Abstract
Background
Patterns of poor nutritional intake may exacerbate elevated morbidity experienced by cancer survivors. It remains unclear whether cancer survivors adhere to existing dietary guidelines, and whether survivors’ diet differs from individuals without cancer long-term.
Methods
We evaluated dietary intake and quality in 1,533 adult cancer survivors in the National Health and Nutrition Examination Survey (NHANES) 1999–2010 and compared that to 3,075 individuals without a history of cancer who were matched to cancer survivors by age, gender, and race/ethnicity. Dietary intake was assessed using 24-hour dietary recalls. The Healthy Eating Index (HEI)-2010 was used to evaluate diet quality.
Results
The mean HEI-2010 total score was 47.2 (SD=0.5) in cancer survivors and 48.3 (SD=0.4) in non-cancer individuals (p=0.03). Compared to non-cancer individuals, cancer survivors had a significantly lower score of empty calories (13.6 vs. 14.4, p=0.001), which corresponds to worse adherence to dietary intake of calories from solid fats, alcohol and added sugars. Cancer survivors also had a significantly lower dietary intake of fiber than non-cancer individuals (15.0 vs. 15.9 grams/day, p=0.02). Survivors’ mean dietary intakes of vitamin D, vitamin E, potassium, fiber, and calcium were 31%, 47%, 55%, 60%, and 73% in relation to the recommended intake whereas the mean dietary intake of saturated fat and sodium was 112% and 133% of the recommended intake.
Conclusions
Cancer survivors had a poor adherence to the 2010 Dietary Guidelines for Americans, and their intake patterns were worse than those in the general population for empty calories and fiber.
Keywords: Nutrition, Diet Quality, Cancer Survivors
INTRODUCTION
With advancements in cancer screening and treatment, cancer mortality rates continue to decline for most major cancer types in the United States.1 This translates to a growing cohort of cancer survivors, estimated to be 13.7 million in 2012.2 However, this success has also brought the recognition that cancer survivors have significantly elevated risk of premature mortality and serious morbidity due to chronic health conditions.3 Nutrition plays an important role in the etiology of chronic health conditions, and is among the few modifiable behaviors that can prevent or delay their onset. Patterns of poor nutritional intake may exacerbate these morbidities in cancer survivors, while healthy dietary patterns may serve a protective function. Identifying nutritional patterns in cancer survivors is a priority for improving the survival and long-term health of this population.
It remains unclear whether cancer survivors adhere to existing dietary guidelines especially long-term, and whether survivors’ age, gender, cancer type, and years from diagnosis affect their dietary intake. In particular, evidence is lacking from a nationally representative sample of cancer survivors in the United States. The primary purpose of this study is to evaluate the extent to which cancer survivors who were included in the National Health and Nutrition Examination Survey (NHANES) adhere to the 2010 Dietary Guidelines for Americans,4 and whether the diet quality of cancer survivors is better or worse than that in the general population.
METHODS
Study population
We selected cancer survivors from persons aged 20–79 years who participated in the six cycles (1999–2000, 2001–2002, 2003–2004, 2005–2006, 2007–2008, and 2009–2010) of NHANES. NHANES is a nationally representative, cross-sectional study conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC), to assess information on health and nutritional status of the non-institutionalized civilian population in the United States.5 Cancer survivors were defined as having cancer if they responded to “Yes” to the interview question, “Have you even been told by a doctor or other health professional that he/she had cancer or a malignancy of any kind?” Individuals with a non-melanoma or unknown skin cancer were excluded (n=438), unless they had an additional form of malignancy. This selection resulted in 1,550 cancer survivors. We then randomly selected 3,100 individuals from those who did not report a diagnosis of cancer using a 2:1 ratio and matched them to cancer survivors by age (20–39, 40–49, 50–59, 60–69, and 70–79 years), gender, and race/ethnicity (non-Hispanic white, and others).
The NCHS obtained IRB approval for all cycles of NHANES. These data have been made available for public use.6
Dietary intake
Dietary intake for NHANES 1999–2010 participants was assessed using 24-hour dietary recalls obtained by trained interviewers. From 1999 to 2002, one 24-hour diet recall was conducted in-person in the NHANES Mobile Examination Center; from 2003 to 2010, a second recall was obtained by telephone interview approximately 3–10 days after the first recall.7 Using the Automated Multiple Pass Method (AMPM), all foods and beverages consumed during the previous day were recorded.7 A standard set of measuring guides were used to help the respondent report the volume and dimensions of the food items consumed. Food intakes were coded and nutrient values were determined using the United States Department of Agriculture (USDA) Food and Nutrient Database for Dietary Studies, versions 1.0–5.0,8 which is based on nutrient values in the USDA National Nutrient Database for Standard Reference, Release 24.9 Intakes of nutrients are based on consumption of food and beverages and do not include intake from supplements. Intake according to food group was calculated using the MyPyramid Equivalents Database, version 2.0.10 When two recalls were available, reported intake was averaged to estimate the mean dietary intake of foods and nutrients.
We estimated diet quality by calculating the Healthy Eating Index (HEI)-2010 using the methods provided by the USDA. HEI-2010 measures adherence to the 2010 Dietary Guidelines for Americans (DGA) and has 12 components.11,12 For each component, intakes of foods and nutrients are represented on a density basis, counted as amount per 1,000 kcal. The 9 adequacy components include total fruit, whole fruit, total vegetables, greens and beans, whole grains, dairy, total protein foods, seafood and plant proteins, and fatty acids, which reflects the ratio of polyunsaturated fatty acids (PUFAs) and monounsaturated fatty acids (MUFAs) to saturated fatty acids (SFAs). Three moderation components include refined grains, sodium, and empty calories, which reflect calories from solid fats, alcohol and added sugars. For adequacy components, a score of 0 is assigned for no intake, and the scores increase proportionately as intakes increase up to the recommended intake. For moderation components, levels of intakes at the recommended level are assigned the maximum score and the scores decrease as intakes increase. The total HEI-2010 score ranges from 0 (non-adherence) to 100 (perfect adherence). A higher score indicates a better adherence to the dietary guidelines.
Statistical analyses
We first identified 42 individuals with potentially unreliable dietary intake, defined as total energy intake exceeding three standard deviations above and below the mean value of the natural log-transformed energy intake in non-cancer individuals. After excluding these 42 individuals (17 cancer survivors and 25 non-cancer individuals), we compared 1,533 cancer survivors and 3,075 non-cancer individuals with regard to demographic and health-related characteristics including age, gender, race/ethnicity, education, family income, alcohol consumption, cigarette smoking, and weight status using analysis of variance (ANOVA) for continuous variables and the chi-square test for categorical variables.13 Race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, and other. Education level was categorized into two groups: high school or less, and some college or college graduate. Income level was categorized based on family income to poverty ratio (FIPR): <130%, 130–299%, 300–499%, and ≥ 500%.14 Alcohol drinkers were defined as participants who reported consuming at least 12 drinks per year. Persons who had consumed at least 12 drinks in any year or in their entire life, and had consumed alcohol on at least one day in the past year, were considered current drinkers; those who had not were considered nondrinkers.15 Smokers were defined as participants who reported smoking at least 100 cigarettes during their lifetime, with former smokers defined as participants who reported smoking at least 100 cigarettes, but not currently smoking, and current smokers were defined otherwise. For former smokers, years of quitting smokers were calculated based on the difference between age of start and age of last smoking cigarettes regularly or how long since quitting smoking cigarettes. Body mass index (BMI) was calculated based on measured weight and height using the standard formula, i.e., BMI = weight (kg)/height (m)2. Weight status was categorized as underweight if BMI<18.5 kg/m2, normal weight if BMI=18.5–24.9 kg/m2, overweight if BMI=25–29.9 kg/m2, and obese if BMI ≥ 30 kg/m2.
We then calculated HEI-2010 total score and its component scores for cancer survivors and non-cancer individuals, and compared the mean scores between the two groups using analysis of covariance (ANCOVA) adjusting age, gender, and race/ethnicity. We further assessed the difference in HEI-2010 between cancer survivors and non-cancer individuals separately in nonsmokers, former smokers, and current smokers. In an attempt to evaluate the extent to which the difference in diet quality was explained by difference in demographic and other risk factors between survivors and non-cancer individuals, we performed multivariate logistic regression adjusting for age, gender, race/ethnicity, education, smoking status and years quitting smoking, and BMI. Diet quality was categorized into two groups (high vs. low) based on the median score in non-cancer individuals. An odds ratio (OR) < 1 corresponds to a lower diet quality in cancer survivors compared to non-cancer individuals. Results were compared in logistic regression models with and without adjusting for smoking status and years quitting smoking.
Among cancer survivors, we assessed whether the HEI-2010 total score differed by survivors’ demographic and health-related characteristics, years from cancer diagnosis, and cancer type using ANOVA. Years from cancer diagnosis was calculated based on the interval between time at diagnosis and time of the interview. We then evaluated whether dietary intake of nutrients (macronutrients, micronutrients, and minerals) differed in cancer survivors and non-cancer individuals using analysis of covariance (ANCOVA) adjusting for age, gender, and race/ethnicity, and further compared dietary intake of nutrients in both survivors and non-cancer individuals to the recommended intake by calculating the percentage of mean intake to the recommended intake × 100. Recommended intake was based on the Dietary Reference Intakes (DRIs).16 Dietary Guidelines recommendations were used when no quantitative DRI values is available.4 Given the recommended intake is age- and gender-specific we estimated a summary recommendation intake using age- and gender-specific DRI weighted by the age and gender distribution of the study population. Sampling weights were adjusted in all analyses to account for the complex sampling design of NHANES. SAS version 9.2 (SAS Institute, Cary, NC) was used for all analyses.
RESULTS
The mean age of the 1,533 cancer survivors was 58.1 (SD=0.6) years, and the mean interval from diagnosis was 10.8 (0.4) years. The four major cancer types (breast, prostate, colorectal, and lung) accounted for 43% of all cancers included. The majority of survivors were female (66%) and non-Hispanic white (83%). About half completed at least some college, and half had family income to poverty ratios of ≥ 300%. About 70% of the survivors reported alcohol consumption on at least one day in the past year; and roughly 60% reported smoking at least 100 cigarettes in life. The mean BMI was 28.9 kg/m2, and 70% of the survivors were overweight or obese. None of the survivors were underweight. Cancer survivors and non-cancer individuals had similar age, gender, race/ethnicity, education, family income, alcohol consumption, and weight status but cancer survivors were significantly more like to be current and former smokers than individuals without a history of cancer (Table 1).
Table 1.
Characteristics of adult cancer survivors and non-cancer individuals, NHANES 1999–20101
| Cancer (N=1,533) | Non-Cancer (N=3,075) | P value | |
|---|---|---|---|
| Age (year), Mean (SD) N (%) | 58.1 (0.6) | 57.8 (0.4) | 0.65 |
| 20–39 | 162 (12.9) | 331 (11.7) | 0.85 |
| 40–49 | 160 (13.8) | 322 (14.3) | |
| 50–59 | 232 (20.2) | 467 (21.5) | |
| 60–69 | 444 (27.0) | 887 (26.1) | |
| 70–79 | 535 (26.0) | 1,068 (26.5) | |
| Gender, N (%) | |||
| Female | 898 (65.6) | 1,799 (64.7) | 0.65 |
| Male | 635 (34.5) | 1,276 (35.3) | |
| Race/Ethnicity, N (%) | |||
| Non-Hispanic white | 983 (83.4) | 1,978 (84.6) | 0.22 |
| Non-Hispanic black | 284 (8.0) | 420 (6.6) | |
| Other | 266 (8.6) | 677 (8.9) | |
| Education, N (%) | |||
| High school graduate or less | 823 (45.3) | 1,708 (46.2) | 0.22 |
| Some college or college graduate | 709 (54.7) | 1,359 (53.8) | |
| Family income to poverty ratio, N (%) | |||
| <130% | 384 (19.0) | 785 (17.3) | 0.08 |
| 130%–299% | 445 (24.4) | 947 (29.4) | |
| 300%–499% | 291 (23.4) | 578 (23.2) | |
| Over 500% | 291 (25.4) | 519 (23.4) | |
| Missing | 122 (7.8) | 246 (6.6) | |
| Alcohol drinking, N (%) | |||
| Drinkers | 993 (69.5) | 1,944 (68.0) | 0.43 |
| Nondrinkers | 478 (30.5) | 973 (32.0) | |
| Smoking, N (%) | |||
| Current smokers | 299 (20.8) | 527 (17.2) | <0.0001 |
| Former smokers | 612 (38.8) | 1,047 (32.3) | |
| Nonsmokers | 621 (40.4) | 1,499 (50.5) | |
| BMI (kg/m2), Mean (SD) N (%) | 28.9 (0.3) | 28.9 (0.2) | 0.88 |
| 18.5 –24.9 | 400 (30.0) | 776 (28.7) | 0.65 |
| 25–29.9 | 539 (34.2) | 1,106 (36.0) | |
| ≥ 30 | 594 (35.8) | 1,102 (35.3) | |
Frequencies presented were weighted frequencies.
After adjusting for age, gender, and race/ethnicity, cancer survivors had a significantly lower mean HEI-2010 total score than non-cancer individuals (47.2 vs. 48.3, P=0.03) (Table 2). For individual component, cancer survivors had a significantly lower mean score for empty calories (13.6 vs. 14.4, p=0.001), which is aligned with higher consumptions of calories from solid fats, alcohol, and added sugars. In stratified analyses where the difference in HEI score between cancer survivors and non-cancer individuals were evaluated separately by smoking status (Table 3), no significant difference was found in nonsmokers and former smokers (in nonsmokers, mean difference in HEI-2010 total score = 0.5, p = 0.59; in former smokers, mean difference = −0.8, p=0.28). However, in current smokers, cancer survivors had significantly lower diet quality than non-cancer individuals, and the difference was statistically significant (mean difference = −2.9, p=0.002).
Table 2.
Healthy Eating Index (HEI)-2010 in adult cancer survivors and non-cancer individuals, NHANES 1999–2010
| HEI-2010 | Max.Score | Standard for Max. Score | Standard for Min. Score of 0 | Cancer (N=1,533) | Non-Cancer (N=3,075) | P1 |
|---|---|---|---|---|---|---|
|
| ||||||
| Mean (SD) | Mean (SD) | |||||
| Adequacy Components | ||||||
| Total vegetables | 5 | ≥1.1 cup equiv. per 1,000 kcal | No vegetables | 2.3 (0.1) | 2.3 (0.1) | 0.94 |
| Greens and beans | 5 | ≥0.2 cup equiv. per 1,000 kcal | No dark green vegetables or beans and peas | 1.1 (0.1) | 1.2 (0.05) | 0.43 |
| Total fruit | 5 | ≥0.8 cup equiv. per 1,000 kcal | No fruit | 1.8 (0.1) | 1.8 (0.1) | 0.74 |
| Whole fruit | 5 | ≥0.4 cup equiv. per 1,000 kcal | No whole fruit | 1.9 (0.1) | 1.9 (0.1) | 0.99 |
| Whole grains | 10 | ≥1.5 oz equiv. per 1,000 kcal | No whole grains | 1.7 (0.1) | 1.8 (0.1) | 0.45 |
| Dairy | 10 | ≥1.3 cup equiv. per 1,000 kcal | No dairy | 3.1 (0.1) | 3.0 (0.1) | 0.81 |
| Total protein foods | 5 | ≥2.5 cup equiv. per 1,000 kcal | No protein foods | 3.1 (0.1) | 3.0 (0.1) | 0.40 |
| Seafood and plant protein | 5 | ≥0.8 cup equiv. per 1,000 kcal | No seafood or plant proteins | 1.7 (0.1) | 1.7 (0.1) | 0.52 |
| Fatty acids | 10 | (PUFAs+MUFAs)/SFAs 2.5 | (PUFAs+MUFAs)/SFAs < 1.2 | 5.0 (0.1) | 5.1 (0.1) | 0.77 |
| Moderation Components | ||||||
| Sodium | 10 | ≤1.1 gram per 1,000 kcal | ≥2.0 gram per 1,000 kcal | 4.4 (0.1) | 4.4 (0.1) | 0.87 |
| Refined grains | 10 | ≤1.8 cup equiv. per 1,000 kcal | ≥4.3 cup equiv. per 1,000 kcal | 7.1 (0.1) | 7.2 (0.1) | 0.53 |
| Empty calories | 20 | ≤19% of energy | ≥50% of energy | 13.6 (0.2) | 14.4 (0.2) | 0.001 |
| Total HEI score | 100 | 47.2 (0.5) | 48.3 (0.4) | 0.03 | ||
Adjusted for age (continuous), sex, and race/ethnicity (non-Hispanic white, non-Hispanic black, and other)
Table 3.
Difference in Healthy Eating Index (HEI)-2010 in adult cancer survivors and non-cancer individuals by smoking status, NHANES 1999–2010
| Nonsmokers (N=2,120) | Former smokers (N=1,659) | Current Smokers (N=826) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean1 | P value | Mean1 | P value | Mean1 | P value | |
| Adequacy Components | ||||||
| Total vegetables | 0.1 | 0.43 | −0.1 | 0.37 | 0.1 | 0.70 |
| Greens and beans | 0.2 | 0.08 | −0.2 | 0.06 | −0.2 | 0.16 |
| Total fruit | 0.1 | 0.57 | 0.1 | 0.56 | −0.1 | 0.37 |
| Whole fruit | 0.2 | 0.23 | 0.1 | 0.51 | −0.2 | 0.22 |
| Whole grains | 0.2 | 0.21 | −0.1 | 0.60 | −0.4 | 0.11 |
| Dairy | 0.2 | 0.31 | −0.1 | 0.48 | 0.1 | 0.83 |
| Total protein foods | 0.1 | 0.55 | 0.1 | 0.65 | 0.1 | 0.62 |
| Seafood and plant protein | −0.03 | 0.79 | 0.02 | 0.85 | −0.1 | 0.44 |
| Fatty acids | −0.02 | 0.93 | 0.1 | 0.52 | −0.2 | 0.52 |
| Moderation Components | ||||||
| Sodium | −0.3 | 0.21 | 0.1 | 0.69 | 0.2 | 0.60 |
| Refined grains | −0.3 | 0.20 | 0.1 | 0.53 | 0.01 | 0.97 |
| Empty calories | −0.02 | 0.94 | −0.9 | 0.02 | −2.1 | 0.005 |
| Total HEI score | 0.5 | 0.59 | −0.8 | 0.28 | −2.9 | 0.002 |
Mean was calculated as the mean difference in HEI-2010 score between cancer survivors and non-cancer individuals; and a mean difference <0 corresponds to a lower score in cancer survivors than non-cancer individuals. The mean difference was adjusted for age (continuous), sex, and race/ethnicity (non-Hispanic white, non-Hispanic black, and other)
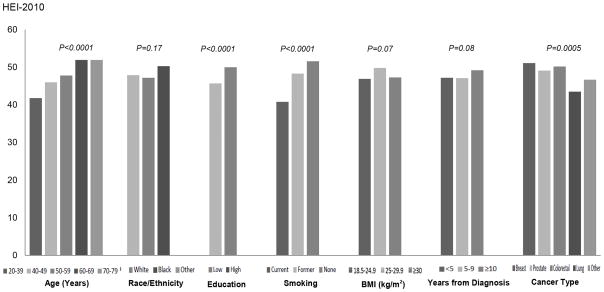
The mean HEI-2010 total score in cancer survivors increased linearly with age (Pearson correlation coefficient = 0.12, p<0.0001). Male and female survivors had similar mean HEI-2010 total score (47.6 vs. 48.3, p=0.49). Survivors who received some college or college education had a significantly higher HEI-2010 total score than those who were less educated (50.0 vs. 45.7, p<0.0001). Current smokers (40.8) had significantly lower diet quality than former (48.3) or non-smokers (51.6) (p<0.0001). For the four major cancer types evaluated, breast cancer survivors (51.1) had the best diet quality while lung cancer survivors had the worst (43.5) compared to other cancer types (p=0.008 and 0.002, respectively). Diet quality did not differ significantly by race/ethnicity, weight status, alcohol consumption, and years from diagnosis (Figure 1).
Figure 1.
HEI-2010 total score in adult cancer survivors NHANES 1999-2010 by age, race/ethnicity, education, cigarette smoking, BMI, years from diagnosis, and cancer type. For race/ethnicity, “white” refers to non-Hispanic white, and “black” refers to non-Hispanic black. For education, “low” refers to high school or less, and “high” refers to some college or college graduate.
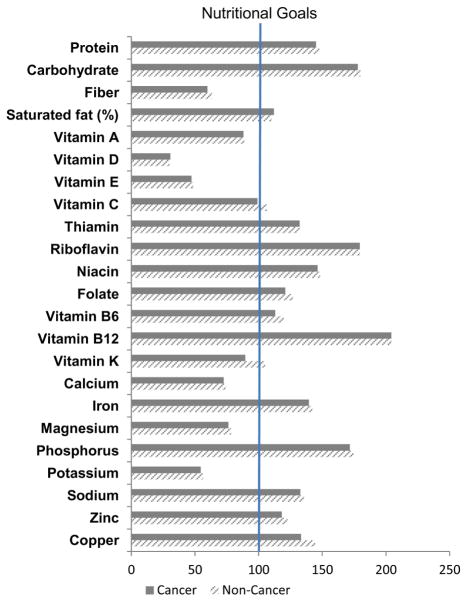
For individual nutrients and minerals, cancer survivors had a significantly lower mean intake of fiber than non-cancer individuals (15.0 vs. 15.9 g/day, p=0.02) (Table 4). However, the percentage of fiber intake in relation to recommendations was low in both cancer survivors and unaffected individuals (59.7 and 63.3%, respectively) (Figure 2). Cancer survivors had significantly lower intake vitamin K (90.3 vs.105.6 mcg/day, p=0.02) than non-cancer individuals but a high percentage from both groups met recommended levels. No significant differences were found between the two groups for other nutrients. However, survivors had low intakes of vitamin D (31%), vitamin E (47%), potassium (55%), and calcium (73%), and high intakes of saturated fat (112%) and sodium (133%) in relation to recommended levels.
Table 4.
Dietary intake in adult cancer survivors and non-cancer individuals, NHANES 1999–2010
| Cancer (N=1,533) | Non-Cancer (N=3,075) | P1 | |
|---|---|---|---|
|
| |||
| Mean (SD) | Mean (SD) | ||
| Macronutrients | |||
| Protein (g) | 71.8 (1.1) | 73.1 (0.8) | 0.15 |
| (% calories) | 15.7 (0.1) | 15.9 (0.1) | 0.14 |
| Carbohydrate (g) | 231.2 (3.6) | 234.0 (2.2) | 0.53 |
| (% calories) | 49.2 (0.4) | 49.7 (0.2) | 0.23 |
| Fiber (g) | 15.0 (0.3) | 15.9 (0.3) | 0.02 |
| Total fat (% calories) | 34.1 (0.3) | 34.0 (0.2) | 0.88 |
| Saturated fat (% calories) | 11.2 (0.1) | 11.0 (0.1) | 0.36 |
| Cholesterol (mg) | 261.3 (6.6) | 257.2 (4.9) | 0.63 |
| Micronutrients | |||
| Vitamin A (mcg RAE) | 679.2 (20.9) | 684.0 (16.8) | 0.86 |
| Vitamin D (mcg)2 | 4.6 (0.2) | 4.5 (0.2) | 0.78 |
| Vitamin E (mg AT) | 7.1 (0.2) | 7.3 (0.1) | 0.59 |
| Vitamin C (mg) | 79.5 (2.5) | 85.4 (2.4) | 0.09 |
| Thiamin (mg) | 1.5 (0.03) | 1.5 (0.02) | 0.70 |
| Riboflavin (mg) | 2.1 (0.03) | 2.1 (0.04) | 0.44 |
| Niacin (mg) | 21.5 (0.3) | 21.8 (0.3) | 0.50 |
| Folate (mcg)2 | 483.6 (11.2) | 507.1 (9.2) | 0.17 |
| Vitamin B6 (mg) | 1.7 (0.03) | 1.8 (0.03) | 0.30 |
| Vitamin B12 (mcg) | 4.9 (0.2) | 4.9 (0.1) | 1.00 |
| Vitamin K (mcg)2 | 90.3 (4.4) | 105.6 (4.7) | 0.02 |
| Minerals | |||
| Calcium (mg) | 829.5 (15.9) | 846.5 (13.3) | 0.16 |
| Iron (mg) | 14.4 (0.2) | 14.7 (0.2) | 0.44 |
| Magnesium (mg) | 270.6 (3.9) | 278.0 (3.7) | 0.13 |
| Phosphorus (mg) | 1,202.0 (18.1) | 1,221.5 (13.3) | 0.34 |
| Potassium (mg) | 2,565.6 (35.0) | 2,647.4 (28.8) | 0.05 |
| Sodium (mg) | 3,054.3 (46.2) | 3,118.3 (34.5) | 0.28 |
| Zinc (mg) | 10.7 (0.2) | 11.1 (0.2) | 0.09 |
| Copper (mg) | 1.2 (0.02) | 1.3 (0.02) | 0.17 |
| Selenium (mcg) | 96.6 (1.8) | 98.7 (1.3) | 0.27 |
Adjusted for age (continuous), sex, and race/ethnicity (non-Hispanic white, non-Hispanic black, and other)
Dietary intakes for folate and vitamin K were estimated from NHANES cycles 2003–2010, and dietary intake of vitamin D was estimated from NHANES cycles 2007–2010 because data on dietary intake of these nutrients were not available in previous NAHNES cycles.
Figure 2. Dietary Intake of Adult Cancer Survivors and Individuals without a History of Cancer in NHANES 1999-2010 Compared to Recommended Intake.
The dark grey bars indicate cancer survivors, and the line-shaded bars indicate non-cancer individuals. The lengths of bars for each nutrient correspond to the percentage of mean intake to the recommended intake × 100. Recommended intake is based on the Dietary Reference Intake (DRI) published by Institute of Medicine. Dietary Guidelines recommendations are used when no quantitative DRI value is available. Nutritional goals are set at 100 when mean intake reaches the recommended intake.
DISCUSSION
In this study which represents one of the few population-based studies of nutrient intake in cancer survivors as compared to unaffected matched controls, data suggest that cancer survivors have poor adherence to the 2010 Dietary Guidelines for Americans. Cancer survivors consumed significantly more empty calories than individuals without a history of cancer. Cancer survivors also had low dietary intakes of fiber, vitamin D, vitamin E, potassium, and calcium, but high intakes of saturated fat and sodium in relation to recommended intake levels in the dietary guidelines.
Poor dietary intake has been previously reported in other cohorts of cancer survivors.17,18 For example, less than 15% of the 9,105 cancer survivors in the American Cancer Society’s Studies of Cancer Survivors II met the recommended intake of five daily servings of fruits and vegetables.17 Other than intakes of vegetables and fruits, the adequacy of nutrients intake has not been extensively studied. Accurately assessing nutrient intake is hindered by measurement errors often associated with the use of food frequency questionnaires.19,20 Our study is one of the few studies that assessed diet quality and detailed nutrients intake in this population using 24-hour dietary recalls. Moreover, the NHANES offers a detailed examination for nutrient intake in a representative sample of the US population, thereby assuring the generalizability of our findings. Specifically, we found cancer survivors had worse diet quality and adherence to empty calories than the general population. If replicated, this may suggest reducing consumption of empty calories is an important target for dietary interventions in cancer survivors. Although other components of diet quality appeared similar between cancer survivors and non-cancer individuals, the component score was below 50% of the maximum score for several components such as greens and beans (22% of the maximum score) and whole grains (17% of the maximum score). Cancer survivors also had inadequate intakes of fiber, vitamin D, calcium, magnesium, and potassium, but excessive intakes of saturated fat and sodium. Because some of these intake patterns are established risk factors for cardiovascular disease, obesity, and bone health, it is important to further investigate whether these intake patterns contribute to elevated morbidity and mortality in this population, so that targeted dietary interventions can be implemented. Cancer survivors experience elevated morbidity and excessive morality than the general population. Interventions improving diet quality, even to a small extent, may play important roles in improving the long-term health of this vulnerable population.
We also found that cancer survivors had poor diet quality regardless of the interval from diagnosis. However, NHANES data are cross-sectional. Longitudinal studies are required to further assess whether positive dietary changes that occur soon after cancer diagnosis persist into long-term survivorship. Whether survivors with various cancer types have different diet quality has not been previously investigated. Among the four major cancer types in the US, we found breast cancer survivors had the best diet quality whereas lung cancer survivors had the worse diet quality compared to survivors with other cancer types. While the existing evidence focuses mostly on breast cancer survivors, there is clearly a need to further study dietary intake and nutritional status of survivors with other caner type.
Because current smokers had poor diet quality; and cancer survivors were more likely to be current smokers than non-cancer individuals, we further evaluated whether the difference in diet quality between cancer survivors and non-cancer individuals remained after controlling for smoking status. In multivariate logistic regression analyses adjusted for age, sex, race/ethnicity, education, and BMI, additionally controlling smoking status slightly reduced the associations indicating worse diet quality in cancer survivors than non-cancer individuals (before adjusting for smoking: OR=0.82, 95% CI: 0.69–0.96, p=0.01; after adjusting for smoking: OR=0.86, 95% CI: 0.73–1.01, p=0.06) (Supplemental Table 1). This suggests that the difference in diet quality between cancer survivors and non-cancer individuals may be partly, but not completely, explained by the difference in smoking status. In stratified analysis, cancer survivors who were current smokers had significantly lower diet quality than non-cancer individuals. They may represent a particular high-risk population who will benefit from dietary intervention.
Older survivors had a significantly better diet quality than younger survivors. This age effect has been supported by previous studies in cancer survivors,21–23 which may partly reflect better dietary habits in older than younger generations (i.e. cohort effect). Younger survivors have longer life expectancy; and the impact of a healthy diet on their long-term health is likely to be greater than older survivors. As expected, survivors with lower education had lower diet quality than those of higher education, and survivors who were current smokers had a lower diet quality than non-smokers or former smokers. These findings corroborate those of other studies which show similar trends,18,24 suggesting the needs of improving diet quality in young survivors, survivors coming from low socioeconomic background, and those who are currently smoking.
Overweight survivors appeared to have better diet quality than survivors who either had a normal weight or were obese but the difference was not statistically significant. In contrast, two previous studies in breast cancer survivors reported a linear relationship between diet quality and body mass index.21,25 However, our results were not completely surprising given advanced cancer stage can cause weight loss and being overweight may partly reflect an overall better health status in cancer survivors. Different from previous studies that reported differences in diet quality by gender and race/ethnicity in cancer survivors (i.e., women tended to have a better diet quality than men, and non-Hispanic whites tended to have a better diet quality than other racial/ethnic groups),21,26 we did not find diet quality of cancer survivors significantly differed by sex and race/ethnicity.
Although our study has numerous strengths, e.g., a population-based study with a large sample size and collection of data using validated measures, there are some limitations that need to be considered. First, cancer diagnosis is based on self-report and may be associated with misclassification. However, we expect any reporting errors to be minimal given cancer is a life threatening life event. Second, cancer stage and treatment may affect survivors’ dietary intake through complex pathways. Such information was not captured by NHANES and we were not able to evaluate the impact of cancer stage and treatment on diet quality in cancer survivors. Third, because of the cross-sectional nature of the NHANES study design we were not able to assess whether cancer survivors changed their dietary intake after cancer diagnosis and whether changes in dietary intake persist into later years. In addition, the sample size limitation did not allow us to evaluate the trend in dietary intake and diet quality over various cycles of NHANES in cancer survivors.
Despite these limitations, our study is the first study assessing dietary intake in cancer survivors from a nationally representative sample of the US population. We found cancer survivors had poor adherence to the 2010 Dietary Guidelines for Americans, and their intake patterns were worse than those in the general population for empty calories and fiber. This finding reinforces the need for dietary interventions in this vulnerable patient population. Oncology care providers play an important role in reinforcing the importance of a healthful diet, and can refer patients to registered dietitians who are experts in oncology care or to other reputable sources in order to improve survivors' overall health.27
Supplementary Material
Acknowledgments
Sources of Funding: All phases of this study were supported by the Boston Nutrition Obesity Research Center Grant Number P30DK46200, the National Center for Research Resources Grant Number UL1 RR025752, the National Center for Advancing Translational Sciences and the National Institutes of Health Grant Number UL1 TR000073. The funding source had no role in the design, conduct, or analysis of this study or the decision to submit the manuscript for publication.
Footnotes
Conflict of Interests Statement: The authors have no conflicts of interests to disclose.
REFERNECES
- 1.Lerro CC Office ACSNH. A systematic review of large-scale surveys of cancer survivors conducted in North America, 2000–2011. Journal of Cancer Survivorship. 2014;6(2):115–145. doi: 10.1007/s11764-012-0214-1. catherine.lerro@cancer.org, et al. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, DeSantis C, Virgo K, Stein K, et al. Cancer treatment and survivorship statistics, 2012. 20120710 DCOM- 20121002 (1542–4863 (Electronic)) [Google Scholar]
- 3.Edwards BK, Noone A-M, Mariotto AB, et al. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2013:n/a–n/a. doi: 10.1002/cncr.28509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Department of Agriculture (USDA) Dietary Guidelines for Americans. Vol. 2010 Washington, DC: Dec, 2010. [Google Scholar]
- 5.NHANES - About the National Health and Nutrition Examination Survey. 2014 http://www.cdc.gov/nchs/nhanes/about_nhanes.htm.
- 6.NHANES - NCHS Research Ethics Review Board Approval. 2014 http://www.cdc.gov/nchs/nhanes/irba98.htm.
- 7.Moshfegh AJ, Rhodes DG, Baer DJ, et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. The American journal of clinical nutrition. 2008 Aug;88(2):324–332. doi: 10.1093/ajcn/88.2.324. [DOI] [PubMed] [Google Scholar]
- 8.Food Surveys Research Group. [Accessed January 5, 2015];Food and Nutrient Database for Dietary Studies. 2013 http://www.ars.usda.gov/News/docs.htm?docid=12089. 2014.
- 9.U.S. Department of Agriculture (USDA); Agriculture USDo, editor. Nutrient Intakes from Food: Mean Amounts Consumed per Individual, by Gender and Age, What We Eat in America, NHANES 2009–2010. Agricultural Research Service; 2012. [Google Scholar]
- 10.Bowman SA, Friday JE, Moshfegh A. (USDA) USDoA, editor. MyPyramid Equivalents Database. 2.0 for USDA Survey Foods 2003–2004. Beltsville: USDA Agricultral Research Service; 2008. [Google Scholar]
- 11.Developing the Healthy Eating Index–2010. 2014 http://appliedresearch.cancer.gov/hei/developing.html?&url=/tools/hei/developing.html.
- 12.Guenther PM, Kirkpatrick SI, Reedy J, et al. The Healthy Eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 Dietary Guidelines for Americans. J Nutr. 2014 Mar;144(3):399–407. doi: 10.3945/jn.113.183079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vittinghoff E, Shiboski SC, Glidden DV, McCulloch CE. Regression Methods in Biostatistics. 2005. [Google Scholar]
- 14.Alaimo K, Olson CM, Frongillo EA, Jr, Briefel RR. Food insufficiency, family income, and health in US preschool and school-aged children. American journal of public health. 2001 May;91(5):781–786. doi: 10.2105/ajph.91.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breslow RA, Guenther PM, Smothers BA. Alcohol drinking patterns and diet quality: the 1999–2000 National Health and Nutrition Examination Survey. Am J Epidemiol. 2006 Feb 15;163(4):359–366. doi: 10.1093/aje/kwj050. [DOI] [PubMed] [Google Scholar]
- 16.Intakes SCoTSEoDR. Food and Nutrition Board; Institute of Medicine NAoS, editor. Dietary Reference Intakes. Washington, DC: National Academy Press; 1997. [Google Scholar]
- 17.Blanchard CM, Courneya KS, Stein K. Cancer survivors' adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society's SCS-II. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008 May 1;26(13):2198–2204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 18.Coups EJ, Ostroff JS. A population-based estimate of the prevalence of behavioral risk factors among adult cancer survivors and noncancer controls. Preventive medicine. 2005 Jun;40(6):702–711. doi: 10.1016/j.ypmed.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Livingstone MB, Black AE. Markers of the validity of reported energy intake. J Nutr. 2003 Mar;133 (Suppl 3):895S–920S. doi: 10.1093/jn/133.3.895S. [DOI] [PubMed] [Google Scholar]
- 20.Willett WC, editor. Nutritional Epidemiology. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 21.George SM, Neuhouser ML, Mayne ST, et al. Postdiagnosis diet quality is inversely related to a biomarker of inflammation among breast cancer survivors. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010 Sep;19(9):2220–2228. doi: 10.1158/1055-9965.EPI-10-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humpel N, Magee C, Jones SC. The impact of a cancer diagnosis on the health behaviors of cancer survivors and their family and friends. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2007 Jun;15(6):621–630. doi: 10.1007/s00520-006-0207-6. [DOI] [PubMed] [Google Scholar]
- 23.Thomson CA, Flatt SW, Rock CL, Ritenbaugh C, Newman V, Pierce JP. Increased fruit, vegetable and fiber intake and lower fat intake reported among women previously treated for invasive breast cancer. Journal of the American Dietetic Association. 2002 Jun;102(6):801–808. doi: 10.1016/s0002-8223(02)90180-x. [DOI] [PubMed] [Google Scholar]
- 24.George SM, Ballard-Barbash R, Shikany JM, et al. Better Postdiagnosis Diet Quality Is Associated with Reduced Risk of Death among Postmenopausal Women with Invasive Breast Cancer in the Women's Health Initiative. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014 Apr;23(4):575–583. doi: 10.1158/1055-9965.EPI-13-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim EH, Willett WC, Fung T, Rosner B, Holmes MD. Diet quality indices and postmenopausal breast cancer survival. Nutrition and cancer. 2011;63(3):381–388. doi: 10.1080/01635581.2011.535963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patterson RE, Neuhouser ML, Hedderson MM, Schwartz SM, Standish LJ, Bowen DJ. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. Journal of the American Dietetic Association. 2003 Mar;103(3):323–328. doi: 10.1053/jada.2003.50045. [DOI] [PubMed] [Google Scholar]
- 27.Demark-Wahnefried W, Rogers LQ, Alfano CM, et al. Practical clinical interventions for diet, physical activity, and weight control in cancer survivors. CA: a cancer journal for clinicians. 2015 Feb 13; doi: 10.3322/caac.21265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.