Abstract
Background
Since chemosensory genes play key roles in insect behaviour, they can potentially be used as new targets for pest control. The cabbage beetle, Colaphellus bowringi, is a serious insect pest of cruciferous vegetables in China and other Asian countries. However, a systematic identification of the chemosensory genes expressed in the antennae has not been reported.
Results
We assembled the antennal transcriptome of C. bowringi by using Illumina sequencing technology and identified 104 candidate chemosensory genes by analyzing transcriptomic data, which included transcripts encoding 26 odorant-binding proteins (OBPs), 12 chemosensory proteins (CSPs), four sensory neuron membrane proteins (SNMPs), 43 odorant receptors (ORs), nine ionotropic receptors (IRs), and ten gustatory receptors (GRs). The data obtained are similar to those found in other coleopteran species, suggesting that our approach successfully identified the chemosensory genes of C. bowringi. The expression patterns of 43 OR genes, some of which were predominately found in the antenna or associated with sex-biased expression, were analyzed using quantitative real time RT-PCR (qPCR).
Conclusions
Our study revealed that a large number of chemosensory genes are expressed in C. bowringi. These candidate chemosensory genes and their expression profiles in various tissues provide further information on understanding their function in C. bowringi as well as other insects, and identifying potential targets to disrupt the odorant system in C. bowringi so that new methods for pest management can be developed.
Electronic supplementary material
The online version of this article (doi:10.1186/s12864-015-2236-3) contains supplementary material, which is available to authorized users.
Keywords: Transcriptome analysis, Chemosensory gene, Antenna, Tissue expression, Cabbage beetle
Background
The olfactory system plays a key role in various insect behaviours, such as those related to locating suitable hosts, avoiding predators, identifying oviposition sites, and finding sexual partners [1]. The antennae are crucial olfactory organs in this system, and many studies have demonstrated that the system generally involves two main steps. Firstly, odorants penetrate the sensillar lymph through pores, wherein they are recognised and bound by odorant-binding proteins (OBPs) [2–4] or chemosensory proteins (CSPs) [5, 6]. Secondly, it was speculated that the OBPs or CSPs were the transporters that transferred odorants through the sensillar lymph to a family of integral membrane protein, the olfactory receptors (ORs), located on the dendrites of olfactory receptor neurons (ORNs) [7–10]. Additionally, sensory neuron membrane proteins (SNMPs) [11, 12] and ionotropic receptors (IRs) [2, 13–15] have also been proposed to play a role in insect olfaction.
To thoroughly explore the mechanisms of insect olfaction, tissue or sex expression profiling as well as functional analyses of candidate chemosensory genes are the primary important steps that should be performed. Compared with initial techniques such as gene cloning with degenerate primers and Rapid Amplification of cDNA Ends (RACE) [16–19], RNA-seq is considered to be a timesaving, cost effective, and highly efficient method. Therefore, large-scale studies identifying chemosensory genes have been undertaken with distinct insects whose genomes have not been sequenced in recent years, such as Ips typographus (european spruce bark beetle) [20], Dendroctonus ponderosae (mountain pine beetle) [20], Dendroctonus valens (red turpentine beetle) [21], Anomala corpulenta (metallic green beetle) [22], Sesamia inferens (purple stem borer) [23], and Helicoverpa armigera (cotton bollworm) [24].
To date, many chemosensory genes have been identified from insects of almost every insect order. However, their exact functions are largely unknown, as these genes were identified based on sequence similarity to previously reported genes. Examination of gene expression profiles, particularly the tissue or sex distribution, and phylogenetic analyses could potentially provide important information concerning the function of chemosensory genes [25–30].
The cabbage beetle, Colaphellus bowringi Baly (Coleoptera: Chrysomelidae), is a serious insect pest and widely distributed in China as well as some other Asian countries. It primarily feeds on the developing leaves of cruciferous vegetables such as Raphanus sativus, Brassica chinensis, B. pekinensis and B. campestris, and aestivates and hibernates in the soil during the adult stage [31, 32]. There are two distinct infestation peaks annually: one in spring with a single generation and a second in autumn involving three generations. Both sexes copulate an average of five times per day [33–35], and 15-dayold partners have significantly greater mating success in mate choice than other developmental stages [36, 37]. However, highly effective sex attractants and pesticides to control the pest are not available [38, 39].
In this study, we performed a transcriptome analysis of adult antennae of C. bowringi, and identified 104 candidate chemosensory genes comprising 26 OBPs, 12 CSPs, 4 SNMPs, 43 ORs, 9 IRs, and 10 GRs. Furthermore, we conducted a comprehensive and comparative phylogenetic analysis and examined OR gene transcription patterns using quantitative real-time RT-PCR (qPCR). The results clearly revealed a unique feature of sex-biased expression of some ORs, and ultimately allowed us to identify potential targets to disrupt odorant perception in C. bowringi that could lead to new pest management techniques.
Results
Transcriptome sequencing and sequence assembly
We carried out next-generation sequencing on a cDNA library constructed from the adult antennae of C. bowringi using the Illumina HiSeq™ 2500 platform. The transcriptome sequence consisted of approximately 50 million clean reads (5.0 Gb). After clustering and redundancy filtering, we identified 41,761 unigenes with an N50 length of 1510 bp (Table 1). We called these 41,761sequences unigenses, although each might not necessarily represent a unique gene. Of the 41,761 unigenes, those with a sequence length greater than 500 bp accounted for 39.55 % of the transcriptome assembly (Additional file 1: Figure S1).
Table 1.
Summary of C. bowringi transcriptome assembly
| Statistics project | Number |
|---|---|
| Total clean reads | 50,737,524 |
| GC percentage | 41.13 % |
| Q20 percentage | 96.84 % |
| Total unigene nucleotides | 33,080,006 |
| Total unigene | 41,761 |
| N50 of unigenes (nt) | 1510 |
| Min length of unigenes (nt) | 201 |
| Median length of unigenes (nt) | 379 |
| Max length of unigenes (nt) | 21,193 |
| Unigenes with homolog in NR | 18,903 |
Homology analysis and Gene Ontology (GO) annotation
Among the 41,761 unigenes, 18,903 were matched by a Blastx similarity of the entries in the NCBI non-redundant (nr) protein database, with a cut-off E-value of 10−5. The highest match percentage (54.60 %) was to Tribolium castaneum (red flour beetle) sequences followed by Dendroctonus ponderosae (16.70 %), Acyrthosiphon pisum (pea aphid) (2.50 %), Diaphorina citri (asian citrus psyllid) (1.90 %) and Bombyx mori (silkworm) (1.30 %) (Additional file 2: Figure S2).
Gene Ontology (GO) annotation was used to classify transcripts into functional groups according to the GO category. Of the 41,761 unigenes, 14,147 (33.87 %) could be annotated based on sequence similarity. In the molecular function category, the genes expressed in the antenna were mostly associated with binding, catalytic, and transporter activities. In the biological process category, cellular, metabolic, and single-organism processes were the most represented. In the cellular component category, cell, cell part, and organelle were the most abundant groups (Additional file 3: Figure S3).
Identification of candidate chemosensory genes
By similarity analysis, a total of 104 transcripts belonging to gene families putatively involved in insect chemoreception were identified, including OBPs (26 transcripts), CSPs (12 transcripts), SNMPs (four transcripts), ORs (43 transcripts), IRs (9 transcripts) and GRs (10 transcripts) (Tables 2 and 3). Compared with insects where the chemosensory genes had been identified by analyzing either the genome or transcriptome, the number of candidate chemosensory genes identified here in C. bowringi was similar to those in D. ponderosae (111) and more than I. typographus (80), but less than in T. castaneum (642) (Fig. 1).
Table 2.
The Blastx Match of C. bowringi candidate OBPs, CSPs and SNMPs genes
| Gene | Acc. | ORF | Signal | Complete | Best Blastx Match | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Name | No. | (aa) | Peptide | ORF | Name | Acc. No. | Species | E value | Identity (%) | Group |
| Odorant Binding Protein (OBP) | ||||||||||
| OBP1 | KT381483 | 142 | 1–19 | Y | odorant-binding protein 1 | AHA33382.1 | Batocera horsfieldi | 2.00E-61 | 65 | Classic |
| OBP2 | KT381484 | 142 | 1–21 | Y | odorant binding protein 23 | EFA10803.1 | Tribolium castaneum | 1.00E-43 | 59 | Classic |
| OBP3 | KT381485 | 89 | N | N | odorant-binding protein 5 | AHA39270.1 | Monochamus alternatus | 1.00E-20 | 47 | -- |
| OBP4 | KT381486 | 113 | N | N | odorant-binding protein 26 | AGI05179.1 | Dendroctonus ponderosae | 5.00E-19 | 35 | -- |
| OBP5 | KT381487 | 131 | 1–18 | Y | odorant-binding protein 5 | AHA39270.1 | Monochamus alternatus | 5.00E-10 | 35 | Minus-C |
| OBP6 | KT381488 | 133 | 1–16 | Y | odorant-binding protein 2 | AHA39267.1 | Monochamus alternatus | 1.00E-37 | 58 | Minus-C |
| OBP7 | KT381489 | 136 | 1–21 | Y | pheromone binding protein PBP1 | AIV43008.1 | Batocera horsfieldi | 4.00E-48 | 63 | Classic |
| OBP8 | KT381490 | 134 | 1–24 | Y | odorant-binding protein 3 | AHA33381.1 | Batocera horsfieldi | 1.00E-07 | 35 | Classic |
| OBP9 | KT381491 | 151 | 1–19 | Y | odorant-binding protein 1 | AJM71475.1 | Tenebrio molitor | 1.00E-27 | 37 | Classic |
| OBP10 | KT381492 | 134 | 1–27 | Y | odorant binding protein 05 | EFA05677.1 | Tribolium castaneum | 2.00E-06 | 37 | Classic |
| OBP11 | KT381493 | 140 | 1–18 | Y | odorant binding protein 1 | ABR53888.1 | Monochamus alternatus | 2.00E-10 | 33 | Minus-C |
| OBP12 | KT381494 | 248 | 1–18 | Y | odorant binding protein 2 | AKK25130.1 | Dendroctonus valens | 1.00E-31 | 62 | Classic |
| OBP13 | KT381495 | 133 | 1–18 | Y | odorant binding protein | AHE13799.1 | Lissorhoptrus oryzophilus | 1.00E-22 | 37 | Minus-C |
| OBP14 | KT381496 | 123 | 1–23 | Y | odorant-binding protein 16 | AGI05186.1 | Dendroctonus ponderosae | 3.00E-06 | 29 | Classic |
| OBP15 | KT381497 | 140 | 1–19 | Y | minus-C odorant binding protein 3 | ADD82416.1 | Batocera horsfieldi | 8.00E-14 | 40 | Minus-C |
| OBP16 | KT381498 | 141 | 1–18 | Y | minus-C odorant binding protein 2 | ADD70031.1 | Batocera horsfieldi | 3.00E-18 | 32 | Minus-C |
| OBP17 | KT381499 | 136 | 1–18 | Y | odorant-binding protein 2 | AHA33380.1 | Batocera horsfieldi | 4.00E-48 | 57 | Classic |
| OBP18 | KT381500 | 179 | 1–22 | Y | odorant-binding protein 28 | AHF71059.1 | Lygus lineolaris | 2.00E-50 | 50 | Plus-C |
| OBP19 | KT381501 | 130 | 1–22 | Y | odorant-binding protein 5 | AHA39270.1 | Monochamus alternatus | 8.00E-14 | 34 | Minus-C |
| OBP20 | KT381502 | 130 | 1–15 | Y | odorant binding protein 4 | AKK25132.1 | Dendroctonus valens | 2.00E-34 | 44 | Minus-C |
| OBP21 | KT381503 | 142 | 1–20 | Y | odorant binding protein | AHE13800.1 | Lissorhoptrus oryzophilus | 1.00E-07 | 25 | Minus-C |
| OBP22 | KT381504 | 132 | 1–16 | Y | odorant binding protein 10 | AKK25136.1 | Dendroctonus valens | 4.00E-13 | 30 | Minus-C |
| OBP23 | KT381505 | 130 | 1–19 | Y | odorant binding protein C03 | EFA07546.1 | Tribolium castaneum | 9.00E-18 | 37 | Minus-C |
| OBP24 | KT381506 | 103 | N | N | minus-C odorant binding protein 4 | ADD82417.1 | Batocera horsfieldi | 7.00E-12 | 36 | -- |
| OBP25 | KT381507 | 248 | 1–21 | Y | odorant-binding protein 2 | AGI05158.1 | Dendroctonus ponderosae | 2.00E-51 | 41 | Plus-C |
| OBP26 | KT381508 | 150 | 1–16 | Y | odorant binding protein 12 | EFA02857.1 | Tribolium castaneum | 1.00E-22 | 36 | Classic |
| Chemosensory Protein (CSP) | ||||||||||
| CSP1 | KT381509 | 126 | 1–18 | Y | chemosensory protein 12 | NP_001039280.1 | Tribolium castaneum | 1.00E-40 | 56 | |
| CSP2 | KT381510 | 138 | 1–19 | Y | chemosensory protein 11 precursor | NP_001039279.1 | Tribolium castaneum | 2.00E-31 | 52 | |
| CSP3 | KT381511 | 130 | 1–19 | Y | chemosensory protein 6 | AGI05162.1 | Dendroctonus ponderosae | 4.00E-34 | 55 | |
| CSP4 | KT381512 | 124 | 1–18 | Y | CSP11 | AKI84394.1 | Holotrichia parallela | 1.00E-37 | 53 | |
| CSP5 | KT381513 | 126 | 1–17 | Y | chemosensory protein 8 | AHE13803.1 | Lissorhoptrus oryzophilus | 7.00E-45 | 80 | |
| CSP6 | KT381514 | 123 | 1–19 | Y | chemosensory protein 2 | AGI05172.1 | Dendroctonus ponderosae | 7.00E-36 | 45 | |
| CSP7 | KT381515 | 125 | 1–18 | Y | chemosensory protein 7 precursor | NP_001039289.1 | Tribolium castaneum | 1.00E-54 | 69 | |
| CSP8 | KT381516 | 97 | 1–19 | N | chemosensory protein CSP3 | AJO62209.1 | Tenebrio molitor | 7.00E-33 | 59 | |
| CSP9 | KT381517 | 148 | N | Y | chemosensory protein CSP7 | AJO62213.1 | Tenebrio molitor | 9.00E-31 | 46 | |
| CSP10 | KT381518 | 138 | 1–16 | Y | chemosensory protein 8 | AHE13803.1 | Lissorhoptrus oryzophilus | 3.00E-30 | 45 | |
| CSP11 | KT381519 | 113 | N | Y | chemosensory protein 1 isoform X1 | XP_008200934.1 | Tribolium castaneum | 1.00E-42 | 69 | |
| CSP12 | KT381520 | 162 | N | Y | chemosensory protein | AFI45003.1 | Dendroctonus ponderosae | 9.00E-65 | 72 | |
| Sensory Neuron Membrane Protein (SNMP) | ||||||||||
| SNMP1a | KT381536 | 514 | Y | SNMP-1 | AJO62245.1 | Tenebrio molitor | 0.00E + 00 | 59 | ||
| SNMP1b | KT381537 | 534 | Y | sensory neuron membrane protein | AFI45066.1 | Dendroctonus ponderosae | 0.00E + 00 | 52 | ||
| SNMP2 | KT381538 | 522 | Y | SNMP-2 | AJO62246.1 | Tenebrio molitor | 9.00E-119 | 42 | ||
| SNMP3 | KT381539 | 520 | Y | sensory neuron membrane protein 2-like | XP_008198962.1 | Tribolium castaneum | 3.00E-162 | 46 | ||
Table 3.
The Blastx Match of C. bowringi candidate ORs, IRs and GR genes
| Gene | Acc. | ORF | Signal | Complete | Best Blastx Match | ||||
|---|---|---|---|---|---|---|---|---|---|
| Name | No. | (aa) | Peptide | ORF | Name | Acc. No. | Species | E value | Identity (%) |
| Odorant Receptor (OR) | |||||||||
| OR1 | KT381540 | 344 | 4 | N | odorant receptor 127 | EEZ97733.1 | Tribolium castaneum | 1.00E-24 | 26 |
| ORco(OR2) | KT381541 | 479 | 7 | Y | odorant receptor co-receptor | AJF94638.2 | Ambrostoma quadriimpressum | 0.00E + 00 | 92 |
| OR3 | KT381542 | 330 | 5 | N | odorant receptor 43 | EEZ99411.1 | Tribolium castaneum | 6.00E-101 | 44 |
| OR4 | KT381543 | 378 | 6 | Y | odorant receptor 14 | AKC58549.1 | Anomala corpulenta | 3.00E-44 | 30 |
| OR5 | KT381544 | 254 | 4 | N | odorant receptor 18 | AKC58553.1 | Anomala corpulenta | 1.00E-16 | 28 |
| OR6 | KT381545 | 384 | 7 | Y | odorant receptor 89 | EFA10702.1 | Tribolium castaneum | 3.00E-43 | 32 |
| OR7 | KT381546 | 318 | 4 | N | olfactory receptor OR16 | AJO62235.1 | Tenebrio molitor | 2.00E-47 | 32 |
| OR8 | KT381547 | 174 | 3 | N | odorant receptor 44 | EEZ99412.1 | Tribolium castaneum | 3.00E-43 | 40 |
| OR9 | KT381548 | 336 | 5 | N | odorant receptor 59 | EEZ99171.1 | Tribolium castaneum | 1.00E-91 | 44 |
| OR10 | KT381549 | 363 | 5 | Y | odorant receptor 14 | AKC58549.1 | Anomala corpulenta | 1.00E-41 | 27 |
| OR11 | KT381550 | 336 | 5 | N | odorant receptor 64 | EFA10800.1 | Tribolium castaneum | 5.00E-146 | 56 |
| OR12 | KT381551 | 156 | 2 | N | odorant receptor 47 | EFA02940.1 | Tribolium castaneum | 9.00E-24 | 36 |
| OR13 | KT381552 | 420 | 8 | Y | odorant receptor 14 | AKC58549.1 | Anomala corpulenta | 1.00E-33 | 27 |
| OR14 | KT381553 | 356 | 4 | N | odorant receptor 58 | EEZ99414.1 | Tribolium castaneum | 1.00E-49 | 33 |
| OR15 | KT381554 | 389 | 7 | Y | odorant receptor 3 | EFA01310.1 | Tribolium castaneum | 1.00E-66 | 36 |
| OR16 | KT381555 | 196 | 0 | N | odorant receptor 128 | EFA02867.1 | Tribolium castaneum | 9.00E-10 | 21 |
| OR17 | KT381556 | 387 | 7 | Y | odorant receptor 80 | EFA10776.1 | Tribolium castaneum | 3.00E-55 | 30 |
| OR18 | KT381557 | 174 | 0 | N | odorant receptor 49b-like | XP_001812261.1 | Tribolium castaneum | 4.00E-18 | 34 |
| OR19 | KT381558 | 379 | 6 | Y | odorant receptor 89 | EFA10702.1 | Tribolium castaneum | 2.00E-45 | 32 |
| OR20 | KT381559 | 248 | 3 | N | odorant receptor 23 | AGI05173.1 | Dendroctonus ponderosae | 3.00E-12 | 24 |
| OR21 | KT381560 | 355 | 4 | N | odorant receptor 58 | EEZ99414.1 | Tribolium castaneum | 1.00E-48 | 32 |
| OR22 | KT381561 | 390 | 3 | Y | odorant receptor 89 | EFA10702.1 | Tribolium castaneum | 3.00E-41 | 29 |
| OR23 | -- | 42 | 0 | N | odorant receptor 82a | XP_966790.1 | Tribolium castaneum | 3.00E-57 | 45 |
| OR24 | KT381562 | 395 | 4 | Y | odorant receptor 89 | EFA10702.1 | Tribolium castaneum | 2.00E-51 | 29 |
| OR25 | -- | 58 | 0 | N | odorant receptor 35 | EEZ99408.1 | Tribolium castaneum | 2.00E-21 | 73 |
| OR26 | KT381563 | 380 | 6 | Y | odorant receptor 41 | EEZ99227.1 | Tribolium castaneum | 2.00E-46 | 29 |
| OR27 | KT381564 | 398 | 6 | Y | odorant receptor 167 | EFA02801.1 | Tribolium castaneum | 9.00E-70 | 35 |
| OR28 | KT381565 | 368 | 4 | Y | odorant receptor 120 | EEZ99330.1 | Tribolium castaneum | 3.00E-14 | 24 |
| OR29 | KT381566 | 250 | 3 | N | odorant receptor 14 | AKC58549.1 | Anomala corpulenta | 7.00E-29 | 29 |
| OR30 | KT381567 | 194 | 4 | N | odorant receptor 37 | EEZ99229.1 | Tribolium castaneum | 3.00E-29 | 30 |
| OR31 | KT381568 | 418 | 6 | Y | odorant receptor 14 | AKC58549.1 | Anomala corpulenta | 1.00E-36 | 27 |
| OR32 | KT381569 | 393 | 6 | Y | odorant receptor 94a-like | XP_011629601.1 | Pogonomyrmex barbatus | 5.00E-13 | 27 |
| OR33 | KT381570 | 185 | 2 | N | olfactory receptor OR10 | AJO62229.1 | Tenebrio molitor | 3.00E-51 | 37 |
| OR34 | KT381571 | 436 | 5 | Y | odorant receptor Or1-like | XP_008560066.1 | Microplitis demolitor | 3.00E-16 | 27 |
| OR35 | KT381572 | 388 | 4 | Y | odorant receptor 92 | EFA02873.1 | Tribolium castaneum | 6.00E-49 | 30 |
| OR36 | KT381573 | 383 | 7 | Y | odorant receptor 14 | AKC58549.1 | Anomala corpulenta | 7.00E-58 | 32 |
| OR37 | KT381574 | 371 | 4 | Y | odorant receptor 123 | EEZ99420.1 | Tribolium castaneum | 2.00E-14 | 24 |
| OR38 | KT381575 | 271 | 2 | N | odorant receptor 37 | EEZ99229.1 | Tribolium castaneum | 2.00E-82 | 47 |
| OR39 | KT381576 | 121 | 2 | N | olfactory receptor OR60 | AJE25900.1 | Planotortrix excessana | 6.00E-07 | 39 |
| OR40 | KT381577 | 369 | 6 | Y | putative olfactory receptor 10 | BAR43452.1 | Ostrinia furnacalis | 1.00E-19 | 23 |
| OR41 | KT884514 | 79 | 0 | N | odorant receptor 184 | EFA01394.1 | Tribolium castaneum | 1.00E-05 | 33 |
| OR42 | KT884515 | 83 | 0 | N | olfactory receptor 17 | CAM84015.1 | Tribolium castaneum | 3.00E-04 | 27 |
| OR43 | KT884516 | 73 | 0 | N | odorant receptor 3 | EFA01310.1 | Tribolium castaneum | 1.00E-04 | 38 |
| Ionotropic Receptor (IR) | |||||||||
| IR6 | KT381529 | 920 | 3 | Y | chemosensory ionotropic receptor IR6 | AJO62244.1 | Tenebrio molitor | 0.00E + 00 | 80 |
| IR21a | KT381530 | 158 | 1 | N | chemosensory ionotropic receptor 21a | AKC58586.1 | Anomala corpulenta | 1.00E-82 | 58 |
| IR75q | KT381531 | 483 | 3 | N | chemosensory ionotropic receptor 75q | AKC58589.1 | Anomala corpulenta | 3.00E-110 | 46 |
| IR8a | KT381532 | 866 | 3 | Y | ionotropic receptor 8a | AGI05169.1 | Dendroctonus ponderosae | 0.00E + 00 | 60 |
| IR41a | KT381533 | 347 | 2 | N | chemosensory ionotropic receptor 41a | AKC58587.1 | Anomala corpulenta | 5.00E-65 | 40 |
| IR5 | KT381534 | 552 | 3 | Y | chemosensory ionotropic receptor IR5 | AJO62243.1 | Tenebrio molitor | 4.00E-180 | 52 |
| IR2 | KT381535 | 607 | 3 | Y | chemosensory ionotropic receptor IR2 | AJO62240.1 | Tenebrio molitor | 0.00E + 00 | 60 |
| IR64a | KT884510 | 213 | 1 | N | ionotropic receptor | BAR64801.1 | Ostrinia furnacalis | 6.00E-57 | 38 |
| IR68a | KT884511 | 95 | 0 | N | ionotropic receptor | BAR64802.1 | Ostrinia furnacalis | 3.00E-44 | 66 |
| Gustatory Receptor (GR) | |||||||||
| GR1 | KT381521 | 134 | 0 | N | gustatory receptor 99 | EFA02933.1 | Tribolium castaneum | 5.00E-06 | 35 |
| GR2 | KT381522 | 287 | 5 | N | gustatory receptor | ABY40623.1 | Tribolium castaneum | 9.00E-71 | 52 |
| GR3 | KT381523 | 120 | 2 | N | gustatory receptor 6 | EFA04712.1 | Tribolium castaneum | 1.00E-30 | 48 |
| GR4 | KT381524 | 216 | 5 | N | gustatory receptor | ABY40595.1 | Tribolium castaneum | 2.00E-06 | 33 |
| GR5 | KT381525 | 77 | 0 | N | gustatory receptor for sugar taste 43a-like | XP_001813898.1 | Tribolium castaneum | 3.00E-17 | 49 |
| GR6 | KT381526 | 183 | 3 | N | putative gustatory receptor 28b | XP_001813096.2 | Tribolium castaneum | 5.00E-08 | 38 |
| GR7 | KT381527 | 113 | 1 | N | gustatory receptor candidate 10 | CAL23143.2 | Tribolium castaneum | 2.00E-59 | 82 |
| GR8 | KT381528 | 118 | 2 | N | gustatory receptor 2 isoform X1 | XP_008191523.1 | Tribolium castaneum | 2.00E-68 | 82 |
| GR9 | KT884512 | 81 | 1 | N | gustatory receptor 102 | EFA02935.1 | Tribolium castaneum | 2.00E-04 | 29 |
| GR10 | KT884513 | 79 | 1 | N | gustatory receptor | ABY40593.1 | Tribolium castaneum | 4.00E-04 | 33 |
Genes without accession number represent that the gene fragments obtained in this study were less than 200 bp in length. Gene fragments less than 200 bp are unable to be deposited in the GenBank, and thus no accession numbers were provided for these genes
Fig. 1.
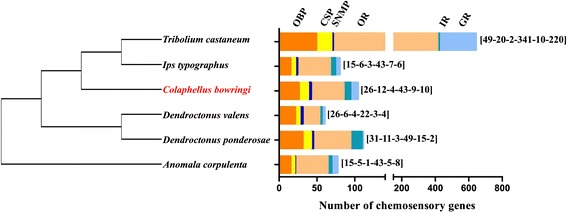
The number of chemosensory genes in different insect species. The digits by the histogram bars represent number of chemosensory genes in different subfamilies. A phylogenetic tree showing the phylogenetic relationships between these species is illustrated on the left. The data are obtained from the current study for C. bowringi and from the references [9, 10, 12, 15] for Tribolium castaneum, [20] for Ips typographus and Dendroctonus ponderosae, [21] for D. valens and [22] for Anomala corpulenta
OBPs
We identified 26 different transcripts encoding candidate OBPs in C. bowringi, which is less than that in D. ponderosae (31), but more than that in I. typographus (15), A. corpulenta (15), and D. valens (21). The results of the sequence analysis revealed 23 transcripts with a full-length open reading frame (ORF) with predicted signal peptide sequences, and CbowOBP3, 4, 24 corresponded to a partial sequence that encoded amino acids from 89 to 113. Except for CbowOBP18, the other 25 CbowOBPs identified were similar to known coleopteran OBPs (Table 2). Among the 26 CbowOBPs, CbowOBP5 showed the highest expression level (RPKM = 18323.68) (Additional file 4: Table S1)
A phylogenetic tree of the OBPs was constructed using the protein sequences from C. bowringi, T. castaneum, D. ponderosae, I. typographus, A. corpulenta, and Drosophila melanogaster (fruit fly) (Fig. 2). As previous reports [4, 40–42] and our results, 23 full-length CbowOBPs could be divided into three groups: Minus-C OBPs (CbowOBP5, 6, 11, 13, 15, 16, 19, 20, 21, 22 and 23), Plus-C OBPs (CbowOBP18 and 25), and the remainder Classic OBPs.
Fig. 2.
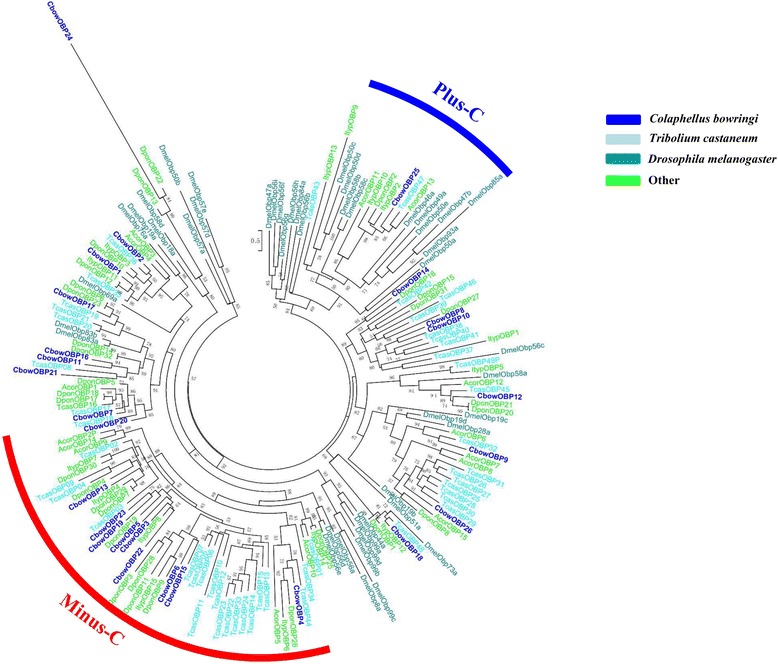
Phylogenetic tree of insect OBP. The C. bowringi translated genes are shown in blue. Amino acid sequences used for the tree are given in Additional file 6: Table S2. Bootstrap values greater than 50 % are shown. The Plus-C subfamily is marked in blue, and the Minus-C subfamily is marked in red
CSPs
In total, 12 different transcripts encoding candidate CSPs with four conserved cysteine profiles were obtained in C. bowringi through bioinformatic analysis, which included 11 sequences predicted to be full length and 8 with a signal peptide (Table 2), with CbowCSP3 harbouring the highest expression level (RPKM = 4155.27) (Additional file 4: Table S1). The phylogenetic tree revealed two branches with high bootstrap values: CbowCSP8 with TcasCSP8 and DponCSP11, and finally CbowCSP11 with AgamCSP8, BmorCSP20, and BmorCSP21 (Fig. 3).
Fig. 3.
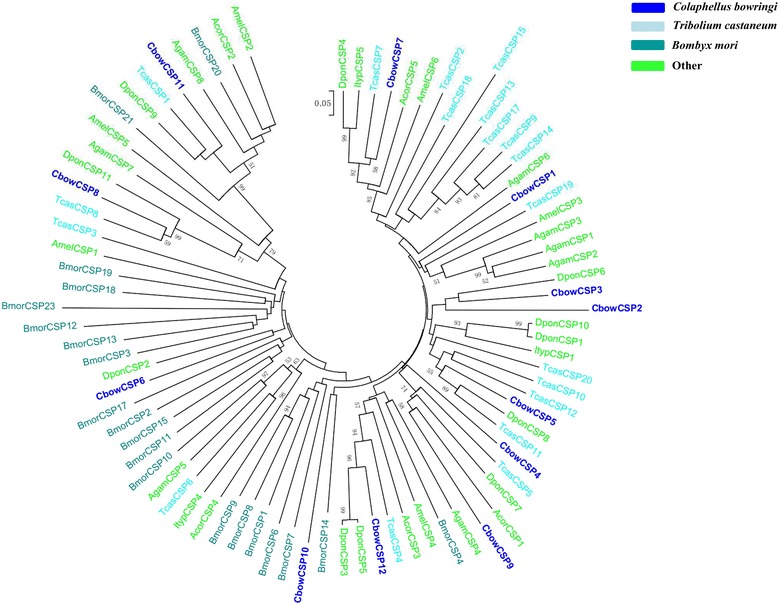
Phylogenetic tree of insect CSP. The C. bowringi translated genes are shown in blue. Amino acid sequences used for the tree are given in Additional file 6: Table S2. Bootstrap values greater than 50 % are shown
SNMPs
Four SNMP homologs with full-length ORFs were also obtained from the C. bowringi transcriptome. This number is consistent with D. valens, but is greater than that in other previously studied coleoptera insects (Fig. 1). The Blastx results demonstrated that CbowSNMPs encoding proteins harboured a 42–59 % identity to those of other reported insects (Table 2). The RPKM results showed that CbowSNMPs displayed the highest expression level (RPKM = 90.28) (Additional file 4: Table S1). Based on the phylogenetic analysis, we found that CbowSNMP1a and CbowSNMP1b clustered with the coleoptera SNMP1 group, while CbowSNMP2 and CbowSNMP3 clustered with high support with DponSNMP2 and ItypSNMP2Fix, respectively (Additional file 5: Figure S4).
ORs
Forty-three different transcripts for candidate ORs were identified based on the antennal transcriptome data for C. bowringi, among which 20 sequences contained a full-length ORF that encoded 363 to 479 amino acids. We identified one OR sequence that shared a high level of identity with the conserved ORco proteins of other insect species and labelled it CbowORco. The amino-acid sequence of CbowORco shared 92 % identity with the co-receptor of Ambrostoma quadriimpressum (leaf beetle) (AJF94638.2). More than 80 % of the CbowORs were highly divergent, and had low levels of identity (21–40 %) with other reported insect ORs. Based on prediction and comparison with other insect ORs [20, 22], we found full-length CbowORs had 3 to 8 TMD (transmembrane domains) (Table 3).
A phylogenetic analysis was conducted using a data set containing the sequences of the 36 ORs longer than 160 amino acids in C. bowringi and 192 ORs from four other coleopteran species (Fig. 4). The OR sequences were clustered into several subgroups according to previous studies. CbowORs were only present within the previously defined coleopteran OR subgroups 1, 2, 3, and 7 as well as the ORco subgroup. We found that 6 CbowORs (CbowOR6, 17, 19, 22, 24 and 35) and a functionally characterized McarOR20 [43] were clustered in subgroup 1. A total of17 CbowORs (OR3, 4, 7–11, 13–15, 21, 26, 29, 31, 33, 36 and 38) and 2 functionally characterized McarORs (OR3 and 5) [43] belong to subgroup 2 (Fig. 4).
Fig. 4.
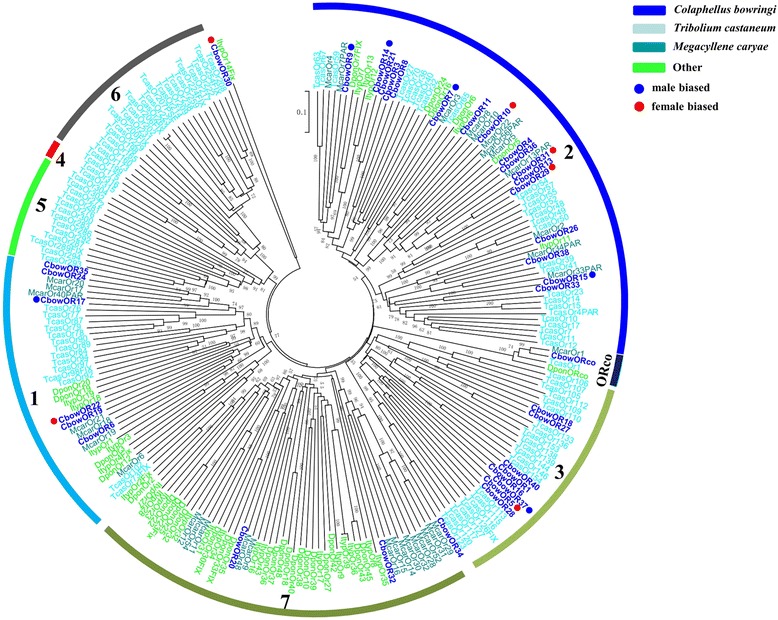
Phylogenetic tree of insect OR. The C. bowringi translated genes are shown in blue. Amino acid sequences used for the tree are given in Additional file 6: Table S2. Bootstrap values greater than 50 % are shown
The transcriptional profiles of CbowOR genes were characterized using qPCR, and the results revealed that all of the 43 CbowORs displayed predominately antenna linked or otherwise biased expression levels. Although we did not identify apparent sex-specific genes in these C. bowringi olfactory receptors, there were six (CbowOR7, 9, 14, 15, 17 and 37) and10 (CbowOR5 10, 12, 22, 25, 29, 30,31, 41 and 42) with significantly higher expression in the male and female antennae, respectively (Fig. 5).
Fig. 5.
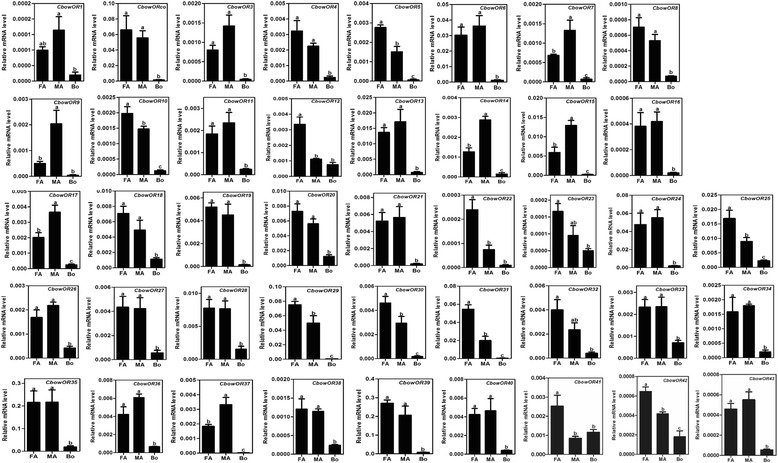
Relative expression levels of all ORs in adult antennae and whole body, using qPCR. FA, female antennae; MA, male antennae; Bo, whole insect body (without antennae). The relative expression level is indicated as mean ± SE (N = 3). Different capital letters mean significant difference between tissues (P <0.05, ANOVA, LSD)
IRs and GRs
In total, we identified nine IR and ten GR candidates in C. bowringi, which is similar to that reported in other recent antennal transcriptomic studies of coleoptera insects [20, 22] (Fig. 1). Only four of these likely represented a full-length ORF (CbowIR2, 5, 6 and 8a), among which we also found three TMDs. The RPKM results showed that CbowIR6 (RPKM = 74.73) and CbowGR1 (RPKM = 53.07) displayed the highest expression levels (Additional file 4: Table S1). According to the phylogenetic tree of the IRs from D. melanogaster and various coleopterans, we observed all nine CbowIRs were clustered into antennal IRs and IR25a/IR8a clades (Fig. 6).
Fig. 6.
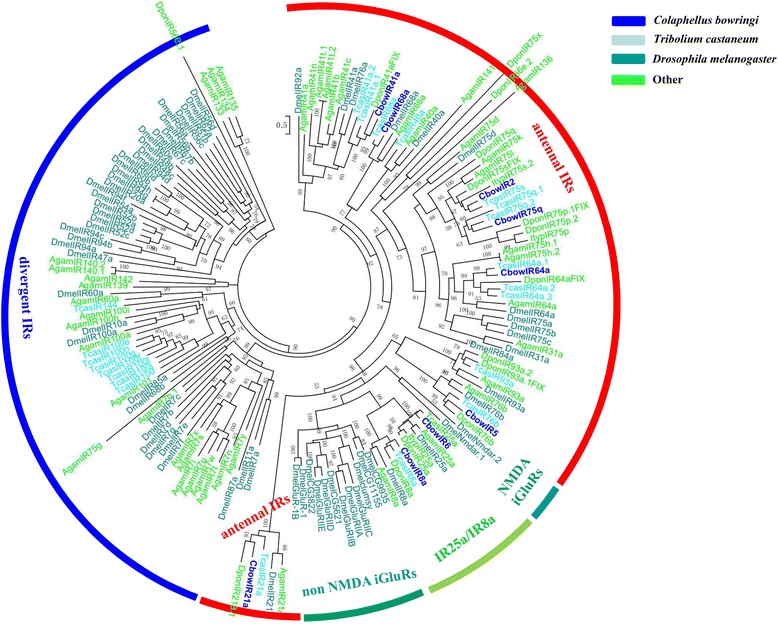
Phylogenetic tree of insect IR. The C. bowringi translated genes are shown in blue. Amino acid sequences used for the tree are given in Additional file 6: Table S2. Bootstrap values greater than 50 % are shown
Discussion
Compared to dipterans and lepidopterans, the molecular basis of chemoreception in coleopterans is relatively poorly understood. In the current study, we sequenced and analyzed the transcriptome of antennae from C. bowringi. Among the 41,761 unigenes identified, only 45.26 % gene translations shared significant similarity with entries in the NCBI non-redundant (nr) protein database, and only 33.87 % could be annotated to one or more GO term, which is similar to that reported in other coleopteran species [20–22], indicating that a large number of C. bowringi genes are non-coding or homologous to genes that do not have any GO term, or perhaps some are C. bowringi-specific or fast-evolving genes. Importantly, we identified 104 novel chemosensory genes in C. bowringi. Our results not only establish a means to further elucidate the molecular mechanisms of chemosensation, but also provide insight into insect physiology and the development of additional pest control strategies [44].
The total number (104) of chemosensory transcripts identified in C. bowringi is different from what has been reported in D. ponderosae (111) and I. typographus (80), This phenomenon may be due to the evolution of divergent physiological behaviours (such as: herbivory, mating, and oviposition) of different insects during the process of adaptation to various environments [45–47]. Specific environments might lead to divergent evolutionary trajectories of the same ancestral chemosensory genes, resulting in different functional genes among species.
In total, 26 OBPs were identified in the antennal transcriptome of C. bowringi. This is close to the number of OBPs in the antennae of D. ponderosae (31) and D. valens (21), however less than in T. castaneum (49). The number of CbowCSPs (12) is similar to D. ponderosae (11) while less than T. castaneum (20). Previous studies showed that some insect OBPs and CSPs are expressed primarily or exclusively in non-antennae tissues or in larvae [23, 48–50], thus we may not have obtained these types of genes.
Currently, the general mechanism of insect SNMP function is still poorly understood. While DmelSNMP1 is essential for the detection of the pheromone (Z)-11-octadecenyl acetate (a volatile male-specific fatty-acid-derived pheromone) in D. melanogaster, and it is thought that SNMP acts in concert with odorant receptors to capture pheromone molecules on the surface of olfactory dendrites [51, 52]. In this study, SNMP transcripts were identified in C. bowringi (4) and were found to be more numerous than those in the T. castaneum genome (2). The expression of antennal SNMPs in C. bowringi, similar to what was previously reported for other known coleopteran insects, suggests that SNMPs in coleopteran insects may have same role as in D. melanogaster.
In comparison with the lepidopterans, although the coleopterans ORs have been focused on in recent years [20–22, 43, 53], species richness and function analyses are still lacking. For this reason, it is necessary to identify additional coleopteran ORs to further elucidate the mechanisms of coleopteran chemosensation. In insects, gene duplications and deletion events may be the major contributors to high levels of diversity in OR genes and variability in gene number among species. Forty-three ORs were first identified in the antennal transcriptome of C. bowringi, which is less than the number of ORs in the complete genome of T. castaneum (341). However, it is same as the number of ORs identified in the antennal transcriptome of I. typographus (43) and A. corpulenta (43), suggesting we may missed some larvae-biased ORs or those with lower expression levels. Remarkably, similar to what has been observed in T. castaneum, M. caryae, and A. corpulenta, a species-specific expansion of ORs (CbowOR1/5/16/28/37/40 and 3/8/14/21) was also found in C. bowringi, which may reflect that these distinct species inhabit different ecological niches. M. caryae was the first beetle in which the function of the ORs was characterized [43]. For this reason, we are only able to speculate on the possible functions of CbowORs by examining those of the orthologous McarORs. McarOR3 can bind the pheromone component (S)-2-methyl-1-butanol and additional structurally related chemicals using functional analysis in vitro. CbowOR7 displayed a male-biased transcriptional profile characteristic and could be clustered into the same subgroup with McarOR3, indicating that it may have a similar function to McarOR3 as well as other lepidopteran pheromone receptors (PRs) [54–57]. In total, we identified 6 (CbowOR7, 9, 14, 15, 17 and 37) and 10 (CbowOR5, 10, 12, 22, 25, 29, 30, 31, 41 and 42) genes with significantly higher expression levels in male and female antennae, respectively. Based on previous studies of the insect OR functions [57–60], the male-biased CbowORs may be involved in the detection of the sex pheromone or other male-specific behaviours, while female-biased CbowORs may detect odours critical to female behaviour, such as oviposition cues or male-produced courtship pheromones. The sex-specific functions of these CbowORs need to be further investigated in the future.
Furthermore, we identified 9 IRs from the antennal transcriptome assembly in C. bowringi, which is fewer than that in T. castaneum (10) and D. ponderosae (15). This may be due to the possibility that some transcripts were missing from our antennal transcriptome. Like ORco, both IR8a and IR25a were thought to act as co-receptors since they are co-expressed along with other IRs [61]. Sequence alignments and the phylogenetic tree revealed that CbowIR8a and CbowIR6 (25a) belong to the co-expression IR group. To date, multiple GRs have also been identified in different insect species [20, 62–65]. While only ten CbowGRs were found in C. bowringi, this was expected since GRs are primarily expressed in gustatory organs, such as the proboscis and maxillary palps, rather than the antennae [65–67].
Conclusions
In conclusion, we identified an extensive set of candidate genes that may be related to odorant perception of C. bowringi by analyzing transcriptomic sequence data. As the first step towards understanding gene functions, we conducted a comprehensive and comparative phylogenetic analysis and examined OR gene transcription patterns, some of which were sex-biased. Further analysis is needed to explore the function of these genes using integrated functional studies.
Methods
Insect rearing and collection
C. bowringi were collected in April 2015 from a B. campestris field in the Pollution-Free Planting Base of Huaibei City, Huaibei, China. The field studies did not involve endangered or protected species, and no specific permissions were required for these research activities at these locations. Specimens were separated into females and males, and were reared on fresh leaves of B. campestris. The rearing conditions were 25 °C ± 1 °C, a 12 h light : 12 h dark photoperiod, and 70 ± 10 % relative humidity [68]. For transcriptome sequencing, the antennae of 800 adults (400 males and 400 females) were collected. For the expression study of different tissues, 150–200 female antennae (FA), 150–200 male antennae (MA), and 10–15 whole insect body without antennae (Bo) were also collected. All samples were immediately frozen in liquid nitrogen and stored at −80 °C until use.
cDNA library construction
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), cDNA library construction and Illumina sequencing of the samples were performed at Novogene Bioinformatics Technology Co., Ltd., Beijing, China. The mRNA was purified from 3 μg of total RNA using oligo (dT) magnetic beads and fragmented into short sequences in the presence of divalent cations at 94 °C for 5 min. Then, the first-strand cDNA was generated using random hexamer-primed reverse transcription, followed by synthesis of the second-strand cDNA using RNaseH and DNA polymerase I. After the end repair and ligation of adaptors, the products were amplified by PCR and purified using the QIAquick PCR Purification Kit (Qiagen, Valencia, CA, USA) to create a cDNA library, which was assessed on the Agilent Bioanalyzer 2100 system.
Clustering and sequencing
Clustering of the index-coded samples was performed on a cBot Cluster Generation System using TruSeq PE Cluster Kit v3-cBot-HS (Illumina) according to the manufacturer’s instructions. After cluster generation, the libraries were sequenced on an Illumina HiSeq™ 2500 platform and paired-end reads were generated.
De novo assembly of short reads and gene annotation
Clean short reads were obtained by removing those containing an adapter or poly-N and of low quality from the raw reads. Transcriptome de novo assembly was carried out with the short read assembling program Trinity (r20140413p1) [69, 70] with min_kmer_cov set to two by default and all other parameters also set as default. The resulting sequences were the unigenes. The unigenes larger than 150 bp were first aligned by Blastx to protein databases, including Nr, Swiss-Prot, KEGG, and COG (E-value < 10−5), retrieving proteins with the highest sequence similarity with the given unigenes along with their protein functional annotations. Then, we used the Blast2GO program [71] to obtain a GO annotation of the unigenes, and GO functional classification with the WEGO software [72].
Expression abundance analysis of the Unigenes
The expression abundance of these unigenes were calculated based on the reads per kilobase per million mapped reads (RPKM) method [73], using the formula: RPKM (A) = (10,00,000 × C × 1000)/(N × L), where RPKM (A) is the abundance of gene A, C is the number of reads that uniquely aligned to gene A, N is the total number of reads that uniquely aligned to all genes, and L is the number of bases in gene A. The RPKM method was able to eliminate the influence of different gene lengths and sequencing discrepancies in the calculation of expression abundance.
RNA isolation and cDNA synthesis
Total RNA was extracted with the SV 96 Total RNA Isolation System (Promega, Madison, WI, USA) following the manufacturer’s instructions, in which a DNaseI digestion was included to avoid contamination of genomic DNA. RNA quality was checked with a spectrophotometer (NanoDropTM 1000, Thermo Fisher Scientific, USA). The single-stranded cDNA templates were synthesized from 1 μg of total RNA from the various tissue samples using the PrimeScript™ RT Master Mix (TaKaRa, Dalian, China).
Sequence analysis
The open reading frames (ORFs) of the chemosensory genes were predicted using ORF finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). The similarity searches were performed with the NCBI-BLAST network server (http://blast.ncbi.nlm.nih.gov/). Transmembrane domains of both CbowORs and CbowIRs were predicted with the TMHMM Server Version 2.0 (http://www.cbs.dtu.dk/services/TMHMM). Putative N-terminal signal peptides of CbowOBPs and CbowCSPs were predicted by Signal IP 4.1 (http://www.cbs.dtu.dk/services/SignalP/) [74].
Nomenclature of all genes
We adopted nomenclature for the CbowORco, CbowIRs and CbowSNMPs that are analogous to those deposited in GenBank (http://www.ncbi.nlm.nih.gov/genbank/). Based on previous studies, CbowOBPs were divided into three groups [3, 4]: Classic OBPs, characterized by 6 cysteine residues at conserved positions; Plus-C OBPs, which have 4–6 additional cysteines and one characteristic proline; and Minus-C OBPs, which are missing cysteine residues, generally C2 and C5. The rest of the chemosensory genes of C. bowringi were named based on their order in the antennal transcriptome data.
Phylogenetic analysis
The phylogenetic trees were reconstructed for the analyses of CbowOBPs, CbowCSPs, CbowSNMP, CbowORs, and CbowIRs, using these genes (the signal peptides of sequences were removed from OBPs and CSPs) as well as the sequences in other insects. The OBP data set contained 26 sequences from C. bowringi and 150 from other insects. The CSP data set contained 12 sequences from C. bowringi and 72 from other insects. The SNMP data set contained 4 sequences from C. bowringi and 17 from other insects. The OR data set contained 36 sequences from C. bowringi (amino acids > 160 aa), and 192 from other insects. The IR data set contained 9 sequences from C. bowringi and 108 from other insects. The amino acid sequences of the genes used for phylogenetic tree construction are listed in Additional file 6: Table S2. Amino acid sequences were aligned with ClustalX 1.83 [75] and unrooted trees were constructed with MEGA5.0 [76] using the neighbour-joining method, with Poisson correction of distances (CSP, SNMP, and OR) and FastTree 2.1.7 [77] using maximum-likelihood method (OBP and IR). The species phylogenetic tree was constructed based on the alignment result of cytochrome oxidase subunit I (COI) genes, from different species (T. castaneum: KJ003352.1, I. typographus: KF846151.1, D. ponderosae: JQ308497.1, D. valens: EU404100.1 and A. corpulenta: the reference [19]) using MEGA5.0.
Quantitative real time-PCR validation
The expression profiles of 43 OR genes were analyzed using quantitative real time-PCR (qPCR) experiments. The qPCR was performed on an ABI 7300 (Applied Biosystems, Foster City, CA, USA) using a mixture of 10 μl 2 × TransStart Top Green qPCR SuperMix (TransGen Biotech, Beijing, China), 0.4 μl of each primer (10 μM), 2.5 ng of sample cDNA, and 6.8 μl sterilized ultrapure H2O. The reaction programs were 30 s at 94 °C, 40 cycles of 94 °C for 5 s and 60 °C for 31 s. This was followed by the measurement of fluorescence during 55–95 °C melting curve in order to detect a single gene-specific peak and to check the absence of primer dimer peaks. A single and discrete peak was detected for all primers tested. Negative controls were non-template reactions (replacing cDNA with H2O). The results were analyzed using the ABI 7300 analysis software SDS 1.4. The qPCR primers (Additional file 7: Table S3) were designed using Beacon Designer 7.9 (PREMIER Biosoft International, CA, USA).
According to a previous study [68], expression levels of these genes were calculated relative to the two most stable reference genes CbowEF1α and CbowACT1 using the Q-Gene method in Microsoft Excel-based software of Visual Basic [78, 79]. For each sample, three biological replications were performed with each biological replication measured in three technique replications.
Statistical analysis
Data (mean ± SE) form various samples were subjected to a one-way nested analysis of variance (ANOVA) followed by the least significant difference test (LSD) for mean comparison using SPSS Statistics 17.0 (SPSS Inc., Chicago, IL, USA).
Supporting information
All the Illumina sequencing data are available from the SRA database (accession number: SRX1309381), and all of the chemosensory genes of Colaphellus bowringi were submitted to the GenBank (accession numbers: KT381483 - KT381577 and KT884510 - KT884516).
Acknowledgements
We thank Bachelor students Wei-Wei Zhou, Tian Tian, Xiao-Tong Zhu and Xiao-Xue Xu (Huaibei Normal University, China) for help collecting insects. We are grateful to Dr. Xiao Li (Shandong Peanut Research Institute, China) for providing the COI sequence of A. corpulenta. This work was supported by grants from the National Natural Science Foundation (NO. 31240075 and 31501647) of China, Natural Science Fund of Education Department of Anhui province (NO. KJ2013A233), and Doctor Scientific Research Foundation of Huaibei Normal University, China (NO. 1500969).
Abbreviations
- RNA-seq
High-throughput mRNA sequencing
- RPKM
Reads per kilobase per million mapped reads
- GO
Gene ontology
- KEGG
Kyoto encyclopedia of genes and genomes
- COG
Clusters of orthologous groups
- OR
Odorant receptor
- GR
Gustatory receptor
- IR
Ionotropic receptor
- OBP
Odorant-binding protein
- CSP
Chemosensory protein
- SNMP
Sensory neuron membrane protein
- ORF
Open reading frame
- TMD
Transmembrane domain
- PCR
Polymerase chain reaction
- qPCR
Quantitative real time RT-PCR
- cDNA
Complementary DNA
- ANOVA
Analysis of variance
- SE
Standard error
Additional files
Distribution of unigene size in the C. bowringi transcriptome assembly. (TIF 190 kb)
Percentage of homologous hits of the C. bowringi transcripts to other insect species. The C. bowringi transcripts were searched by Blastx against the non-redundancy protein database with a cutoff E-value 10−5. Species that have more than 1 % matching hits to the C. bowringi transcripts are shown. (TIF 221 kb)
Gene ontology (GO) classification of the C. bowringi transcripts with Blast2GO program. (TIF 1678 kb)
The Reads Per Kilobase per Million mapped reads (RPKM) values of CbowOBPs, CSPs, SNMPs, IRs and GRs. (XLSX 10 kb)
Phylogenetic tree of insect SNMP. The C. bowringi translated genes are shown in blue. Amino acid sequences used for the tree are given in Additional file 6: Table S2. Values at the nodes are results of bootstrap with 1000 replicates and values greater than 50 % are shown. (TIF 141 kb)
Amino acid sequences of C. bowringi and other insect used in phylogenetic analyses. (PDF 814 kb)
Primers used for qPCR. (XLS 29 kb)
Footnotes
Competing interests
The authors declare no conflict of interests.
Authors’ contributions
XML and YNZ conceived and designed the experimental plan. ZQW, YW and GC preformed the experiments. XML, XYZ, PH, LS, DGD and YNZ analyzed and interpreted the sequence data. YNZ drafted the manuscript. All authors read and approved the final manuscript.
Contributor Information
Xiao-Ming Li, Email: lixiaomingchnu@126.com.
Xiu-Yun Zhu, Email: 805121031@qq.com.
Zhi-Qiang Wang, Email: 2249543361@qq.com.
Yi Wang, Email: chrissy96@163.com.
Peng He, Email: fcc.phe@gzu.edu.cn.
Geng Chen, Email: 2360528717@qq.com.
Liang Sun, Email: liangsun@tricaas.com.
Dao-Gui Deng, Email: dengdg@263.net.
Ya-Nan Zhang, Email: ynzhang_insect@163.com.
References
- 1.Leal WS. Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu Rev Entomol. 2013;58(1):373–91. doi: 10.1146/annurev-ento-120811-153635. [DOI] [PubMed] [Google Scholar]
- 2.Vogt RG. Biochemical diversity of odor detection:OBPs, ODEs and SNMPs. In: Blomquist GJ, Vogt RG, editors. Insect pheromone biochemistry and molecular biology. California: Elsevier; 2003. pp. 397–451. [Google Scholar]
- 3.Zhou JJ. Odorant-binding proteins in insects. Vitam Horm. 2010;83:241–72. doi: 10.1016/S0083-6729(10)83010-9. [DOI] [PubMed] [Google Scholar]
- 4.Xu YL, He P, Zhang L, Fang SQ, Dong SL, Zhang YJ, et al. Large-scale identification of odorant-binding proteins and chemosensory proteins from expressed sequence tags in insects. BMC Genomics. 2009;10(25):632. doi: 10.1186/1471-2164-10-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pelosi P, Zhou JJ, Ban LP, Calvello M. Soluble proteins in insect chemical communication. Cell Mol Life Sci. 2006;63(14):1658–76. doi: 10.1007/s00018-005-5607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelosi P, Calvello M, Ban L. Diversity of odorant-binding proteins and chemosensory proteins in insects. Chem Senses. 2005;30 Suppl 1:i291–2. doi: 10.1093/chemse/bjh229. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Walker WB, Wang G. Pheromone reception in moths: from molecules to behaviors. Prog Mol Biol Transl Sci. 2015;130:109–28. doi: 10.1016/bs.pmbts.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Crasto CJ. Olfactory Receptors. In: Walker JM, editor. Methods in molecular biologyTM. New York: Springer; 2013. [Google Scholar]
- 9.Robertson HM, Warr CG, Carlson JR. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2003;100(Suppl 2):14537–42. doi: 10.1073/pnas.2335847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez-Gracia A, Vieira FG, Rozas J. Molecular evolution of the major chemosensory gene families in insects. Heredity (Edinb) 2009;103(3):208–16. doi: 10.1038/hdy.2009.55. [DOI] [PubMed] [Google Scholar]
- 11.Rogers ME, Sun M, Lerner MR, Vogt RG. Snmp-1, a novel membrane protein of olfactory neurons of the silk moth Antheraea polyphemus with homology to the CD36 family of membrane proteins. J Biol Chem. 1997;272(23):14792–9. doi: 10.1074/jbc.272.23.14792. [DOI] [PubMed] [Google Scholar]
- 12.Vogt RG, Miller NE, Litvack R, Fandino RA, Sparks J, Staples J, et al. The insect SNMP gene family. Insect Biochem Mol Biol. 2009;39(7):448–56. doi: 10.1016/j.ibmb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Tunstall NE, Warr CG. Chemical communication in insects: the peripheral odour coding system of Drosophila melanogaster. Adv Exp Med Biol. 2012;739:59–77. doi: 10.1007/978-1-4614-1704-0_4. [DOI] [PubMed] [Google Scholar]
- 14.Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136(1):149–62. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Croset V, Rytz R, Cummins SF, Budd A, Brawand D, Kaessmann H, et al. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet. 2010;6(8):e1001064. doi: 10.1371/journal.pgen.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKenna MP, Hekmat-Scafe DS, Gaines P, Carlson JR. Putative Drosophila pheromone-binding proteins expressed in a subregion of the olfactory system. J Biol Chem. 1994;269(23):16340–7. [PubMed] [Google Scholar]
- 17.Picimbon JF, Gadenne C. Evolution of noctuid pheromone binding proteins: identification of PBP in the black cutworm moth, Agrotis ipsilon. Insect Biochem Mol Biol. 2002;32(8):839–46. doi: 10.1016/S0965-1748(01)00172-2. [DOI] [PubMed] [Google Scholar]
- 18.Xiu WM, Zhou YZ, Dong SL. Molecular characterization and expression pattern of two pheromone-binding proteins from Spodoptera litura (Fabricius) J Chem Ecol. 2008;34(4):487–98. doi: 10.1007/s10886-008-9452-0. [DOI] [PubMed] [Google Scholar]
- 19.Calvello M, Brandazza A, Navarrini A, Dani FR, Turillazzi S, Felicioli A, et al. Expression of odorant-binding proteins and chemosensory proteins in some Hymenoptera. Insect Biochem Mol Biol. 2005;35(4):297–307. doi: 10.1016/j.ibmb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Andersson MN, Grosse-Wilde E, Keeling CI, Bengtsson JM, Yuen MM, Li M, et al. Antennal transcriptome analysis of the chemosensory gene families in the tree killing bark beetles, Ips typographus and Dendroctonus ponderosae (Coleoptera: Curculionidae: Scolytinae) BMC Genomics. 2013;14:198. doi: 10.1186/1471-2164-14-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu XC, Zhang YN, Kang K, Dong SL, Zhang LW. Antennal transcriptome analysis of odorant reception genes in the Red Turpentine Beetle (RTB), Dendroctonus valens. PLoS One. 2015;10(5):e0125159. doi: 10.1371/journal.pone.0125159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Ju Q, Jie W, Li F, Jiang X, Hu J, et al. Chemosensory gene families in adult antennae of Anomala corpulenta Motschulsky (Coleoptera: Scarabaeidae: Rutelinae) PLoS ONE. 2015;10(4):e0121504. doi: 10.1371/journal.pone.0121504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang YN, Jin JY, Jin R, Xia YH, Zhou JJ, Deng JY, et al. Differential expression patterns in chemosensory and non-chemosensory tissues of putative chemosensory genes identified by transcriptome analysis of insect pest the purple stem borer Sesamia inferens (Walker) PLoS ONE. 2013;8(7):e69715. doi: 10.1371/journal.pone.0069715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Gu S, Zhang Y, Guo Y, Wang G. Candidate olfaction genes identified within the Helicoverpa armigera antennal transcriptome. PLoS ONE. 2012;7(10):e48260. doi: 10.1371/journal.pone.0048260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong DP, Zhang HJ, Zhao P, Xia QY, Xiang ZH. The odorant binding protein gene family from the genome of silkworm, Bombyx mori. BMC Genomics. 2009;10:332. doi: 10.1186/1471-2164-10-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelletier J, Leal WS. Characterization of olfactory genes in the antennae of the Southern house mosquito, Culex quinquefasciatus. J Insect Physiol. 2011;57(7):915–29. doi: 10.1016/j.jinsphys.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Gu SH, Wang SP, Zhang XY, Wu KM, Guo YY, Zhou JJ, et al. Identification and tissue distribution of odorant binding protein genes in the lucerne plant bug Adelphocoris lineolatus (Goeze) Insect Biochem Mol Biol. 2011;41(4):254–63. doi: 10.1016/j.ibmb.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Robertson HM, Wanner KW. The chemoreceptor superfamily in the honey bee, Apis mellifera: expansion of the odorant, but not gustatory, receptor family. Genome Res. 2006;16(11):1395–403. doi: 10.1101/gr.5057506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olivier V, Monsempes C, Francois MC, Poivet E, Jacquin-Joly E. Candidate chemosensory ionotropic receptors in a Lepidoptera. Insect Mol Biol. 2011;20(2):189–99. doi: 10.1111/j.1365-2583.2010.01057.x. [DOI] [PubMed] [Google Scholar]
- 30.Krieger J, Grosse-Wilde E, Gohl T, Dewer YM, Raming K, Breer H. Genes encoding candidate pheromone receptors in a moth (Heliothis virescens) Proc Natl Acad Sci U S A. 2004;101(32):11845–50. doi: 10.1073/pnas.0403052101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue F, Li A, Zhu X, Gui A, Jiang P, Liu X. Diversity in life history of the leaf beetle, Colaphellus bowringi. Baly Acta Entom Sin. 2002;45(4):494–8. [Google Scholar]
- 32.Wang XP. Diapause induction and post-diapause life-history traits in the cabbage beetle, Colaphellus bowringi (Coleoptera: Chrysomelidae) Phd thesis, Hunan Agricultural University. 2004. [DOI] [PubMed] [Google Scholar]
- 33.Liu XP, He HM, Kuang XJ, Xue FS. Mating behavior of the cabbage beetle Colaphellus bowringi Baly (Coleoptera: Chrysomelidae) Insect Sci. 2010;17(1):61–6. doi: 10.1111/j.1744-7917.2009.01285.x. [DOI] [Google Scholar]
- 34.Liu XP, He HM, Kuang XJ, Xue FS. A comparison of female fitness between monogamy and polyandry in the cabbage beetle, Colaphellus bowringi. Anim Behav. 2010;79(6):1391–5. doi: 10.1016/j.anbehav.2010.03.019. [DOI] [Google Scholar]
- 35.Liu XP, He HM, Xue FS. The effect of mating frequency and mating pattern on female reproductive fitness in cabbage beetle, Colaphellus bowringi. Entomol Exp Appl. 2013;146(3):379–85. doi: 10.1111/eea.12037. [DOI] [Google Scholar]
- 36.Liu XP, Xu J, He HM, Xue FS. Male age affects female mate preference and reproductive performance in the cabbage beetle, Colaphellus bowringi. J Insect Behav. 2011;24(2):83–93. doi: 10.1007/s10905-010-9237-5. [DOI] [Google Scholar]
- 37.Liu XP, He HM, Xue FS. The influence of female age on male mating preference and reproductive success in cabbage beetle, Colaphellus bowringi. Insect Sci. 2014;21(4):515–22. doi: 10.1111/1744-7917.12051. [DOI] [PubMed] [Google Scholar]
- 38.Gao Y, Jurat-Fuentes JL, Oppert B, Fabrick JA, Liu C, Gao J, et al. Increased toxicity of Bacillus thuringiensis Cry3Aa against Crioceris quatuordecimpunctata, Phaedon brassicae and Colaphellus bowringi by a Tenebrio molitor cadherin fragment. Pest Manag Sci. 2011;67(9):1076–81. doi: 10.1002/ps.2149. [DOI] [PubMed] [Google Scholar]
- 39.Shu C, Su H, Zhang J, He K, Huang D, Song F. Characterization of cry9Da4, cry9Eb2, and cry9Ee1 genes from Bacillus thuringiensis strain T03B001. Appl Microbiol Biotechnol. 2013;97(22):9705–13. doi: 10.1007/s00253-013-4781-5. [DOI] [PubMed] [Google Scholar]
- 40.Hull JJ, Perera OP, Snodgrass GL. Cloning and expression profiling of odorant-binding proteins in the tarnished plant bug, Lygus lineolaris. Insect Mol Biol. 2014;23(1):78–97. doi: 10.1111/imb.12064. [DOI] [PubMed] [Google Scholar]
- 41.Zhou JJ, Huang W, Zhang GA, Pickett JA, Field LM. “Plus-C” odorant-binding protein genes in two Drosophila species and the malaria mosquito Anopheles gambiae. Gene. 2004;327(1):117–29. doi: 10.1016/j.gene.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Spinelli S, Lagarde A, Iovinella I, Legrand P, Tegoni M, Pelosi P, et al. Crystal structure of Apis mellifera OBP14, a C-minus odorant-binding protein, and its complexes with odorant molecules. Insect Biochem Mol Biol. 2012;42(1):41–50. doi: 10.1016/j.ibmb.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell RF, Hughes DT, Luetje CW, Millar JG, Soriano-Agaton F, Hanks LM, et al. Sequencing and characterizing odorant receptors of the cerambycid beetle Megacyllene caryae. Insect Biochem Mol Biol. 2012;42(7):499–505. doi: 10.1016/j.ibmb.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou JJ, Field LM, He XL. Insect odorant-binding proteins: Do they offer an alternative pest control strategy? Outlooks Pest Manag. 2010;21(1):31–4. doi: 10.1564/21feb08. [DOI] [Google Scholar]
- 45.Goldman-Huertas B, Mitchell RF, Lapoint RT, Faucher CP, Hildebrand JG, Whiteman NK. Evolution of herbivory in Drosophilidae linked to loss of behaviors, antennal responses, odorant receptors, and ancestral diet. Proc Natl Acad Sci U S A. 2015;112(10):3026–31. doi: 10.1073/pnas.1424656112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou X, Slone JD, Rokas A, Berger SL, Liebig J, Ray A, et al. Phylogenetic and transcriptomic analysis of chemosensory receptors in a pair of divergent ant species reveals sex-specific signatures of odor coding. PLoS Genet. 2012;8(8):e1002930. doi: 10.1371/journal.pgen.1002930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lavagnino N, Serra F, Arbiza L, Dopazo H, Hasson E. Evolutionary enomics of genes involved in olfactory behavior in the Drosophila melanogaster species group. Evol Bioinform Online. 2012;8:89–104. doi: 10.4137/EBO.S8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poivet E, Gallot A, Montagne N, Glaser N, Legeai F, Jacquin-Joly E. A comparison of the olfactory gene repertoires of adults and larvae in the noctuid moth Spodoptera littoralis. PLoS ONE. 2013;8(4):e60263. doi: 10.1371/journal.pone.0060263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou SS, Sun Z, Ma W, Chen W, Wang MQ. De novo analysis of the Nilaparvata lugens (Stål) antenna transcriptome and expression patterns of olfactory genes. Comp Biochem Physiol Part D Genomics Proteomics. 2014;9:31–9. doi: 10.1016/j.cbd.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 50.Dippel S, Oberhofer G, Kahnt J, Gerischer L, Opitz L, Schachtner J, et al. Tissue-specific transcriptomics, chromosomal localization, and phylogeny of chemosensory and odorant binding proteins from the red flour beetle Tribolium castaneum reveal subgroup specificities for olfaction or more general functions. BMC Genomics. 2014;15(1):1141. doi: 10.1186/1471-2164-15-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benton R, Vannice KS, Vosshall LB. An essential role for a CD36-related receptor in pheromone detection in Drosophila. Nature. 2007;450(7167):289–93. doi: 10.1038/nature06328. [DOI] [PubMed] [Google Scholar]
- 52.Jin X, Ha TS, Smith DP. SNMP is a signaling component required for pheromone sensitivity in Drosophila. Proc Natl Acad Sci U S A. 2008;105(31):10996–1001. doi: 10.1073/pnas.0803309105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tribolium Genome Sequencing C, Richards S, Gibbs RA, Weinstock GM, Brown SJ, Denell R, et al. The genome of the model beetle and pest Tribolium castaneum. Nature. 2008;452(7190):949–55. doi: 10.1038/nature06784. [DOI] [PubMed] [Google Scholar]
- 54.Jiang XJ, Guo H, Di C, Yu S, Zhu L, Huang LQ, et al. Sequence similarity and functional comparisons of pheromone receptor orthologs in two closely related Helicoverpa species. Insect Biochem Mol Biol. 2014;48:63–74. doi: 10.1016/j.ibmb.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 55.Mitsuno H, Sakurai T, Murai M, Yasuda T, Kugimiya S, Ozawa R, et al. Identification of receptors of main sex-pheromone components of three Lepidopteran species. Eur J Neurosci. 2008;28(5):893–902. doi: 10.1111/j.1460-9568.2008.06429.x. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, Yan S, Liu Y, Jacquin-Joly E, Dong S, Wang G. Identification and functional characterization of sex pheromone receptors in the common cutworm (Spodoptera litura) Chem Senses. 2015;40(1):7–16. doi: 10.1093/chemse/bju052. [DOI] [PubMed] [Google Scholar]
- 57.Zhang YN, Zhang J, Yan SW, Chang HT, Liu Y, Wang GR, et al. Functional characterization of sex pheromone receptors in the purple stem borer, Sesamia inferens (Walker) Insect Mol Biol. 2014;23(5):611–20. doi: 10.1111/imb.12109. [DOI] [PubMed] [Google Scholar]
- 58.Steinwender B, Thrimawithana AH, Crowhurst RN, Newcomb RD. Pheromone receptor evolution in the cryptic leafroller species, Ctenopseustis obliquana and C. herana. J Mol Evol. 2015;80(1):42–56. doi: 10.1007/s00239-014-9650-z. [DOI] [PubMed] [Google Scholar]
- 59.Pelletier J, Hughes DT, Luetje CW, Leal WS. An odorant receptor from the southern house mosquito Culex pipiens quinquefasciatus sensitive to oviposition attractants. PLoS ONE. 2010;5(4):e10090. doi: 10.1371/journal.pone.0010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson AR, Wanner KW, Trowell SC, Warr CG, Jaquin-Joly E, Zagatti P, et al. Molecular basis of female-specific odorant responses in Bombyx mori. Insect Biochem Mol Biol. 2009;39(3):189–97. doi: 10.1016/j.ibmb.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 61.Abuin L, Bargeton B, Ulbrich MH, Isacoff EY, Kellenberger S, Benton R. Functional architecture of olfactory ionotropic glutamate receptors. Neuron. 2011;69(1):44–60. doi: 10.1016/j.neuron.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu NY, Xu W, Papanicolaou A, Dong SL, Anderson A. Identification and characterization of three chemosensory receptor families in the cotton bollworm Helicoverpa armigera. BMC Genomics. 2014;15:597. doi: 10.1186/1471-2164-15-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu W, Papanicolaou A, Liu NY, Dong SL, Anderson A. Chemosensory receptor genes in the Oriental tobacco budworm Helicoverpa assulta. Insect Mol Biol. 2015;24(2):253–63. doi: 10.1111/imb.12153. [DOI] [PubMed] [Google Scholar]
- 64.Leitch O, Papanicolaou A, Lennard C, Kirkbride KP, Anderson A. Chemosensory genes identified in the antennal transcriptome of the blowfly Calliphora stygia. BMC Genomics. 2015;16:255. doi: 10.1186/s12864-015-1466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sparks JT, Vinyard BT, Dickens JC. Gustatory receptor expression in the labella and tarsi of Aedes aegypti. Insect Biochem Mol Biol. 2013;43(12):1161–71. doi: 10.1016/j.ibmb.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 66.Clyne PJ, Warr CG, Carlson JR. Candidate taste receptors in Drosophila. Science. 2000;287(5459):1830–4. doi: 10.1126/science.287.5459.1830. [DOI] [PubMed] [Google Scholar]
- 67.Obiero GF, Mireji PO, Nyanjom SR, Christoffels A, Robertson HM, Masiga DK. Odorant and gustatory receptors in the tsetse fly Glossina morsitans morsitans. PLoS Negl Trop Dis. 2014;8(4):e2663. doi: 10.1371/journal.pntd.0002663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tan QQ, Zhu L, Li Y, Liu W, Ma WH, Lei CL, et al. A de novo transcriptome and valid reference genes for quantitative real-time PCR in Colaphellus bowringi. PLoS ONE. 2015;10(2):e0118693. doi: 10.1371/journal.pone.0118693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29(7):644–52. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li R, Zhu H, Ruan J, Qian W, Fang X, Shi Z, et al. De novo assembly of human genomes with massively parallel short read sequencing. Genome Res. 2010;20(2):265–72. doi: 10.1101/gr.097261.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–6. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 72.Ye J, Fang L, Zheng H, Zhang Y, Chen J, Zhang Z, et al. WEGO: a web tool for plotting GO annotations. Nucleic Acids Res. 2006;34(Web Server issue):W293–7. doi: 10.1093/nar/gkl031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5(7):621–8. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 74.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8(10):785–6. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 75.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–8. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 76.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–9. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Price MN, Dehal PS, Arkin AP. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS ONE. 2010;5(3):e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simon P. Q-Gene: processing quantitative real-time RT-PCR data. Bioinformatics. 2003;19(11):1439–40. doi: 10.1093/bioinformatics/btg157. [DOI] [PubMed] [Google Scholar]
- 79.Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques. 2002;32(6):1372–4, 6, 8–9. [PubMed] [Google Scholar]


