Abstract
Background
The comparative effectiveness of sulfonylureas and metformin on cardiovascular disease (CVD) outcomes in type 2 diabetes are not well characterized.
Objective
To compare the effectiveness of sulfonylureas and metformin on the outcome of CVD (acute myocardial infarction, stroke) or death
Design
Retrospective cohort study
Setting
National Veterans Health Administration (VHA) databases linked to Medicare files
Patients
Veterans who initiated metformin or sulfonylureas for diabetes. Patients with chronic kidney disease or serious medical illness were excluded.
Measurements
Composite outcome of hospitalizations for acute myocardial infarction, stroke, or death. Cox regression analyses compared the incidence of the composite outcome between groups, adjusting for baseline demographics, medications, cholesterol, glycated hemoglobin, creatinine, blood pressure, body mass index, healthcare utilization and co-morbidities.
Results
Among 253,690 patients (98,665 sulfonylurea and 155,025 metformin initiators) the crude outcome rates were 18.2 and 10.4 per 1000 person-years in sulfonylurea and metformin users, respectively (adjusted hazard ratio [aHR] 1.21, 95% Confidence Intervals [CI] 1.13, 1.30). Results were consistent for both glyburide (aHR 1.26, 95% CI 1.16, 1.37) and glipizide (aHR 1.15, 95% CI 1.06, 1.26) as well as for those with prior history of CVD (aHR 1.25, 95% CI 1.13, 1.55) and without history of CVD (aHR: 1.16, 95% CI: 1.06, 1.29). Results were also consistent in a propensity score-matched analysis. For patients initiating sulfonylureas rather than metformin, we estimated an excess of 1 and 4 CVD events per 1000 person-years for those without and with a CVD history, respectively.
Limitations
Data on women and minorities is limited but reflective of the VHA population.
Conclusions
Use of sulfonylureas compared to metformin for initial treatment of diabetes was associated with an increased hazard of CVD events or death.
Keywords: Diabetes mellitus, myocardial infarction, stroke, cardiovascular disease, comparative effectiveness
Cardiovascular disease (CVD) accounts for approximately 65% of deaths in patients with diabetes mellitus (DM). (1–3) Although randomized trials have evaluated risk of CVD associated with selected glycemic control thresholds,(4, 5) the role of specific antidiabetic drugs on CVD risk is less clear. Recent controversy surrounded thiazolidinedione use and the risk for CVD;(6–8) however, the comparative effectiveness of the two most commonly used drugs metformin and sulfonylurea, is not well characterized.
In 1970, the University Group Diabetes Program (UGDP) raised questions about the cardiovascular safety of sulfonylureas. They reported an increased risk of cardiovascular death among patients randomized to tolbutamide (sulfonylurea) compared to placebo and insulin arms.(9–11) As a result of the UGDP, the Food and Drug Administration mandated a black-box warning for all sulfonylureas, despite controversial study results.(11–14) In 1998, the United Kingdom Prospective Diabetes Study (UKPDS) allayed concerns about increased cardiovascular risk associated with sulfonylureas. Among 3867 newly diagnosed diabetic patients, those randomized to sulfonylureas and insulin had superior glucose control and fewer microvascular outcomes compared to diet, but surprisingly, diabetes-related and all-cause mortality at 10 years was similar in those randomized to sulfonylurea, insulin, and diet only. Nevertheless, in a sub-study of overweight patients, those randomized to metformin experienced 42% fewer diabetes-related deaths and 36% fewer all-cause deaths compared to the diet alone arm. Compared to overweight patients randomized to sulfonylureas or insulin, there was an advantage of metformin on mortality. However, this sub-analysis included only 342 patients on metformin and all patients were overweight.(15, 16) The ADOPT trial (A Diabetes Outcome Prevention Trial),(17) randomized 4,360 patients to metformin, rosiglitazone, or glyburide. Cardiovascular events (fatal/non fatal acute myocardial infarction and stroke) were a secondary (adverse) outcome, and after a median of 4 years were low overall, with no differences between the 3 arms (2.9% metformin vs. 2.9% rosiglitazone vs. 2.4% glyburide).
Compared with metformin, sulfonylurea use is associated with detrimental changes in weight, lipids, and greater risk of hypoglycemia, but similar glycemic control.(4, 18–20) Thus, metformin is recommended as first line therapy for patients without contraindications.(21, 22) Nonetheless, sulfonylureas are sometimes preferred because little titration is required and there are fewer gastrointestinal side effects compared with metformin. In 2007, more than 10.1 million Americans (~34% of patients with treated diabetes) used a sulfonylurea as part of their diabetes treatment.(23) Although available evidence suggests there may also be cardiovascular advantages of metformin compared to sulfonylureas, the evidence is considered weak and imprecise.(5, 24, 25)
We sought to determine the comparative effectiveness of sulfonylureas and metformin on the hazard of CVD outcomes and all-cause mortality using a national cohort and data that allowed control for important patient characteristics including glycated hemoglobin, body mass index (BMI), and blood pressure. We also quantified differences in CVD outcomes between metformin and sulfonylureas among patients with and without CVD history.
METHODS
Study Design and Data Sources
We constructed a retrospective cohort of patients initiating oral monotherapy for diabetes from national Veterans Health Administration (VHA) databases (October 1, 2001 through September 30, 2008). Pharmacy datasets contain data on prescriptions dispensed by a VHA or consolidated mail outpatient pharmacy, including medication name, date filled, days supplied, pill number and dosage.(26) VHA medical datasets contain patient demographics, and ICD9-CM coded diagnostic and procedure information from inpatient and outpatient encounters.(27) Laboratory test results are collected from standard VHA clinical sources. Data on vital signs included all outpatient measurements of height, weight and blood pressure. Date of death was obtained from VHA vital status files. For veterans who were also Medicare or Medicaid eligible, supplemental encounter and race data from the Centers for Medicaid and Medicare Services were obtained.(28) The institutional review boards of Vanderbilt University and the VA Tennessee Valley Healthcare System approved this study.
Study Population
The study population included veterans who filled a first prescription for an oral antidiabetic drug (inception cohort) during the study period, were ≥18 years old and received regular care in a VHA center (defined as a VHA encounter or prescription fill at least every 180 days). Incident users with known birth date and gender, and ≥365 days of baseline data preceding their first eligible prescription fill were identified. Patients with incident prescriptions (new-users)(29) were defined as active users of the VA medical system for at least 365 days with their first fill of an oral hypoglycemic after ≥365 days without any prior oral or injectable antidiabetic drug fill. Ninety percent of patients also had an ICD9-CM coded encounter for diabetes. We excluded patients with serious medical conditions identified at baseline (congestive heart failure, HIV, cancer except for non-melanoma skin cancer, organ transplant, end stage kidney or liver disease, or respiratory failure), cocaine use, or baseline serum creatinine ≥1.5mg/dL as these conditions may influence the prescription of specific antidiabetic drugs and risk of outcomes.
Exposures
Incident exposures were metformin and sulfonylureas (glyburide, glipizide). Thiazolidinediones and combination metformin+sulfonylurea prescriptions were excluded because they are uncommon incident regimens within VHA. Using pharmacy information, we calculated “days’ supply in hand,” and accounted for patients who “stockpile" medications. Follow-up began on the incident prescription date and continued through the first of: switch or addition of another antidiabetic drug, the 90th day with no drug in hand, an outcome or a censoring event. Censoring events were: reaching a creatinine value of ≥1.5 mg/dL, (because metformin use is not recommended); the 181st day of no contact with any VHA facility (inpatient, outpatient or pharmacy use); or end of study (September 30, 2008).
Outcomes: Cardiovascular Disease and Death
The primary composite outcome included hospitalization for acute myocardial infarction, stroke, or death. Acute myocardial infarction was defined as an ICD9-CM primary discharge diagnosis for fatal and non-fatal acute myocardial infarction (410.x). This strategy had a positive predictive value (PPV) of 67%–97% using chart review for case confirmation.(30–32) Stroke included ischemic stroke (433.x1, 434 [excluding 434.x0], or 436), intracerebral hemorrhage (431), and subarachnoid hemorrhage (430) but excluded traumatic brain injury (800–804, and 850–854). The PPV for this algorithm was 97%. (33) Mortality was determined using the VHA Vital Status file, which combines information from multiple sources (Medicare, VHA, Social Security and VHA compensation and pension benefits) to determine date of death and is highly accurate (sensitivity and specificity of 98.3% and 99.8%). (34)
Covariates
Study covariates included: age, sex, race, fiscal year, physiologic variables closest to cohort entry [blood pressure, creatinine, glycated hemoglobin (HbA1c), low density lipoprotein levels (LDL) and body mass index (BMI)], indicators of healthcare utilization (number of outpatient visits and active medications, hospitalization during baseline [yes/no]), selected medications indicative of CVD, smoking related illness, and presence of co-morbidities (Online material: Appendix Table 1). We initially stratified patients by CVD history defined by diagnoses or procedure codes for myocardial infarction, coronary artery disease, transient ischemic attack, stroke, or surgical procedures for repair of peripheral or carotid artery disease in the baseline period. We considered a potential interaction between CVD history and oral antidiabetic treatment effects; however, the formal test for interaction was not statistically significant (p=0.98). Therefore we describe patients with and without CVD combined for simplicity of presentation but the subgroup analyses by CVD history are also reported. For patients with missing covariates, a multiple imputation procedure was conducted using the Markov Chain Monte Carlo method and an non-informative Jeffreys prior.(35) All covariates from the primary analysis, survival time and a censoring indicator were included in twenty imputation models, and used to compute the final estimates.
Statistical Analysis
The primary analysis was time to the composite outcome of acute myocardial infarction, stroke or all-cause death. A secondary analysis used a composite that included acute myocardial infarction and stroke events only, with death as a censoring event rather than an outcome. Cox proportional hazards models were used to compare time-to-composite outcomes for sulfonylurea versus metformin, adjusting for covariates. Adjusted hazard ratios (aHR) and 95% confidence intervals (CI) were calculated. The proportional hazard assumptions were examined using log-log plots. We adjusted for clustering of observations within patients calculating robust standard errors based on the sandwich variance estimator because some patients (about 5%) re-entered the cohort when they had a second qualifying exposure.(36) Continuous covariates were modeled with third degree polynomials to account for non-linearity (age, BMI, HbA1c, LDL, creatinine, BP, number of medications and visits).
We also performed a propensity score matched analysis. The propensity score modeled the probability of metformin use given all other study covariates and the VHA facility of care (additional information and logistic regression model available in Appendix Table 2). The visual inspection of the distributions of propensity scores between exposure groups showed good overlap (Appendix Figure 1) and the model yielded a c statistic of 0.71, reflecting that imbalance in baseline covariates was small. Sulfonylurea and metformin observations were matched using a 1 to 1 greedy matching algorithm, yielding 80,648 propensity score-matched observations.(37, 38)
Sensitivity and subgroup analyses
Planned sensitivity analyses assessed if our definitions affected study findings. First, we used the incident prescription to define exposure groups and ignored subsequent changes in regimens (persistent exposure not required). This approach is similar to the intention to treat analysis in clinical trials and includes all person time after the initial exposure, regardless of adherence to the original regimen. Second, to determine the sensitivity of our findings to the multiple imputation strategy, we restricted the analysis to patients with complete covariates.(39–41) Third, we conducted stratified analyses by CVD history, age (<65 vs. ≥65 years), and BMI (<30 and ≥30 kg/m2). Fourth, information on baseline urine protein testing was available in the form of microalbumin to creatinine ratio (ACR) in a subset of patients (n=36,425). Patients were considered to have microalbuminuria if their ACR was ≥30 mg/g. In this subgroup, we performed a stratified analysis based on the presence of microalbuminuria.
Finally, to explore the potential for residual confounding (42) we quantified the strength of the association of an unmeasured confounder that would be required to explain our findings. Because most of our covariates are binary, we considered an unmeasured binary confounder. The bias from an unmeasured confounder is determined by its association with CVD and its prevalence in sulfonylurea versus metformin users. For this assessment, we assumed a confounder-outcome association similar to that observed among measured covariates (Hazard Ratio= 1.25) and considered a broad range of confounder prevalence, to adjust our estimated hazard ratios and 95% confidence intervals for the presence of an unmeasured confounder. Statistical analyses were conducted using R (available at: http://www.r-project.org) and SAS for Windows 11.0. (SAS Institute, Cary, NC).
RESULTS
Study Cohort and Patient Characteristics
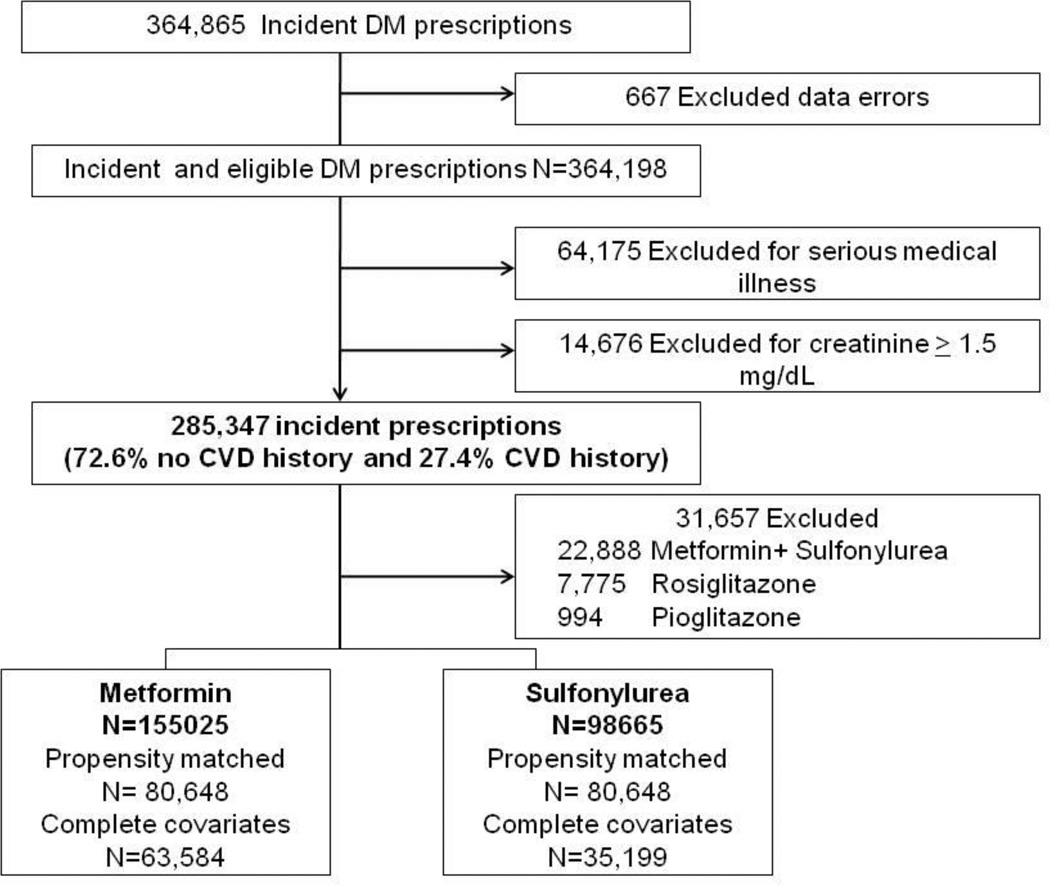
We identified 364,865 incident prescriptions for oral antidiabetic drugs. Of these, we excluded 667 (<0.2 %) for missing date of birth, gender, age <18 years or data errors; 64,175 (17.6%) for serious medical illness/cocaine use during baseline; and 14,676 (4.0%) for creatinine ≥1.5mg/dL. Of the remaining, 285,347 prescriptions among 269,921 patients, 72.6% were for patients without CVD history. Our analysis focused on incident prescriptions for metformin (50%) and sulfonylureas (40%: 55% glyburide, 45% glipizide), and excluded combination metformin+sulfonylurea (8%), rosiglitazone (3%) and pioglitazone (<1%). Five percent of the cohort contributed more than one episode of care (maximum 4) (Figure 1).
Figure 1.
Flow of eligible prescriptions
Patients were seen at 128 VHA facilities. Each facility contributed a median of 1768 prescriptions to the analysis (interquartile range [IQR] 1131, 2306; minimum 410 and maximum 6544). In the propensity matched cohort the median was1030 prescriptions per facility [IQR 696, 1554]. The median length of follow up by exposure group was 0.78 years for metformin (range 0.003 to 5.5 years [IQR 0.25, 1.71], 0.61 years for sulfonylureas (range 0.003 to 5.5 years [IQR 0.25, 1.50]. Among metformin and sulfonylurea users respectively, 73% vs. 66% were censored for stopping therapy; 18% vs. 21% for changing therapy; 5% vs. 7% were censored for leaving VHA/study end; and 2% vs. 4% were censored for reaching a creatinine of 1.5mg/dL. Censoring results were similar among the propensity matched cohort.
Patients were 97% male, and 75% white (Table 1). Median age was 62 years (interquartile range [IQR] 54, 75). Baseline HbA1c was 7.1 (6.5, 7.9) among metformin initiators and 7.3 (6.6, 8.4) among sulfonylurea initiators. Metformin patients were heavier (BMI 32.1 vs. 31.3) and more often used statins (55% vs. 47%) compared to sulfonylurea users. The distribution of baseline characteristics after propensity score matching is also shown in Table 1, and as expected, characteristics of the two matched cohorts are more similar. When evaluating baseline differences between metformin and sulfonylurea users, the standardized difference represents an absolute difference in means or percents divided by an evenly weighted pooled standard deviation. For example, the standardized difference demonstrates the difference between groups in number of standard deviations. As demonstrated in table 1, the standardized differences were small prior to propensity score matching, yet with the very large sample size, all were statistically significant. After propensity score matching the standardized differences became negligible for all covariates, with a few statistically significant differences due to the large sample size. Characteristics of the subset with complete covariates were also similar (Appendix Table 3).
Table 1.
Patient characteristics in full and propensity matched cohorts, by new metformin or sulfonylurea exposure
| Characteristic | Full Cohort | Propensity Matched Cohort | ||||
|---|---|---|---|---|---|---|
| Metformin N=155,025 |
Sulfonylurea N=98,665 |
Standardized differences † |
Metformin N=80,648 |
Sulfonylurea N=80, 648 |
Standardized differences ‡ |
|
| Age, median (IQR)* | 62 (56,71) | 67 (57, 76) | 0.33 | 65 (57, 74) | 64 (56, 74) | 0.03 ‡ |
| Male (%) | 95 | 97 | 0.12 | 97 | 97 | 0.01 |
| Race, (%) | ||||||
| White | 74 | 75 | 0.04 | 75 | 75 | 0.01 |
| Black | 12 | 13 | 0.04 | 13 | 13 | 0.00 |
| Hispanic/ Other | 6 | 6 | 0.03 | 6 | 6 | 0.00 |
| Available % | 91 | 95 | 0.13 | 94 | 94 | 0.01 |
| HbA1c (%), median (IQR) | 7.0 (6.4, 7.8) | 7.3 (6.6, 8.2) | 0.17 | 7.2 (6.5, 8.2) | 7.2 (6.6, 8.2) | 0.02 |
| Available % | 67 | 61 | 0.14 | 63 | 63 | 0.01 |
| Low Density Lipoprotein (mg/dL), median (IQR) | 103 (81, 128) | 101 (80,127) | 0.03 | 102(80, 127) | 102 (81, 127) | 0.01 |
| Available % | 63 | 55 | 0.17 | 57 | 57 | 0.02 |
| Creatinine (mg/dL), median (IQR) | 1.0 (0.9, 1.1) | 1.1 (0.9, 1.2) | 0.29 | 1.1 (0.9, 1.2) | 1.0 (0.9, 1.2) | 0.03‡ |
| Available % | 80 | 72 | 0.18 | 74 | 74 | 0.02 |
| Systolic Blood pressure (mm/Hg), median (IQR) | 134 (124, 144) | 135 (124,146) | 0.08 | 135 (124,146) | 135 (124, 146) | 0.01 |
| Diastolic Blood pressure (mm/Hg), median (IQR) | 77 (70, 84) | 76 (68, 83) | 0.09 | 76 (69, 83) | 76 (69, 83) | 0.00 |
| Available % | 95 | 94 | 0.07 | 94 | 94 | 0.00 |
| Body Mass Index (kilograms/meter2), median (IQR) | 31.9 (28.5,36.2) | 30.2 (26.9, 34.2) | 0.30 | 30.7 (27.4, 34.6) | 30.7 (27.5, 34.7) | 0.02‡ |
| Available % | 93 | 91 | 0.09 | 91 | 91 | 0.01 |
| Number of Medications median (IQR) | 5 (3, 7) | 5 (3, 7) | 0.03 | 5 (3, 7) | 5 (3, 7) | 0.00 |
| Outpatient visits, median (IQR) | 4 (2, 7) | 3 (2, 7) | 0.02 | 4 (2, 7) | 4 (2, 7) | 0.00 |
| Hospitalized (%) | 6 | 7 | 0.04 | 7 | 7 | 0.00 |
| Baseline Co-morbidities (%) § | ||||||
| Myocardial infarction/coronary disease | 20 | 23 | 0.09 | 22 | 22 | 0.01 |
| Stroke/Transient ischemic attack/carotid revascularization | 8 | 9 | 0.05 | 9 | 9 | 0.00 |
| Peripheral artery disease | 3 | 3 | 0.05 | 3 | 3 | 0.00 |
| Tobacco use/dependence | 10 | 8 | 0.07 | 9 | 9 | 0.00 |
| Obstructive pulmonary disease/Emphysema | 9 | 9 | 0.02 | 9 | 9 | 0.00 |
| Atrial Fibrillation/Flutter | 3 | 4 | 0.06 | 3 | 3 | 0.01 |
| Fiscal year | ||||||
| 2003 | 13 | 19 | 0.17 | 18 | 17 | 0.02‡ |
| 2004 | 17 | 22 | 0.11 | 21 | 21 | 0.01 |
| 2005 | 21 | 21 | 0.01 | 21 | 21 | 0.00 |
| 2006 | 24 | 21 | 0.07 | 22 | 22 | 0.01 |
| 2007 | 25 | 17 | 0.19 | 18 | 19 | 0.02 |
| Use of Medications (%) | ||||||
| ACE Inhibitors or ARBs ‖ | 58 | 57 | 0.01† | 57 | 58 | 0.00 |
| Beta-blockers | 36 | 38 | 0.04 | 37 | 37 | 0.00 |
| Calcium Channel Blockers | 23 | 25 | 0.06 | 25 | 24 | 0.01 |
| Other Antihypertensives | 16 | 18 | 0.06 | 17 | 17 | 0.01 |
| Statin lipid lowering agents | 61 | 55 | 0.12 | 56 | 56 | 0.01 |
| Other lipid lowering agents | 13 | 11 | 0.05 | 11 | 11 | 0 |
| Anti-arrhythmics | 1 | 1 | 0.06 | 1 | 1 | 0 |
| Anticoagulants | 4 | 6 | 0.08 | 5 | 5 | 0.01 |
| Antipsychotic medications | 7 | 7 | 0.01† | 7 | 7 | 0.01 |
| Digoxin | 3 | 6 | 0.14 | 5 | 5 | 0.01 |
| Thiazide and other diuretics | 33 | 30 | 0.05 | 30 | 31 | 0 |
| Loop Diuretics | 9 | 14 | 0.17 | 11 | 11 | 0.01‡ |
| Nitrates | 11 | 14 | 0.09 | 13 | 13 | 0.01 |
| Aspirin | 17 | 17 | 0.01† | 17 | 17 | 0 |
| Platelet inhibitors | 6 | 8 | 0.07 | 8 | 7 | 0.01 |
Interquartile range (IQR)
All P values for the comparison of metformin and sulfonylurea users were statistically significant at P<0.001, except ACE/ARB p=0.059; antipsychotic use p=0.006; aspirin use p=0.002
All P values for the comparison of metformin and sulfonylurea users in propensity score matched cohort were not statistically significant except age p<0.001; creatinine p <0.001; fiscal year p<0.001; body mass index p=0.003; loop diuretics p=0.006
Definitions of co morbidities and medications available in supplemental table 1
angiotensin converting enzyme inhibitors (ACE); angiotensin receptor blockers (ARB)
Time to Cardiovascular Event or Death
Among 98,665 sulfonylurea and 155,025 metformin initiators the crude CVD/death rates were 18.2 and 10.4 per 1000 person-years, respectively (adjusted hazard ratio [aHR] 1.21, 95% Confidence Intervals [CI] 1.13, 1.30). Results were consistent for glyburide (aHR 1.26, 95% CI 1.16, 1.37) and for glipizide (aHR 1.15, 95% CI 1.06, 1.26). We also examined the secondary outcome of cardiovascular events. The crude cardiovascular event rates were 13.5 and 8.2 per 1000 person-years, respectively (aHR 1.16, 95% CI 1.06, 1.25). (Table 2)
Table 2.
Rates and adjusted hazard ratios (95% confidence interval [CI]) for hazard of cardiovascular disease or death (primary composite outcome) and cardiovascular events (secondary outcome) among full cohort and propensity matched cohort in new users of sulfonylureas compared to metformin
| Full Cohort | Propensity matched Cohort | |||
|---|---|---|---|---|
| Persistent Exposure required* | Metformin N=155,025 |
Sulfonylurea N=98,665 |
Metformin N=80,648 |
Sulfonylurea N=80,648 |
| Person Years | 179,351 | 101,125 | 94,970 | 83,848 |
| Cardiovascular events or death | 1871 | 1844 | 1239 | 1284 |
| Rate/1000 person-years | 10.4 | 18.2 | 13.0 | 15.2 |
| Adjusted Hazard ratio† (95% Confidence Intervals) | Reference | 1.21 (1.13, 1.30) | Reference | 1.15 (1.07, 1.25) |
| Cardiovascular events | 1467 | 1367 | 958 | 969 |
| Rate/1000 person-years | 8.2 | 13.5 | 10.1 | 11.6 |
| Adjusted Hazard ratio† (95% Confidence Intervals) | Reference | 1.16 (1.06, 1.25) | Reference | 1.13 (1.03, 1.23) |
| Persistent exposure not required ‡ | ||||
| Person Years | 361,929 | 244,804 | 204,286 | 198,517 |
| Cardiovascular events or death | 4818 | 5572 | 3550 | 3816 |
| Rate/1000 person-years | 13.3 | 22.8 | 17.4 | 19.2 |
| Adjusted Hazard ratio† (95% Confidence Intervals) | Reference | 1.21 (1.16, 1.27) | Reference | 1.20 (1.14, 1.26) |
| Cardiovascular events | 3194 | 3422 | 2202 | 2378 |
| Rate/1000 person-years | 8.8 | 14.0 | 10.8 | 12.0 |
| Adjusted Hazard ratio† (95% Confidence Intervals) | Reference | 1.14 (1.08, 1.20) | Reference | 1.13 (1.07, 1.20) |
Primary analysis considers patients persistent on incident regimen until they do not have oral antidiabetic medications for 90 days.
Cox Proportional Hazards model for time to cardiovascular disease with sandwich variance estimate. Adjusted for age, sex, race, fiscal year of cohort entry, number of medications, number of outpatient visits, history of hospitalization, baseline HbA1c, Body mass index, serum creatinine, Low density lipoprotein cholesterol, blood pressure, use of medications (see Appendix table 1), smoking-related illness, myocardial infarction; obstructive coronary disease or prescription for a long acting nitrate; stroke/ transient ischemic attack; atrial fibrillation/ flutter; mitral/ aortic or rheumatic heart disease; asthma/obstructive pulmonary disease; procedures for carotid/ peripheral artery revascularization or bypass or lower extremity amputation. Propensity score matched models also include facility of care. All continuous variables were modeled as third degree polynomials.
Persistent exposure not required analysis- In which patients remain in their exposure group, regardless of persistence on drug therapy, until outcome or end of the study.
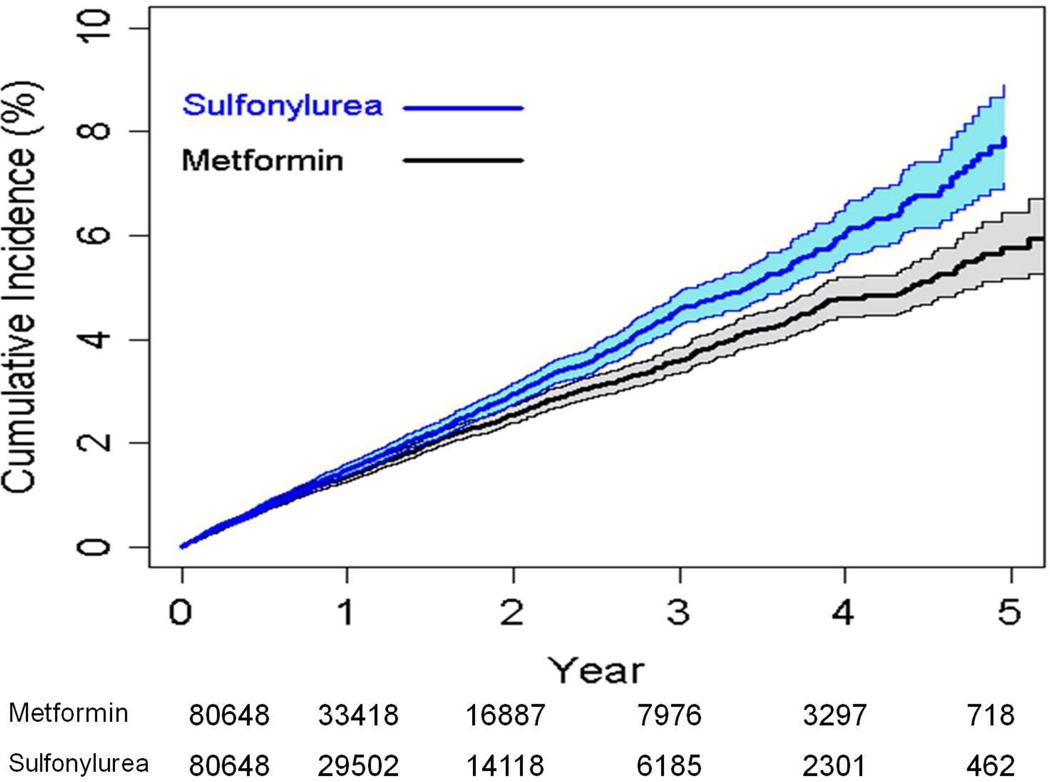
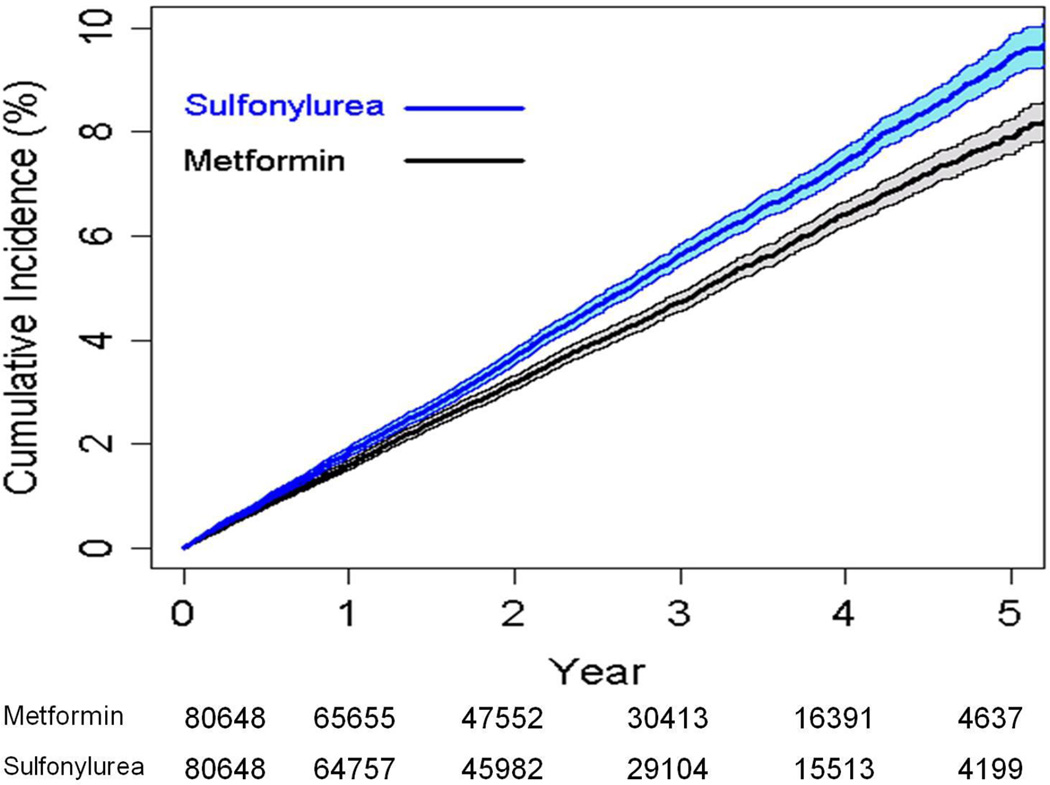
We conducted a propensity matched analysis included matching on facility of care to control for differences in prescribing and cardiovascular care at each facility. The results were consistent with those of the full cohort. Among the 161,296 patients in the propensity matched cohort (80648 sulfonylurea and 80648 metformin initiators), the crude CVD/death rates were 15.2 and 13.0 per 1000 person-years, among sulfonylurea and metformin initiators, respectively (aHR 1.15, 95% CI 1.07, 1.25) (Table 2 and Figure 2). Among the propensity matched cohort the crude cardiovascular event rates were 11.6 and 10.1 per 1000 person-years, among sulfonylurea and metformin initiators, respectively (aHR 1.13, 95% CI 1.03, 1.23).
Figure 2.
Cumulative incidence and 95% confidence intervals of cardiovascular disease or death among the propensity matched cohort
Sensitivity and Subgroup Analyses
Our primary analysis required that patients persist on their initial monotherapy, with gaps up to 90 days, and with no additional antidiabetic treatments. When patients remained in their incident group throughout follow-up regardless of changes, results were similar to the main analysis (Table 2). Important subgroups analyses are displayed in Figure 3 and Appendix Table 4. The crude CVD/death rates among the subgroup without CVD were 13.3 and 7.9 per 1000 person-years for sulfonylurea and metformin users, respectively (aHR 1.16, 95% CI 1.06, 1.29). The 16% excess hazard associated with sulfonylurea use translates to 1 excess CVD/death per 1000 person years of follow-up. The crude CVD/death rates among those with CVD were 28.7 and 17.1 per 1000 person-years for sulfonylurea and metformin users, respectively (aHR 1.25, 95% CI 1.13, 1.39). The 25% excess hazard associated with sulfonylurea use translates to 4 excess CVD/deaths per 1000 person years of follow-up. Results stratified by age (<65 vs. ≥65 years) and BMI (<30 and ≥30 kg/m2) were similar to the main findings. Results among those with microalbuminuria were similar but confidence intervals were wide (aHR 1.13 [95% CI 0.74, 1.73] for composite outcome). Results restricted to patients with complete covariates were also consistent (Appendix Table 5).
Figure 3.
Adjusted hazard ratios for cardiovascular disease (CVD) or death composite outcome and secondary outcome (CVD alone) among subgroups of patients
* CVD Cardiovascular disease
† BMI Body Mass Index
Our final adjusted hazard ratio of 1.21 for sulfonylurea users compared with metformin users could still result from an unmeasured confounder that increased both the hazard of CVD and had a sufficiently greater prevalence among sulfonylurea compared to metformin users. We calculated that an unmeasured binary confounder with a HR =1.25 on CVD (such as smoking) would need to be at least 53% more prevalent among sulfonylurea users compared with metformin users to fully explain our main findings. Unmeasured confounders with HRs for CVD of 1.50, 1.75, and 2.00 would need to be at least 27%, 18%, and 14% more prevalent in sulfonylurea compared to metformin users, respectively (Appendix Table 6).
DISCUSSION
CVD remains the major complication of DM and leading cause of death. Since CVD risk is associated with glycemic control, several studies have evaluated the effects of achieving glycemic targets on CVD, without regard to the medications used. However, whether CVD risk varies by incident agent used remains unclear. Our national cohort study of veterans initiating oral treatments for DM, found that sulfonylurea use was associated with an increased hazard of acute myocardial infarction, stroke or death compared with metformin. Alternatively, metformin may have protective effects on myocardial infarction, stroke and death compared to sulfonylurea, as was demonstrated among a small subgroup in the UKPDS study.(15) Recent comparative effectiveness reviews and meta-analyses(4, 5, 24) concluded that metformin had a slightly lower risk of all-cause mortality compared with sulfonylureas, but results were inconsistent and imprecise. Our large study further strengthens those main conclusions, and provides quantification of the differences in these outcomes for those initiating sulfonylureas versus metformin.
Our study results are consistent with those of the UKPDS and extend them by inclusion of non-overweight persons. Our results are also consistent with several observational studies in diabetic patients. In a smaller propensity score-matched cohort (n= 8,977), McAfee et al. (43) demonstrated a 23% decrease in acute myocardial infarction or revascularization with metformin compared to sulfonylurea initiation (aHR 0.77 [0.62, 0.96]). Using the UK general practice research database (n=91,000), Tzoulaki and colleagues (44) found sulfonylurea compared to metformin use was associated with an increase in all-cause mortality (aHR 1.24 [1.14, 1.35]), but not first acute myocardial infarction (aHR 1.09 [0.94, 1.27]). Another study by Corrao and colleagues(45) found that those initiating sulfonylureas were at a higher hazard for hospitalization (aHR 1.15 [1.08, 1.21]) and death (aHR1.37 [1.26, 1.49]). Finally, the VHA Diabetes Epidemiology Cohort reported all-cause mortality of 2.7% in 2,988 metformin users compared to 5.3% among 19,053 sulfonylureas users (adjusted odds ratio 0.87 [0.68, 1.10]).(46)
Our study has findings consistent with UKPDS and previous observational studies and has multiple additional strengths. First, it includes more than 250,000 patients and the largest number of initiators of metformin and sulfonylureas to date. We were able to measure and adjust for relevant clinical variables, such as HbA1c, cholesterol, blood pressure, creatinine and BMI, lacking in both the McAfee (43) and the Corrao study, (45) which relied on administrative data alone. Few observational studies have included these variables, (44, 46) because they were often missing and reduce the patient sample available for analysis. We used multiple imputation techniques to preserve our sample size for those with missing covariates. Notably, our sensitivity analyses and propensity matched analyses demonstrated that our findings were not sensitive to the imputation strategy.(47–49)
Second, our prior studies evaluating the association of oral antidiabetic medications and intermediate outcomes in a regional VHA cohort reported results similar to those reported in a recent comparative effectiveness review and determined to be “high quality evidence.” In that review of clinical trial data and observational studies, metformin compared to sulfonylureas resulted in 2.7 kg lower weight; 10 mg/dL lower LDL; 8.6 mg/dL lower triglycerides, and no difference in HbA1c.(4) Our group estimated that after one year, compared with sulfonylurea use, metformin initiators had 3.2 kg lower weight; a non-significant 5 mg/dL lower LDL, 8.7 mg/dL lower triglycerides; and no difference in HbA1c.(18, 19) Our prior studies also found metformin to be associated with 1.2 mmHg lower systolic blood pressure and a modest advantage on changes in kidney function.(50) These results are similar to those from clinical trials and suggest a lack of systematic bias in our cohort. Whether the minor advantages in cholesterol, weight and blood pressure among metformin users could account for the differences observed in CVD and death or whether there is another mechanism accounting for the risk difference observed, such as ischemic preconditioning(51) is currently unknown.
Third, our large cohort allowed quantification of the excess risk associated with choice of therapy, and a number of planned sensitivity and subgroup analyses gave consistent results highlighting the robustness of our main findings.(39–41)
Despite these strengths, our findings must be interpreted in light of limitations. First, confounding by indication could occur if patients with certain characteristics which increase CVD risk were also more likely to use metformin or sulfonylureas. We collected an extensive list of covariates, and accounted for them in our regression models. Because of the use of multiple imputation; we only included baseline clinical variables and did not account for time varying covariates given the time span of the study. Furthermore, the laboratory results which we used were done at each individual VHA facility and not performed centrally which could lead to potential variation. Since sulfonylurea prescribing declined over time,(23, 52) we also accounted for this secular trend in all analyses. Although we could not rule out some residual confounding, we estimated that an unmeasured confounder, or an underreported confounder such as smoking related illness, would need to have a very large prevalence imbalance between exposure groups to fully explain our findings. These analyses demonstrated that an unmeasured confounder with an increased hazard of 1.25 would need prevalence 53% higher among sulfonylurea compared to metformin users to explain our study findings. Our large sample size allowed us to control for covariates directly in a multivariable regression as well as to conduct secondary propensity score-matched analyses. Notably, both analyses yielded virtually identical results. Second, refill data were used as a proxy for medication taking, and may result in exposure misclassification. Nevertheless, prescription fills appear to be a good proxy for medication use.(53) Third, if individuals were admitted to non-VHA facilities for outcomes, those events could be missed by our definitions and outcome misclassification could occur. Nevertheless, we supplemented our VHA data with national Medicaid/Medicare data to minimize this concern. Furthermore, the use of non-VHA facilities is unlikely to be differential by exposure group. The analysis restricted to patients aged <65 years, in which missing events are more likely, yielded similar results. Finally our patients are reflective of a typical veteran population, with the majority of patients being white and male.
In conclusion, our study suggests a modest, but clinically important 21% increased hazard of acute myocardial infarction, stroke or death associated with initiation of sulfonylureas, compared with metformin. This translates into an excess of 1 and 4 events per 1000 person-years in patients without and with CVD history. These observations support the use of metformin for first line diabetes therapy, and strengthen the evidence regarding the cardiovascular advantages of metformin compared with sulfonylureas. These results also call into question the current practice of stopping metformin at the onset of modest kidney disease, because of concern about lactic acidosis, a rare adverse event.(5, 25) This practice should be re-examined in light of the benefits of metformin compared with sulfonylurea on important patient cardiovascular outcomes.
Supplementary Material
Acknowledgments
Funding Source
This project was funded under Contract No. 290-05-0042 from the Agency for Healthcare Research and Quality, US Department of Health and Human Services as part of the Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) program. The authors of this report are responsible for its content. Statements in the report should not be construed as endorsement by the Agency for Healthcare Research and Quality, the US Department of Health and Human Services or the Department of Veterans Affairs. Drs. Roumie (04-2-2) and Hung (2-031-09S) were supported by VA Career Development Awards. Dr Roumie was also supported in part by the Vanderbilt Clinical Translational Scientist Award UL1 RR024975-01 from NCRR/NIH.
Footnotes
Disclosures
None
Author Contributions
Design (Roumie, Elasy, Grijalva, Griffin)
Conduct/data collection (Roumie, Liu, Greevy, Murff, Griffin)
Analysis (Liu, Greevy)
Drafting manuscript (Roumie)
Critical revision of manuscript (Roumie, Hung, Liu, Greevy, Grijalva, Murff, Elasy, Griffin)
Protocol and Statistical Code available from Dr. Roumie on request
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Executive summary: heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125(1):188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. [Accessed on: March 27, 2012];2011 Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf.
- 4.Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med. 2007;147(6):386–399. doi: 10.7326/0003-4819-147-6-200709180-00178. [DOI] [PubMed] [Google Scholar]
- 5.Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2010;154(9):602–613. doi: 10.7326/0003-4819-154-9-201105030-00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA. 2007;298(10):1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 7.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 8.Nissen SE, Wolski K. Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch Intern Med. 170(14):1191–1201. doi: 10.1001/archinternmed.2010.207. [DOI] [PubMed] [Google Scholar]
- 9.Goldner MG, Knatterud GL, Prout TE. Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. 3. Clinical implications of UGDP results. JAMA. 1971;218(9):1400–1410. [PubMed] [Google Scholar]
- 10.Knatterud GL, Klimt CR, Jacobson ME, Goldner MG. The UGDP and insulin therapy: a reply. Diabetes Care. 1979;2(2):247–248. doi: 10.2337/diacare.2.2.247. [DOI] [PubMed] [Google Scholar]
- 11.Prout TE, Knatterud GL, Meinert CL, Klimt CR. The UGDP controversy. Clinical trials versus clinical impressions. Diabetes. 1972;21(10):1035–1040. doi: 10.2337/diab.21.10.1035. [DOI] [PubMed] [Google Scholar]
- 12.Seltzer HS. A summary of criticisms of the findings and conclusions of the University Group Diabetes Program (UGDP) Diabetes. 1972;21(9):976–979. doi: 10.2337/diab.21.9.976. [DOI] [PubMed] [Google Scholar]
- 13.Schor SS. Editorials: Statistical problems in clinical trials: the UGDP study revisited. Am J Med. 1973;55(6):727–732. doi: 10.1016/0002-9343(73)90252-0. [DOI] [PubMed] [Google Scholar]
- 14.Bradley RF, Dolger H, Forsham PH, Seltzer H. "Settling the UGDP controversy"? JAMA. 1975;232(8):813–817. [PubMed] [Google Scholar]
- 15.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854–865. [PubMed] [Google Scholar]
- 16.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 17.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355(23):2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 18.Roumie CL, Huizinga MM, Liu X, et al. The effect of incident antidiabetic regimens on lipid profiles in veterans with type 2 diabetes: a retrospective cohort. Pharmacoepidemiol Drug Saf. 2010;20(1):36–44. doi: 10.1002/pds.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huizinga MM, Roumie CL, Greevy RA, et al. Glycemic and weight changes after persistent use of incident oral diabetes therapy: a Veterans Administration retrospective cohort study. Pharmacoepidemiol Drug Saf. 2010;19(11):1108–1112. doi: 10.1002/pds.2035. [DOI] [PubMed] [Google Scholar]
- 20.Phung OJ, Scholle JM, Talwar M, Coleman CI. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303(14):1410–1418. doi: 10.1001/jama.2010.405. [DOI] [PubMed] [Google Scholar]
- 21.Executive summary: standards of medical care in diabetes-2011. Diabetes Care. 34(Suppl 1):S4–S10. doi: 10.2337/dc11-S004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Standards of medical care in diabetes-2011. Diabetes Care. 34(Suppl 1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med. 2008;168(19):2088–2094. doi: 10.1001/archinte.168.19.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selvin E, Bolen S, Yeh HC, et al. Cardiovascular outcomes in trials of oral diabetes medications: a systematic review. Arch Intern Med. 2008;168(19):2070–2080. doi: 10.1001/archinte.168.19.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cryer DR, Nicholas SP, Henry DH, Mills DJ, Stadel BV. Comparative outcomes study of metformin intervention versus conventional approach the COSMIC Approach Study. Diabetes Care. 2005;28(3):539–543. doi: 10.2337/diacare.28.3.539. [DOI] [PubMed] [Google Scholar]
- 26.Arnold N, Hines D, Stroupe K. VIReC Technical Report #1 Comparison of VA Outpatient Prescriptions in the DSS Datasets and the PBM Database. [Accessed on: November 11, 2010];2006 Jan 15; Available at: http://www.virec.research.va.gov/References/TechnicalReports/VIReCTechnicalReport1.pdf.
- 27.US Dept of Health and Human Services. Public Health Service International Classification of Diseases, Ninth Revision, Clinical Modification. 1988 [Google Scholar]
- 28.Hynes DM, Koelling K, Stroupe K, et al. Veterans' access to and use of Medicare and Veterans Affairs health care. Med Care. 2007;45(3):214–223. doi: 10.1097/01.mlr.0000244657.90074.b7. [DOI] [PubMed] [Google Scholar]
- 29.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 30.Choma NN, Griffin MR, Huang RL, et al. An algorithm to identify incident myocardial infarction using Medicaid data. Pharmacoepidemiol Drug Saf. 2009 doi: 10.1002/pds.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosamond WD, Chambless LE, Sorlie PD, et al. Trends in the sensitivity, positive predictive value, false-positive rate, and comparability ratio of hospital discharge diagnosis codes for acute myocardial infarction in four US communities, 1987–2000. Am J Epidemiol. 2004;160(12):1137–1146. doi: 10.1093/aje/kwh341. [DOI] [PubMed] [Google Scholar]
- 32.Petersen LA, Wright S, Normand SL, Daley J. Positive predictive value of the diagnosis of acute myocardial infarction in an administrative database. J Gen Intern Med. 1999;14(9):555–558. doi: 10.1046/j.1525-1497.1999.10198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roumie CL, Mitchel E, Gideon PS, Varas-Lorenzo C, Castellsague J, Griffin MR. Validation of ICD-9 codes with a high positive predictive value for incident strokes resulting in hospitalization using Medicaid health data. Pharmacoepidemiol Drug Saf. 2008;17(1):20–26. doi: 10.1002/pds.1518. [DOI] [PubMed] [Google Scholar]
- 34.Arnold N, Sohn M, Maynard C, DM H. Edward Hines, Jr. VA Hospital VA Information Resource Center. VIReC Technical Report 2: VA NDI Mortality Data Merge Project. 2006 Apr 9; [Google Scholar]
- 35.Yuan YC. Multiple Imputation for Missing Data: Concepts and New Development (Version 9.0) SAS reference documents. 2011 Vol. version 3.0. [Google Scholar]
- 36.Lin D, Wei L. The Robust Inference for the Cox Proportional Hazards Model. Journal of the American Statistical Association. 1989;84(408):1074–1078. [Google Scholar]
- 37.Parsons L. Reducing Bias in a Propensity Score Matched-Pair Sample Using Greedy Matching Techniques. :214–226. [Google Scholar]
- 38.D'Agostino R, Rubin D. Estimating and using Propensity Scores with partially missing data. Journal of the American Statistical Association. 2000;95(451):749–759. [Google Scholar]
- 39.Psaty BM, Koepsell TD, Lin D, et al. Assessment and control for confounding by indication in observational studies. J Am Geriatr Soc. 1999;47(6):749–754. doi: 10.1111/j.1532-5415.1999.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 40.Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15(5):291–303. doi: 10.1002/pds.1200. [DOI] [PubMed] [Google Scholar]
- 41.Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics. 1998;54:948–963. [PubMed] [Google Scholar]
- 42.Schneeweiss S, Glynn RJ, Tsai EH, Avorn J, Solomon DH. Adjusting for unmeasured confounders in pharmacoepidemiologic claims data using external information: the example of COX2 inhibitors and myocardial infarction. Epidemiology. 2005;16(1):17–24. doi: 10.1097/01.ede.0000147164.11879.b5. [DOI] [PubMed] [Google Scholar]
- 43.McAfee AT, Koro C, Landon J, Ziyadeh N, Walker AM. Coronary heart disease outcomes in patients receiving antidiabetic agents. Pharmacoepidemiol Drug Saf. 2007;16(7):711–725. doi: 10.1002/pds.1443. [DOI] [PubMed] [Google Scholar]
- 44.Tzoulaki I, Molokhia M, Curcin V, et al. Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ. 2009;339:b4731. doi: 10.1136/bmj.b4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corrao G, Romio SA, Zambon A, Merlino L, Bosi E, Scavini M. Multiple outcomes associated with the use of metformin and sulphonylureas in type 2 diabetes: a population-based cohort study in Italy. Eur J Clin Pharmacol. 67(3):289–299. doi: 10.1007/s00228-010-0939-6. [DOI] [PubMed] [Google Scholar]
- 46.Kahler KH, Rajan M, Rhoads GG, et al. Impact of oral antihyperglycemic therapy on all-cause mortality among patients with diabetes in the Veterans Health Administration. Diabetes Care. 2007;30(7):1689–1693. doi: 10.2337/dc06-2272. [DOI] [PubMed] [Google Scholar]
- 47.White IR, Carlin JB. Bias and efficiency of multiple imputation compared with complete-case analysis for missing covariate values. Stat Med. 29(28):2920–2931. doi: 10.1002/sim.3944. [DOI] [PubMed] [Google Scholar]
- 48.Mackinnon A. The use and reporting of multiple imputation in medical research - a review. J Intern Med. 268(6):586–593. doi: 10.1111/j.1365-2796.2010.02274.x. [DOI] [PubMed] [Google Scholar]
- 49.Spratt M, Carpenter J, Sterne JA, et al. Strategies for multiple imputation in longitudinal studies. Am J Epidemiol. 172(4):478–487. doi: 10.1093/aje/kwq137. [DOI] [PubMed] [Google Scholar]
- 50.Hung AM, Roumie CL, Greevy RA, et al. Comparative effectiveness of incident oral antidiabetic drugs on kidney function. Kidney Int. 2012;81(7):698–706. doi: 10.1038/ki.2011.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pantalone KM, Kattan MW, Yu C, et al. The Risk of Overall Mortality in Patients with Type 2 Diabetes Receiving Glipizide, Glyburide, or Glimepiride Monotherapy: A Retrospective Analysis. Diabetes Care. 2010;33(6):1224–1229. doi: 10.2337/dc10-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huizinga MM, Roumie CL, Elasy TA, et al. Changing incident diabetes regimens: a Veterans Administration cohort study from 2000 to 2005. Diabetes Care. 2007;30(8):e85. doi: 10.2337/dc07-0650. [DOI] [PubMed] [Google Scholar]
- 53.Grymonpre R, Cheang M, Fraser M, Metge C, Sitar DS. Validity of a prescription claims database to estimate medication adherence in older persons. Med Care. 2006;44(5):471–477. doi: 10.1097/01.mlr.0000207817.32496.cb. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.