Abstract
Objective
Anesthetics have been linked to widespread neuronal cell death in neonatal animals. Epidemiological human studies have associated early childhood anesthesia with long-term neurobehavioral abnormalities, raising substantial concerns that anesthetics may cause similar cell death in young children. However, key aspects of the phenomenon remain unclear, such as why certain neurons die, whereas immediately adjacent neurons are seemingly unaffected, and why the immature brain is exquisitely vulnerable, whereas the mature brain seems resistant. Elucidating these questions is critical for assessing the phenomenon’s applicability to humans, defining the susceptible age, predicting vulnerable neuronal populations, and devising mitigating strategies.
Methods
This study examines the effects of anesthetic exposure on late- and adult-generated neurons in newborn, juvenile, and adult mice, and characterizes vulnerable cells using birth-dating and immunohistochemical techniques.
Results
We identify a critical period of cellular developmental during which neurons are susceptible to anesthesia-induced apoptosis. Importantly, we demonstrate that anesthetic neurotoxicity can extend into adulthood in brain regions with ongoing neurogenesis, such as dentate gyrus and olfactory bulb.
Interpretation
Our findings suggest that anesthetic vulnerability reflects the age of the neuron, not the age of the organism, and therefore may potentially not only be relevant to children but also to adults undergoing anesthesia. This observation further predicts differential heightened regional vulnerability to anesthetic neuroapoptosis to closely follow the distinct regional peaks in neurogenesis. This knowledge may help guide neurocognitive testing of specific neurological domains in humans following exposure to anesthesia, dependent on the individual’s age during exposure.
Hundreds of thousands of patients undergo anesthesia every day, spanning from premature infants to octogenarians.1 The anesthetic state, while producing a powerful disruption of central nervous system function, has always been thought to be reversible and without long-term consequences for the brain. This belief has recently been challenged by animal studies demonstrating widespread apoptotic neuronal cell death following anesthetic exposure early in life.2–6 This phenomenon has been observed in numerous studies for all anesthetics and sedatives acting at N-Methyl-D-aspartic acid glutamate (NMDA) and/or γ-aminobutyric acid type A (GABAA) receptors, across a wide range of species, from nematodes to nonhuman primates, whereas initial studies suggest that the sedative and α2-agonist dexmedetomidine may be devoid of deleterious effects (see Istaphanous et al7 for review).
Importantly, although the phenomenon’s mechanism is unknown, it was felt to represent an inherent vulnerability of the developing brain and to be limited by the age of the organism, for example, only occurring between 4 and 14 days of age (P4–P14) in small rodents.8 Older animals were seemingly unaffected. Alarmingly, in humans, several epidemiological studies have now detected long-term learning abnormalities and language impairment in children exposed to surgery with anesthesia early in life.9,10 These findings have raised significant concern among neuroscientists, pediatric neurologists, anesthesiologists, and government regulators that exposure to anesthesia and prolonged sedation may induce neurological injury in humans similar to that observed in animals; however, this risk was felt to be limited to very young children.11,12
Importantly, however, it remained unresolved why the immature brain is specifically vulnerable to this phenomenon and the mature brain seemingly resistant, and why certain neurons die whereas immediately adjacent cells are apparently unaffected. To start addressing these questions, in the present study we explored an alternative hypothesis, that anesthetic vulnerability reflects the age of the neuron, not the age of the organism, thereby predicting that neurons exhibit a critical period during which they may succumb to anesthesia-induced cell death, but following which they become resistant. This hypothesis is largely consistent with the existing literature, as the majority of neurons are generated early in development; therefore, the peak of vulnerability is invariably expected to occur early in life. This is particularly true for the brain regions of primary focus thus far, neocortex and thalamus, where neurogenesis peaks during mid to late gestation.13 However, our hypothesis predicts that late- and adult-generated neurons, such as dentate granule cells (DGCs) and olfactory bulb neurons,13 will be vulnerable beyond the early postnatal period. If correct, this hypothesis would provide vital new insights into the selectivity of anesthesia-induced neurotoxicity, and may predict differential vulnerability of particular brain regions in children as well as adults.
Materials and Methods
Animals
C57BL6/J breeding pairs were housed on a 12/12-hour light/dark cycle at 22°C. Their offspring either remained with the parents until treatment as newborns (P7, n = 22) or as juveniles (P21, n = 94), with P0 considered the day of birth, or were weaned from the dam on P28 and allowed to spontaneously exercise in running wheels prior to isoflurane treatment as young adults (P49, n = 44). Similar to previously published mouse exercise paradigms,14 animals were housed in regular cages that accommodated the running wheel without additional enrichment. Two animals of the same gender were housed per cage to maximize access to the running wheel and were frequently observed to run in pairs. Immediately following the anesthetic exposures at P7, P21, or P49, animals were euthanized and brains were removed for further analysis. All experiments followed the National Institutes of Health guidelines and were approved by the Animal Care and Use Committee of the Cincinnati Children’s Research Foundation. Efforts were made to minimize the number of animals used.
Bromodeoxyuridine Injections
The thymidine analogue bromodeoxyuridine (BrdU; Sigma Life Sciences, B5002-1G; Sigma-Aldrich, St Louis, MO) was dissolved in phosphate-buffered saline (PBS) and sodium hydroxide in a warm water bath. Animals were divided into 4 different groups to receive subcutaneous BrdU injections (75 mg/kg) for 3 consecutive days, on P1–P3, P6–P8, P11–P13, or P16–P18 prior to treatment on P21. This injection schema allowed for identification of neurons that were 3 to 5, 8 to 10, 13 to 15, or 18 to 20 days old during anesthetic exposure, respectively. A similar injection schema was performed in adult animals prior to anesthetic exposure on P49.
Anesthesia
On P7, P21, or P49, mice were randomly assigned to either a 6-hour exposure to 1.5% isoflurane in 30% oxygen (anesthesia) or to fasting in room air (no anesthesia). Both female and male littermates were used, because preliminary experiments revealed no sex differences in neuroapoptosis. Anesthetic, oxygen, and carbon dioxide concentrations were monitored using a gas analyzer (RGM 5250; Datex-Ohmeda, Louisville, CO). During exposure, animals were housed in padded acrylic containers placed in incubators warmed to 35.5°C. Immediately following treatment, mice were euthanized with an intraperitoneal injection of ketamine (20 mg/kg), acepromazine (0.5 mg/kg), and xylazine (1 mg/kg). Animals were then transcardially perfused with normal saline followed by 4% paraformaldehyde in PBS (PFA, pH = 7.4). Brains were removed, fixed in PFA, and cryoprotected in 20% and 30% sucrose solutions in PBS.
Histology
Brain were cryosectioned in the sagittal plane at 40µm (Thermo Electronics, Kalamazoo, MI). Sections between 0.84 and 1.32mm lateral to the midline in accord with Paxinos and Franklin’s Mouse Brain Atlas15 were used for analysis in P21 animals; the entire hemibrain was used at P49. For BrdU immunohistochemistry, sections were incubated for 5 minutes at 100°C in a 1:10 dilution of sodium citrate buffer (pH = 6.0; CB910m; Biocare, Concorde, CA) in Coplin jars at 6psi. Following this step, the tissue was incubated in 0.025% trypsin for 3 minutes and then in 2M HCl at room temperature for 30 minutes. The slides were washed twice in phosphate buffer (pH = 8.5). Slide-mounted sections were permeabilized for 4 hours in 3% Tween-20 and 0.75% glycine in PBS. Sections were blocked for 1 hour in 5% normal goat serum or 5% normal donkey serum before primary antibodies were added.
Slide-mounted sections were incubated overnight at room temperature with up to 3 primary antibodies to accommodate combinations of compatible secondary antibodies. Sections were stained with either 1:100 rabbit anti–caspase 3 antibodies (9661L; Cell Signaling Technology, Danvers, MA) and 1:200 sheep anti-BrdU (GeneTex, Irvine, CA) for birth-dating studies, 1:200 mouse anti-Sox2 (4900S; Cell Signaling Technology) for early progenitors, goat anti-NeuroD1 (sc-1084; Santa Cruz Biotechnology, Santa Cruz, CA) for late progenitors, 1:500 mouse anticalretinin (MAB1568; Millipore, Billerica, MA) or 1:500 guinea pig anticalretinin (214 104; Synaptic Systems, Göttingen, Germany) for immature granule cells, or 1:200 guinea pig anticalbindin D28k (214 004; Synaptic Systems) for mature granule cells. Sections were rinsed in blocker and were then, as appropriate, incubated in 1:250 dilutions of Alexa Fluor 488 donkey antirabbit, 594 donkey antisheep, 594 donkey antigoat, 647 donkey antimouse, 488 goat antirabbit, 594 goat anti–guinea pig, 594 goat antirat, or 647 goat antimouse (all Life Technologies, Grand Island, NY). After 4 hours of exposure, slides were washed in PBS and mounted with Fluoro-Gel (Electron Microscopy Sciences, Hatfield, PA).
Image Collection and Analysis
Confocal images were collected through the z-depth of the dentate gyrus at 0.5µm increments to generate 3-dimensional confocal image stacks (Leica TCS SP5 with LAS software; Leica Microsystems, Wetzlar, Germany). The fluorochromes were excited using 488, 543, or 633nm laser lines, as appropriate, and emission wavelengths between 493 and 550, 582 and 622, or 655 and 706nm, respectively, were collected. The resulting image stacks were transferred to image analysis software (Neurolucida v10.31; MBF Bioscience, Williston, VT) for assessment by an observer unaware of group assignment. Apoptotic, caspase 3–positive cells and cells staining for the respective maturational markers or BrdU were quantified using the optical dissector method. Data from a minimum of 8 animals were pooled for each marker to obtain a sufficient number of cells for analysis. Cell counts for maturational markers are expressed as a fraction of all caspase 3–positive cells, indicating the percentage of apoptotic neurons in a particular maturational stage. Conversely, for birth-dating experiments, caspase 3–positive cell counts are expressed as a percentage of all BrdU-positive cells to adjust for changing rates of neurogenesis over the 4 time periods during which BrdU was administered.16
Neurogenesis rates in dentate decline with age,17 as previously found and consistent with our qualitative observations of BrdU labeling. To compensate for the expected reduction in potentially vulnerable newborn granule cells, quantification of caspase 3–positive cells at P49 was therefore performed by stereological cell counts through the entire rostral–caudal extent of the dentate gyrus. The granule cell layer area was traced (Stereo Investigator v7.50.4; MBF Bioscience) at ×63 magnification, and all caspase 3–positive cells were quantified in every eighth section through the entire z-stack using the optical dissector method (n = 4 for anesthesia and for no anesthesia, respectively). Density of caspase 3–positive cells was calculated using the Cavalieri method.18
Statistical Analysis
All data are presented as means ± standard error and were analyzed using Stata v10.1 (StataCorp, College Station, TX). Normality was tested with the Shapiro–Wilk test, and statistical analysis was performed using Student t test for parametric data, Mann–Whitney U test for nonparametric data, and Pearson chi-square test for categorical data. Significance was accepted at p < 0.05.
Figure Preparation
Tissue images presented in the paper are maximum projections exported as TIFF files and imported into Adobe Photoshop (Adobe Systems, San Jose, CA). Some images were adjusted using a Leica morphological erosion filter (radius = 3; iterations = 1). Brightness and contrast of entire digital images were adjusted to optimize cellular detail. Identical adjustments were performed on all images meant for comparison.
Results
Vulnerability to Anesthesia-Induced Neuroapoptosis in DGCs Is Delayed and Continues into Adulthood
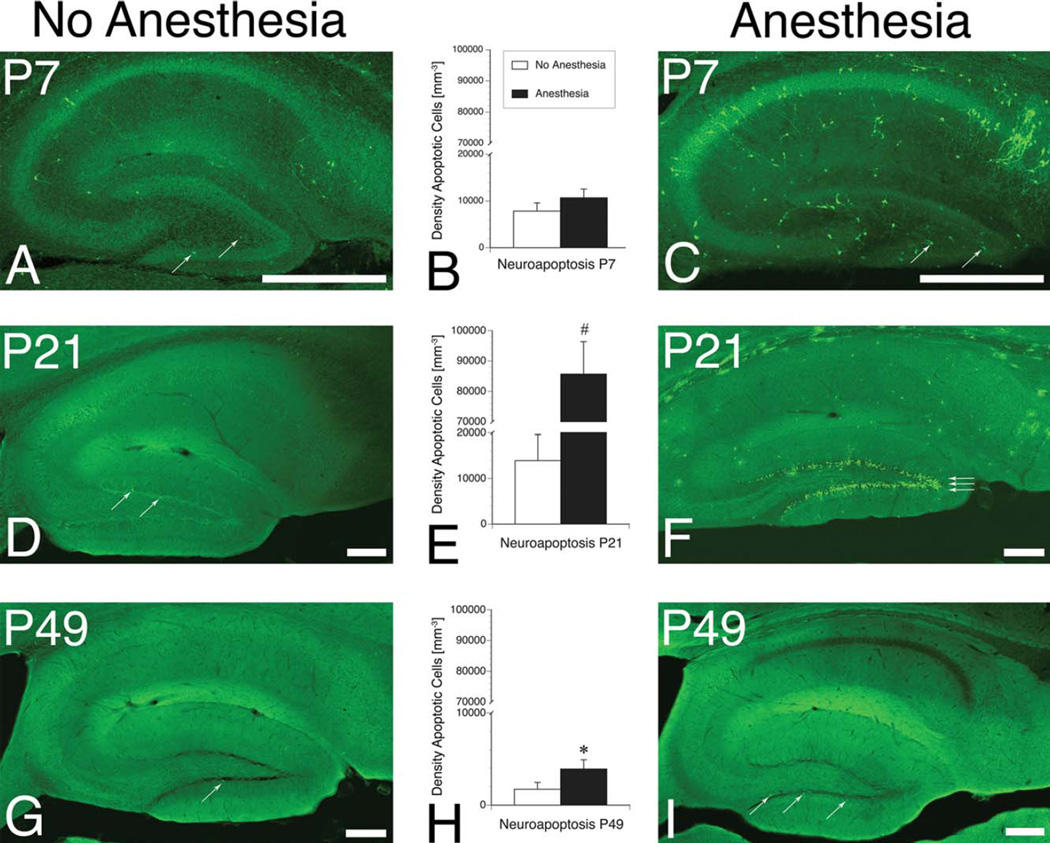
Newborn (P7), juvenile (P21), and young adult (P49) mice were exposed to isoflurane for 6 hours and sacrificed immediately thereafter. The density of apoptotic neurons was quantified in dentate gyrus using stereological techniques and compared with unanesthetized littermates of the same age. In agreement with previous studies,3,8,19,20 qualitatively, P7 animals exhibited widespread neuronal loss in several forebrain structures. Among dentate granule cells, however, which are just reaching peak neurogenesis at this age, no significant increase in apoptosis was observed in these newborn animals (Fig 1A–C). In juvenile animals, by contrast, the pattern reversed. Cortex and midbrain were largely spared, whereas granule cell death increased significantly relative to unanesthetized littermates (see Fig 1D–F). This delayed vulnerability in dentate—outside of the previously observed window of susceptibility for neocortex—parallels the delayed neurogenesis in this region, raising the possibility that adult-generated granule cells might show similar susceptibility. Accordingly, neuroapoptosis was quantified in young adult animals following anesthetic exposure and, strikingly, DGC death was also significantly increased in these young adult animals relative to unanesthetized littermates (see Fig 1G–I). DGC neurogenesis was stimulated in these animals by voluntary wheel running for 3 weeks for an average of 2.6 ± 0.2km per day prior to the anesthetic exposure on P49. Although the absolute number of dying cells was less than in juvenile mice—consistent with reduced rates of neurogenesis in older animals—the finding demonstrates that vulnerability persisted into adulthood.
FIGURE 1.
Vulnerability to anesthesia-induced neuroapoptosis is delayed in dentate granule cells and continues into adulthood. Representative hippocampal photomicrographs stained for activated caspase 3 (positive cells are seen as bright green puncta) from newborn (postnatal day 7 [P7]; A, C), juvenile (P21; D, F), and young adult (P49; G, I) mice exposed to 1.5% of isoflurane (anesthesia) for 6 hours, compared with fasted, unanesthetized littermates (no anesthesia). Arrows mark apoptotic dentate granule cells. Bar graphs (B, E, H) represent quantification of apoptotic neuronal density (n = 6–8 animals per group for each time point, mean ± standard error of the mean). Stereological cell counts of caspase 3+ cellular density reveal that neuroapoptosis is not significantly increased, compared with unanesthetized littermates, following anesthetic exposure at P7, but is increased following anesthetic exposure at P21 (note y-axis break) and P49; #p < 0.001, *p < 0.05, Mann–Whitney U test. All scale bars = 200µm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com]
DGC Vulnerability Peaks Approximately 2 Weeks after the Cells Are Born
The temporal association between peak neurogenesis in the dentate around P7 and maximum vulnerability to anesthesia at P21 suggested a particularly vulnerable neuronal age.13 To test this hypothesis, a separate group of animals was injected with the S-phase marker BrdU to birth-date neurons at 4 time points: 3 to 5, 8 to 10, 13 to 15, or 18 to 20 days prior to the anesthetic exposure on P21. Among these 4 cellular ages, vulnerability was highest in the youngest granule cells, with ≈7 to 14% of 3- to 5-, 8- to 10-, and 13- to 15-day-old cells undergoing apoptosis, but declined significantly between the 13- to 15- and 18- to 20-day-old groups, to only 4.2 ± 1.3% of granule cells (Fig 2A–F). BrdU birth-dating in P49 mice confirmed that dying cells were adult-generated (see Fig 2G), although the smaller number of double-labeled cells in these animals—reflecting the reduced neurogenesis rates in adults relative to juveniles—precluded developing an accurate age–response curve.
FIGURE 2.
Dentate granule cell vulnerability to anesthesia-induced neuroapoptosis is dependent on neuronal age and peaks approximately 2 weeks after the cells are born. Animals were injected with the S-phase marker bromodeoxyuridine (BrdU) at 4 time points: 3 to 5, 8 to 10, 13 to 15, or 18 to 20 days prior to an anesthetic exposure of 1.5% isoflurane for 6 hours on postnatal day 21 (P21) or P49. (A) Graph depicts the fraction of birth-dated cells from each injection point that were vulnerable to anesthesia-induced apoptosis by dividing the number of caspase 3+/BrdU+ double-positive cells by the number of all BrdU+ cells (n = 17–32 animals for each time point; *p = 0.008 compared with cells older than 18 days; other comparisons were not significant, Mann–Whitney U test). (B–D) Representative high-magnification photomicrograph composite images of dentate granule cells from a juvenile mouse following anesthetic exposure shows neurons positive for the apoptotic marker activated caspase 3 (green, B), the S-phase marker BrdU (red, C), and a merged image (D). Arrows mark 2 cells double-positive for caspase 3 and BrdU, as quantified in A. (E–G) Similar representative images from a young adult, P49 mouse, which was injected with BrdU from P39 to P41, identifying the double-labeled neuron (arrows) as being an adult-generated dentate granule cell, between 8 and 10 days old during the anesthetic exposure on P49; note 2 neighboring neurons of similar age (BrdU+), but not colocalizing with caspase 3. Scale bars = 30µm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com]
DGC Vulnerable to Anesthesia Express Immature Phenotypic Markers
Interestingly, caspase 3–positive DGCs were predominantly localized to the subgranular zone (SGZ) and the inner third of the granule cell body layer, both at P21 and P49 (Fig 3D and K, respectively). This suggested that the affected cells were recently generated, as granule cells are born in the SGZ (see Fig 3F) and take up residence in the inner third of the granule cell layer.17,21 To further define the vulnerable population of cells, the maturational stage of apoptotic, caspase 3–positive neurons was characterized immunohistochemically. During their maturation, DGCs transiently express (1) Sox2 as radial glialike progenitors, (2) NeuroD1 as late progenitors, (3) calretinin as immature granule cells, and (4) calbindin as mature granule cells.22–24 Both in juvenile and adult mice (see Fig 3E and K, respectively), apoptotic neurons were most frequently identified as late progenitors (NeuroD1+) and immature granule cells (calretinin+). Regardless of the age of the animal, earlier stages, such as Sox2-positive, radial glialike progenitors (type 1) or more mature calbindin-expressing granule cells, were not substantially affected. These results suggest that vulnerability to anesthetic neurotoxicity is determined by neuronal age and maturational stage, not by the age of the organism.
FIGURE 3.
Anesthesia triggers cell death in newly generated dentate granule cells (DGCs), as identified by their location and phenotypic markers, irrespective of the age of the animal. (A–D) Representative photomicrograph composites of dentate gyrus from a 21-day-old (P21) mouse, following an isoflurane anesthetic, stained for the apoptotic marker caspase 3 (green, A), the mature granule cell marker calbindin (red, B), the immature granule cell marker calretinin (blue, C), and a merged image (D). Caspase 3+, apoptotic neurons are predominantly located in the subgranular zone (arrows), and express the immature marker calretinin but not the mature marker calbindin. (E) Bar graphs represent the fraction of apoptotic, caspase 3+ DGCs colocalizing the respective maturational stage-specific markers (caspase 3+ cells colabeled with 1 of the 4 respective markers divided by the total number of caspase 3+ cells). Apoptotic DGCs are identified as late progenitors (349 of 586 caspase+ cells were NeuroD1+; *p < 0.0001 compared with Sox2+ by Pearson chi-square test) and immature granule cells (849 of 1,587 caspase+ cells were calretinin+; #p < 0.0001 compared with calbindin+ by Pearson chi-square test), but not as radial glialike progenitors (0 of 279 caspase+ cells were Sox2+) or mature neurons (11 of 1587 caspase+ cells were calbindin+; n = 18 animals). (F) Schematic demonstrating DGC maturation progressing from radial glialike progenitor cells, located close to the hilus and expressing Sox2, to transient amplifying cells and neuroblasts, located in the subgranular zone (SGZ) and expressing NeuroD1, to immature neurons with small dendritic trees, located in the SGZ and expressing calretinin, and lastly to calbindin-expressing mature neurons migrating into the granule cell layer (GCL) and extending dendrites into the molecular layer (ML). (G–J) Similar composite photomicrographs from a young adult mouse (P49), and (K) bar graphs of characterization identifying apoptotic cells as late progenitors (NeuroD1 [116 of 358]; *p < 0.0001 compared with Sox2+ [0 of 160] by Pearson chi-square test) and immature granule cells (calretinin+ [76 of 288]; #p < 0.0001, compared with calbindin+ [3 of 288] by Pearson chi-square test; n = 16 animals). All scale bars = 50µm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com]
Anesthetic Neurotoxicity Similarly Affects Young Neurons in Olfactory Bulb
To determine whether vulnerability to anesthesia-induced cell death is similarly delayed in another brain region exhibiting significant neurogenesis beyond the neonatal period,13,25 we quantified apoptotic cells in olfactory bulb for all 3 age groups. Consistent with our hypothesis, similar to the cell death pattern observed in dentate gyrus, anesthesia substantially increased neuroapoptosis in juvenile, 21-day-old (Fig 4F–J), and young adult, 49-day-old mice (see Fig 4K–O) in olfactory bulb. However, unlike in dentate, anesthesia-induced neuronal cell death in olfactory bulb neurons was also increased in newborn animals (see Fig 4A–E), which could be due to the heterogeneity of olfactory bulb neurons compared with dentate granule cells. Although, similar to dentate granule cells, neurogenesis in olfactory bulb granule cells peaks between P0 and P7, other cell populations not present in dentate, such as mitral and tufted cells, are predominantly formed during embryonic development, suggesting that they might represent some of the cells affected by anesthetic exposure at P7. These findings nonetheless support our hypotheses that anesthesia-induced neuroapoptosis targets neurons irrespective of the age of the animal and that vulnerability in brain regions with ongoing neurogenesis extends to adult animals.
FIGURE 4.
Vulnerability to anesthesia-induced neuroapoptosis extends into adulthood in olfactory bulb, another brain region incorporating adult-generated neurons. Representative photomicrographs of olfactory bulbs and quantification of stereological cell counts from unanesthetized, fasted animals, compared with their respective littermates exposed to 1.5% isoflurane for 6 hours on (A–E) postnatal day 7 (P7), (F–J) P21, or (K–O) P49 demonstrating caspase 3+, apoptotic neurons (bright green puncta) and neurons staining for the neuronal marker NeuN (blue areas). White boxes on the left (A, D, F, I, K, N) correspond to respective high-resolution images on the right (B, E, G, J, L, O). Quantification of stereological cell counts of apoptotic neurons in newborn (C), juvenile (H), and young adult mice (M) demonstrate a significant increase of caspase 3+ olfactory bulb neurons (arrows in B, E, G, J, L, O) following anesthetic exposure (#p < 0.01, *p < 0.001, †p < 0.05 compared with their respective, unanesthetized littermates; Mann–Whitney U test; n = 6–8 animals per group). All scale bars = 250µm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com]
Discussion
Neuronal cell death has previously been observed in a wide variety of neonatal animal species following exposure to all commonly used clinical anesthetics and sedatives that act as NMDA antagonists, GABAA agonists, or a combination of both, but seemingly not the α2-agonist dexmedetomidine, leading to concerns by professionals and the public that these findings could apply to children undergoing anesthesia and sedation.12 Previous research has exclusively focused on the newborn brain, because vulnerability seemed restricted to newborn rodents, at least for neocortex and thalamus,8 suggesting that the phenomenon represented an inherent vulnerability of the developing brain to anesthetics. By contrast, here, we demonstrate that brain cells in 2 brain regions with predominantly postnatal neurogenesis are subject to cell death beyond this previously observed window of susceptibility—to at least young adulthood. Moreover, we utilized BrdU birth-dating and the transient expression of maturational markers in dentate granule cells to illustrate that the majority of DGCs eliminated by anesthesia-induced neuroapoptosis are young and relatively immature, suggesting that anesthetics may induce neuroapoptosis by interfering with key stages of neuronal maturation and natural apoptosis.
Anesthesia Eliminates Brain Cells in Adult Animals
The present study, although also observing the previously demonstrated vulnerability of neocortical neurons in animals <14 days of age,8 expands on these findings by revealing that anesthesia-induced brain cell death occurs in 2 brain regions—dentate gyrus and olfactory bulb—beyond this age. These findings contrast with previous work that did not observe hippocampal cell loss in adult animals,26 probably reflecting methodological differences, as our animals were allowed to voluntarily exercise in running wheels; a treatment that increases neurogenesis in the adult dentate gyrus and has been shown to generate new neurons that are structurally and functionally indistinguishable from naturally born sister cells.14,27 We observed anesthesia-induced, adult neuronal cell death in both dentate gyrus and olfactory bulb, indicating that this phenomenon is not restricted to neurons induced by exercise, because voluntary wheel running only increases the number of immature neurons in dentate, but not in olfactory bulb.28 Moreover, previous experiments have demonstrated that voluntary exercise, when initiated after an anesthetic exposure, may alleviate the postanesthetic neurocognitive impairment observed in animals raised in standard housing,29 potentially by increasing neurogenesis. Our findings suggest that these new, exercise-induced neurons may still be vulnerable to anesthetics, such as during repeated exposures, which are not uncommon in clinical practice. Although the average running distance for our mice was on the lower end of previously reported data, probably due to inertia of the metal running wheels, which has previously been found to substantially alter running distances,27,30 it is worth noting that exercised animals better reflect the natural rodent environment, and may have more relevance to the human condition than rodents raised in standard laboratory housing.
Critical Period for Anesthesia-Induced Neuronal Cell Death
The present study demonstrates that exposure to isoflurane does not induce random cell death in dentate granule cells, but rather selective apoptosis among immature neurons, as demonstrated by location, phenotype, and age of the cell. We demonstrate that apoptotic cell death was largely restricted to late progenitor cells (type 2) and immature granule cells expressing NeuroD1 and calretinin, respectively, localized in the subgranular zone, where hippocampal neurogenesis occurs. Conversely, earlier stages, such as Sox2-positive, radial glialike progenitors (type 1) or more mature calbindin-expressing granule cells, were not substantially affected,22–24 which is consistent with previous studies.26,31 Moreover, BrdU birthdating of affected neurons confirmed their immature state, indicating that cells <15 days old were most vulnerable. Interestingly, this maturational stage for dentate granule cells corresponds to the period of naturally occurring cell death for these neurons in the neonatal and adult brain.32,33 The mechanisms regulating this naturally occurring cell death among granule cells are still being explored, but numerous studies implicate NMDA and GABA receptors.34,35 Because all commonly used anesthetics interact with GABAA and/or NMDA receptors,36 and isoflurane represents a relatively potent GABAA agonist and comparatively weaker NMDA antagonist,36 it is conceivable that anesthetics, including isoflurane, induce apoptosis by interfering with the balance of neuronal activity, thereby affecting neuronal survival.37
Newborn granule cells express GABA receptors throughout their development,38 whereas NMDA receptor expression begins around 1 to 2 weeks after cell birth, roughly corresponding to the period of isoflurane vulnerability observed here.39 Moreover, concurrently a change occurs in the chloride gradient, mediated by a switch from predominant Na+–K+–2Cl− cotransporter expression in very young cells to K+–Cl− expression in older cells,40 implying that the former might be excited by the anesthetic’s GABA agonistic effects, whereas the latter inhibited. However, mapping these effects will most likely be complicated by anesthetics acting on a wide variety of ion channels and receptors beyond GABA and NMDA receptors, as well as the dramatic regional differences in brain activity during anesthesia, with some regions being activated, whereas activity in others is depressed.36 Clearly, sophisticated experimental approaches will be needed to explore these questions; nonetheless, the present findings provide the first intriguing hints as to the possible mechanism of anesthesia-induced neuronal loss.
Brain Developmental Patterns May Predict Regional Vulnerability to Anesthetics
Newborn, juvenile, and adult mice were anesthetized with 1.5% isoflurane for 6 hours. This dose represents approximately 0.6 to 1.1 minimum alveolar concentration,6,41 within the range of clinically utilized doses during pediatric and adult anesthesia. Exposure time was at the upper end of the range typically seen in clinical practice, although surgical procedures lasting 6 hours or longer are not uncommon for major cardiothoracic, orthopedic, or neurosurgical procedures, for example. Although small animal models have several limitations precluding their direct applicability to clinical practice,42 cautiously translating the present results to humans would predict regional differences in susceptibility in line with the respective regional peaks in neurogenesis at the time of exposure. Prenatal anesthetic exposures for fetal surgeries, for example, would be expected to have the greatest impact on cortical and forebrain neurons, which are generated earlier in development. Conversely, according to our present findings, postnatal exposures could alter brain structure in dentate gyrus, a brain region involved in learning and memory, and lead to postexposure learning and memory impairment, as has been demonstrated in some children as well as adults following surgical procedures.9,10,43 As an extension, differential regional susceptibility following anesthetic exposures in young children may therefore result in differing neurobehavioral correlates, depending on the critical period of brain development during exposure.44 Language development, for example, starts early in neonatal life,45 and exposure to surgery with anesthesia during this early age has been found to interfere with proper subsequent language function.10 Due to this field’s serious implications for millions of patients, both children and adults, undergoing surgical procedures with anesthesia every year, it is imperative to further advance our knowledge in future clinical and laboratory studies.
Acknowledgment
This study was supported in part by a Mentored Research Training Grant–Basic Science to C.G.W. (mentor, A.W.L.) from the Foundation for Anesthesia Education and Research, by a Masimo-China Pediatric Anesthesia Research Fellow Program Grant to M.D. (mentor, A.W.L.) from the Masimo Foundation, by a Summer Undergraduate Research Fellowship to C.J. (mentor, A.W.L.) sponsored by the Center for Clinical and Translational Science and Training at the University of Cincinnati, and by departmental funds. S.C.D. is supported by the NIH National Institute of Neurological Disorders and Stroke (1R01-NS-065020, 1R01-NS-062806).
We thank Dr J. D. Molkentin for lending us the exercise equipment and Dr K. Seroogy for allowing us the use of the Stereo Investigator system.
Footnotes
Potential Conflicts of Interest
S.C.D.: travel expenses, Yuying Children Hospital, Wenzhou Medical University.
References
- 1.Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372:139–144. doi: 10.1016/S0140-6736(08)60878-8. [DOI] [PubMed] [Google Scholar]
- 2.Ikonomidou C, Bosch F, Miksa M, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 3.Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stefovska VG, Uckermann O, Czuczwar M, et al. Sedative and anticonvulsant drugs suppress postnatal neurogenesis. Ann Neurol. 2008;64:434–445. doi: 10.1002/ana.21463. [DOI] [PubMed] [Google Scholar]
- 5.Brambrink AM, Evers AS, Avidan MS, et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–841. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Istaphanous GK, Howard J, Nan X, et al. Comparison of the neuroapoptotic properties of equipotent anesthetic concentrations of desflurane, isoflurane, or sevoflurane in neonatal mice. Anesthesiology. 2011;114:578–587. doi: 10.1097/ALN.0b013e3182084a70. [DOI] [PubMed] [Google Scholar]
- 7.Istaphanous GK, Ward CG, Loepke AW. The impact of the perioperative period on neurocognitive development, with a focus on pharmacological concerns. Best Pract Res Clin Anaesthesiol. 2010;24:433–449. doi: 10.1016/j.bpa.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience. 2005;135:815–827. doi: 10.1016/j.neuroscience.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 9.Wilder RT, Flick RP, Sprung J, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ing C, DiMaggio C, Whitehouse A, et al. Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics. 2012;130:e476–e478. doi: 10.1542/peds.2011-3822. [DOI] [PubMed] [Google Scholar]
- 11.Loepke AW. Developmental neurotoxicity of sedatives and anesthetics: a concern for neonatal and pediatric critical care medicine? Pediatr Crit Care Med. 2010;11:217–226. doi: 10.1097/PCC.0b013e3181b80383. [DOI] [PubMed] [Google Scholar]
- 12.Rappaport B, Mellon RD, Simone A, Woodcock J. Defining safe use of anesthesia in children. N Engl J Med. 2011;364:1387–1390. doi: 10.1056/NEJMp1102155. [DOI] [PubMed] [Google Scholar]
- 13.Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- 14.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 15.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2nd ed. San Diego, CA: Academic Press; 2001. [Google Scholar]
- 16.Bayer SA. Development of the hippocampal region in the rat. I. Neurogenesis examined with 3H-thymidine autoradiography. J Comp Neurol. 1980;190:87–114. doi: 10.1002/cne.901900107. [DOI] [PubMed] [Google Scholar]
- 17.Mathews EA, Morgenstern NA, Piatti VC, et al. A distinctive layering pattern of mouse dentate granule cells is generated by developmental and adult neurogenesis. J Comp Neurol. 2010;518:4479–4490. doi: 10.1002/cne.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gundersen HJ, Bendtsen TF, Korbo L, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 19.Loepke AW, Istaphanous GK, McAuliffe JJ, III, et al. The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth Analg. 2009;108:90–104. doi: 10.1213/ane.0b013e31818cdb29. [DOI] [PubMed] [Google Scholar]
- 20.Istaphanous GK, Ward CG, Nan X, et al. Characterization and quantification of isoflurane-induced developmental apoptotic cell death in mouse cerebral cortex. Anesth Analg. 2013;116:845–854. doi: 10.1213/ANE.0b013e318281e988. [DOI] [PubMed] [Google Scholar]
- 21.Schlessinger AR, Cowan WM, Gottlieb DI. An autoradiographic study of the time of origin and the pattern of granule cell migration in the dentate gyrus of the rat. J Comp Neurol. 1975;159:149–175. doi: 10.1002/cne.901590202. [DOI] [PubMed] [Google Scholar]
- 22.Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Overstreet-Wadiche LS, Westbrook GL. Functional maturation of adult-generated granule cells. Hippocampus. 2006;16:208–215. doi: 10.1002/hipo.20152. [DOI] [PubMed] [Google Scholar]
- 24.Hodge RD, Hevner RF. Expression and actions of transcription factors in adult hippocampal neurogenesis. Dev Neurobiol. 2011;71:680–689. doi: 10.1002/dneu.20882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barber PC, Raisman G. Cell division in the vomeronasal organ of the adult mouse. Brain Res. 1978;141:57–66. doi: 10.1016/0006-8993(78)90616-9. [DOI] [PubMed] [Google Scholar]
- 26.Stratmann G, Sall JW, Bell JS, et al. Isoflurane does not affect brain cell death, hippocampal neurogenesis, or long-term neurocognitive outcome in aged rats. Anesthesiology. 2010;112:305–315. doi: 10.1097/ALN.0b013e3181ca33a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Creer DJ, Romberg C, Saksida LM, et al. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 2010;107:2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown J, Cooper-Kuhn CM, Kempermann G, et al. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- 29.Shih J, May LD, Gonzalez HE, et al. Delayed environmental enrichment reverses sevoflurane-induced memory impairment in rats. Anesthesiology. 2012;116:586–602. doi: 10.1097/ALN.0b013e318247564d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sall JW, Stratmann G, Leong J, et al. Isoflurane inhibits growth but does not cause cell death in hippocampal neural precursor cells grown in culture. Anesthesiology. 2009;110:826–833. doi: 10.1097/ALN.0b013e31819b62e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gould E, Woolley CS, McEwen BS. Naturally occurring cell death in the developing dentate gyrus of the rat. J Comp Neurol. 1991;304:408–418. doi: 10.1002/cne.903040306. [DOI] [PubMed] [Google Scholar]
- 33.Jabes A, Lavenex PB, Amaral DG, Lavenex P. Quantitative analysis of postnatal neurogenesis and neuron number in the macaque monkey dentate gyrus. Eur J Neurosci. 2010;31:273–285. doi: 10.1111/j.1460-9568.2009.07061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tozuka Y, Fukuda S, Namba T, et al. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 35.Petrus DS, Fabel K, Kronenberg G, et al. NMDA and benzodiazepine receptors have synergistic and antagonistic effects on precursor cells in adult hippocampal neurogenesis. Eur J Neurosci. 2009;29:244–252. doi: 10.1111/j.1460-9568.2008.06579.x. [DOI] [PubMed] [Google Scholar]
- 36.Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–720. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- 37.Tashiro A, Sandler VM, Toni N, et al. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- 38.Overstreet-Wadiche LS, Bromberg DA, Bensen AL, Westbrook GL. GABAergic signaling to newborn neurons in dentate gyrus. J Neurophysiol. 2005;94:4528–4532. doi: 10.1152/jn.00633.2005. [DOI] [PubMed] [Google Scholar]
- 39.Nácher J, Varea E, Miguel Blasco-Ibáñez J, et al. N-methyl-d-aspartate receptor expression during adult neurogenesis in the rat dentate gyrus. Neuroscience. 2007;144:855–864. doi: 10.1016/j.neuroscience.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 40.Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 41.Sonner JM, Gong D, Eger EI., Jr Naturally occurring variability in anesthetic potency among inbred mouse strains. Anesth Analg. 2000;91:720–726. doi: 10.1097/00000539-200009000-00042. [DOI] [PubMed] [Google Scholar]
- 42.Mintz CD, Wagner M, Loepke AW. Preclinical research into the effects of anesthetics on the developing brain: promises and pitfalls. J Neurosurg Anesthesiol. 2012;24:362–367. doi: 10.1097/ANA.0b013e31826a0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 44.Neville H, Bavelier D. Human brain plasticity: evidence from sensory deprivation and altered language experience. Prog Brain Res. 2002;138:177–188. doi: 10.1016/S0079-6123(02)38078-6. [DOI] [PubMed] [Google Scholar]
- 45.Pena M, Maki A, Kovacic D, et al. Sounds and silence: an optical topography study of language recognition at birth. Proc Natl Acad Sci U S A. 2003;100:11702–11705. doi: 10.1073/pnas.1934290100. [DOI] [PMC free article] [PubMed] [Google Scholar]