Abstract
Human brain development is a complex process that evolves from early childhood to young adulthood. Major advances in brain imaging are increasingly being used to characterize the developing brain. These advances have further helped to elucidate the dynamic maturational processes that lead to the emergence of complex cognitive abilities in both typical and atypical development. However, conventional approaches involve categorical group comparison models and tend to disregard the role of widespread interindividual variability in brain development. This review highlights how this variability can inform our understanding of developmental processes. The latest studies in the field of brain development are reviewed, with a particular focus on the role of individual variability and the consequent heterogeneity in brain structural and functional development. This review also highlights how such heterogeneity might be utilized to inform our understanding of complex neuropsychiatric disorders and recommends the use of more dimensional approaches to study brain development.
Keywords: brain development, cortical trajectories, variability, individual differences
Introduction
Human brain development is a complex and dynamic process that evolves from early childhood to adolescence and young adulthood. Over the past two decades, major advances in magnetic resonance (MR)-based structural and functional neuroimaging have greatly enhanced our understanding of brain development across the lifespan.1–3 Brain imaging analyses are increasingly being used to characterize the developing brain and to understand the dynamic maturational processes that lead to the emergence of sophisticated cognitive abilities. Such processes are not only complex but also heterogeneous, and, as such, there are significant individual differences in the emergence of these abilities and their underlying brain architecture.4,5
Despite frequent reports of individual variability, there has been little examination of its neural underpinnings and its implications for understanding clinical heterogeneity in psychiatric disorders. The presence of such individual differences has typically been treated as “noise” and a challenge to the statistical validity and generalizability of findings.6 Conventional analytical approaches that are used to study neurodevelopmental disorders involve comparison with a “neurotypical” control using one-dimensional, categorical methods.7,8 However, most neuropsychiatric disorders defy simple biological boundaries and diagnostic categories and are rife with variability.9,10 This variability can stem from the underlying genetic, environmental, and developmental factors and can manifest as differences both within and across individuals. Intraindividual variability refers to within-person changes in a measurable individual characteristic, over time and over the course of cognitive and brain development. Such variability is often observed when a study makes repeated measurements of a particular variable within an individual. For example, the level of cortisol in the body collected at different times during the day or brain activity during repeated occurrences of a particular task will each vary due to factors that are intrinsic to that individual at a given point in time. On the other hand, interindividual variability reflects differences across individuals. Such variability often occurs due to differences in factors such as age, sex, intellectual functioning, and the presence of a neurodevelopmental disorder in addition to previously mentioned intrinsic factors. Interindividual variability can often affect findings of a study that is trying to make generalized inferences by averaging groups of individuals under specific conditions. For instance, differences between boys and girls in performing a spatial reasoning task may be influenced by the age at which they are measured; likewise, the differences in brain structure between individuals with and without schizophrenia may relate to whether they respond to a particular medication, rather than merely their diagnosis.11,12 While categorical approaches may lead to common findings between groups of individuals, they often tend to disregard the role of various other interacting factors (such as age, sex, and intellectual functioning) that may be contributing to the outcomes at hand.
Variability is of special interest in the context of brain development, where it is well known that multiple interacting factors influence brain structure and function and result in the emergence of complex cognitive traits. Some brain imaging studies have begun to combine multimodal measurements of brain structure and function to address this complexity.13,14 Combining such methods that employ various measurement modalities with analytical approaches that take into account the effect of numerous interacting dimensions such as age, sex, and intellectual functioning on brain architecture is critical for the study of complex brain processes. It is crucial to adopt such multidimensional approaches because of the flexibility they offer in accounting for the numerous factors that influence brain development, especially at the level of the individual. Current one-dimensional, categorical group difference-based approaches are insufficient, not only for neurodevelopmental disorders but also for typical development. Consequently, recent efforts have been made toward a more fine-grained approach to determine the cognitive and behavioral phenotypes and their neural correlates, focusing on the role of individual differences.4,15,16 These efforts have been further supported by genetic evidence that suggests that psychiatric diagnoses are not categorically distinct but comprise various dimensions. As such, one-dimensional categorical models are inadequate to describe neurodevelopmental outcomes.
This article reviews the latest advances in the methods of brain development, specifically highlighting objectives, models, and principles for the study of typical development and neurodevelopmental disorders. The main goal is to summarize the findings and highlight the role of individual variability and the consequent heterogeneity in brain structural and functional development. There is a particular focus on moving away from group difference-based categorical approaches to more dimensional approaches that take interindividual variability into account. Employing such approaches in future studies of brain development may permit more sensitive measurements of neurodevelopmental mechanisms. Moreover, such approaches may also provide better statistical models and define clinically relevant biomarkers that better predict behavioral outcomes over time.
Methods and Principles of Studying Brain Development
The development of the human brain occurs through the interaction of multiple synchronized processes, some of which are complete before birth, while others continue into adulthood.17–19 The first two years of life are an exceptionally dynamic period of structural and functional development in the brain. The infant brain reaches 80% of adult volume by two years of age with a doubling of cortical gray matter volume in the first year of life.20 Both cross-sectional and longitudinal studies have demonstrated that brain development is dynamic and spatially heterogeneous1,18,19,21–23 with different brain regions following temporally distinct developmental trajectories over time.22,24,25 Increasing evidence suggests that many neurodevelopmental and psychiatric disorders are the result of atypical brain development in this stage of rapid cortical growth in early childhood.24,26 As such, studying brain structure and function during this early period, and following its trajectory to young adulthood, has been critical for understanding the mechanisms underlying typical and atypical development.27
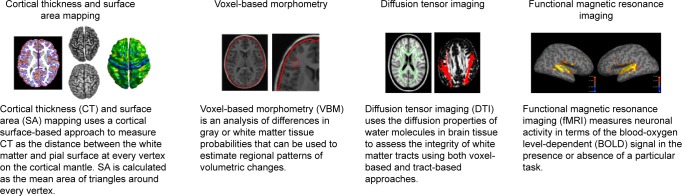
The increase in brain volume from birth to adolescence is not uniform; there is differential growth between subcortical and cortical regions and between different regions of the cortex.18 Brain structure in infants resembles the adult brain by two years of age, and the main fiber tracts can be observed by three years of age.20 Brain volume continues to develop as a function of age and cognitive function and is used as a common metric to study cortical development over time.2,27,28 Cortical thickness (CT) and surface area (SA), which are constituents of brain volume, are known to reflect independent components of cortical morphology.29 Both CT and SA are used as regional metrics of typical brain development. Additional methods such as voxel-based morphometry (VBM) and diffusion tensor imaging (DTI) offer complementary structural imaging approaches (Fig. 1). Given the significant heterogeneity in the trajectories of brain structure in both typical and atypical development,8,30,31 a combination of these methods has proven useful for the study of brain development in terms of aging, cognitive functioning, as well as atypical development in disorders such as schizophrenia,1,32 autism spectrum disorder (ASD),33–35 and attention deficit hyperactivity disorder (ADHD).25 Using a multidimensional approach that brings together complementary imaging methods, while accounting for contributing factors such as intelligence, language, and social functioning, can lead to more informative models of typical and atypical brain development.
Figure 1.
Methods for the study of brain development. This figure illustrates some of the methods used for studying brain development. These include cortical thickness and surface area mapping, voxel-based morphometry, diffusion tensor imaging, and functional magnetic resonance imaging.
Complementary to the study of brain structure, many studies use functional brain imaging to examine brain activity in specific regions of the brain in relation to sensory, motor, and cognitive functions during development.28,36 The most common methods for studying functional brain development are functional magnetic resonance imaging (fMRI), electroencephalography (EEG), and magnetoencephalography (MEG). All these methods are based on the measurement of brain activity (as measured by blood oxygenation response of neurons in the case of fMRI and local electrical or magnetic signals in the case of EEG/MEG) and their correlation with specific tasks designed to measure a particular cognitive function.14 Incorporating structural and functional imaging methods into a combined multidimensional approach, while taking into account the role of individual differences, offers a powerful strategy to address questions about brain development.
As adults, we have brains that are highly structurally and functionally specialized.37 For example, discrete regions of our cortex support cognitive functions such as language and face processing.2,28 Understanding the developing brain ultimately depends on understanding how distributed brain regions interact and develop with age to produce such sophisticated cognitive functions.38 This process can be examined on various levels, from cellular development up to the brain-wide organization of large-scale neural systems, as well as by examining the connections within and between each of those levels. Neuroimaging and histological evidence from the last decade suggests that cortical connections are fine-tuned through pruning of overabundant synapses and strengthening of relevant connections, as a function of genetics and experience.17–19,25 Changes at this microstructural level further influence the maturation of brain structure at a more macrostructural level, which can be measured by techniques such as CT/SA mapping and VBM. Furthermore, the development of brain structure mirrors the functional development of an individual in a bidirectional fashion and reflects the cognitive and behavioral processes. Consequently, brain development results due to interactions both within and across brain structure and function. It is the integration of these various domains that in turn helps in the emergence of complex brain systems. Understanding the role of this integration is critical to the study of brain development.14,38,39 As such, the field of functional neuroimaging has extended beyond task-based functional activation studies, which determined regionally defined functional specializations, to a more distributed systems approach to study functional integration.38 New acquisition methods, such as resting-state functional connectivity and novel data-analytic approaches, allow for the study of large-scale brain networks and connectivity, even in very young populations such as infants who may be at high risk for neurodevelopmental disorders such as ASD.40,41
Connectivity is an umbrella term that encompasses a variety of approaches to measure both physical (structural) and statistical (functional) relationships among brain regions.38,42 Structural connectivity can be studied using DTI, which allows the investigation of the morphology of white matter pathways in the brain.43 Specifically, DTI is sensitive to the directionality (anisotropy) of diffusion of water molecules in brain tissues, which in turn provides information about the integrity and orientation of white matter tracts. Studies using DTI have shown increased anisotropy, decreased overall diffusion, and increased myelination in major white matter fiber tracts with age.44 Additional approaches, such as structural covariance network (SCN) analyses, have recently been used to study the development of large-scale anatomical networks in both typical and atypical development.39,45 SCN analysis is an anatomical correlation-based approach that measures the interrelationships among brain regions based on structural covariation (e.g., of CT) across individuals. SCNs can be used to study coordinated maturation of brain regions, reflective of intrinsic functional networks. Functional connectivity offers a complementary approach that applies similar covariation analyses to resting-state fMRI data.38 This method detects interregional correlations in spontaneous blood oxygen level-dependent signal fluctuations. As such, it has been used to investigate large-scale brain networks involved in motor, sensory, attention, salience, and cognitive control and memory systems.40,46
The analysis of such large-scale networks using multi-modal approaches not only reflects the link between structural and functional aspects of brain development but is also critical for gaining insight into the emergence of complex cognitive functions. Alongside such methods, the use of multidimensional analytical approaches can offer ways to understand brain network architecture in the context of age, sex, and intellectual functioning-related changes as opposed to one-dimensional, categorical approaches. Not only will such multidimensional models take into account the role of individual variability, but they will also allow more sensitive statistical measurements by taking into account the temporal nature of development. Moreover, such approaches are biologically meaningful and clinically relevant, especially in the framework of neurodevelopmental disorders such as ASD, ADHD, and schizophrenia, where typical developmental processes are disrupted.
Neurodevelopmental Disorders
The study of atypical brain development facilitates the understanding of typical development itself.26 Neurodevelopmental disorders are characterized by atypical brain development leading to cognitive, neurological, or psychiatric dysfunction. Such disorders encompass several disease classifications including intellectual disability, developmental delay, autism, schizophrenia, and depression.5,47,48 Despite seemingly distinct primary diagnoses, considerable heterogeneity as well as clinical overlap exists among individuals affected by these disorders.
As highlighted above, there is a significant variability, both biological and behavioral, in development. This variability can manifest in the presentation of behavioral symptoms or abilities as well as in the underlying brain structure and function. In the case of atypical development, this variability includes both etiology and phenotypic presentation—in terms of diagnostic characteristics as well as outcome over time. Individuals with ASD, for example, can present with or without cognitive impairment or intellectual disability.49,50 In fact, comorbidity with other disorders such as ADHD is often presented in individuals with ASD.8 Such clinical overlap has also been observed for psychiatric disorders, such as bipolar disorder and schizophrenia.51 Similarly, it is well known that individuals with schizophrenia also show comorbidity with cognitive impairments of varying severity.52 Many of these psychiatric disorders have been associated with specific neuroanatomical differences.53,54 However, these brain-related differences are not found in all individuals with the disorder and are more often than not reflective of a particular behavioral outcome rather than their shared diagnosis.1 As such, similar atypicalities can be seen in individuals who meet the diagnostic criteria for different psychiatric disorders. On the other hand, these characteristics are not necessarily seen in each and every individual who meets the diagnostic criteria for a particular disorder. Another important aspect in understanding heterogeneity comes from the recognition that these atypicalities, once considered a particular characteristic of a few rare individuals, are now seen as a broad dimension of individual difference that is widely distributed in the general population.55
Such heterogeneity presents significant challenges in the interpretation of findings and is likely a primary contributor to inconsistencies across different studies. For instance, in ASD, there have been reports of both cortical thickening and cortical thinning, and several cases without any difference in CT have also been reported.56–58 Similarly, cross-sectional studies of ADHD show extreme variability in the findings of children and adults in terms of changes in the prefrontal cortex. However, consideration of both age and ADHD subtype (persisters vs remitters) has helped in unifying such findings.59 In the field of schizophrenia, research is moving away from simply investigating group differences in visual processing or executive functions toward identifying brain correlates of individual responsiveness to a particular medication.11
Thus, there is an urgent need for identification of clinically relevant “biomarkers” to stratify broad disorder phenotypes into treatment-relevant subgroups.5 Brain-related measurements may offer a potential to develop such biomarkers. Such biomarkers will help in the development of better diagnostic specificity and sensitivity and translate into clinical practice as reliable measures to monitor individual outcomes.4 In doing so, we may better understand the inconsistencies in previous findings and arrive at more conclusive results at the individual level. Moreover, such an approach will help in a more comprehensive understanding of neurodevelopment, without depending on the complete understanding of a particular disorder and the search for a universal etiology.
Variability in Brain Development
As described above, the maturation of the human brain is a dynamic and complex process, with age- and sex-related differences, structural asymmetry, and uneven developmental trajectories across the lifespan.1,24 Past research indicates that the relationship between genes, environment, brain, and behavior is complex and very indirect.60 Rather than identifying mere snapshots of developmental outcomes, the onto-genetic basis of development must be taken into account by investigating trajectories of these various factors.24,61 Information about brain development, complete with its points of susceptibility or windows of opportunity, provides a starting point for understanding the development and progression of psychopathology. Thus, there is a need to progress from investigating average differences between individuals with and without a diagnosis toward identifying key dimensions of individual differences within the diagnosed group. In this context, this review highlights the specific role of a number of factors on brain development such as age, sex, intelligence quotient (IQ), and language abilities.
Some of this variability is already typically accounted for in current research designs. For instance, the effect of developmental age is a research topic of substantial interest. In combination with advances in imaging technology, this has led to the study of neurodevelopmental disorders early in life and, longitudinally, to understand how alternative developmental pathways might lead to different phenotypic outcomes.17 For example, in typical development, the total brain volume follows an inverted U-shaped trajectory but peaks at different ages across the sexes.18 Similarly, age-related changes in disorders such as ASD also manifest as distinct trajectories early in development.34 In adolescence, core cognitive processes continue to develop and mature, parallel to structural brain changes.2 It is important to study disorder-related differences in the context of such developmental trajectories.
Recent findings have shown significant associations between regional patterns of structural brain change and cognitive development.17,22,23 Shaw et al23 demonstrated that the trajectory of change in the thickness of the cortex is highly related to intelligence level. The authors found a marked developmental shift from a predominantly negative correlation between intelligence and CT in early childhood to a positive correlation in late childhood and beyond. Additionally, the level of intelligence was associated with the trajectory of change in CT, especially in the frontal cortex, a region implicated in the maturation of intelligence. Gogtay et al22 reported accelerated maturation of the frontal lobe during adolescence and found this maturation to be related to better executive function. Reviewing 37 neuroimaging studies, Jung and Haier62 reported a remarkable agreement among findings that relate individual intelligence test scores to variations in brain structure and function in a large network of parieto-frontal regions. More recently, Karama et al63 analyzed a large normative sample of children and adolescents (N = 216) and found associations between intelligence scores and CT in lateral prefrontal, occipital extrastriate, and parahippocampal areas. Variations in these structures and functions may be biomarkers for intelligence.64,65 Furthermore, variations in these brain structures might be predictive of cognitive abilities at different ages. This has most recently been illustrated in the study by Karama et al,66 which analyzed 588 participants who had IQs available at both 11 and 70 years of age and structural MRI data obtained at approximately 73 years of age. It was found that childhood IQ accounted for more than two-thirds of the association between IQ at 70 years and CT measured at 73 years. These findings suggest that prior measures of cognitive functioning can be useful in explaining individual differences in brain structure at later ages.
Another domain of interest has been the role of sex differences in brain development. Morphologically, men have larger brains than women.67 Previous studies have suggested that focal differences in gray matter between males and females might account for the behavioral differences in spatial and verbal abilities between men and women.68 Numerous studies have reported the role of sex differences in white matter connectivity as well. Gong et al69 investigated the effects of sex on the topology of anatomical networks using SCN analysis combined with DTI tractography in 95 normal subjects aged 19–85 years. After controlling for age and brain size, women showed greater overall cortical connectivity and higher values in the efficiency of network organization. Notably, there was a clear hemispheric asymmetry of sex differences in regional efficiency. These differences have been further substantiated by subsequent studies of structural and functional connectivity that have identified sex differences. It has also been shown that there are age-dependent sex differences in brain maturational processes.61,67 The study of age-related sex differences in cerebral pruning and myelination may aid in understanding the mechanism of several developmental neuropsychiatric disorders that show sex-specific incidence and clinical features. For example, ASD has a higher prevalence in males.70,71 In schizophrenia, male and female individuals, on average, show different symptoms, age of onset, and time course of the illness.72 It is possible that the differences in the underlying brain structure and function may account for the sex-specific nature of these disorders.
As highlighted above, multiple factors such as age, sex, and IQ contribute to individual differences in brain development. It is crucial to account for these, and any other relevant factors, in current analytical models. The following section describes some approaches that have been taken toward this goal to convert heterogeneity into an opportunity, rather than a limitation. Furthermore, accounting for factors that contribute to phenotypic variability can better inform models of analysis while still maintaining the generalizability of findings to broader groups.
Categorical versus Dimensional Approaches in Studies of Brain Development
As reviewed above, variability is a characteristic feature of brain development and a potential indicator of ongoing maturational processes. Heterogeneity should therefore be treated as an important source of information. The quantification of natural variability due to these above-mentioned factors (age, IQ, gender, etc) can be a useful tool to characterize between-and within-subject deviations in brain metrics for both typical and atypical development.
The conventional analytical approach in the studies of brain development has been the group comparison method. Consequently, group-averaging techniques are well developed and commonly accepted, and in this context, variability within groups is generally considered to represent error or noise. Even in models that include multiple covariates, the objective is to account for that variability to further increase sensitivity to averaged group differences. However, including measures of variability in statistical models of analysis as covariates of interest can be very informative in understanding developmental trajectories. Such analyses not only complement categorical approaches but could also lead to further hypotheses about the etiology and phenotypic presentation of complex neuropsychiatric disorders. Furthermore, the very concept of “categorical” psychiatric disorders is questionable, given that most disorders defy a simple description defined by diagnostic boundaries. As such, a dimensional spectrum may provide a better account of the clinical reality and may help in the development of clinically useful biomarkers4,16 while still not limiting generalizability of results.
Toward this goal, a few recent studies have taken a multidimensional approach for better understanding individual differences in brain structural and functional development. The main objective of these multidimensional approaches has been to use metrics that can explain brain imaging data from clinical samples in a biomedically relevant perspective. For example, Lombardo et al73 examined the role of functional brain responses to speech as a neural predictor of language outcome in toddlers at risk for ASD. They found that this outcome measure in prediagnosed ASD toddlers having good language outcome was very similar to non-ASD comparison groups. This result was in contrast to toddlers with ASD having poor language outcomes and decreased brain response to speech. In this way, comparing distinct functional neuroimaging phenotypes can provide insight on an ASD toddler’s later outcome before ASD diagnoses become clinically clear, as opposed to merely identifying group differences in functional neuroimaging responses to speech in ASD versus non-ASD toddlers.74 In another study by Lai et al,75 the authors showed that high-functioning adult males with ASD showed neuroanatomical variations that were best explained by their developmental and current language characteristics, not merely the diagnosis of ASD, further suggesting that such characteristics significantly contribute to neuroanatomical differences between ASD and typical individuals. In a similar approach, an ongoing study in our laboratory is investigating how within-group differences in structural language abilities are related to anatomical covariance patterns in high-functioning, school-age children with ASD, rather than simply comparing covariance patterns in a group-wise manner between ASD and a typically developing comparison group.76 More specifically, our findings show that alterations in cortical structure and covariance in children with ASD are related to their structural language abilities. They also suggest that diagnostic specifiers, such as language, can be useful tools for understanding heterogeneity in ASD, much more than either symptom severity or cognitive ability. In another study by Mueller et al,15 the authors explicitly studied the role of interindividual variability on functional connectivity across the cortex by taking measurements at multiple repeated time points and subsequently controlling for the effects of intra-individual variability. Their results showed that this variability was best explained by the extent of evolutionary cortical expansion between macaques and humans, further suggesting that individual differences can provide valuable insight, not just about ontogenetic development but also about brain evolution. Together, these recent studies highlight approaches that directly benefit from individual variability while examining typical and atypical development.
There is an urgent need to follow such studies with more detailed characterizations of brain and behavioral phenotypes across the lifespan. Identifying the intrinsic biological factors underlying different developmental outcomes and neuropsychiatric subtypes is crucial for better understanding the complex mechanisms underlying neurodevelopment.77 The above-outlined dimensional approaches can provide a way to do so, in particular, to better explain variable clinical change.7 This will aid the formation of more clear and consistent theories about the pathophysiology of neurodevelopmental disorders, especially in the context of high variability.
Conclusions
Brain development across the lifespan is a complex and dynamic process that should be considered from early infancy onward and simultaneously through multiple modalities. The treatment of individual variability in development as merely an expression of measurement error or noise is limiting, especially when it precludes its consideration as a metric of interest, either as an indicator of development or as the reason of change. Thus, there is a need to move forward from investigating average differences between groups of individuals toward identifying key dimensions of individual differences within the groups, based on interacting factors such language abilities, IQ, and age. Dimensional approaches to brain development that specifically take individual variability into account can provide more sensitive measurements of neurodevelopmental principles, as well as improved statistical models. This is critical for understanding the mechanisms underlying the development of brain structure and the emergence of cognitive functions. As such, these dimensional approaches can provide an important tool to identify clinically relevant phenotypes that are accurate predictors of outcome over time, without depending on a complete understanding of the etiology of disorders. It is critical that brain development research includes studies of individual trajectories, considering not just age but also cognitive development as a whole. As outlined in this review, this has significant implications for both research and clinical settings.
Acknowledgments
The authors would like to thank Ms Ana Tryfon for comments on the manuscript.
Footnotes
ACADEMIC EDITOR: Lora Talley Watts, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1338 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: MS and KLH. Analyzed the data: MS. Wrote the first draft of the manuscript: MS. Contributed to the writing of the manuscript: MS, NEVF and KLH. Agree with manuscript results and conclusions: MS, NEVF and KLH. Jointly developed the structure and arguments for the paper: MS, NEVF and KLH. Made critical revisions and approved final version: MS, NEVF and KLH. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67(5):728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blakemore SJ. Imaging brain development: the adolescent brain. Neuroimage. 2012;61(2):397–406. doi: 10.1016/j.neuroimage.2011.11.080. [DOI] [PubMed] [Google Scholar]
- 3.Dennis EL, Thompson PM. Typical and atypical brain development: a review of neuroimaging studies. Dialogues Clin Neurosci. 2013;15(3):359–384. doi: 10.31887/DCNS.2013.15.3/edennis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Insel T, Cuthbert B, Garvey M, Heinssen R, DS P, Quinn K. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 5.Kapur S, Phillips AG, Insel TR. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry. 2012;17(12):1174–1179. doi: 10.1038/mp.2012.105. [DOI] [PubMed] [Google Scholar]
- 6.Zilles K, Amunts K. Individual variability is not noise. Trends Cogn Sci. 2013;17(4):153–155. doi: 10.1016/j.tics.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Jones TB, Bandettini PA, Kenworthy L, et al. Sources of group differences in functional connectivity: an investigation applied to autism spectrum disorder. Neuroimage. 2010;49(1):401–414. doi: 10.1016/j.neuroimage.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeste SS, Geschwind DH. Disentangling the heterogeneity of autism spectrum disorder through genetic findings. Nat Rev Neurol. 2014;10(2):74–81. doi: 10.1038/nrneurol.2013.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galaburda AM, Rosen GD, Sherman GF. Individual variability in cortical organization: its relationship to brain laterality and implications to function. Neuropsychologia. 1990;28(6):529–546. doi: 10.1016/0028-3932(90)90032-j. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald SW, Nyberg L, Bäckman L. Intra-individual variability in behavior: links to brain structure, neurotransmission and neuronal activity. Trends Neurosci. 2006;29(8):474–480. doi: 10.1016/j.tins.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Cao H, Dixson L, Meyer-Lindenberg A, Tost H. Functional connectivity measures as schizophrenia intermediate phenotypes: advances, limitations, and future directions. Curr Opin Neurobiol. 2015;36:7–14. doi: 10.1016/j.conb.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Campbell DB, Ebert PJ, Skelly T, et al. Ethnic stratification of the association of RGS4 variants with antipsychotic treatment response in schizophrenia. Biol Psychiatry. 2008;63(1):32–41. doi: 10.1016/j.biopsych.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sui J, Huster R, Yu Q, Segall JM, Calhoun VD. Function-structure associations of the brain: evidence from multimodal connectivity and covariance studies. Neuroimage. 2013;102(pt 1):11–23. doi: 10.1016/j.neuroimage.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bandettini PA. Twenty years of functional MRI: the science and the stories. Neuroimage. 2012;62(2):575–588. doi: 10.1016/j.neuroimage.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 15.Mueller S, Wang D, Fox MD, et al. Individual variability in functional connectivity architecture of the human brain. Neuron. 2013;77(3):586–595. doi: 10.1016/j.neuron.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ousley O, Cermak T. Autism spectrum disorder: defining dimensions and subgroups. Curr Dev Disord Reports. 2013;1(1):20–28. doi: 10.1007/s40474-013-0003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9(2):60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Wallace GL, Eisenberg IW, Robustelli B, et al. Longitudinal cortical development during adolescence and young adulthood in autism spectrum disorders: increased cortical thinning but comparable surface area changes. J Am Acad Child Adolesc Psychiatry. 2015;54(6):464–469. doi: 10.1016/j.jaac.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knickmeyer RC, Gouttard S, Kang C, et al. A structural MRI study of human brain development from birth to 2 years. J Neurosci. 2008;28(47):12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 22.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw P, Greenstein D, Lerch J, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 24.Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27(1–2):3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 25.Shaw P, Kabani NJ, Lerch JP, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karmiloff-Smith A. Development itself is the key to understanding developmental disorders. Trends Cogn Sci. 1998;2(10):389–398. doi: 10.1016/s1364-6613(98)01230-3. [DOI] [PubMed] [Google Scholar]
- 27.Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci. 2005;9(3):104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54(1–3):241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 29.Ecker C, Bookheimer SY, Murphy DG. Neuroimaging in autism spectrum disorder: brain structure and function across the lifespan. Lancet Neurol. 2015;14(11):1121–1134. doi: 10.1016/S1474-4422(15)00050-2. [DOI] [PubMed] [Google Scholar]
- 30.Coe BP, Girirajan S, Eichler EE. The genetic variability and commonality of neurodevelopmental disease. Am J Med Genet C Semin Med Genet. 2012;160C(2):118–129. doi: 10.1002/ajmg.c.31327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pacheco A, Reis A, Araújo S, Inácio F, Petersson KM, Faísca L. Dyslexia heterogeneity: cognitive profiling of Portuguese children with dyslexia. Read Writ. 2014;27:1529–1545. [Google Scholar]
- 32.Rimol LM, Panizzon MS, Fennema-Notestine C, et al. Cortical thickness is influenced by regionally specific genetic factors. Biol Psychiatry. 2010;67(5):493–499. doi: 10.1016/j.biopsych.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr Opin Neurobiol. 2005;15(2):225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Courchesne E, Pierce K, Schumann CM, et al. Mapping early brain development in autism. Neuron. 2007;56(2):399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 35.Hazlett HC, Poe MD, Gerig G, et al. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch Gen Psychiatry. 2011;68(5):467–476. doi: 10.1001/archgenpsychiatry.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson MH. Functional brain development in humans. Nat Rev Neurosci. 2001;2(7):475–483. doi: 10.1038/35081509. [DOI] [PubMed] [Google Scholar]
- 37.Johnson MH. Functional brain development in infants: elements of an interactive specialization framework. Child Dev. 2000;71(1):75–81. doi: 10.1111/1467-8624.00120. [DOI] [PubMed] [Google Scholar]
- 38.Friston KJ. Functional and effective connectivity: a review. Brain Connect. 2011;1(1):13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- 39.Evans AC. Networks of anatomical covariance. Neuroimage. 2013;80:489–504. doi: 10.1016/j.neuroimage.2013.05.054. [DOI] [PubMed] [Google Scholar]
- 40.Biswal B, Mennes M, Zuo XN, et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uddin LQ, Supekar K, Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front Hum Neurosci. 2013;7:458. doi: 10.3389/fnhum.2013.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fornito A, Bullmore ET. Connectomics: a new paradigm for understanding brain disease. Eur Neuropsychopharmacol. 2014;25(5):733–748. doi: 10.1016/j.euroneuro.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 43.Jones DK, Leemans A. Diffusion tensor imaging. Methods Mol Biol. 2011;711:127–144. doi: 10.1007/978-1-61737-992-5_6. [DOI] [PubMed] [Google Scholar]
- 44.Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2008;34(1):51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- 45.Alexander-Bloch A, Giedd JN, Bullmore E. Imaging structural co-variance between human brain regions. Nat Rev Neurosci. 2013;14(5):322–336. doi: 10.1038/nrn3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Biswal BB. Resting state fMRI: a personal history. Neuroimage. 2012;62(2):938–944. doi: 10.1016/j.neuroimage.2012.01.090. [DOI] [PubMed] [Google Scholar]
- 47.Mouga S, Almeida J, Café C, Duque F, Oliveira G. Adaptive profiles in autism and other neurodevelopmental disorders. J Autism Dev Disord. 2015;45(4):1001–1012. doi: 10.1007/s10803-014-2256-x. [DOI] [PubMed] [Google Scholar]
- 48.Kendler KS. Explanatory models for psychiatric illness. Am J Psychiatry. 2008;165(6):695–702. doi: 10.1176/appi.ajp.2008.07071061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Black DO, Wallace GL, Sokoloff JL, Kenworthy L. Brief report: IQ split predicts social symptoms and communication abilities in high-functioning children with autism spectrum disorders. J Autism Dev Disord. 2009;39(11):1613–1619. doi: 10.1007/s10803-009-0795-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Charman T, Pickles A, Simonoff E, Chandler S, Loucas T, Baird G. IQ in children with autism spectrum disorders: data from the Special Needs and Autism Project (SNAP) Psychol Med. 2011;41(3):619–627. doi: 10.1017/S0033291710000991. [DOI] [PubMed] [Google Scholar]
- 51.Malhotra D, McCarthy S, Michaelson JJ, et al. High frequencies of de novo CNVs in bipolar disorder and schizophrenia. Neuron. 2011;72(6):951–963. doi: 10.1016/j.neuron.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. Am J Psychiatry. 2008;165(5):579–587. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
- 53.Tost H, Bilek E, Meyer-Lindenberg A. Brain connectivity in psychiatric imaging genetics. Neuroimage. 2012;62(4):2250–2260. doi: 10.1016/j.neuroimage.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 54.Ecker C, Murphy D. Neuroimaging in autism-from basic science to translational research. Nat Rev Neurol. 2014;10(2):82–91. doi: 10.1038/nrneurol.2013.276. [DOI] [PubMed] [Google Scholar]
- 55.Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Arch Gen Psychiatry. 2003;60(5):524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- 56.Haar S, Berman S, Behrmann M, Dinstein I. Anatomical Abnormalities in Autism? Cereb Cortex. 2014 doi: 10.1093/cercor/bhu242. [DOI] [PubMed] [Google Scholar]
- 57.Foster NE, Doyle-Thomas KA, Tryfon A, et al. Structural gray matter differences during childhood development in autism spectrum disorder: a multi-metric approach. Pediatr Neurol. 2015;53(4):350–359. doi: 10.1016/j.pediatrneurol.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 58.Osipowicz K, Bosenbark DD, Patrick KE. Cortical Changes Across the Autism Lifespan. Autism Res. 2015;8:379–385. doi: 10.1002/aur.1453. [DOI] [PubMed] [Google Scholar]
- 59.Friedman LA, Rapoport JL. Brain development in ADHD. Curr Opin Neurobiol. 2015;30:106–111. doi: 10.1016/j.conb.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 60.Berardi N, Sale A, Maffei L. Brain structural and functional development: genetics and experience. Dev Med Child Neurol. 2015;57(suppl 2):4–9. doi: 10.1111/dmcn.12691. [DOI] [PubMed] [Google Scholar]
- 61.Knickmeyer RC, Styner M, Short SJ, et al. Maturational trajectories of cortical brain development through the pubertal transition: unique species and sex differences in the monkey revealed through structural magnetic resonance imaging. Cereb Cortex. 2010;20(5):1053–1063. doi: 10.1093/cercor/bhp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jung RE, Haier RJ. The parieto-frontal integration theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav Brain Sci. 2007;30(2):135–154. doi: 10.1017/S0140525X07001185. discussion 154–187. [DOI] [PubMed] [Google Scholar]
- 63.Karama S, Colom R, Johnson W, et al. Brain Development Cooperative Group. Cortical thickness correlates of specific cognitive performance accounted for by the general factor of intelligence in healthy children aged 6 to 18. Neuroimage. 2011;55(4):1443–1453. doi: 10.1016/j.neuroimage.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Colom R, Karama S, Jung RE, Haier RJ. Human intelligence and brain networks. Dialogues Clin Neurosci. 2010;12(4):489–501. doi: 10.31887/DCNS.2010.12.4/rcolom. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deary IJ, Penke L, Johnson W. The neuroscience of human intelligence differences. Nat Rev Neurosci. 2010;11(3):201–211. doi: 10.1038/nrn2793. [DOI] [PubMed] [Google Scholar]
- 66.Karama S, Bastin ME, Murray C, et al. Childhood cognitive ability accounts for associations between cognitive ability and brain cortical thickness in old age. Mol Psychiatry. 2014;19(5):555–559. doi: 10.1038/mp.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Bellis MD. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11(6):552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- 68.Luders E, Narr KL, Thompson PM, et al. Gender effects on cortical thickness and the influence of scaling. Hum Brain Mapp. 2006;27(4):314–324. doi: 10.1002/hbm.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gong G, He Y, Evans AC. Brain connectivity: gender makes a difference. Neuroscientist. 2011;17(5):575–591. doi: 10.1177/1073858410386492. [DOI] [PubMed] [Google Scholar]
- 70.Centers for Disease Control and Prevention . Prevalence of Autism Spectrum Disorders: Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States, 2008. 3. Vol. 61. Surveillance Summaries; Atlanta, USA: 2012. pp. 1–19. (Morbidity and Mortality Weekly Report). [PubMed] [Google Scholar]
- 71.Miller JS, Bilder D, Farley M, et al. Autism spectrum disorder reclassified: a second look at the 1980s Utah/UCLA Autism Epidemiologic Study. J Autism Dev Disord. 2013;43(1):200–210. doi: 10.1007/s10803-012-1566-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chase KA, Rosen C, Rubin LH, et al. Evidence of a sex-dependent restrictive epigenome in schizophrenia. J Psychiatr Res. 2015;65:87–94. doi: 10.1016/j.jpsychires.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lombardo MV, Pierce K, Eyler LT, et al. Different functional neural substrates for good and poor language outcome in autism. Neuron. 2015;86(2):567–577. doi: 10.1016/j.neuron.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim SH, Macari S, Koller J, Chawarska K. Examining the phenotypic heterogeneity of early Autism Spectrum Disorder: subtypes and short-term outcomes. J Child Psychol Psychiatry. 2015:12448. doi: 10.1111/jcpp.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lai MC, Lombardo MV, Ecker C, et al. MRC AIMS Consortium. Neuroanatomy of individual differences in language in adult males with autism. Cereb Cortex. 2015;25(10):3613–3628. doi: 10.1093/cercor/bhu211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sharda M, Foster N, Tryfon A, NA Imaging Group Structural covariance networks of language and communication in autism spectrum disorders; Annual Meeting of the Organization of Human Brain Mapping; Honolulu, HI, USA: 2015. pp. 5–7. [Google Scholar]
- 77.Lord C, Luyster R, Guthrie W, Pickles A. Patterns of developmental trajectories in toddlers with autism spectrum disorder. J Consult Clin Psychol. 2012;80(3):477–489. doi: 10.1037/a0027214. [DOI] [PMC free article] [PubMed] [Google Scholar]