Abstract
Purpose
To determine if incident oral antidiabetic drugs (OAD) use was associated with 12-month systolic blood pressure (BP) and if this was mediated through body mass index (BMI) changes.
Methods
A retrospective cohort of veterans with hypertension who initiated metformin (n=2057) or sulfonylurea (n=1494) between January 1, 2000 and December 31, 2007 in the Veterans Administration Mid-South Network was assembled. Patients were included if they had complete covariates, including 12 month BP and BMI, and persisted on therapy for 12 months. Linear regression was conducted to investigate the effect of OADs on 12-month systolic BP adjusting for demographics, glycated hemoglobin, creatinine, body mass index, healthcare utilization and co-morbidities including cardiovascular disease. A second analysis examined if these effects were mediated by BMI change. The secondary outcome was the proportion of patients who had a controlled BP (≤140/90 mmHg) at 12 months adjusted for baseline BP and covariates.
Results
Patients were white (82%) males (97%) with median age of 64 years (Interquartile range [IQR] 57, 72) and 27% had history of CVD. Sulfonylurea users had a 1.33 mmHg [0.16, 2.50 p=0.03] higher 12-month systolic BP than metformin users. The median change in BMI from OAD initiation to 12 months was −0.76 (IQR −1.78, 0.07) and 0.21 (IQR −0.57, 1.03) among metformin and sulfonylurea users, respectively. In a model adjusting for BMI change, the difference in 12-month systolic BP between sulfonylurea and metformin users became insignificant [0.23 (−1.00, 1.45) p=0.72], while one BMI unit change was associated with an increase in 12-month systolic BP of 1.07 mmHg (0.74, 1.40 p<0.0001). At 12 months 68.3% of metformin patients had controlled BP versus 64.2% of sulfonylurea patients (p=0.01).
Conclusions
Compared with metformin, sulfonylurea initiation was associated with increased systolic BP at 12 months which appears to be mediated by the differential effects of these drugs on BMI.
Keywords: Hypertension, Diabetes, Obesity
INTRODUCTION
Diabetes mellitus (DM) is an established risk factor for macrovascular disease including myocardial infarction, stroke and microvascular disease, including nephropathy, neuropathy, and retinopathy. The prevalence of cardiovascular disease (CVD) in patients with DM is 2–4 times that of patients without DM, and CVD is the most common cause of mortality among diabetics.[1, 2] The majority of patients with DM have hypertension and there is a strong linear relationship between increasing systolic blood pressure (BP) and the risk for diabetic microvascular and macrovascular complications.[3, 4] In order to evaluate if specific oral antidiabetic drugs (OADs) are associated with development of CVD, first it is important to understand if each OAD category might have differential effects on BP.
Although weight change, BP and diabetes are interrelated, limited data exist on BP changes associated with the initiation of OADs.[5, 6] In a retrospective cohort of 2,574 patients with a new diagnosis of DM, Feldstein et al. [7] estimated 3-year weight trajectories. Those with higher stable weight or weight-gain patterns were more likely than those who lost weight to have above-goal blood pressure (estimated relative risk: 1.83 [1.31–2.57] and 1.47 [1.03–2.10], respectively). Similarly, two classic trials of hypertension prevention demonstrated that weight loss of 2–4 kg led to an average decrease in systolic BP of 4–6 mmHg and decrease in diastolic BP of 3 mmHg.[8, 9] Recent systematic reviews have estimated that weight gain associated with use of sulfonylureas is approximately 3.5 kg compared to metformin.[6, 10] Consistent with these observations, we recently reported that veterans who persisted on sulfonylureas for one year compared to similar veterans who persisted on metformin, had a 1 unit BMI difference, equivalent to 3.2 kg for a population with a median height of 5’10”.[11] We were now interested in evaluating if this weight change had an effect on BP.
Mediation analysis is conducted in order to indirectly assess the effect of a proposed cause or exposure on some outcome through a proposed mediator.[12, 13] The proposed mediator is considered a mediator if three criteria are satisfied. First, the exposure significantly predicts the outcome; second, the exposure significantly predicts the mediator; and third, the mediator significantly predicts the outcome while controlling for the exposure. Our aim was to determine whether choice of incident OAD was associated with 12-month systolic BP among veterans with hypertension who had newly treated DM. The hypothesis was that patients started on sulfonylureas would be more likely to have higher 12-month systolic BP compared with metformin given the favorable effects of metformin on BMI. We postulated that changes in BMI would mediate the change in 12-month systolic BP.
METHODS
Study Design, Setting and Data Sources
A retrospective cohort study of veterans with DM and pre-existing hypertension seen in the Veterans Affairs (VA) Mid South Healthcare Network (VISN 9) for their care between January 1, 2000 and December 31, 2007 was constructed from the VA administrative and pharmacy databases. The administrative data files contain patient demographics, vital signs, laboratory results, and coded diagnostic and procedure information from inpatient and outpatient encounters. Diagnoses associated with health care visits were coded according to the International Classification of Diseases, Ninth Revision; Clinical Modification (ICD9-CM).[14] The pharmacy files contain data from each prescription filled through the VA pharmacy including medication name, date filled, days supplied, pill number and dosage. For veterans who were also Medicare eligible, Medicare data were obtained (through December 31, 2004) through the VA Information Resource Center (VIReC) and merged with our VA databases.[15] VIReC data were also used to supplement data on co-morbidities and race.
Population
The study population included veterans’ aged 18 years or older receiving care in the VA VISN9 who filled an OAD prescription from January 2000 through December 2007 and had a pre-existing diagnosis of hypertension. We selected patients who predominantly utilized the VA as their healthcare provider by selecting patients who had an encounter with the VA or a prescription fill at least every 180 days before cohort entry. The analysis focused on patients with hypertension who became incident users of OADs with known birth date and gender and at least 365 days of baseline data to avoid methodological issues related to the inclusion of prevalent users in assessments of medication effects.[16] Incident prescriptions were defined as the first OAD filled after ≥365 days of active use of the VA pharmacy services without any antidiabetic drug. Only patients with a pre-existing diagnosis of hypertension were included. These patients were identified by fulfilling at least one of three possible hypertension definitions during a baseline period (see Appendix 1): 1) fill of an antihypertensive medication and an outpatient ICD-9 code for hypertension (401.xx–405.99) 2) two outpatient ICD-9 codes for hypertension separated by at least 30 days; or, 3) a single inpatient ICD-9 code for hypertension. Patients with any serious medical conditions (HIV/AIDS, cancer except for non-melanoma skin cancer, end stage kidney or liver disease, organ transplant or respiratory failure) identified during the baseline period were excluded, as these conditions may influence the provider's decision to control BP and may have independent effects on BMI. Patients were followed from the index date (date of incident prescription) until the study outcome: 12-month BP, a censoring event (see below) or the end of the study. The cohort was restricted to patients with complete baseline covariate information, who remained persistent on their incident drug until their 12 month BP measurement, and had available BP and BMI measurements at baseline and at 12-months. However a sensitivity analysis included those who were missing baseline covariates by using multiple imputation.
Censoring events were hospitalization for any cause- because important medication changes could potentially be made at this time; death; discontinuation of VA services (180 days without a visit or prescription fill); ending use of the incident OAD medication (90 days without drugs in hand or switch to a new regimen) or the end of the study (December 31, 2007). Once eligibility ended a person could re-enter the cohort if all inclusion criteria were again fulfilled.
Exposures
The study OADs were: metformin and sulfonylurea. Users of thiazolidinediones and combination metformin + sulfonylurea were excluded because of small numbers. Using pharmacy information, a “days supply in hand” estimate was calculated. Given that patients may "stockpile" medications, we estimated how many pills a patient had on each follow-up day. For example, if a patient filled a 90 day supply of metformin and refilled it on day 80, then on day 80 the patient had 100 days supply in hand (90 from the new fill plus 10 leftover from initial fill). Days supply in hand was reset to 0 with a change in OAD dose. Episodes of use for a specific OAD began on the index date and stopped at the end of the persistence period, defined as the first of: switch to or addition of another hypoglycemic medication, a medication gap of 90 days, or an outcome or censoring event, as described above.
Outcomes
The primary outcome was the systolic BP 12 months after initiation of OADs. Secondary outcomes were the proportion with controlled BP at 12 months and the diastolic BP at 12 months. The VA/Department of Defense (DOD) definition for BP control is ≤140/90 mmHg including patients with diabetes. Only BPs measured in the outpatient setting were used. If more than one value was available within the time frame, then the measurement closest to 12 months was used (range 9–15 months). To avoid inclusion of erroneous outliers, systolic BP values <60 and >250 mmHg and diastolic BP values <30 or >130 mmHg were excluded. Probable data entry errors where the systolic minus diastolic values were <10 mmHg were also excluded. Excluded BP values were 0.2% of all BP values in the dataset and not differential across exposures.
Covariates
Important co-morbidities were determined a priori using demographic data, health care coded diagnoses, procedures and prescriptions filled in the 365-day baseline period. The study covariates included: age, sex, race (white, black, other), calendar year, marital status, cardiovascular disease (Supplemental table 1), physiologic variables collected closest to cohort entry [systolic BP, serum creatinine, glycosylated hemoglobin (HbA1c), and body mass index (BMI)], and variables indicative of healthcare utilization (number of outpatient visits, hospitalization during baseline (yes/no), and number of unique active prescription medications on the index date). We also accounted for use of medications that might affect weight or blood pressure including antipsychotics, glucocorticoids, thiazide diuretics, angiotensin receptor blockers (ARB), angiotensin converting enzyme inhibitors (ACE), and other diuretics.
Statistical Analysis
The unit of analysis was the episode of use beginning with an incident OAD prescription. Since some patients contributed more than one episode of use, adjustment for clustering of episodes within individual patients calculating robust standard errors was done using the sandwich variance estimator.[17]
Three main multivariate analyses were conducted to evaluate if change in weight mediated the effect of sulfonylureas on BP. All three models adjusted for the a priori defined covariates. The first analysis was a linear regression that compared the12-month systolic BPs between incident OAD categories (sulfonylurea versus metformin users). The second linear regression determined if change in BMI was associated with systolic BP at 12 months. The third linear regression added both incident OAD category and a term for the change in BMI to determine if the effect of OAD category on systolic BP at 12 months was mediated through the change in BMI which had occurred since initiating the OAD.
For the secondary outcome, proportion with controlled BP at 12 months, we calculated adjusted proportions for each OAD exposure group while accounting for the above covariates. Regression diagnostics were performed to assess the fulfillment of model assumptions. Statistical analyses were conducted using R Statistical Program (R Foundation, available at: http://www.r-project.org.) and SAS for Windows 11.0. (SAS Institute, Cary, NC).
Sensitivity and Subgroup Analyses
Because patients may have important changes to their medications during hospitalizations with resulting changes to the exposure-outcome (OAD/BP) relationship, the primary analysis censored at the time of hospitalization. A sensitivity analysis did not censor follow-up at hospitalization. Because many of the patients in the cohort were missing baseline covariates and were excluded from the primary analysis, we also conducted a sensitivity analysis using a multiple imputation procedure. We utilized the Markov Chain Monte Carlo method and a non-informative Jeffreys prior.[18] All covariates from the primary analysis, and a censoring indicator were included in twenty imputation models, and used to compute the final estimates.
The robustness of findings was also tested by examining effects in pre-defined subgroups: restricted to those with an estimated glomerular filtration rate (eGFR) greater than 60 ml/min/1.73 m2; those with no congestive heart failure (to exclude possible weight changes secondary to decompensated heart failure) those with no prior hospitalization in the past year and white versus black race.
The institutional review boards of Vanderbilt University and the VA Tennessee Valley Healthcare System—Research and Development Committee approved this study. The U.S. Department of Veterans Affairs had the opportunity to comment on this manuscript before submission.
RESULTS
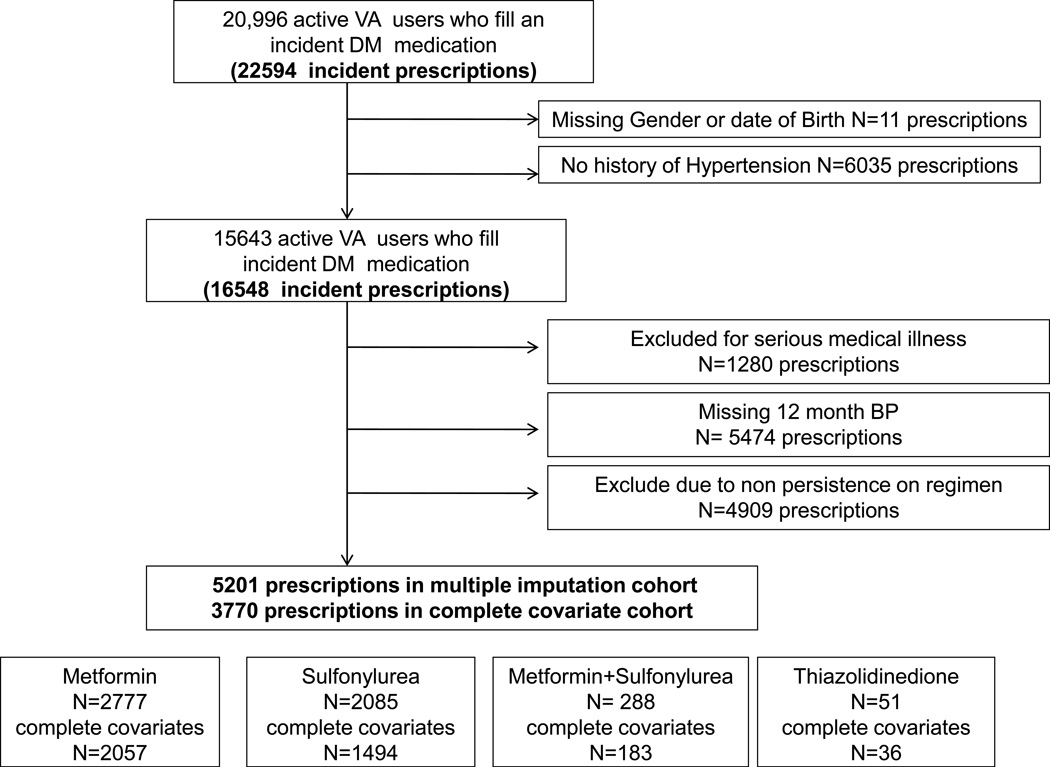
Of the 22594 new episodes of OAD use identified, a total of 6046 (27%) were ineligible because they had no history of hypertension (N=6035) or were missing gender or date of birth (<1%). Of the remaining 16,559 potentially eligible episodes, 1280 were excluded for serious illness identified at baseline; 6589 due to missing covariates or 12 month BP; and 4909 due to non-persistence on their OAD at the time of the 12-month BP measurement. Thus, our final study sample consisted of 3770 episodes among 3760 patients (10 patients re-entered the cohort and had more than 1 episode). Fifty four percent of incident episodes were for metformin (N=2057); 40% were for a sulfonylurea (N=1494). The remaining 219 episodes were for either thiazolidinediones or combination metformin+ sulfonylurea and were excluded. For our sensitivity analysis, the use of multiple imputation for missing covariates increased the sample size to 5201 episodes (2777 metformin; 2085 sulfonylurea; 339 either thiazolidinediones or combination metformin+ sulfonylurea) (Figure 1).
Figure 1.
Flow of patients and prescriptions included in the analysis of hypertensive diabetic patients initiating an OAD
Characteristics
The baseline characteristics of study patients are shown in Table 1. The patients were 97% male, 82% white, had a median age of 64 years (Interquartile range [IQR] 57, 72) and 27% had a history of CVD. Sulfonylurea users were older than metformin users and more likely to have CVD and have been hospitalized in the baseline year. Baseline median BMI was 32.3 (IQR 28.9, 35.9) for metformin users and lower for sulfonylurea users (median 30.4 [IQR 27.3, 34.1]). At baseline, 63.4 % of Metformin patients had a BP that was controlled (≤140/90 mmHg) and 60.3% of sulfonylurea patients were controlled. Data for those excluded from analyses for missing covariates, 12 month BP, or non-persistence are shown for comparison (Supplemental table 2). Characteristics of the 5201 patients included in the sensitivity analysis which used multiple imputation were similar to the complete covariate cohort and are shown in Supplemental table 3.
Table 1.
Characteristics of patients initiating OAD and included in analysis of 12 month Systolic BP
| Characteristic | Metformin N=2057 |
Sulfonylurea N=1494 |
|---|---|---|
| Age Median (IQR) | 62 (56, 69) | 68 (58, 76) |
| Gender (% Male) | 97 | 98 |
| Race (%) | ||
| White | 82.6 | 81.1 |
| Black | 8.2 | 12.7 |
| Other | 1.0 | 0.7 |
| Unknown | 8.2 | 5.5 |
| Cardiovascular Disease (%) | 24.8 | 30.0 |
| Marital Status (%) | ||
| Married | 67.3 | 66.3 |
| Divorced/Separated | 20.8 | 17.7 |
| Single | 4.2 | 4.4 |
| Unknown | 7.7 | 11.6 |
| % Hospitalized | 8.4 | 12.4 |
| Outpatient Meds Median (IQR) | 5 (3,8) | 6 (3,9) |
| Outpatient Visits Median (IQR) | 5 (3,8) | 6 (3,9) |
| Body Mass Index kg/m2 Median (IQR) | 32.3 (28.9, 35.9) | 30.4 (27.3, 34.1) |
| HgbA1c Median (IQR) | 7.0 (6.4, 7.7) | 7.2 (6.6, 8.0) |
| Creatinine (mg/dL) Median (IQR) | 1.0 (0.9, 1.2) | 1.2 (1.0, 1.4) |
| SBP (mm/Hg) Median (IQR) | 136 (126, 147) | 137 (126, 150) |
| DBP (mm/Hg) Median (IQR) | 77 (70, 84) | 76 (68, 83) |
| Proportion with controlled BP* | 63.4 | 60.3 |
| Use of Medications (%) | ||
| ACEI | 65.6 | 65.0 |
| ARB | 5.0 | 4.6 |
| Thiazide and other diuretics | 40.2 | 38.0 |
| Loop Diuretics | 12.4 | 19.6 |
| Antipsychotics | 6.9 | 6.3 |
| Glucocorticoids | 6.2 | 7.4 |
BP control defined according to VA/Department of Defense as ≤140/90 mmHg
Systolic BP at 12 months
Among metformin users, the median [IQR] baseline and 12-month Systolic BP was 136 (126, 147) mmHg and 133 (123, 142) mmHg, respectively. Among sulfonylurea users, the median [IQR] baseline and 12 month systolic BP was 137 (126, 150) mmHg and 135 (124, 147) mmHg, respectively. Therefore, the median difference between 12-month and baseline systolic BP was −3 mmHg (IQR −15, 9) among metformin users and −2mmHg (IQR −16, 10) among sulfonylurea users.
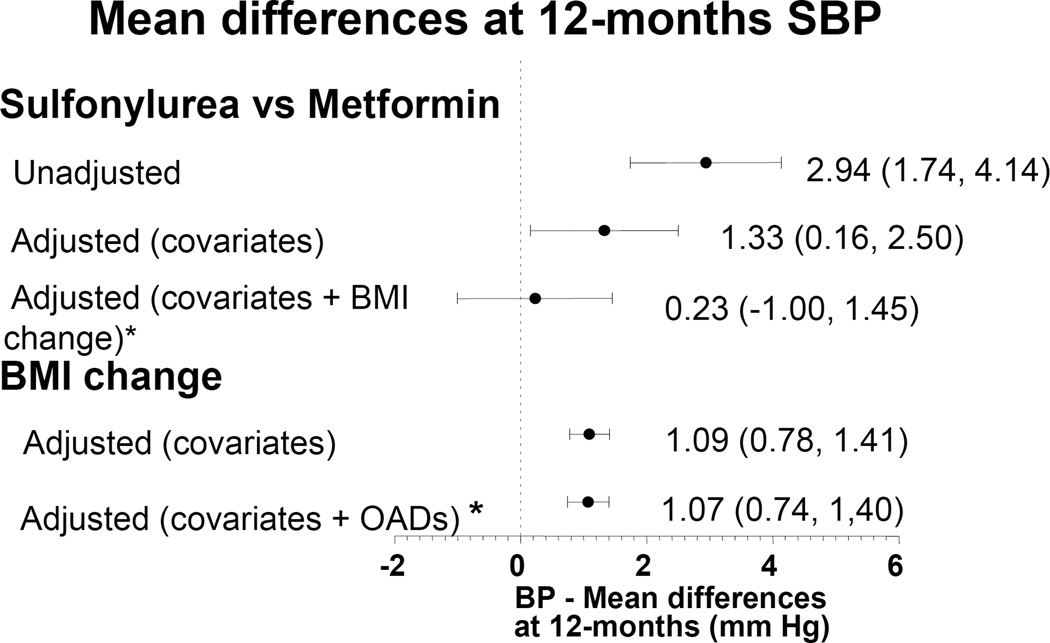
The linear regression model results are reported in table 2 and figure 2. Incident sulfonylurea users compared to metformin users had higher systolic BP values at 12 months (2.94 mmHg [1.74, 4.14 p<0.0001]) in the unadjusted analysis. When adjusted for all pre-specified covariates, incident sulfonylurea users compared with metformin users continued to have higher systolic BP values (1.40 mmHg [0.23, 2.50] p=0.02). Among metformin initiators, the median and mean changes in BMI during the 12 month follow-up were −0.76 (IQR −1.78, 0.07) and −0.95 SD± 1.90. Median and mean changes in BMI among sulfonylurea initiators were +0.21 (IQR −0.57, 1.03) and +0.17 SD ±1.57 (p=0.0001). In the second linear regression model that evaluated the effect of change in BMI (during follow-up) on 12-month systolic BP, each 1 BMI unit increase resulted in a 1.09 (0.77, 1.40 p<0.0001) mmHg increase in 12-month systolic BP. In the third model, which evaluated if the effects of OADs on 12-month systolic BP were mediated by changes in BMI, the difference in 12-month systolic BP between sulfonylurea and metformin users was no longer significant [0.31 (−0.92, 1.53) p=0.62] after adjustment for BMI change during the 12-month follow-up. There was, however, a statistically significant relationship between one unit change in BMI and the 12-month systolic BP measurement [1.05 mmHg (0.73, 1.38 p<0.0001)], indicating that the effects of OADs on 12-months systolic BP were indeed mediated through changes in BMI (Figure 2).
Table 2.
Difference in 12 month BP among patients with hypertension started on a new oral diabetic medication
| Systolic BP at 12 months among cohort | Model 1 Unadjusted |
Model 2 Association of OAD on 12 month BP adjusted for covariates † |
Model 3 Association of BMI change on 12 month BP adjusted for covariates ‡ |
Model 4 Association of OAD and BMI change on 12 month BP adjusted for covariates § |
|---|---|---|---|---|
| Total patients (N=3770) | ||||
| Intercept* | 133.40 | 135.24 | 136.40 | 136.14 |
| Sulfonylurea | 2.94 (1.74, 4.14) | 1.40 (0.23, 2.57) | -- | 0.31 (−0.92, 1.53) |
| BMI change | -- | -- | 1.09 (0.77, 1.40) | 1.05 (0.73, 1.38) |
| Sensitivity Analysis, multiple imputation cohort (N=5201) | ||||
| Intercept* | 134.00 | 136.93 | 137.85 | 137.87 |
| Sulfonylurea | 2.34 (1.31, 3.38) | 1.02 (0.01, 2.02) | -- | −0.09 (−1.13, 0.96) |
| BMI change | -- | -- | 1.16 (0.88, 1.44) | 1.16 (0.86, 1.45) |
| Systolic BP at 12 months among subgroups eGFR >60ml/min/1.73m2 (N=3006) | ||||
| Intercept * | 133.55 | 135.12 | 136.15 | 136.02 |
| Sulfonylurea | 3.01 (1.62, 4.39) | 1.38 (0.09, 2.68) | -- | 0.27 (−1.09, 1.62) |
| BMI change | -- | -- | 1.06 (0.71, 1.40) | 1.04 (0.68, 1.40) |
| No Congestive Heart Failure (N=3564) | ||||
| Intercept * | 133.59 | 135.73 | 136.86 | 136.61 |
| Sulfonylurea | 3.08 (1.85, 4.31) | 1.43 (0.24, 2.63) | -- | 0.25 (−1.00, 1.50) |
| BMI change | -- | -- | 1.17 (0.85, 1.49) | 1.14 (0.81, 1.48) |
| No hospitalization (N=3392) | ||||
| Intercept * | 133.47 | 135.68 | 136.70 | 136.49 |
| Sulfonylurea | 3.22 (1.95, 4.49) | 1.43 (0.20, 2.67) | -- | 0.26 (−1.04, 1.56) |
| BMI change | -- | -- | 1.19 (0.84, 1.53) | 1.16 (0.80, 1.52) |
| White (N=3084) | ||||
| Intercept * | 133.05 | 135.91 | 137.04 | 136.55 |
| Sulfonylurea | 2.87 (1.56, 4.17) | 1.36 (0.09, 2.63) | -- | 0.18 (−1.13, 1.50) |
| BMI change | -- | -- | 1.15 (0.82, 1.49) | 1.13 (0.78, 1.48) |
| Black (N=385) | ||||
| Intercept * | 135.91 | 130.24 | 131.3 | 130.74 |
| Sulfonylurea | 2.19 (−1.94, 6.33) | 1.54 (−2.76, 5.84) | -- | 1.02 (−3.41, 5.44) |
| BMI change | -- | -- | 0.68 (−0.73, 2.10) | 0.58 (−0.87, 2.04) |
Intercept represents 12 month systolic BP among those who are metformin users.
Model 2-Considers patient persistent on incident regimen until they do not have OAD medications for 90 days. Adjusted for age, sex, race, year of index prescription, baseline HbA1c, BMI, Systolic BP (third degree polynomial), serum creatinine, history of cardiovascular disease, marital status, baseline number of medications, number of outpatient visits, history of hospitalization; Model for entire cohort and sensitivity analysis also adjust for antipsychotic medications, corticosteroid use, thiazide diuretics, angiotensin receptor blockers (ARB), angiotensin converting enzyme inhibitors (ACE), and other diuretics.
Model 3-No OAD in model. Adjusted for age, sex, race, year of index prescription, baseline HbA1c, BMI, Systolic BP (third degree polynomial), serum creatinine, history of cardiovascular disease, marital status, baseline number of medications, number of outpatient visits, history of hospitalization and change in BMI between cohort entry and 12 month systolic BP. Model for entire cohort and sensitivity analysis also adjust for antipsychotic medications, corticosteroid use, thiazide diuretics, angiotensin receptor blockers (ARB), angiotensin converting enzyme inhibitors (ACE), and other diuretics.
Model 4 Considers patients persistent on incident regimen until they do not have OAD medications for 90 days. Adjusted for age, sex, race, year of index prescription, baseline HbA1c, BMI, Systolic BP (third degree polynomial), serum creatinine, history of cardiovascular disease, marital status, baseline number of medications, number of outpatient visits, history of hospitalization and change in BMI between cohort entry and 12-month systolic BP. Model for entire cohort and sensitivity analysis also adjust for antipsychotic medications, corticosteroid use, thiazide diuretics, angiotensin receptor blockers (ARB), angiotensin converting enzyme inhibitors (ACE), and other diuretics.
Figure 2.
Mean difference in 12-month systolic BP among hypertensive diabetic patients initiated on a new OAD
Adjusted represents model 2 or 3 evaluating the effects of either sulfonylurea on Systolic BP or BMI change on systolic BP and adjusts for baseline covariates
* Adjusted + covariates + BMI change represents model 4 and includes both the OAD and the change in BMI.
Proportion with BP controlled and Diastolic BP
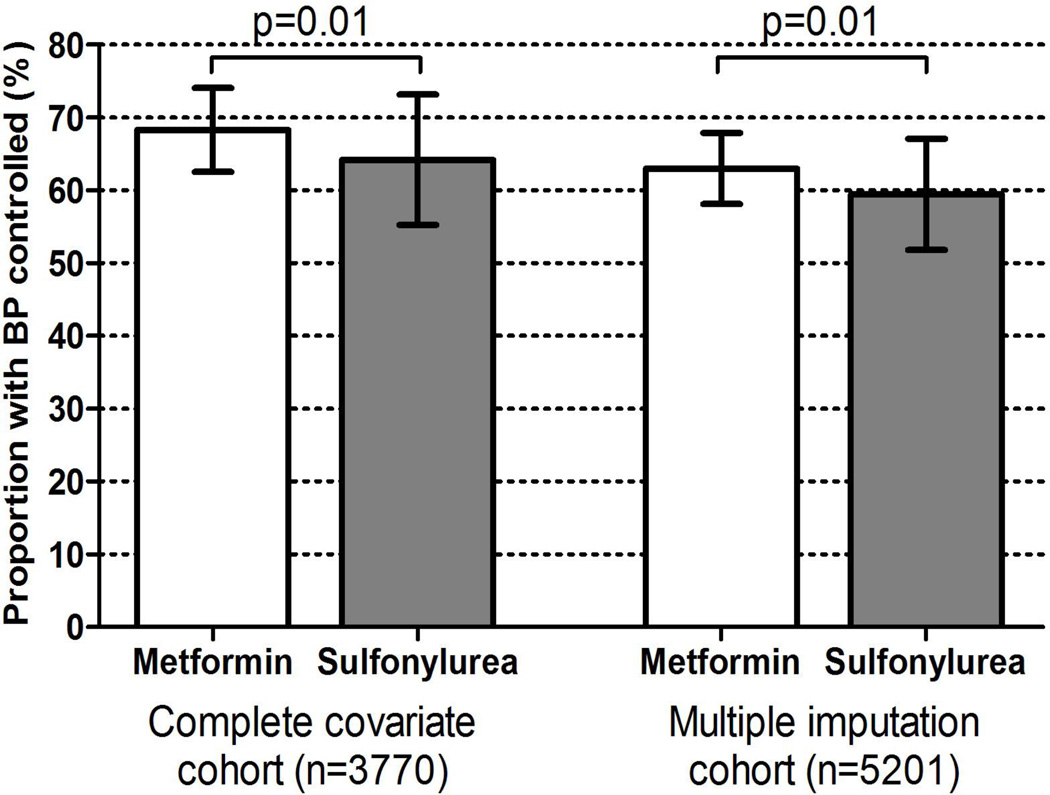
We also calculated the proportion of both metformin and sulfonylurea users who had a controlled BP at 12 months adjusted for baseline BP and all covariates. At 12 months, 68.3% of metformin patients versus 64.2% of sulfonylurea patients had a controlled BP (p=0.01) (Figure 3).
Figure 3.
Adjusted proportion of patients who achieved BP control (<140/90 mmHg) at 12 months among cohort with complete covariates (left panel) and multiple imputation cohort (right panel)
Among metformin initiators, the median baseline diastolic BP was 77 mmHg (IQR 70, 84) and 12-month diastolic BP was 75 mmHg (68, 82). Among sulfonylurea initiators, median baseline and 12-month diastolic BP was 76 (IQR 68, 83) and 73 mmHg (66, 80), respectively. Users of sulfonylurea did not have a statistically higher diastolic BP at 12 months compared to metformin users [0.40 mmHg (−0.33, 1.13) p=0.29]. However, when we evaluated for the effects of change in BMI on diastolic BP, a 1 BMI unit change influenced the 12-month diastolic BP [0.51 mmHg (0.31, 0.71) p<.0001].
Sensitivity and Subgroup Analyses
In a sensitivity analysis that did not censor on hospitalization, there were 4288 observations among 4272 patients (metformin N=2284 [53.3%]; sulfonylurea N=1760 [41.0%]; 244 excluded because they were either thiazolidendione or metformin and sulfonylurea combination users). Results of this analysis were consistent with those from the main model and sulfonylureas continued to have a positive difference in systolic BP compared to metformin [1.20 mmHg (95% CI 0.09, 2.30 p=0.03)]. These results were again attenuated after adjusting for the change in BMI that occurred after OAD initiation [difference in 12-month systolic BP among sulfonylurea users 0.10 mmHg (−1.04, 1.26 p=0.86); difference in 12-month systolic BP for each one point change in BMI 1.07 mmHg (0.77, 1.38 p<0.0001)].
In a sensitivity analysis that used multiple imputations among those who were missing covariates there were 5201 observations. Results were consistent in all 3 models (Table 2). When evaluating the adjusted proportion of patients with controlled BP at 12 months, 63% of metformin patients were controlled versus 59.5% of sulfonylurea patients (p=0.01) (Figure 3).
Clinically important subgroups were also evaluated, including those with an eGFR >60ml/min/1.73 m2; those with no history of hospitalization or congestive heart failure in the prior year and white versus black race. All results demonstrated a consistent effect with sulfonylurea users having an increased systolic BP at 12 months, which were statistically significant except in the black race subgroup. In each mediation model, inclusion of BMI change, significantly attenuated the increase in 12-month systolic BP. (Table 2)
DISCUSSION
Few studies have investigated the effects of OADs on BP. Although differential effects of OADs on weight have been documented, it is unclear if weight changes result in meaningful changes in BP. Several lines of evidence suggest this to be biologically plausible. Both randomized trials of hypertension prevention [8, 9] and the observational Framingham cohort [19] have demonstrated that weight loss improves hypertension control and prevention.
In this study, we demonstrated that use of sulfonylureas increased systolic BP at 12 months compared to use of metformin on average 1 mmHg. This change in systolic BP led to meaningful differences in the proportion of patients who achieved BP control at 12 months (64.2 % of sulfonylurea users versus 68.3% of metformin users). These results were consistent among the cohort of patients with hypertension who had complete covariates and among the cohort who were missing covariates (multiple imputation cohort). Although elevation of systolic BP by 1–2 mmHg may seem trivial; from a population standpoint, the effects of a 1.2 mmHg reduction in systolic BP from dietary sodium reduction was projected to reduce the incidence of coronary heart disease by 4.7%, myocardial infarction by 3.7%, stroke by 2.4% and cardiovascular mortality in the US by 3.3%. It was estimated that such a change in BP would save approximately 4.1 billion dollars in US healthcare costs.[20] With more than 65% of adults with DM using OADs alone or in combination, an increase in systolic BP among those using sulfonylureas has the potential to lead to significant population increases in BP and related adverse health effects.
The Atherosclerosis Risk in Communities (ARIC) cohort study demonstrated that over six years, an average weight gain of 1 kg/year among white men was associated with an increase of 0.64 mmHg and 0.40 mmHg in systolic and diastolic BP, respectively.[21, 22] In comparison, among sulfonylurea users in our study who gained an average of 3 kg compared to metformin users in the first year, the average increase in systolic BP of 1.09 mmHg seems plausible. Nevertheless, the mechanisms by which weight gain is associated with worsening of hypertension are unclear. It has been suggested that increased alterations in the autonomic nervous system may contribute to increases in BP along with insulin resistance[23], sodium retention, and vascular reactivity. Sulfonylureas an insulin secretogogue has also been described to produce hyper-insulinemia which in turn can produce hypertension.[24] Hyper-insulinemia has been described to stimulate the sympathetic nervous system and the renin-angiotensin-aldosterone system. This in turn can cause renal and vascular damage through hemodynamic and nonhemodynamic pathways leading to subsequent hypertension. Our study cannot separate out with certainty whether it was the weight gain caused by the sulfonylurea or the resultant hyperinsulinemia which resulted in the worsening hypertension.
Other limitations also exist. We excluded a large number of potential study subjects because of non persistence on their incident regimen and missing 12 month BP values. However, these selection criteria were necessary for the analyses and similar criteria were applied to metformin and sulfonylurea users to assure internal validity. We also performed multiple imputations to include a larger cohort of patients who were missing baseline clinical variables, and obtained similar results. Additionally, the characteristics of subjects with and without these measurements appeared similar (Supplemental table 2). Refill data were used as a proxy for medication taking, and may result in exposure misclassification. Nevertheless, prescription fills have been shown to be a good proxy for medication use.[25] Lastly, the use of a VA population may limit the generalizability of our findings as the VA population composition is unique and the VA system has a structured formulary which limits the use of certain newer medications. For this reason, and due to low number of patients initiating thiazolidinediones, these OADs were not included in the analyses.
Future research is needed to determine if the effects of OADs on BP control will also lead to differences in cardiovascular disease incidence. In addition, whether the choice of OAD affects mortality or cardiovascular disease through other mechanisms requires further study. In summary, compared with patients treated with metformin as initial therapy, patients treated with sulfonylureas had a 1.3 mmHg higher systolic BP and were 4.1% less likely to have a controlled BP at 12 months. These clinically important effects make metformin a more favorable initial choice for eligible patients.
Supplementary Material
Acknowledgements
This project was funded in part by Contract No. 290-05-0042 from the Agency for Healthcare Research and Quality, US Department of Health and Human Services as part of the Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) program. The authors of this report are responsible for its content. Statements in the report should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services. The funding sources did not participate in the planning, collection, analysis or interpretation of data or in the decision to submit for publication. The investigators had full access to the data and were responsible for the study protocol, statistical analysis plan, progress of the study, analysis, reporting of the study and the decision to publish. Drs. Roumie (04-342-2) and Hung (2-031-09S) were supported by a VA Career Development Awards.
Footnotes
No financial disclosures were reported by the authors of this paper
Author Contributions
Design (Roumie, Greevy, Grijalva, Griffin)
Conduct/data collection (Roumie, Choma, Liu, Greevy)
Analysis (Roumie, Choma, Liu, Greevy)
Writing manuscript (Roumie, Choma, Liu, Hung, Greevy, Grijalva, Griffin)
References
- 1.Gilmer TP, et al. The cost to health plans of poor glycemic control. Diabetes Care. 1997;20(12):1847–1853. doi: 10.2337/diacare.20.12.1847. [DOI] [PubMed] [Google Scholar]
- 2.Gilmer TP, et al. Predictors of health care costs in adults with diabetes. Diabetes Care. 2005;28(1):59–64. doi: 10.2337/diacare.28.1.59. [DOI] [PubMed] [Google Scholar]
- 3.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854–865. [PubMed] [Google Scholar]
- 4.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 5.Bennett WL, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2010;154(9):602–613. doi: 10.7326/0003-4819-154-9-201105030-00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolen S, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med. 2007;147(6):386–399. doi: 10.7326/0003-4819-147-6-200709180-00178. [DOI] [PubMed] [Google Scholar]
- 7.Feldstein AC, et al. Weight change in diabetes and glycemic and blood pressure control. Diabetes Care. 2008;31(10):1960–1965. doi: 10.2337/dc08-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. The Trials of Hypertension Prevention Collaborative Research Group. Arch Intern Med. 1997;157(6):657–667. [PubMed] [Google Scholar]
- 9.He J, et al. Long-term effects of weight loss and dietary sodium reduction on incidence of hypertension. Hypertension. 2000;35(2):544–549. doi: 10.1161/01.hyp.35.2.544. [DOI] [PubMed] [Google Scholar]
- 10.Phung OJ, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. Jama. 303(14):1410–1418. doi: 10.1001/jama.2010.405. [DOI] [PubMed] [Google Scholar]
- 11.Huizinga MM, et al. Glycemic and weight changes after persistent use of incident oral diabetes therapy: A Veterans Administration Retrospective Cohort Study. Pharmacoepidemiology and Drug Safety. 2010 doi: 10.1002/pds.2035. In press. [DOI] [PubMed] [Google Scholar]
- 12.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackinnon DP, Fairchild AJ. Current Directions in Mediation Analysis. Curr Dir Psychol Sci. 2009;18(1):16. doi: 10.1111/j.1467-8721.2009.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Classification of Diseases, Ninth Revision, Clinical Modification. Washington, DC: Public Health Service, US Dept of Health and Human Services; 1988. [Google Scholar]
- 15.Hynes DM, et al. Veterans' Access to and Use of Medicare and Veterans Affairs Health Care. Med Care. 2007;45(3):214–223. doi: 10.1097/01.mlr.0000244657.90074.b7. [DOI] [PubMed] [Google Scholar]
- 16.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 17.Lin D, Wei L. The Robust Inference for the Cox Proportional Hazards Model. Journal of the American Statistical Association. 1989;84(408):1074–1078. [Google Scholar]
- 18.Yuan YC. Multiple Imputation for Missing Data: Concepts and New Development (Version 9.0) SAS reference documents; 2011. Volume. [Google Scholar]
- 19.Moore LL, et al. Weight loss in overweight adults and the long-term risk of hypertension: the Framingham study. Arch Intern Med. 2005;165(11):1298–1303. doi: 10.1001/archinte.165.11.1298. [DOI] [PubMed] [Google Scholar]
- 20.Bibbins-Domingo K, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 362(7):590–599. doi: 10.1056/NEJMoa0907355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juhaeri, et al. Associations of weight loss and changes in fat distribution with the remission of hypertension in a bi-ethnic cohort: the Atherosclerosis Risk in Communities Study. Prev Med. 2003;36(3):330–339. doi: 10.1016/s0091-7435(02)00063-4. [DOI] [PubMed] [Google Scholar]
- 22.Juhaeri, et al. Associations between weight gain and incident hypertension in a bi-ethnic cohort: the Atherosclerosis Risk in Communities Study. Int J Obes Relat Metab Disord. 2002;26(1):58–64. doi: 10.1038/sj.ijo.0801846. [DOI] [PubMed] [Google Scholar]
- 23.Shibao C, et al. Acarbose, an alpha-glucosidase inhibitor, attenuates postprandial hypotension in autonomic failure. Hypertension. 2007;50(1):54–61. doi: 10.1161/HYPERTENSIONAHA.107.091355. [DOI] [PubMed] [Google Scholar]
- 24.Levin G, et al. Glucose, insulin, and incident hypertension in the multi-ethnic study of atherosclerosis. Am J Epidemiol. 172(10):1144–1154. doi: 10.1093/aje/kwq266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grymonpre R, et al. Validity of a prescription claims database to estimate medication adherence in older persons. Med Care. 2006;44(5):471–477. doi: 10.1097/01.mlr.0000207817.32496.cb. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.