257 | 5.44 | Inorganic ion transport and metabolism |
| Q | 142 | 3.01 | Secondary metabolites biosynthesis, transport and catabolism |
| R | 430 | 9.11 | General function prediction only |
| S | 260 | 5.51 | Function unknown |
| - | 1464 | 26.35 | Not in COGs |
The total is based on the total number of protein coding genes in the genome
Fig. 3.
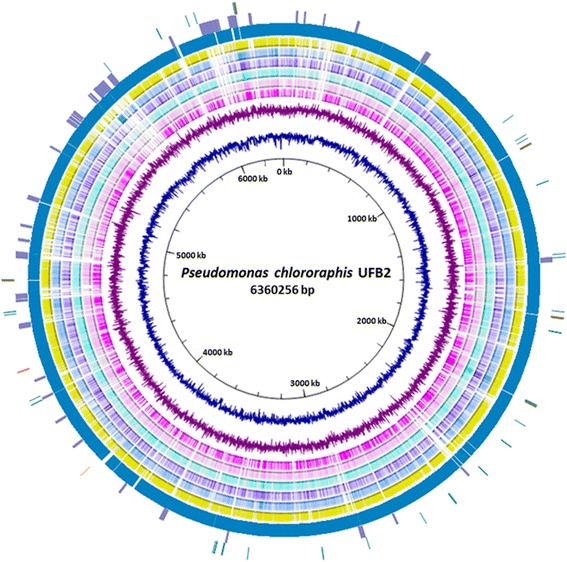
Circular representation of the P. chlororaphis UFB2 genome compared with six sequenced Pseudomonas whole genomes. Rings from inside to outside: (1) Scale, (2) GC content (navy), (3) GC skew (purple), (4) BLAST comparison with P. syringae pv. syringae B728a (deep pink), (5) BLAST comparison with P. putida KT2440 (pink), (6) BLAST comparison with P. chlororaphis strain PA23 (cyan), (7) BLAST comparison with P. aeruginosa PAO1 (violet), (8) BLAST comparison with P. fluorescens Pf0-1 (skyblue), (9) BLAST comparison with P. sp. UW4 (yellow), (10) Coding sequences of P. chlororaphis UFB2 genome (dark cyan), (11) Gene islands (medium purple), (12) rRNA genes (orange), tRNA genes (dark green) and ncRNA (red). BLASTn comparison of genomes was visualized by BRIG [53] and UFB2 genome the image was generated with Circos [54]
Insights from the genome sequence
Blast research of P. chlororaphis strain UFB2 genome against P. syringae pv. syringae B728a (NC_007005), P. putida KT2440 (NC_002947), P. chlororaphis strain PA23 (NZ_CP008696), P. aeruginosa PAO1 (NC_002516), P. fluorescens Pf0-1 (NC_007492) and P. sp. UW4 (NC_019670) genome revealed multiple unique gene regions which were only found in the strain UFB2 genome (Fig. 3). The BLASTn atlas showed noticeable genome diversity of strain UFB2 when compared to other Pseudomonas species. Seventy genomic islands ranging from 4 kbp to 30 kbp were also identified throughout the strain UFB2 genome, indicating significant horizontal gene transfers occurred during the evolution of strain UFB2 to better adapt the environment it inhabited.
P. chlororaphis strain UFB2 harbors an intact phl gene cluster (VM99_23970-23995), which is responsible for biosynthesis of the antimicrobial compound 2,4-diacetylphloroglucinol [33, 34]. 2,4-diacetylphloroglucinol is an especially efficient agent against soil borne fungal plant pathogens [35]. The phl gene cluster is involved in the Pseudomonas antifungal activity against Clavibacter michiganensis 1–07 [36]. Hydrogen cyanide [37, 38] biosynthesis gene homologs were also identified in strain UFB2 genome. The production of hydrogen cyanide by Pseudomonas species helps protect plants from soil-borne fungal pathogens [39, 40]. Biosynthetic gene clusters of common Pseudomonas species-produced antibiotics such as phenazine [41], pyrrolnitrin [42] and pyoluteorin [43] were not identified in strain UFB2 genome. Biosynthetic gene clusters of common toxins that contribute to plant and animal pathogenicity and/or virulence of Pseudomonas species were also searched for within strain UFB2 genome. The toxin biosynthetic gene cluster that were not identified in strain UFB2 genome include the phytotoxin lipopeptide syringomycin [44], tobacco wildfire spotting causal agent tabtoxin [45], bacterial canker of kiwifruit causal agent phaseolotoxin [46], plant-hormone-mimic toxin coronatine [47], and cytotoxic agent pederin [48]. Strain UFB2 genome harbors homolog genes to those in the bacterial apical necrosis causal agent mangotoxin [49] biosynthesis gene cluster. However, mboC gene homolog that is required for mangotoxin production [50] was not identified in strain UFB2 genome. Overall, the lack of the key pathogenicity/virulence genes in strain UFB2 further indicates that strain UFB2 has a great potential as a biocontrol agent.
Conclusions
The complete genome sequence of P. chlororaphis strain UFB2 is described in this report. The strain UFB2 was originally isolated from the rhizosphere of a healthy soybean plant growing in a group of plants exhibiting charcoal rot disease in Mississippi. This strain possesses significant antimicrobial activities against a wide range of plant pathogenic bacteria and fungi. It is evident that the genome of P. chlororaphis strain UFB2 harbors the complete gene set for production of the antimicrobial compounds 2,4-DAPG and HCN, which may largely contribute to its antimicrobial activities. However, gene homologs required for biosynthesis of all the known toxins to plants, such as syringomycin, tabtoxin, phaseolotoxin, tolaasin, coronatine, or pederin, were absent from the strain UFB2 genome. The genome sequence of P. chlororaphis strain UFB2 will help in understanding genetic mechanisms of the antimicrobial activity studies that are useful for development of biologically-based disease management in agriculture.
Acknowledgements
We thank Chuan-Yu Hsu and Kurt C. Showmaker for sequencing services and Richard Baird, Sead Sabanadzovic and Justin Thornton for helpful discussion. This research was funded by USDA NIFA to SL (MIS-401170).
Footnotes
Competing interests
The authors declare no competing interests.
Authors’ contributions
PD and SL designed the experiments; PD, XW, and SB performed the experiments; PD and SL wrote the manuscript and all authors read, critiqued and edited the manuscript.
References
- 1.Calderon CE, Ramos C, de Vicente A, Cazorla FM. Comparative genomic analysis of Pseudomonas chlororaphis PCL1606 reveals new insight into antifungal compounds involved in biocontrol. Mol Plant Microbe Interact. 2015;28(3):249–260. doi: 10.1094/MPMI-10-14-0326-FI. [DOI] [PubMed] [Google Scholar]
- 2.Loewen PC, Villenueva J, Fernando WG, de Kievit T. Genome Sequence of Pseudomonas chlororaphis Strain PA23. Genome Announc 2014; 2(4), doi: 10.1128/genomeA.00689-14. [DOI] [PMC free article] [PubMed]
- 3.Loper JE, Hassan KA, Mavrodi DV, Davis EW, 2nd, Lim CK, Shaffer BT, et al. Comparative genomics of plant-associated Pseudomonas spp.: insights into diversity and inheritance of traits involved in multitrophic interactions. PLoS Genet. 2012;8(7):e1002784. doi: 10.1371/journal.pgen.1002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen X, Chen M, Hu H, Wang W, Peng H, Xu P, et al. Genome sequence of Pseudomonas chlororaphis GP72, a root-colonizing biocontrol strain. J Bacteriol. 2012;194(5):1269–1270. doi: 10.1128/JB.06713-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim MS, Kim YC, Cho BH. Gene expression analysis in cucumber leaves primed by root colonization with Pseudomonas chlororaphis O6 upon challenge-inoculation with Corynespora cassiicola. Plant Biol (Stuttg) 2004;6(2):105–108. doi: 10.1055/s-2004-817803. [DOI] [PubMed] [Google Scholar]
- 6.Bloemberg GV, Lugtenberg BJ. Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr Opin Plant Biol. 2001;4(4):343–350. doi: 10.1016/S1369-5266(00)00183-7. [DOI] [PubMed] [Google Scholar]
- 7.Chin AWTF, Bloemberg GV, Mulders IH, Dekkers LC, Lugtenberg BJ. Root colonization by phenazine-1-carboxamide-producing bacterium Pseudomonas chlororaphis PCL1391 is essential for biocontrol of tomato foot and root rot. Mol Plant Microbe Interact. 2000;13(12):1340–1345. doi: 10.1094/MPMI.2000.13.12.1340. [DOI] [PubMed] [Google Scholar]
- 8.Selin C, Habibian R, Poritsanos N, Athukorala SN, Fernando D, de Kievit TR. Phenazines are not essential for Pseudomonas chlororaphis PA23 biocontrol of Sclerotinia sclerotiorum, but do play a role in biofilm formation. FEMS Microbiol Ecol. 2010;71(1):73–83. doi: 10.1111/j.1574-6941.2009.00792.x. [DOI] [PubMed] [Google Scholar]
- 9.Pseudomonas chlororaphis strain 63–28 (006478) Fact Sheet [http://www.epa.gov/opp00001/chem_search/reg_actions/registration/fs_PC-006478_01-Apr-01.pdf]
- 10.Tombolini R, van der Gaag DJ, Gerhardson B, Jansson JK. Colonization pattern of the biocontrol strain Pseudomonas chlororaphis MA 342 on barley seeds visualized by using green fluorescent protein. Appl Environ Microbiol. 1999;65(8):3674–3680. doi: 10.1128/aem.65.8.3674-3680.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savchuk S, Dilantha Fernando WG. Effect of timing of application and population dynamics on the degree of biological control of Sclerotinia sclerotiorum by bacterial antagonists. FEMS Microbiol Ecol. 2004;49(3):379–388. doi: 10.1016/j.femsec.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Eichenlaub R, Gartemann KH. The Clavibacter michiganensis subspecies: molecular investigation of gram-positive bacterial plant pathogens. Annu Rev Phytopathol. 2011;49:445–464. doi: 10.1146/annurev-phyto-072910-095258. [DOI] [PubMed] [Google Scholar]
- 13.Davis MJ, Gillaspie AG, Vidaver AK, Harris RW. Clavibacter: a new genus containing some phytopathogenic coryneform bacteria, including Clavibacter xyli subsp. xyli sp. nov., subsp. nov. and Clavibacter xyli subsp. cynodontis subsp. nov., pathogens that cause ratoon stunting disease of sugarcane and bermudagrass stunting disease. Int J Syst Bacteriol. 1984;34:107–117. doi: 10.1099/00207713-34-2-107. [DOI] [Google Scholar]
- 14.Maiden MC. Multilocus sequence typing of bacteria. Annu Rev Microbiol. 2006;60:561–588. doi: 10.1146/annurev.micro.59.030804.121325. [DOI] [PubMed] [Google Scholar]
- 15.Vidaver AK. Synthetic and complex media for the rapid detection of fluorescence of phytopathogenic pseudomonads: effect of the carbon source. Appl Microbiol. 1967;15(6):1523–1524. doi: 10.1128/am.15.6.1523-1524.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang CH, Koch AL. Constancy of growth on simple and complex media. J Bacteriol. 1978;136(3):969–975. doi: 10.1128/jb.136.3.969-975.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winslow CE, Broadhurst J, Buchanan RE, Krumwiede C, Rogers LA, Smith GH. The families and genera of the bacteria: final report of the committee of the society of american bacteriologists on characterization and classification of bacterial types. J Bacteriol. 1920;5(3):191–229. doi: 10.1128/jb.5.3.191-229.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skerman VBD, McGowan V, Sneath PHA. Approved lists of bacterial names. Int J Syst Bacteriol. 1980;30:225–420. doi: 10.1099/00207713-30-1-225. [DOI] [PubMed] [Google Scholar]
- 20.Urakami T, Ito-Yoshida C, Araki H, Kijima T, Suzuki KI, Komagata K. Transfer of Pseudomonas plantarii and Pseudomonas glumae to Burkholderia as Burkholderia spp. and description of Burkholderia vandii sp. nov. Int J Syst Bacteriol. 1994;44:235–245. doi: 10.1099/00207713-44-2-235. [DOI] [Google Scholar]
- 21.Yabuuchi E, Kosako Y, Yano I, Hotta H, Nishiuchi Y. Transfer of two Burkholderia and an Alcaligenes species to Ralstonia gen. Nov.: Proposal of Ralstonia pickettii (Ralston, Palleroni and Doudoroff 1973) comb. Nov., Ralstonia solanacearum (Smith 1896) comb. Nov. and Rals tonia eutropha (Davis 1969) comb. Nov. Microbiol Immunol. 1995;39(11):897–904. doi: 10.1111/j.1348-0421.1995.tb03275.x. [DOI] [PubMed] [Google Scholar]
- 22.Validation of the publication of new names and new combinations previously effectively published outside the IJSB. Int J Syst Bacteriol. 1996;46(2):625–626. [DOI] [PubMed]
- 23.Waldee EL. Comparative studies of some peritrichous phytopathogenic bacteria; 1945.
- 24.Gartemann KH, Kirchner O, Engemann J, Grafen I, Eichenlaub R, Burger A. Clavibacter michiganensis subsp. michiganensis: first steps in the understanding of virulence of a Gram-positive phytopathogenic bacterium. J Biotechnol. 2003;106(2–3):179–191. doi: 10.1016/j.jbiotec.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Thornton CR, Slaughter DC, Davis RM. Detection of the sour-rot pathogen Geotrichum candidum in tomato fruit and juice by using a highly specific monoclonal antibody-based ELISA. Int J Food Microbiol. 2010;143(3):166–172. doi: 10.1016/j.ijfoodmicro.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Ingram DM, Lu S-E. Evaluation of Foliar Sprays of Bacteriophages for the Management of Bacterial Canker in Greenhouse Tomatoes. [http://www.plantmanagementnetwork.org/pub/php/research/2009/tomato/].
- 27.Pagani I, Liolios K, Jansson J, Chen IM, Smirnova T, Nosrat B, et al. The Genomes OnLine Database (GOLD) v.4: status of genomic and metagenomic projects and their associated metadata. Nucleic Acids Res. 2012;40:D571–D579. doi: 10.1093/nar/gkr1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrett T, Clark K, Gevorgyan R, Gorelenkov V, Gribov E, Karsch-Mizrachi I, et al. BioProject and BioSample databases at NCBI: facilitating capture and organization of metadata. Nucleic Acids Res. 2012;40(Database issue):D57–D63. doi: 10.1093/nar/gkr1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen IM, Palaniappan K, Chu K, Szeto E, Grechkin Y, Ratner A, et al. IMG: the Integrated Microbial Genomes database and comparative analysis system. Nucleic Acids Res. 2012;40:D115–D122. doi: 10.1093/nar/gkr1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durfee T, Nelson R, Baldwin S, Plunkett G, 3rd, Burland V, Mau B, et al. The complete genome sequence of Escherichia coli DH10B: insights into the biology of a laboratory workhorse. J Bacteriol. 2008;190(7):2597–2606. doi: 10.1128/JB.01695-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langille MG, Brinkman FS. IslandViewer: an integrated interface for computational identification and visualization of genomic islands. Bioinformatics. 2009;25(5):664–665. doi: 10.1093/bioinformatics/btp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tatiana T, Mike D, Azat B, Vyacheslav C, Stacy C, Wenjun L. Prokaryotic Genome Annotation Pipeline. The NCBI Handbook [Internet]. 2nd edition. 2013.
- 33.Bangera MG, Thomashow LS. Identification and characterization of a gene cluster for synthesis of the polyketide antibiotic 2,4-diacetylphloroglucinol from Pseudomonas fluorescens Q2-87. J Bacteriol. 1999;181(10):3155–3163. doi: 10.1128/jb.181.10.3155-3163.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cronin D, Moenne-Loccoz Y, Fenton A, Dunne C, Dowling DN, O’Gara F. Role of 2,4-Diacetylphloroglucinol in the Interactions of the Biocontrol Pseudomonad Strain F113 with the Potato Cyst Nematode Globodera rostochiensis. Appl Environ Microbiol. 1997;63(4):1357–1361. doi: 10.1128/aem.63.4.1357-1361.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shanahan P, O’Sullivan DJ, Simpson P, Glennon JD, O’Gara F. Isolation of 2,4-diacetylphloroglucinol from a fluorescent pseudomonad and investigation of physiological parameters influencing its production. Appl Environ Microbiol. 1992;58(1):353–358. doi: 10.1128/aem.58.1.353-358.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lanteigne C, Gadkar VJ, Wallon T, Novinscak A, Filion M. Production of DAPG and HCN by Pseudomonas sp. LBUM300 contributes to the biological control of bacterial canker of tomato. Phytopathology. 2012;102(10):967–973. doi: 10.1094/PHYTO-11-11-0312. [DOI] [PubMed] [Google Scholar]
- 37.Laville J, Blumer C, Von Schroetter C, Gaia V, Defago G, Keel C, et al. Characterization of the hcnABC gene cluster encoding hydrogen cyanide synthase and anaerobic regulation by ANR in the strictly aerobic biocontrol agent Pseudomonas fluorescens CHA0. J Bacteriol. 1998;180(12):3187–3196. doi: 10.1128/jb.180.12.3187-3196.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gross H, Loper JE. Genomics of secondary metabolite production by Pseudomonas spp. Nat Prod Rep. 2009;26(11):1408–1446. doi: 10.1039/b817075b. [DOI] [PubMed] [Google Scholar]
- 39.Voisard C, Keel C, Haas D, Defago G. Cyanide production by Pseudomonas fluorescens helps suppress black root rot of tobacco under gnotobiotic conditions. EMBO J. 1989;8(2):351–358. doi: 10.1002/j.1460-2075.1989.tb03384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haas D, Blumer C, Keel C. Biocontrol ability of fluorescent pseudomonads genetically dissected: importance of positive feedback regulation. Curr Opin Biotechnol. 2000;11(3):290–297. doi: 10.1016/S0958-1669(00)00098-7. [DOI] [PubMed] [Google Scholar]
- 41.Mavrodi DV, Bonsall RF, Delaney SM, Soule MJ, Phillips G, Thomashow LS. Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J Bacteriol. 2001;183(21):6454–6465. doi: 10.1128/JB.183.21.6454-6465.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costa R, van Aarle IM, Mendes R, van Elsas JD. Genomics of pyrrolnitrin biosynthetic loci: evidence for conservation and whole-operon mobility within gram-negative bacteria. Environ Microbiol. 2009;11(1):159–175. doi: 10.1111/j.1462-2920.2008.01750.x. [DOI] [PubMed] [Google Scholar]
- 43.Souza JT, Raaijmakers JM. Polymorphisms within the prnD and pltC genes from pyrrolnitrin and pyoluteorin-producing Pseudomonas and Burkholderia spp. FEMS Microbiol Ecol. 2003;43(1):21–34. doi: 10.1111/j.1574-6941.2003.tb01042.x. [DOI] [PubMed] [Google Scholar]
- 44.Scholz-Schroeder BK, Hutchison ML, Grgurina I, Gross DC. The contribution of syringopeptin and syringomycin to virulence of Pseudomonas syringae pv. syringae strain B301D on the basis of sypA and syrB1 biosynthesis mutant analysis. Mol Plant Microbe Interact. 2001;14(3):336–348. doi: 10.1094/MPMI.2001.14.3.336. [DOI] [PubMed] [Google Scholar]
- 45.Kinscherf TG, Coleman RH, Barta TM, Willis DK. Cloning and expression of the tabtoxin biosynthetic region from Pseudomonas syringae. J Bacteriol. 1991;173(13):4124–4132. doi: 10.1128/jb.173.13.4124-4132.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hwang MS, Morgan RL, Sarkar SF, Wang PW, Guttman DS. Phylogenetic characterization of virulence and resistance phenotypes of Pseudomonas syringae. Appl Environ Microbiol. 2005;71(9):5182–5191. doi: 10.1128/AEM.71.9.5182-5191.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng XY, Spivey NW, Zeng W, Liu PP, Fu ZQ, Klessig DF, et al. Coronatine promotes Pseudomonas syringae virulence in plants by activating a signaling cascade that inhibits salicylic acid accumulation. Cell Host Microbe. 2012;11(6):587–596. doi: 10.1016/j.chom.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piel J, Hofer I, Hui D. Evidence for a symbiosis island involved in horizontal acquisition of pederin biosynthetic capabilities by the bacterial symbiont of Paederus fuscipes beetles. J Bacteriol. 2004;186(5):1280–1286. doi: 10.1128/JB.186.5.1280-1286.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arrebola E, Cazorla FM, Codina JC, Gutierrez-Barranquero JA, Perez-Garcia A, de Vicente A. Contribution of mangotoxin to the virulence and epiphytic fitness of Pseudomonas syringae pv. syringae. Int Microbiol. 2009;12(2):87–95. [PubMed] [Google Scholar]
- 50.Carrion VJ, Arrebola E, Cazorla FM, Murillo J, de Vicente A. The mbo operon is specific and essential for biosynthesis of mangotoxin in Pseudomonas syringae. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0036709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 53.Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics. 2011;12:402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19(9):1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Field D, Garrity G, Gray T, Morrison N, Selengut J, Sterk P, et al. The minimum information about a genome sequence (MIGS) specification. Nat Biotechnol. 2008;26(5):541–547. doi: 10.1038/nbt1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garrity GM, Bell JA, Lilburn T. Phylum XIV. Proteobacteria phyl. nov. In: Garrity GM, Brenner DJ, Krieg NR, Staley JT, editors. Bergey’s Manual of Systematic Bacteriology. 2. New York: Springer; 2005. p. 2. [Google Scholar]
- 58.Validation of publication of new names and new combinations previously effectively published outside the IJSEM. List no. 106. Int J Syst Evol Microbiol. 2005; 55:2235–2238. [DOI] [PubMed]
- 59.Garrity GM, Bell JA, Lilburn T. Class III. Gammaproteobacteria class. nov. In: Garrity GM, Brenner DJ, Krieg NR, Staley JT, editors. Bergey’s Manual of Systematic Bacteriology. 2. New York: Springer; 2005. p. 2. [Google Scholar]
- 60.Orla-Jensen S. The main lines of the natural bacterial system. J Bacteriol. 1921;6(3):263–273. doi: 10.1128/jb.6.3.263-273.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winslow CEA, Broadhurst J, Buchanan RE, Krumwiede C, Rogers LA, Smith GH. The families and genera of the bacteria: preliminary report of the committee of the society of american bacteriologists on characterization and classification of bacterial types. J Bacteriol. 1917;2(5):505–566. doi: 10.1128/jb.2.5.505-566.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Commission J. Opinion 5: Conservation of the generic name Migula 1894 and designation of aeruginosa (Schroeter) Migula 1900 as type species. Int Bull Bacteriol Nomencl Taxon. 1952;2:121–122. [Google Scholar]
- 63.Migula W. Über ein neues System der Bakterien. Arb Bakteriol Inst Karlsruhe. 1894;1:235–238. [Google Scholar]
- 64.Anzai Y, Kim H, Park JY, Wakabayashi H, Oyaizu H. Phylogenetic affiliation of the pseudomonads based on 16S rRNA sequence. Int J Syst Evol Microbiol. 2000;50(Pt 4):1563–1589. doi: 10.1099/00207713-50-4-1563. [DOI] [PubMed] [Google Scholar]
- 65.Bergey DH, Harrison FC, Breed RS, Hammer BW, Huntoon FM, et al. Pseudomonas chlororaphis (Guignard and Sauvageau) In: Bergey, et al., editors. Bergey’s Manual of Determinative Bacteriology. 1930. p. 166. [Google Scholar]
- 66.Palleroni NJ. Pseudomonadaceae. In: Krieg NR, Holt JG, editors. Bergey’s Manual of Systematic Bacteriology. Baltimore: The Williams and Wilkins Co; 1984. pp. 141–199. [Google Scholar]
- 67.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25(1):25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]


