Abstract
OBJECTIVE
The aim of this study was to estimate the accuracy of prenatal assessment of interventricular septum (IVS) thickness, right myocardial wall thickness (RMWT), and left myocardial wall thickness (LMWT) by two-dimensional (2D) ultrasound for the prediction of perinatal mortality and postnatal diagnosis of hypertrophic cardiomyopathy (HCM) among diabetic pregnant women.
SUBJECTS AND METHODS
A total of 120 diabetic pregnant women at 35 weeks or more were enrolled in this study from January 1, 2012, to June 30, 2014, at Ain Shams Maternity Hospital, Cairo, Egypt. The 2D ultrasound was done once for all the participants at the time of recruitment; IVS thickness, RMWT, and LMWT were measured. The glycosylated hemoglobin (HbA1c) levels of the participants were recorded. Neonatal assessment including postnatal echocardiography was done after 48 hours. Postnatal results were compared with the prenatal predictive results.
RESULTS
Higher thickness values for IVS, RMW, and LMW were obtained in the uncontrolled diabetic cases (HbA1c > 6.5%) than in the controlled diabetic cases (HbA1c < 6.5%; P < 0.01). Of the included 120 neonates, 10 (8.3%) were stillborn, 99 (82.5%) had a five-minute Apgar score ≥7, and 4 (3.3%) had a five-minute Apgar score ≤3. The four neonates with severe neonatal distress died after admission to neonatal intensive care unit within one week after delivery. Out of 110 live-born neonates, 4 (3.6%) neonates had a low ejection fraction (EF) (<50%) due to HCM; of them 2 (1.8%) died within one week after delivery, while 2 (1.8%) survived. Another two (1.8%) neonates died from severe respiratory distress syndrome. A cutoff value of ≥4.5 mm for prenatal IVS thickness was predictive of neonatal distress due to HCM with a sensitivity of 82%, specificity of 68%, and diagnostic accuracy of 72%. A cutoff value of <1.18 for the ratio of IVS thickness to LMWT had a sensitivity of 82%, specificity of 72%, and diagnostic accuracy of 74% for the prediction of neonatal distress due to HCM. In this study, 8 of the 10 fetuses with intrauterine demise and the 2 neonates who died within one week after delivery due to heart failure had a prenatal IVS thickness of ≥4.5 mm, while 7 of the 10 fetuses with intrauterine demise and the 2 neonates who died postnatal from heart failure had a prenatal IVS thickness to LMWT ratio of ≤1.18.
CONCLUSION
A prenatal IVS thickness of ≥4.5 mm or an IVS/LMWT ratio of ≤1.18 seems to be predictive of HCM and is associated with almost twofold higher risk of intrauterine fetal death and almost threefold higher risk of possibly relevant perinatal mortality.
Keywords: interventricular septum (IVS) thickness, left myocardial wall thickness (LMWT), two-dimensional ultrasound, hypertrophic cardiomyopathy (HCM), diabetes mellitus (DM) with pregnancy, echocardiography, infant of diabetic mother
Introduction
Diabetes mellitus (DM) affects the fetal heart during early and late gestation. During early gestation, it hinders the proper expression of genes needed for the correct development of the fetal heart during embryogenesis, causing structural cardiac defects, for example, ventricular septal defects.1 Moreover, during late gestation, fetal hyperinsulinemia due to inadequate maternal glycemic control increases the expression of fetal insulin cardiac receptors. Insulin, an anabolic hormone, causes hyperplasia and hypertrophy of the fetal myocardium, leading to hypertrophic cardiomyopathy (HCM).2,3 Microscopically, there is also a disarray of the myofibrils.4 Right and left posterior ventricular walls become hypertrophied, but the most prominent hypertrophy is of the interventricular septum (IVS) due to its abundance of insulin receptors.5
Approximately 3–6% of infants of diabetic mothers (IDMs) have congenital cardiac malformations, of which 40% are with HCM that may or may not be symptomatic. A major finding is hypertrophy of the ventricular and septal walls of the neonatal heart. In all, 5% of neonates born to diabetic mothers suffer from congestive heart failure due to left ventricular outflow obstruction. Fortunately, in most cases, cardiac hypertrophy is transient with spontaneous echocardiographic resolution within the early months after birth, requiring no therapy.6,7
Doppler echocardiography has evolved over the last few years as a noninvasive tool to evaluate the structure and the function of the fetal heart through the assessment of systolic and diastolic functions.8 This has assisted obstetricians to take decisions such as stoppage of digoxin in fetuses with heart failure due to structural cardiac defects when diagnosed with HCM antenatal.9 However, obstetricians have rarely studied about prenatally measured IVS thickness and its correlation with the postnatal presentation of HCM. The prediction of such a complication in an infant of a diabetic mother can allow the attending obstetrician to prepare proper facilities to care for such neonates especially in low-resource health-care systems.
The aim of the current study was to estimate the accuracy of prenatal assessment of IVS thickness right myocardial wall thickness (RMWT), and left myocardial wall thickness (LMWT) by two-dimensional (2D) ultrasound for the prediction of perinatal mortality and postnatal diagnosis of HCM among diabetic pregnant women.
Patients and Methods
A total of 120 diabetic pregnant women at 35 weeks or more were enrolled in this study from January 1, 2012, to June 30, 2014, in Ain Shams Maternity Hospital, Cairo, Egypt. Participants with congenital fetal malformations, multiple pregnancies, or other obstetrical complications, or pregnant women with medical disorders with pregnancy other than DM were excluded from this study. This research complied with the principles of Declaration of Helsinki. After obtaining the hospital’s ethical committee approval, the participants signed the written informed consents for participation in this study. Demographic data were recorded. The 2D ultrasound was done once for all the participants by the second author at the time of recruitment. The glycosylated hemoglobin (HbA1c) levels of the participants were recorded; the mean cutoff value is 6.5%, where a level of <6.5% indicates good glycemic control and a level of >6.5% indicates poor glycemic control. Neonatal assessment including Apgar score; signs of respiratory distress, such as apnea, grunting, nasal flaring, rapid shallow breathing, and abnormal chest movement during breathing and cyanosis; difficulty in feeding; tachycardia (heart rate > 180 beats/minute) and arrhythmias; hepatomegaly (length below costal margin > 3 cm); increased cardiothoracic ratio (>60%); and need for neonatal intensive care admission were recorded. A postnatal cardiac echocardiography of the neonates was done after 48 hours to determine the IVS thickness, RMWT, LMWT, and ejection fraction (EF) using M-mode and 2D echocardiography as described by the Japan Society of Ultrasonics in Medicine.10 IVS, RMWT, and LMWT with a standard deviation (SD) of >2 for age with the EF of <50% are considered abnormal.10–12
Routine fetal biometry via 2D ultrasound was performed by the second author (GMM.) using Voluson E6 expert machine (GE Healthcare). After obtaining the transverse section of the fetal chest, a four chamber view of the fetal heart was obtained and the IVS thickness, RMWT, and LMWT were measured in millimeters.
Sample size was calculated according to a previous study,13 which stated that the expected prevalence of thick IVS among fetuses of well-controlled diabetic mothers is 33% and among fetuses of poorly controlled diabetic mothers is 75%. Calculation according to these values produces an average sample size of 120 patients.
Analysis was performed by a statistician using the Statistical Package for the Social Sciences (Version 15). Data were expressed as mean ± SD (range) or as number (%) of cases. The comparison of proportions and means between both groups was done by using the χ2 test and independent t-test, respectively. The Fisher’s exact test was used when applicable. Odds ratio, 95% confidence interval, and paired t-test were also used in comparison between pre- and postnatal IVS in good and poor glycemic control. P < 0.05 is considered the cutoff value for significance. The differences in mean of the previous variables against the type of diabetes were tested using one-way analysis of variance. Kaplan-Meier technique was used to estimate the time of distress producing a survival curve (log-rank test compared the survival rates of the developing distress in infants of good and poor glycemic control). Multivariate analysis was performed between the significant variables. Receiver operating characteristic (ROC) curve was used to define the best cutoff values for the prenatal assessment of IVS thickness, RMWT, and LMWT to predict distress.
Results
A total of 120 pregnant women with DM were included in this study. The mean age of the included women was 32.58 ± 6.72 years (range: 18–46 years). The mean parity was 1.76 ± 1.2 (range: 0–5). The mean body mass index was 28.33 ± 4.01 kg/m2 (range: 23.4–43.1 kg/m2). The mean gestational age at recruitment was 36.07 ± 0.86 weeks (range: 35–38 weeks). The mean HbA1c was 6.16 ± 1.1% (range: 4–8%). Higher thickness values for IVS, RMW, and LMW were obtained in the uncontrolled diabetic cases (HbA1c > 6.5%) than in the controlled diabetic cases (HbA1c < 6.5%) (Table 1). Of the included 120 women, 74 (61.7%) had pregestational DM, while 46 (38.3%) had gestational DM. The mean duration of DM (in women with pregestational DM) was 7.01 ± 5.61 years (range: 1–25 years).
Table 1.
Comparison between good and poor glycemic control on prenatal and postnatal ultrasound measurements.
| GLYCEMIC CONTROL (110) | P VALUE | ||
|---|---|---|---|
| GOOD (78) | POOR (32) | ||
| Interventricular septum (mm) | |||
| by prenatal ultrasound | 3.78 ± 0.78 | 5.09 ± 0.96 | <0.0001 |
| by postnatal ultrasound | 3.81 ± 0.78 | 5.20 ± 0.93 | <0.0001 |
| Right myocardial thickness (mm) | |||
| by prenatal ultrasound | 4.36 ± 0.58 | 5.25 ± 0.76 | <0.0001 |
| by postnatal ultrasound | 4.38 ± 0.59 | 5.25 ± 0.76 | <0.0001 |
| Left myocardial thickness (mm) | |||
| by prenatal ultrasound | 4.97 ± 0.62 | 5.66 ± 0.75 | <0.0001 |
| by postnatal ultrasound | 5.01 ± 0.63 | 5.78 ± 0.81 | <0.0001 |
| Ejection fraction (%) | |||
| by postnatal echocardiography | 61.79 ± 3.17 | 57.50 ± 4.02 | <0.0001 |
The mean gestational age at delivery was 37.11 ± 0.9 weeks (range: 37–38.29 weeks of gestation). A total of 73 patients (60.8%) had cesarean delivery and 47 patients (39.1%) had vaginal delivery. The indications of cesarean sections were previous cesarean sections, failed induction of labor, failed progress of labor, fetal macrosomia, and nonreassuring fetal cardiotocography. The mean birth weight was 3.22 ± 2.19 kg (range: 2.3–4.9 kg). Of the included 120 neonates, 43 (35.8%) were macrosomic (birth weight > 4 kg), while 15 (12.5%) had low birth weight (<2.5 kg). Of the included 120 neonates, 10 (8.3%) were stillborn (3 patients suffered diabetic ketoacidosis, 4 patients had previous history of intrauterine fetal demise (IUFD), and the condition of 3 patients could not be explained), 99 (82.5%) had a five-minute Apgar score of ≥7, 11 (9.2%) had a five-minute Apgar score of <7, and 4 (3.3%) had a five-minute Apgar score of <3. The four neonates with five-minute Apgar score of ≤3 died after admission to neonatal intensive care unit (NICU) within one week after delivery.
There was a slight, yet statistically significant, mean paired difference between postnatal and prenatal ultrasound assessment of IVS thickness, RMWT, and LMWT. Prenatal assessment tends to have slightly lower measurements for the IVS thickness and slightly higher measurements for the RMWT and LMWT (Table 2).
Table 2.
Comparison between prenatal and postnatal assessments of the IVS thickness, RMWT, and LMWT.
| PRENATAL | POSTNATAL | MPD ± SD | P VALUE | |
|---|---|---|---|---|
| IVS thickness (mm) | 4.16 ± 1.02 | 4.21 ± 1.03 | 0.04 ± 0.18 | 0.004 |
| Right myocardial thickness (mm) | 4.73 ± 0.84 | 4.63 ± 0.76 | 0.08 ± 0.21 | 0.002 |
| Left myocardial thickness (mm) | 5.30 ± 8.06 | 5.23 ± 0.77 | 0.09 ± 0.19 | 0.001 |
Notes: Data are presented as mean ± SD. *Analysis is done using paired student’s t-test.
Abbreviations: IVS, interventricular septum; MPD, mean paired difference; SD, standard deviation.
The mean EF measured postnatally in the included neonates was 60.55 ± 3.94% (range: 40–65%). Of the 110 live-born neonates, 4 (3.6%) neonates had a low EF (<50%) with hypertrophied IVS (>4.5 mm) and suffered from cyanosis, weak suckling, tachypnea, and tachycardia. Of them, two (1.8%) neonates died within one week after delivery due to heart failure, while two (1.8%) survived. They received the following treatment in the NICU: positive pressure ventilation, intravenous diuretics, correction of electrolyte disturbance and metabolic acidosis, intravenous fluid therapy according to urinary output, positive inotropics, and control of dysrhythmias. Another two (1.8%) neonates died from severe respiratory distress syndrome. More importantly, all cases that had poor perinatal outcome had a poor glycemic control (with HbA1c > 6.5%) and had pregestational DM.
ROC curves were constructed for estimating the association between the prenatal IVS thickness, RMWT, and LMWT and the postnatal diagnosis of HCM. All of these measurements showed significant predictability, with the prenatal IVS thickness being the most predictable, having the largest area under the curve (Fig. 1). A prenatal IVS thickness of ≥4.5 mm was associated with a postnatal diagnosis of HCM at a sensitivity of 82%, a specificity of 68%, a positive predictive value (PPV) of 37%, a negative predictive value (NPV) of 94%, a positive likelihood ratio (LR+) of 2.6, and an overall accuracy of 72%.
Figure 1.
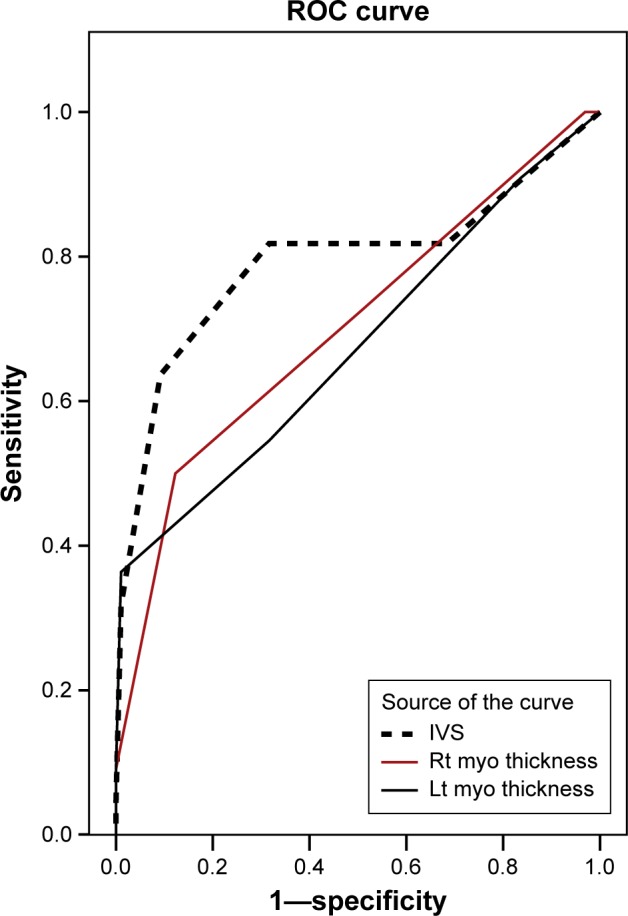
ROC curves for estimating the association between prenatal IVS thickness, right and left myocardial thicknesses, and postnatal diagnosis of HCM.
Notes: All of those measurements showed significant predictability of HCM, with the prenatal IVS thickness being the most predictable, having the largest area under the curve (AUC). A prenatal IVS thickness ≥4.5 mm was associated with a postnatal diagnosis of HCM at a sensitivity of 82%, a specificity of 68%, a positive predictive value (PPV) of 37%, a negative predictive value (NPV) of 94%, a positive likelihood ratio (LR+) of 2.6, and an overall accuracy of 72%. AUC for prenatal IVS thickness: 0.80, 95% CI (0.66 to 0.93), p < 0.001. AUC for prenatal right myocardial thickness: 0.70, 95% CI (0.57 to 0.84), p = 0.003. AUC for prenatal left myocardial thickness: 0.68, 95% CI (0.53 to 0.82), p = 0.01. AUC, 95% CI: area under the curve and its 95% confidence interval.
ROC curve, which was constructed for estimating the association between the prenatal IVS thickness to left myocardial thickness (IVS/LMWT) ratio and the postnatal diagnosis of HCM, showed a significant association (Fig. 2). A prenatal IVS/LMWT ratio of ≤1.18 was associated with a postnatal diagnosis of HCM at a sensitivity of 82%, a specificity of 72%, a PPV of 40%, an NPV of 95%, an LR+ of 3, and an overall accuracy of 74%.
Figure 2.
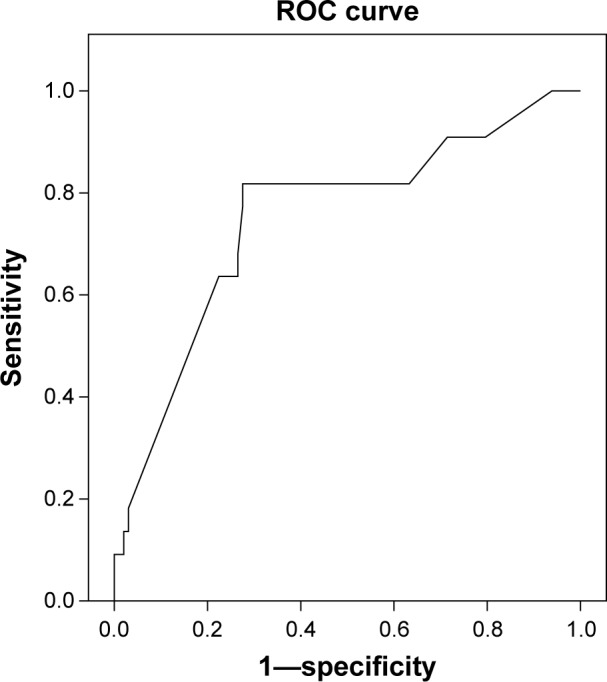
ROC curve for estimating the association between prenatal IVS thickness-to-left myocardial thickness (IVS:LMWT) ratio, and postnatal diagnosis of HCM.
Notes: A prenatal IVS: LMWT ratio ≤1.18 was associated with a postnatal diagnosis of HCM at a sensitivity of 82%, a specificity of 72%, a PPV of 40%, a NPV of 95%, a LR+ of 3, and an overall accuracy of 74%. AUC: 0.75, 95% CI (0.63 to 0.87), p = 0.003. AUC, 95% CI: area under the curve and its 95% confidence interval.
Multivariate regression analysis showed that both prenatal IVS thickness and prenatal IVS/LMWT ratio were independently and significantly predictive of the postnatal diagnosis of HCM. A prenatal IVS thickness of ≥4.5 mm was associated with almost threefold higher risk of having postnatal HCM, while a prenatal IVS/LMWT ratio of ≤1.18 was associated with almost fourfold higher risk of having postnatal HCM (Table 3).
Table 3.
Multivariate regression analysis for association between prenatal IVS thickness and IVS/LMWT ratio and postnatal diagnosis of HCM.
| UNADJUSTED OR (95% CI) | ADJUSTED OR (95% CI) | |
|---|---|---|
| Prenatal IVS thickness ≥4.5 mm | 9.73 (4.04 to 31.15) | 3.02 (1.1 to 11.65) |
| Prenatal IVS:LMWT ratio ≤1.18 | 11.83 (3.67 to 38.15) | 3.78 (1.2 to 14.80) |
Abbreviations: IVS, interventricular septum; LMWT, left myocardial wall thickness; OR (95% CI), odds ratio and its 95% confidence interval.
Finally, 8 of the 10 fetuses who had intrauterine demise and the 2 neonates who died within one week after delivery due to heart failure had a prenatal IVS thickness of ≥4.5 mm, while 7 of the 10 fetuses who had intrauterine demise and the 2 neonates who died within one week after delivery due to heart failure had a prenatal IVS/LMWT ratio of ≤1.18. A prenatal IVS thickness of ≥4.5 mm (Fig. 3) or an IVS/LMWT ratio of ≤1.18 was associated with almost twofold higher risk of IUFD and almost threefold higher risk of possibly relevant perinatal mortality (Table 4).
Figure 3.
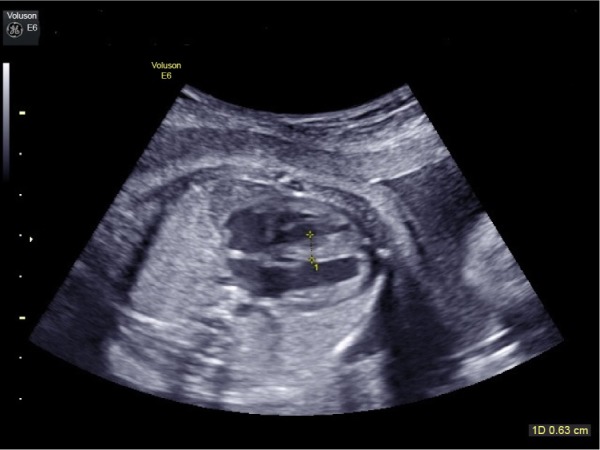
Fetal interventricular septal thickness 6 mm in a case of uncontrolled pregestational diabetes mellitus with pregnancy by 2D ultrasound.
Table 4.
Association between prenatal IVS thickness and IVS/LMWT ratio, and IUFD and relevant perinatal mortality.
| IUFD (n = 10) RR (95% CI) | POSSIBLY RELEVANT PERINATAL MORTALITY* (n = 12) RR (95% CI) | |
|---|---|---|
| Prenatal IVS thickness ≥4.5 mm | 2.15 (1.45 to 3.18) | 2.63 (2.07 to 3.35) |
| Prenatal IVS:LMWT ratio ≤1.18 | 1.88 (1.17 to 3.01) | 2.63 (2.07 to 3.35) |
Notes:
Possibly relevant perinatal mortality includes both the cases of IUFD and neonatal death due to heart failure.
Abbreviations: IVS, interventricular septum; LMWT, left myocardial wall thickness; IUFD, intrauterine fetal demise; RR (95% CI), relative risk and its 95% confidence interval.
Discussion
Left ventricular mass and contractility in fetuses and neonates of diabetic mothers are exaggerated, which leads to left ventricular outflow tract obstruction due to apposition of the anterior leaflet of the mitral valve to the IVS during systole. As a result, cardiac output decreases as does the stroke volume. This effect is proportionate with the severity of septal hypertrophy. A disproportionally hypertrophic IVS in utero is an anabolic response due to fetal hyperinsulinemia caused by maternal hyperglycemia, which could be directly affected by the tightness of glycemic control.14,15 Furthermore, Vural et al16 stated that symptomatic HCM affects 12.1% of IDMs, while it is diagnosed in 30% of IDMs by routine echocardiography. In the current study, four (3.6%) neonates had a low EF (<50%) due to HCM; of them two (1.8%) neonates died within one week after delivery and had a prenatal IVS thickness of ≥4.5 mm and a prenatal IVS/LMWT ratio of ≤1.18.
Cooper et al17 concluded that high levels of HbA1c in the third trimester and maternal hyperglycemia were associated with thick neonatal IVS and macrosomia. Similarly, in the current study, all cases that had poor perinatal outcome had poor glycemic control (with HbA1c > 6.5%) and had pregestational DM. Yet, Vela-Huerta et al18 showed that pregnant diabetic women who achieved a low HbA1c <6.5% indicating tight maternal blood glucose control were not guaranteed a normal sized fetal heart or a normal IVS thickness.
Jaeggi et al19 studied different cardiac functions and interventricular septal thicknesses of the fetuses of tightly controlled diabetic mothers in comparison to normal fetuses of uncomplicated pregnancies with matched gestational age. The interventricular septa of fetuses of diabetic mothers were significantly thicker than the healthy fetuses, yet cardiac functions in both groups were within the normal range in utero. Their results regarding the IVS thickness were similar to the current study, yet they did not correlate their results with postnatal cardiac functions as in the current study. Moreover, their study included exclusively well-controlled diabetic women in the study group.
Some limitations of the present study should be acknowledged. Detailed prenatal echocardiography of the fetus was not done, which would have added more value to the relationship between prenatal predictive results and postnatal outcome. In addition, the autopsy of stillborn fetuses and postmortem analysis of neonates were not done, as it is not done routinely and parents did not grant permission for the same; hence, the exact cause of death could not be confirmed. In addition, a larger study with a greater number of participants should be conducted to confirm the conclusion reached. Yet, one important strength of the current study is the correlation of prenatal ultrasound features with postnatal outcome, which is infrequently encountered in the published literature.
Conclusion
A prenatal IVS thickness of ≥4.5 mm or an IVS/LMWT ratio of ≤1.18 seems to be predictive of HCM and is associated with almost twofold higher risk of intrauterine fetal death and almost threefold higher risk of possibly relevant perinatal mortality.
Footnotes
ACADEMIC EDITOR: Nicole Powell-Dunford, Editor in Chief
PEER REVIEW: Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1397 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE). Provenance: the authors were invited to submit this paper.
Author Contributions
Conceived and designed the experiments: SFE and GMM. Analyzed the data: MSEE. Wrote the first draft: ML and ASH. Contributed to the writing of the manuscript: HME. Agree with manuscript results and conclusions: All authors. Jointly developed the structure and arguments for the paper: ML and ASH. Made critical revisions and approved final version: ML. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Molin DG, Roest PA, Nordstrand H, et al. Disturbed morphogenesis of cardiac outflow tract and increased rate of aortic arch anomalies in the offspring of diabetic rats. Birth Defects Res A Clin Mol Teratol. 2004;70(12):927–938. doi: 10.1002/bdra.20101. [DOI] [PubMed] [Google Scholar]
- 2.Allan L, Hornberger L, Sharland G. Textbook of Fetal Cardiology. Cambridge: Greenwich Medical Media Limited; 2000. [Google Scholar]
- 3.Menezes HS, Barra M, Belló AR, Martins CB, Zielinsky P. Fetal myocardial hypertrophy in an experimental model of gestational diabetes. Cardiol Young. 2001;11(6):609–613. doi: 10.1017/s1047951101000956. [DOI] [PubMed] [Google Scholar]
- 4.Mehta A, Hussain K. Transient hyperinsulinism associated with macrosomia, hypertrophic obstructive cardiomyopathy, hepatomegaly, and nephromegaly. Arch Dis Child. 2003;88(9):822–824. doi: 10.1136/adc.88.9.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thorsson AV, Hintz RL. Insulin receptors in the newborn. Increase in receptor affinity and number. N Engl J Med. 1977;297(17):908–912. doi: 10.1056/NEJM197710272971704. [DOI] [PubMed] [Google Scholar]
- 6.Mormile R, De Michele M, Squarcia U, Quaini F. And what about epidermal growth factor (EGF) as the bridge between survivin and cardiac remodeling? Int J Cardiol. 2011;148:116. doi: 10.1016/j.ijcard.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 7.Leipold H, Worda C, Schwindt J, Kautzky-Willer A, Bancher-Todesca D, Husslein PW. Severe diabetic fetopathy despite strict metabolic control. Wien Kiln Wochenschr. 2005;117(15–16):561–564. doi: 10.1007/s00508-005-0412-1. [DOI] [PubMed] [Google Scholar]
- 8.Zielinsky P. Role of prenatal echocardiography in the study of hypertrophic cardiomyopathy in the fetus. Echocardiography. 1991;8(6):661–668. doi: 10.1111/j.1540-8175.1991.tb01029.x. [DOI] [PubMed] [Google Scholar]
- 9.Narchi H, Kulaylat N. Heart disease in infants of diabetic mothers. Images Paediatr Cardiol. 2000;2(2):17–23. [PMC free article] [PubMed] [Google Scholar]
- 10.The Terminology and Diagnostic Criteria Committee of the Japan Society of Ultrasonics in Medicine Standard measurement of cardiac function indexes. J Med Ultrasonics. 2006;33:123–127. doi: 10.1007/s10396-006-0100-4. [DOI] [PubMed] [Google Scholar]
- 11.Sharma M, Nair MNG, Jatana SK, Shahi BN. Congestive heart failure in infants and children. Armed Forces Med J India. 2003;59:228–233. doi: 10.1016/S0377-1237(03)80014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Treatment of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2011;58(25):e212–e260. doi: 10.1016/j.jacc.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Ullmo S, Vial Y, Di Bernardo S, et al. Pathologic ventricular hypertrophy in the offspring of diabetic mothers: a retrospective study. Eur Heart J. 2007;28(11):1319–1325. doi: 10.1093/eurheartj/ehl416. [DOI] [PubMed] [Google Scholar]
- 14.Walther FJ, Siassi B, King J, Wu PY. Cardiac output in infants of insulin-dependent diabetic mothers. J Pediatr. 1985;107(1):109–114. doi: 10.1016/s0022-3476(85)80630-2. [DOI] [PubMed] [Google Scholar]
- 15.Hornberger LK. Maternal diabetes and the fetal heart. Heart. 2006;92:1019–1021. doi: 10.1136/hrt.2005.083840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vural M, Leke L, Mahomedaly H, Maingourd Y, Kremp O, Risbourg B. Should an echocardiographic scan be done routinely for infants of diabetic mothers? Turk J Pediatr. 1995;37(4):351–356. [PubMed] [Google Scholar]
- 17.Cooper MJ, Enderlein MA, Tarnoff H, Rogé CL. Asymmetric septal hypertrophy in infants of diabetic mothers. Fetal echocardiography and the impact of maternal diabetic control. Am J Dis Child. 1992;146(2):226–229. doi: 10.1001/archpedi.1992.02160140092027. [DOI] [PubMed] [Google Scholar]
- 18.Vela-Huerta MM, Vargas-Origel A, Olvera-López A. Asymmetrical septal hypertrophy in newborn infants of diabetic mothers. Am J Perinatol. 2000;17:89–94. doi: 10.1055/s-2000-9267. [DOI] [PubMed] [Google Scholar]
- 19.Jaeggi ET, Fouron JC, Proulx F. Fetal cardiac performance in uncomplicated and well-controlled maternal type I diabetes. Ultrasound Obstet Gynecol. 2001;17(4):311–315. doi: 10.1046/j.1469-0705.2001.00365.x. [DOI] [PubMed] [Google Scholar]


