Introduction
Early detection of melanoma is crucial to improve survival [1]. Melanoma may be clinically but also dermoscopically indistinguishable from other pigmented skin lesions, especially in incipient and small lesions [2].
Several strategies such as the ABCD(E) acronym [3,4], the “ugly duckling sign” [5] or the EFG [6], just to mention a few, have been proposed to enhance clinical recognition of atypical lesions that should undergo excision or close monitoring, but it usefulness in the detection of melanoma at a curable stage is questionable since only evolved lesions fulfil clinical criteria [7,8].
Dermoscopy has shown to improve melanoma detection by allowing the visualization of diagnostic criteria not visible to the naked eye, and its routine use for the evaluation of skin lesions is recommended in most of the clinical guidelines worldwide [9]. The use of sequential digital dermoscopy for the comparison of current and previous images in search of subtle changes over time has shown to be helpful in the diagnosis of early melanomas that might lack of specific criteria for malignancy [10].
Herein we present a series of melanomas located on the leg with a diameter less than 5 mm measured on relaxed skin before excision. This study reports the dermoscopic clues for early recognition of these lesions and highlights importance of weighing the personal and familial history as well as the clinical context.
Case presentations
The cases are presented in Table 1. The 8 melanomas corresponded to 7 patients, most females (7/8), with a mean age of 42.5 years (range 35–53 years). Four melanomas were detected during routine nevi control and 4 were detected due to changes during digital follow-up; the melanoma was not the reason for the patient´s consultation. Cases 6 and 7 corresponded to the same patient, a 35-year-old female carrier of G101W mutation in CDKN2A.
TABLE 1.
Cases presentations
| Case | Age | Gender | Form of diagnosis | FH MM | PH MM | Multiple MM | AMS | ||
|---|---|---|---|---|---|---|---|---|---|
| MMC | MMRC | MMDD | |||||||
| 1 | 44 | F | ✓ | ✓ | ✓ | ||||
| 2 | 52 | F | ✓ | ||||||
| 3 | 35 | F | ✓ | ✓ | |||||
| 4 | 45 | M | ✓ | ||||||
| 5 | 43 | F | ✓ | ✓ | ✓ | ✓ | |||
| 6 | 35 | F | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| 7 | 35 | F | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| 8 | 53 | F | ✓ | ✓ | |||||
MMC: Melanoma reason for consultation; MMRC: Melanoma routine control; MMDD: melanoma digital dermoscopy; FH MM: familial history of melanoma; PH MM: personal history of melanoma; AMS: Atypical mole syndrome
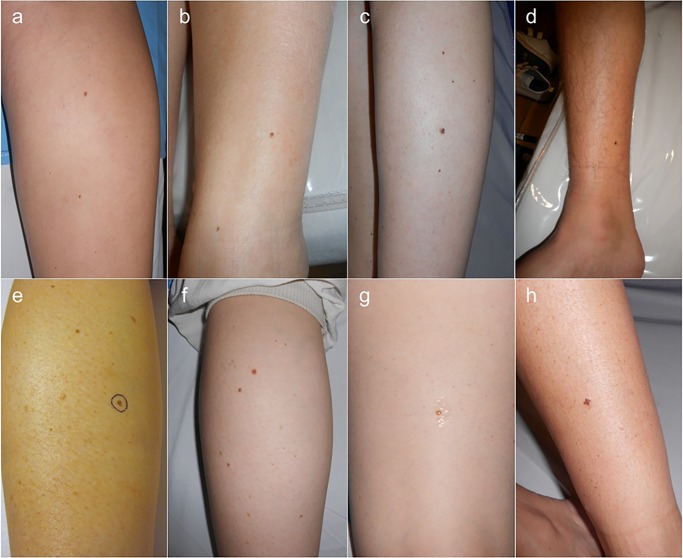
Clinical characteristics are shown in Table 2. All melanomas were located on the legs, 7 in the calf and 1 in the thigh. All melanomas have a diameter lower than 5 mm, with a mean of 3.7 mm (range 2.5–4.5 mm). According to naked eye examination only 2 of 8 melanomas had irregular borders, and 3 out of 8 were asymmetric and had multiple colors. In only 2 cases the patients were able to report a history of change, in both cases the lesions were reported as new. In 4 cases the patients referred the lesion as stable, and in 2 cases the patients were unaware of the lesion (Figure 1).
TABLE 2.
Clinical characteristics
| Case | ABCD (E) | |||||
|---|---|---|---|---|---|---|
| Asymmetry (A) | Irregular borders (B) | Multiple colours (C) | Diameter (D) | History of change (Evolution, E) | ||
| Diameter >6 mm | Diameter (mm) | |||||
| 1 | 3.1 | ✓ | ||||
| 2 | ✓ | ✓ | 2.5 | |||
| 3 | ✓ | ✓ | ✓ | 4.5 | Unknown | |
| 4 | 3.6 | ✓ | ||||
| 5 | 3.3 | |||||
| 6 | 4.3 | |||||
| 7 | 4.0 | |||||
| 8 | ✓ | ✓ | ✓ | 4.5 | Unknown | |
Figure 1.
Clinical images of cases 1 to 8 (a to h). Cases 1 to 4 (a to d) corresponded to melanomas detected during routine nevi control while cases 5 to 8 (e to h) corresponded to melanomas detected during digital follow-up; cases 6 and 7 (f and g) corresponded to the same patient. None of the lesions fulfilled clinical criteria for suspicion of melanoma. [Copyright: ©2015 Salerni et al.]
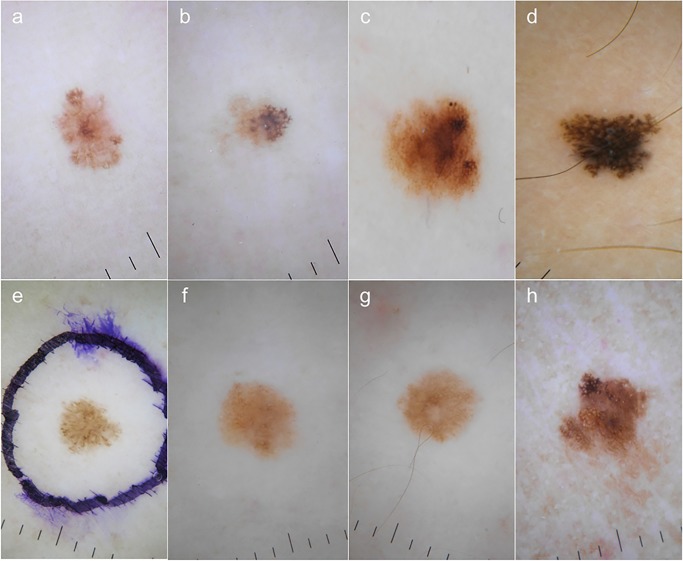
Dermoscopy evaluation was based on pattern analysis; dermoscopic features are shown in Table 3. Upon dermoscopy 5 melanomas were asymmetric; 3 lesions displayed only 1 color, 2 melanomas had 2 colors and 3 displayed 2 or more colors. Reticular pattern and the presence of atypical pigment network were the most frequent features, seen in 7 of 8 melanomas (Figure 2).
TABLE 3.
Dermoscopy evaluation
| Case | Asymmetry | Colors | Global pattern | Dermoscopic features | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | >2 | Reticular | Globular | non-specific | atypical network | Irregular globules | Radial streaks / pseudopods | Hyper/hypo pigmented irregular areas | Irregular blotches | Dotted vessels | Regression features | Perifollicular pigmentation | Negative / inverse network | ||
| 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| 2 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| 3 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| 4 | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| .5 | ✓ | ✓ | ||||||||||||||
| 6 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| 7 | ✓ | ✓ | ✓ | |||||||||||||
| 8 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
Figure 2.
Dermoscopic features of cases 1 to 8 (a to h). Reticular pattern was seen in all cases but in case 5 (e). In cases 1 to 4 (a to d) and in case 8 (h) the presence of atypical pigment network was observed. Cases 1, 4 and 5 (a, d and e) showed pseudopods at the periphery. Cases 6 and 7 displayed typical pigment network with hypo pigmented irregular areas. [Copyright: ©2015 Salerni et al.]
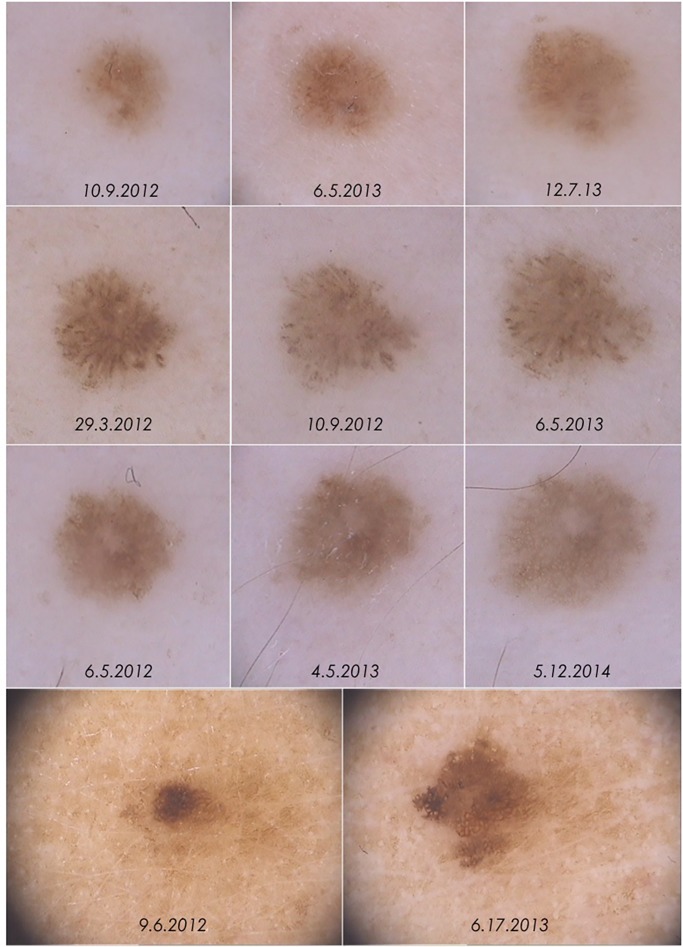
Four melanomas were detected due to changes during digital follow-up (cases 5 to 8). In 3 melanomas (cases 5, 6 and 7) the only change detected was asymmetric enlargement, while case 8 additionally displayed changes in dermoscopic structures and colors (Figure 3).
Figure 3.
Dermoscopic changes leading to excision in cases 5 to 8. Increase in size with no further significant change were observed in cases 5 to 7 (first, second and third row respectively). In case 8 (fourth row) dermoscopic changes were more evident, with changes in shape, structures and pigmentation. [Copyright: ©2015 Salerni et al.]
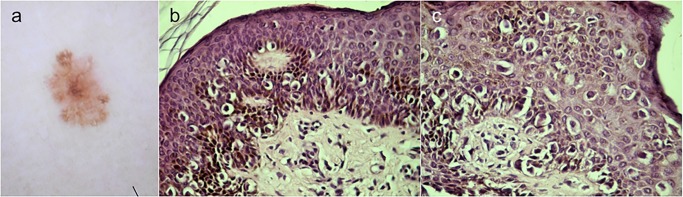
All melanomas corresponded to superficial spreading histologic type. Most melanomas (6/8) were in situ. Two melanomas, corresponding to cases 1 and 8, were invasive with a Breslow thickness of 0.2 (Figure 4) and 0.35 mm respectively.
Figure 4.
Histologic image of case 1, showing proliferation of atypical melanocytes at the dermo-epidermal junction with pagetoid spread and focal invasion of dermis. Diagnosis: superficial spreading melanoma, Breslow 0.2 mm. Histologic images courtesy of Mario Gorosito. [Copyright: ©2015 Salerni et al.]
Discussion
Strategies aimed at recognition of suspicious lesions may have little impact in the diagnosis of small melanomas. The ABCD rule [3], originally designed 30 years ago and revised in 2004 [11] when the E criterion (for Evolution) was included, fails to recognize the existence of small-diameter melanoma, i.e., melanomas less than 6 mm in diameter.
The D criterion has been a matter of controversy since all melanomas are smaller than 6 mm in early stages and a significant proportion of melanomas may be smaller than 6 mm at the time of diagnosis. As stated by Kittler, the limit of 6 mm is not a biological minimum size of melanomas, but the lower limit for the applicability of the ABCD rule [12]. In our series, the melanoma was not the reason for patient’s consultation; the strict application of the ABCD rule for appraisal of lesions that may need to be further examined by a specialist would have led to missing these melanomas. Even though the inclusion of E, for Evolution, has improved the recognition of melanoma by emphasizing change over time as an important additional criterion in the differentiation of melanoma from benign lesions, in only 2 cases the patients were able to report a history of change; in both cases the lesions were reported as new. We found that patients may find it difficult to detect the occurrence of new small lesions as well as clinical changes in pre-existing small lesions.
Unlike nodular type, superficial spreading and lentiginous melanomas usually experiment a variable radial growth phase before invading the underlying dermis. Nevertheless, small-diameter superficial spreading melanomas with vertical growth phase have been reported [13,14]. In our series, 2 invasive melanomas with a diameter of 3.1 and 4.5 mm were observed, high-lighting that even very small lesions may have metastatic potential.
The diagnosis of small-diameter melanomas poses difficulties because the dermoscopic features of small melanomas have been reported infrequently. According to our findings, even in small lesions dermoscopic clues might allow early recognition. Reticular pattern and the presence of atypical pigment network was the most common dermoscopic feature (7/8) followed by radial streaks / pseudopods (2/8). It is recommended that dermoscopy should be performed in all lesions, and not just for those suspicious from the clinical point of view, since clinical pre-selection of lesions for dermoscopic examination is associated with a loss of lesions that might require either surgical excision or follow-up examinations [15].
Four melanomas were detected due to changes during digital of high-risk melanoma patients, increase in size with no further significant change were observed in 3 cases; 1 case displayed changes in shape, structures and pigmentation. This supports the fact that digital follow-up allows for the detection of early melanoma, when specific structures or criteria for malignancy may not be present yet [7,8].
In a recent study Carrera et al. [16] addressed special attention to melanomas of the legs. As in our series, in the dermoscopic analysis none of the cases showed a multi-component pattern, or marked asymmetry in structure or pigmentation, emphasizing the importance of finding other dermoscopic features in these early lesions.
We report a series of small-diameter melanomas of the legs that did not meet the clinical criteria for suspicion. The routinely use of dermoscopy in the evaluation of skin lesions and digital dermoscopy in the monitoring of high-risk individuals provided crucial information for early recognition of these lesions that might have been overlooked assessed solely by the naked eye.
Footnotes
Funding: None.
Competing interests: The authors have no conflicts of interest to disclose.
All authors have contributed significantly to this publication.
References
- 1.Kopf AW, Welkovich B, Frankel RE, et al. Thickness of malignant melanoma: global analysis of related factors. J Dermatol Surg Oncol. 1987;13(4):345–20. doi: 10.1111/j.1524-4725.1987.tb03726.x. [DOI] [PubMed] [Google Scholar]
- 2.Puig S, Argenziano G, Zalaudek I, et al. Melanomas that failed dermoscopic detection: a combined clinicodermoscopic approach for not missing melanoma. Dermatol Surg. 2007;33(10):1262–73. doi: 10.1111/j.1524-4725.2007.33264.x. [DOI] [PubMed] [Google Scholar]
- 3.Friedman RJ, Rigel DS, Kopf AW. Early detection of malignant melanoma: the role of physician examination and self-examination of the skin. CA Cancer J Clin. 1985;35(3):130–51. doi: 10.3322/canjclin.35.3.130. [DOI] [PubMed] [Google Scholar]
- 4.Rigel DS, Friedman RJ. The rationale of the ABCDs of early melanoma. J Am Acad Dermatol. 1993;29:1060–1. doi: 10.1016/s0190-9622(08)82059-2. [DOI] [PubMed] [Google Scholar]
- 5.Grob JJ, Bonerandi JJ. The ‘ugly duckling’ sign: identification of the common characteristics of nevi in an individual as a basis for melanoma screening. Arch Dermatol. 1998;134(1):103–4. doi: 10.1001/archderm.134.1.103-a. [DOI] [PubMed] [Google Scholar]
- 6.Kelly JW, Chamberlain AJ, Staples MP, et al. Nodular melanoma. No longer as simple as ABC. Aust Fam Physician. 2003;32(9):706–9. [PubMed] [Google Scholar]
- 7.Salerni G, Terán T, Alonso C, Fernández-Bussy R. The role of dermoscopy and digital dermoscopy follow-up in the clinical diagnosis of melanoma: clinical and dermoscopic features of 99 consecutive primary melanomas. Dermatol Pract Concept. 2014;4(4):7. doi: 10.5826/dpc.0404a07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salerni G, Lovato L, Carrera C, et al. Melanomas detected in follow-up program compared with melanomas referred to a melanoma unit. Arch Dermatol. 2011;147(5):549–55. doi: 10.1001/archdermatol.2010.430. [DOI] [PubMed] [Google Scholar]
- 9.Australian Cancer Network (ACN) Clinical practice guidelines for the management of melanoma in Australia and New Zealand. 2008. Available from: http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/cp111.pdf. Accessed June 2015.
- 10.Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27(7):805–14. doi: 10.1111/jdv.12032. [DOI] [PubMed] [Google Scholar]
- 11.Abbasi NR, Shaw HM, Rigel DS, et al. Early diagnosis of cutaneous melanoma: revisiting the ABCD criteria. JAMA. 2004;292(22):2771–6. doi: 10.1001/jama.292.22.2771. [DOI] [PubMed] [Google Scholar]
- 12.Kittler H, Rosendahl C, Cameron A, Tschandl P. Dermatoscopy—An Algorithmic Method Based on Pattern Analysis. Austria: Facultas.wuv; 2011. [Google Scholar]
- 13.Pellizzari G, Magee J, Weedon D, et al. A tiny invasive melanoma: a case report with dermatoscopy and dermatopathology. Dermatol Pract Concept. 2013;3(2):6. doi: 10.5826/dpc.0302a06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bono A, Tolomio E, Trincone S, et al. Micro-melanoma detection: a clinical study on 206 consecutive cases of pigmented skin lesions with a diameter < or = 3 mm. Br J Dermatol. 2006;155(3):570–3. doi: 10.1111/j.1365-2133.2006.07396.x. [DOI] [PubMed] [Google Scholar]
- 15.Seidenari S, Longo C, Giusti, et al. Clinical selection of melanocytic lesions for dermoscopy decreases the identification of suspicious lesions in comparison with dermoscopy without clinical preselection. Br J Dermatol. 2006;154(5):873–9. doi: 10.1111/j.1365-2133.2006.07165.x. [DOI] [PubMed] [Google Scholar]
- 16.Carrera C, Palou J, Malvehy J, et al. Early stages of melanoma on the limbs of high-risk patients: clinical, dermoscopic, reflectance confocal microscopy and histopathological characterization for improved recognition. Acta Derm Venereol. 2011;91(2):137–46. doi: 10.2340/00015555-1021. [DOI] [PubMed] [Google Scholar]