Abstract
Specific immune suppression in newly hatched chicks induced by specific maternal antibodies has been reported. Laying hens were immunized with dinitrophenyl-keyhole limpet hemocyanin (DNP-KLH). Purified maternal anti-DNP and non-specific immunoglobulin (Ig) Y antibodies were transferred by yolk sac inoculation to newly hatched chicks, and then, they were immunized with an optimum immunogenic dose of DNP-KLH at 1 and 4 weeks of age. Concentrations of anti-DNP antibodies in serum samples of these chicks were measured by using Enzyme-linked immunosorbent assay (ELISA). Proportions of T-cell subsets in peripheral blood of these chicks were also measured by flow cytometric analysis at 5 weeks of age (one week after the second immunization). Suppression of anti-DNP antibody response and down-regulation of CD3+CD4+ cells were observed in the chicks received high dose of maternal anti-DNP antibodies and immunized with DNP-KLH. On the other hand, normal anti-DNP antibody response and normal proportion of CD3+CD4+ cells were observed in the chicks received high dose of non-specific IgY antibodies and immunized with DNP-KLH. Furthermore, when chicks received high dose of maternal anti-DNP antibodies and immunized with DNP-KLH at 1 and 4 weeks of age and then with rabbit serum albumin (RSA) at 5 and 8 weeks of age, their primary anti-RSA response was also significantly suppressed. We indicate here that specific maternal antibodies can affect both B and T cell responses and induce non-specific suppression against different antigens. However, this non-specific suppression does not continue for a long time.
Keywords: chick, maternal antibody, non-specific, suppression, T-cell
Broiler chickens raised under commercial conditions are susceptible to numerous environmental pathogens. Therefore, disease prevention by vaccination is an integral part of flock health management protocols [43]. Some reports in chickens have revealed that in addition to the protective function of maternal antibodies against environmental pathogens, it may hamper or interfere with the chick immune response to active immunization [1, 11, 12, 28, 29, 51]. Although it has been reported that maternal antibodies suppress the immune response of neonates to active immunization [2, 15, 20, 27, 29, 38,39,40, 45, 46, 52, 53], the immunological mechanisms responsible for immune suppression mediated by these antibodies remain unclear. Specific immune suppression mediated by maternal antibodies on humoral immune response of the newly hatched chicks has been reported [13]. It has been shown that in the presence of high levels of maternal antibodies, B-cell responses were suppressed, but T-cell priming or induction remains largely unaffected by these passively transferred antibodies [16, 21, 45, 47, 48]. In contrast, other reports have demonstrated that maternal derived antibodies suppress both B and T cell responses in newborns to active immunization [5, 6, 37, 41]. There have been relatively few studies evaluating the effects of maternal antibodies on T-cell responses, and as a consequence of the diversity of data obtained from these studies, the effects of maternal antibodies on T-cell response remain unclear. This study investigated the effects of maternally derived antibodies on the T-cell subsets in peripheral blood of newly hatched chicks and examined whether the immune suppressive effect of maternal antibodies is antigen specific.
MATERIALS AND METHODS
Animals: Partially inbred chickens (H-B15 white leghorn; Bu-1a) were used in this study. These chickens were bred in our animal facilities and were provided with food and chlorinated water ad libitum in accordance with the Guidelines for Animal Experiments of Hiroshima University. Eggs derived from these chickens were incubated and hatched at our own facilities. Chicks derived from non-immunized hens were determined to be free from maternal anti-DNP antibodies.
Antibodies: Monoclonal mouse anti-chicken CD4-FITC, monoclonal mouse anti-chicken CD8α-FITC, monoclonal mouse anti-chicken CD8β-FITC, monoclonal mouse anti-chicken CD3-RPE (Southern Biotechnology Associates Inc., Birmingham, AL, U.S.A.) and HRP-labeled goat anti- chicken IgY heavy and light chain (Bethyl Inc., Montgomery, TX, U.S.A.) were used.
Antigen preparation: A classical hapten-carrier protein antigen, dinitrophenylated keyhole limpet hemocyanin (DNP-KLH), was prepared as described previously [19, 22]. Briefly, in a clean, dry and dark container, 200 mg of K2CO3 was dissolved in 6 ml of distilled water and was then placed on a magnetic stirrer, and after that, 200 mg of KLH (Calbiochem Behring Co., Darmstadt, Germany) was slowly added and left at room temperature. At the same time, 200 mg of 2,4-dintrobenzene sulfonic acid sodium salt (DNBS) (Eastman Kodak Co., San Diego, CA, U.S.A.) was dissolved in 4 ml of distilled water. DNBS solution was added into KLH solution. The mixture was then stirred in the dark at room temperature for 18 to 24 hr and was dialyzed against PBS at 4°C until obtaining a zero absorbance value at 360 nm against PBS. Finally, the mixture was passed through a 0.45-µm filter, and the protein content of this antigen was determined based on the OD value measured at 280 nm. The conjugation ratio of hapten to protein was determined as described previously [19, 22]. The final product was DNP32-KLH, and the antigen was kept in a refrigerator at 4°C until use. Dinitrophenylated bovine serum albumin (DNP28-BSA) was prepared in the same manner. Rabbit serum albumin (RSA) was obtained from Sigma Aldrich (St. Louis, MO, U.S.A.).
Immunization of chickens: Six laying hens (age, 1–2 years) were immunized with DNP-KLH (1 mg per hen) emulsified in Freund’s complete adjuvant (FCA) (Wako Pure Chemical Industries, Osaka, Japan) into their peritoneal cavity. Second immunization was performed after 2 weeks, and hens were repeatedly immunized every 3 weeks using Freund’s incomplete adjuvant (FIA) (Wako Pure Chemical Industries). Eggs derived from immunized laying hens were collected daily at one week after the second immunization and were stored in the refrigerator at 4°C until use for extraction of IgY. Chicks receiving maternal anti-DNP antibodies or chicks receiving non-specific maternal IgY or chicks with no maternal anti-DNP antibodies were immunized twice with DNP-KLH (2 mg/kg of body weight: BW) [13, 14] at 1 and 4 weeks of age followed with RSA (2 mg/kg of BW) at 5 and 8 weeks of age. The first immunization was given into the peritoneal cavity with the antigen emulsified with FCA. Second immunization was administered in the same manner, but with FIA instead of FCA.
Purification of chicken IgY: Chicken IgY was extracted from egg yolk by the water dilution method as described previously [3]. Briefly, one volume of egg yolk was mixed slowly and gently with nine volumes of DW. The pH value of diluted egg yolk was adjusted to 5.2 by adding 1 N HCl, and it was then covered and kept in the refrigerator at 4°C for at least 6 hr. Diluted egg yolk was mixed gently before centrifugation (at 10,000 × g for 15 min in a refrigerator centrifuge). The supernatant was decanted into a clean beaker, while stirring gently. Ammonium sulfate (final percentage was 40%) was added gently, and mixing was continued for at least 30 min. The suspension was centrifuged for 15 min at 10,000 × g in a refrigerated centrifuge. Supernatant was discarded. An equal volume of PBS as the original volume of egg yolk was added to the pellet, followed by gentle mixing until the IgY pellet was completely dissolved. The purified IgY solution was dialyzed 4–5 times against PBS until ammonium sulfate was completely removed. The concentration of purified IgY solution was measured after filtration with a 0.45-µm filter. Finally, purified IgY solution was stored in a refrigerator at 4°C until use.
Affinity purification of maternal anti-DNP: DNP28-BSA was conjugated with swollen CNBr-activated Sepharose 4B beads (GE Healthcare, Uppsala, Sweden) in coupling buffer (bicarbonate buffer) (0.5 M NaCl, 0.1 M sodium bicarbonate, pH 8.3). Free-reacted sites on the beads were blocked with 1 M ethanol amine, pH 8.0, and the beads were washed with coupling buffer and low pH buffer (0.5 M NaCl, 0.2 M glycine-HCl, pH 2.5) four times before washing with PBS and were stored in a refrigerator at 4°C until use. The DNP-BSA column was washed gently with 5 ml of low pH buffer and was then gently washed with PBS (20 times the gel volume). Subsequently, IgY solution was added several times to the column. The filtrate was collected. The column was washed with PBS (20 times the gel volume), and 5 ml of elution buffer (0.05M DNP-EACA [ε-amino-n-caproic acid], pH 7.2, Sigma Aldrich) was added to the column. The eluate, which contained anti-DNP antibodies, was collected, then dialyzed against PBS and concentrated using a centrifugal filter device (Amicon ultra-15, Ultracel 100k) (Millipore, Carrigtwohill, County Cork Ireland). Finally, the column was washed with 20 ml of low pH buffer, followed by PBS (20 times the gel volume), and the column was stored in the refrigerator at 4°C with PBS containing sodium azide. The optical density of the concentrated sample was measured at 280 nm in order to calculate anti-DNP antibody concentrations [13].
Injection of purified antibodies: Purified maternal anti-DNP antibody or non-specific IgY (8 mg/200 µl PBS) was injected into yolk sac of the newly hatched chicks directly after hatching, as described previously [13, 14, 50]. Briefly, the abdominal wall of the newly hatched chicks was sterilized with 70% ethanol. One-milliliter syringes with a 30-gauge needle (Becton Dickinson, Rutherford, NJ, U.S.A.) were used for injection of antibodies into the yolk sac. The needle was inserted through the skin and into the yolk sac immediately posterior to the umbilicus where the sac closes to the abdominal wall.
Collection of blood: Blood samples were collected every week from each chick from the wing vein into a 1-ml syringe with a 27 G needle. Serum was separated from clotted blood by centrifugation at 10,000 × g for 5 min and was stored at −80°C until use. Blood samples were also collected from the wing vein into heparin in a 1-ml syringe with a 27 G needle in order to obtain peripheral blood mononuclear leukocytes (PBML).
ELISA: Anti-DNP and anti-RSA antibodies concentrations were measured by ELISA as previously described [54]. Briefly, ELISA plates (NUNC, Roskilde, Denmark) were coated overnight with 55 µl of DNP-BSA solution (50 µg/ml) or RSA solution (50 µg/ml), followed by a 2-hr blocking period at 37°C with 350 µl of 25% Block Ace (Dainippon Sumitomo Pharmaceutical Co., Osaka, Japan) in PBS. Four-fold serum dilutions were then added, and the plates were incubated for 1 hr at 37°C. Each plate contained negative and positive control samples for measuring the concentrations of anti-DNP antibodies. Following incubation, plates were washed five times with PBS-Tween and were then treated with diluted HRP-labeled goat anti-chicken IgY heavy and light chain (1/2,000, diluted in 10% Block Ace in PBS) for 1 hr. Plates were then, washed 5 times with PBS-Tween, and then, the substrate solution was added to the plate; plates were left for 10–20 min in the dark until the appearance of yellow color, and the reaction was stopped using 2N H2SO4. Finally, optical density was measured at 490 nm with a micro plate reader (BIO-RAD Model 680; Bio-Rad, Tokyo, Japan). Plates containing dilution buffer instead of sample acted as negative controls, and standard purified anti-DNP antibodies (1 mg/ml) acted as positive controls. Concentrations of serum anti-DNP antibodies were measured after conversion of ELISA data into mg/ml using standard anti-DNP antibodies samples of known concentration [13]. Concentrations of anti-RSA antibodies were measured using half of plateau dilution units [13].
Flow cytometric analysis: Chicken peripheral blood (0.5 ml) was taken from the wing vein into a heparinized syringe. Live PBML were isolated using Ficoll-paqueTM Plus (GE Healthcare Bio-Sciences AB) with a density gradient of 200 × g at 4°C for 30 min, followed by washing twice with Iscove’s medium (IMDM) containing 1% FBS (1% FBS-IMDM). For double staining, live cells (PBML) were counted (using a hemocytometer slide), and 2 × 106 cells were treated with a mixture of labeled antibodies (PE-labeled anti-CD3 with FITC-labeled anti-CD4 or with FITC-labeled anti-CD8α or FITC-labeled anti-CD8β) for 30 min on ice. Treated cells were then washed twice and resuspended in the same volume of 1% FBS-IMDM. Flow cytometric analysis was carried out with the FACScalibur (Becton Dickinson, Immunocytometry System, San Jose, CA, U.S.A.). Finally, data for 10,000 cells falling within forward scatter (FSC) and side scatter (SSC) gates set for lymphocytes were collected from each sample. All data were then analyzed using CellQuest software.
Statistical analysis: The proportions of T-cell subsets in peripheral blood and the mean of anti-DNP and anti-RSA antibodies titers of the newly hatched chick’s sera were compared using Student’s t-test. All values were expressed as mean ± SD and were considered to be significant at P<0.05 and highly significant at P<0.005.
RESULTS
Effect of maternal antibodies on T-cell subpopulations: Sixteen newly hatched chicks derived from non-immunized laying hens were divided into 3 groups. The first group consisted of 6 chicks that received 8 mg of maternal anti-DNP antibodies per chick. The second group consisted of 6 chicks that received 8 mg of maternal non-specific IgY antibodies per chick. The third group consisted of 4 chicks that did not receive any additional IgY. All chicks were immunized with an optimal dose of DNP-KLH at 1 and 4 weeks of age. Anti-DNP responses of the chicks in the first group were suppressed and significantly lower than those of the chicks in the second group or those of the chicks in the third group [13, 14] (data not shown). The proportions of T-cell subpopulations in the peripheral blood particularly at 5 weeks of age (one week after the second immunization) were measured, and CD3+CD4+:CD3+CD8α+ ratios were also calculated in each animal individually. The proportions of CD3+CD4+ cells in peripheral blood of the chicks in the first group were significantly lower than that of the control groups (the second group and the third group) (P<0.05) (Table 1). In addition, there were no significant differences between these proportions in chicks in the second group and in the third group (P>0.05) (Table 1). The proportions of CD3+CD8α+ cells in the peripheral blood of chicks in the first group were significantly higher than that of the control groups (the second group and the third group) (P<0.05) (Table 1). Moreover, CD3+CD4+:CD3+CD8α+ ratios in chicks in the first group were significantly lower than that of the control groups (the second group and the third group) (P<0.05) (Table 1). However, there were no significant differences between these ratios in chicks in the second group and in the third group (P>0.05) (Table 1). The proportions of CD3+CD8β+ in the peripheral blood of the chicks in all groups were nearly the same (there was no significant difference between all of them) (data not shown).
Table 1. Proportions of CD3+CD4+ cells and CD3+CD8α+cells in peripheral blood of chicks immunized with DNP-KLH.
| Number of chicks | CD3+CD4+ cells% | CD3+CD8α+ cells% | CD3+CD4+: CD3+CD8α+ ratios | |
|---|---|---|---|---|
| Chicks with high maternal anti-DNPa) | 6 | 72.77 ± 1.90* | 21.20 ± 0.77* | 3.44 ± 0.18* |
| Chicks with high maternal IgY (control)b) | 6 | 78.92 ± 3.69 | 16.87 ± 2.73 | 4.79 ± 1.00 |
| Normal chicks (control)c) | 4 | 77.58 ± 1.44 | 12.25 ± 2.70 | 6.54 ± 1.29 |
Data are expressed as means ± SD. *: Significantly different from control groups (P<0.05). a) Chicks received 8 mg of anti-DNP antibodies. b) Chicks received 8 mg of normal IgY antibodies. c) Chicks did not receive additional maternal antibodies.
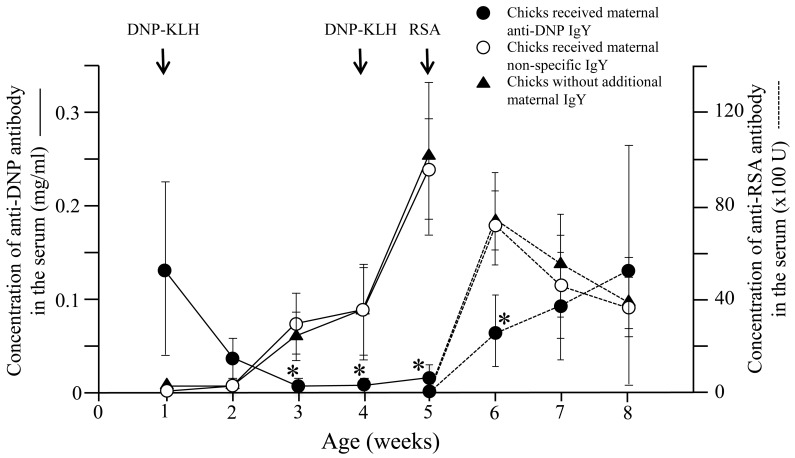
Non-specific immune suppression by maternal antibody and related antigen: Thirty six newly hatched chicks derived from non-immunized hens were divided into 3 groups. The first group consisted of 12 chicks that received 8 mg of maternal anti-DNP antibodies per chick. The second group consisted of 12 chicks that received 8 mg of maternal non-specific IgY antibodies per chick. The third group consisted of 12 chicks that did not receive any additional IgY. All chicks were immunized with an optimal dose of DNP-KLH at 1 and 4 weeks of age, followed by RSA at 5 and 8 weeks of age. Anti-DNP antibody responses of the chicks in the first group were suppressed and significantly lower than those of chicks in the second and third groups (particularly after the second and third weeks of the first immunization and after the first week of the second immunization) (Fig. 1) (P<0.005). Significant suppression was also observed after second and third weeks of second immunization (data not shown).
Fig. 1.
Specific and non-specific immune suppression induced by maternal antibody and related antigen. Anti-DNP and Anti-RSA responses of the newly hatched chicks immunized with of DNP-KLH at 1 and 4 weeks of age, followed by RSA at 5 and 8 weeks of age were measured. Anti-DNP responses of chicks that received maternal anti-DNP antibody were suppressed and significantly lower than those of the chicks received non-specific maternal IgY or those of the chicks that did not receive any additional IgY, especially after the second and third weeks of the first immunization and after the first week of the second immunization with DNP-KLH. Anti-RSA responses of chicks receiving maternal anti-DNP antibodies were suppressed particularly after the first week of the first immunization with RSA when compared to those of the chicks received non-specific maternal IgY or those of the chicks did not receive any additional IgY. Arrows indicate immunization with DNP-KLH and RSA. *; P<0.05.
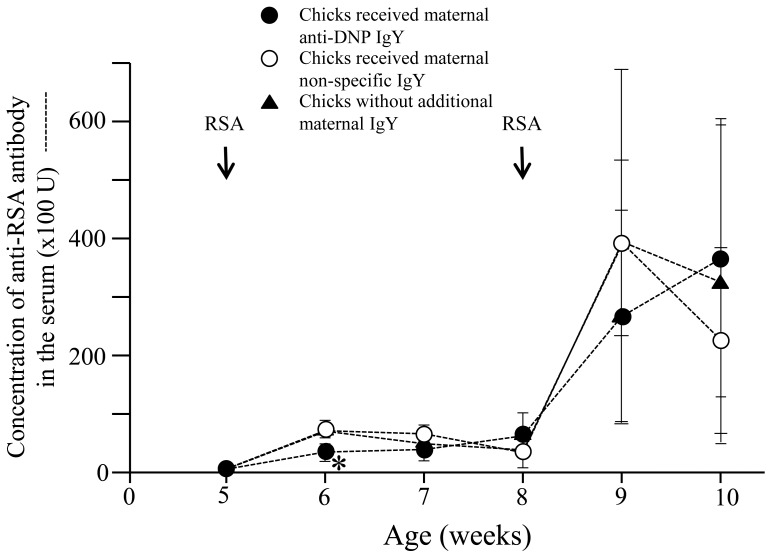
Moreover, anti-RSA responses in the chicks in the first group were also suppressed and were significantly lower than those of the chicks in the second and third groups, particularly one week after the first immunization with RSA (P<0.05) (Fig. 1). However, this non-specific suppression did not continue for long periods, and there were no significant differences between all groups in anti-RSA responses after second immunization with RSA (P>0.05) (Fig. 2).
Fig. 2.
Duration of non-specific immune suppression induced by maternal antibody and related antigen. Anti-RSA responses of the newly hatched chicks immunized with of DNP-KLH at 1 and 4 weeks of age, followed by RSA at 5 and 8 weeks of age were measured. Anti-RSA responses of chicks receiving maternal anti-DNP antibodies were suppressed particularly after first week of the first immunization with RSA when compared to those of the chicks received non-specific maternal IgY or those of the chicks that did not receive any additional IgY. However, Anti-RSA responses of chicks receiving maternal anti-DNP antibodies were normal after second immunization with RSA when compared with those of the chicks received non-specific maternal IgY or those of the chicks that did not receive any additional IgY. Arrows indicate immunization with RSA. *; P<0.05.
DISCUSSION
Nowadays, intensive production of large numbers of chickens is of great economic importance. Vaccines are the most commonly administered veterinary medicines in the poultry industry and are considered to be the most effective tool to control infectious diseases. Vaccination failure occurs when the chickens do not develop adequate antibody titer levels and/or are susceptible to disease outbreaks. Numerous reports have shown that maternal antibodies provide protection against several pathogens to newly hatched chicks; however, they may interfere with their immune response to active vaccination [4, 18, 33, 35, 42]. In addition to immunological immaturity in neonates, the presence of inhibitory concentrations of maternally derived antibodies imposes a further barrier to effective early vaccination [46]. Immune suppression of maternal antibodies on B-cell response has been documented, but the effects of such antibodies on T-cell response remain incompletely defined. Therefore, the goal of this study was to investigate the effects of specific maternal antibodies on the populations of T-cell subsets in peripheral blood of newly hatched chicks immunized with an optimal dose of related antigen and whether the immune suppressive effects of maternal antibodies are antigen specific.
Anti-DNP response of the chicks was suppressed only when they immunized with DNP-KLH in presence of high level of maternal anti-DNP antibodies. Also, the proportions of CD3+CD4+ cells in peripheral blood of the chicks that received high dose of maternal anti-DNP antibodies and immunized with DNP-KLH were significantly lower than that of the control groups (chicks of no-maternal anti-DNP antibodies and immunized with DNP-KLH or chicks of high maternal non-specific IgY and immunized with DNP-KLH). Moreover, the proportions of CD3+CD8α+ cells in their peripheral blood were significantly higher than that of the above mentioned control groups. Also, CD3+CD4+:CD3+CD8α+ ratios in chicks that received a high dose of maternal anti-DNP antibodies and that were immunized with DNP-KLH were significantly lower when compared to controls (chicks with no maternal anti-DNP antibodies and immunized with DNP-KLH or chicks of high maternal non-specific IgY and immunized with DNP-KLH). These observations suggest that only high level of specific maternal antibodies can negatively affect both B and T cell immune responses in the chicks when they immunize with an optimal dose of the specific antigen.
The proposed mechanisms responsible for immune suppression mediated by maternal antibodies may include; inhibition of specific B-cell function by cross-linking of the B-cell receptor with the inhibitory FcγR [7, 8, 10, 17, 26, 34], induction of specific suppressor T lymphocytes that act to inhibit the generation of memory T helper cells involved in IgG production [23], prevention of B-cell receptor from recognizing antigenic epitopes [9, 24, 25, 31, 32, 36, 44] and idiotypic interactions [49].
Some studies [5, 6, 37, 41] have investigated the effects of maternal antibodies on cellular immunity by measuring in vitro proliferation and cytokine production of antigen-specific T cells (either from spleen or from peripheral blood). Significantly lower production of IFNγ and IL-5 was observed in newborns derived from immunized dams when compared with those derived from non-immunized dams. Premenko-Lanier et al. [41] stated that there are 2 possible mechanisms for the inhibition of cellular immunity by maternal antibodies; complete neutralization of antigen (in viral infection), and/or an inhibitory effect of immune complexes on antigen processing and presentation to T cell. Binding of antigen-specific maternal antibody complexes to FcγRIIB on dendritic cells may suppress T cell priming to the antigen [30]. The theory of epitope masking predicts that B cell epitopes on the vaccine will be covered by maternal antibody and therefore will not be recognized by B cells. In consequence, the function of B cells (as an antigen presenting cell), such as phagocytosis and antigen presentation, might be inhibited, resulting in lower frequency of antigen presentation which leads to lower efficiency of T cell activation and different T cell ratios in immunized groups. For further understanding, the phenomenon indicated that specific maternal antibody down regulated the proportion of CD3+CD4+ cells in peripheral blood of the newly hatched chicks when they immunized to its related antigen, anti-DNP and anti-RSA antibodies concentrations were measured in the chicks that received high dose of maternal anti-DNP antibodies at the day of hatching, then immunized with DNP-KLH at 1 and 4 weeks of age and then immunized with RSA at 5 and 8 weeks of age. We surprised that in addition to the suppression of anti-DNP antibody responses, anti-RSA responses in these chicks were also suppressed and significantly lower than those of the chicks of no-maternal anti-DNP (especially one week after the first immunization with RSA). A double antigen immunization may induce a low response to each antigen, respectively. Chicks that received high dose of maternal anti-DNP antibodies and that received high dose of maternal non-specific IgY were immunized with DNP-KLH at 1 and 4 weeks of age and with RSA at 5 weeks of age. Then, there was a low response to the RSA in chicks received high dose of maternal anti-DNP antibodies. Therefore, this low response was not caused by the double antigen immunization. These results reveal that specific maternal antibodies can non-specifically suppress the immune response of the newly hatched chicks to other antigens, if they are either injected together or after its specific antigen. This non-specific suppression may be due to the down regulation of the proportion of CD3+CD4+ cells induced by such antibodies. We previously reported that specific suppression (anti-DNP antibody response) continues for at least 10 weeks of age (14). However, this non-specific suppression does not continue for a long time (Fig. 2). We have to investigate this reason in the next experiments.
In conclusion, maternal antibodies interfere with both B and T cell responses in newly hatched chicks. Moreover, specific maternal antibodies can non-specifically suppress the immune response to other antigens in newly hatched chicks, if they are injected with specific antigen. Therefore, the influence of maternal antibodies should be considered when determining antigen type or minimal dose required for immunization in newly hatched chicks. Moreover, the immunization of the newly hatched chicks with a combination of two antigens should be avoided, if the chicks passively acquired high levels of specific maternal antibodies against one of them. Maternal antibodies may offer an advantage to newly hatched chicks as a neutralizing antibody for antigens. However, this immune reaction may interfere with the immune response to environmental antigens, but only in the short term. Further experiments should be performed in order to understand the mechanisms of this suppression in mice.
Acknowledgments
This study was supported by research project for improving food safety and animal health of the Ministry of Agriculture, Forestry and Fisheries of Japan.
REFERENCES
- 1.Abdelwhab E. M., Grund C., Aly M. M., Beer M., Harder T. C., Hafez H. M.2012. Influence of maternal immunity on vaccine efficacy and susceptibility of one day old chicks against Egyptian highly pathogenic avian influenza H5N1. Vet. Microbiol. 155: 13–20. doi: 10.1016/j.vetmic.2011.08.004 [DOI] [PubMed] [Google Scholar]
- 2.Adkins B., Leclerc C., Marshall-Clarke S.2004. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 4: 553–564. doi: 10.1038/nri1394 [DOI] [PubMed] [Google Scholar]
- 3.Akita E. M., Nakai S.1993. Comparison of four purification methods for the production of immunoglobulin from eggs laid by hens immunized with an enterotoxigenic E.coli strain. J. Immunol. Methods 160: 207–214. doi: 10.1016/0022-1759(93)90179-B [DOI] [PubMed] [Google Scholar]
- 4.Berg T. P.2000. Acute infectious bursal disease in poultry: a review. Avian Pathol. 29: 175–194. doi: 10.1080/03079450050045431 [DOI] [PubMed] [Google Scholar]
- 5.Blomqvist G. A., Lövgren-Bengtsson K., Morein B.2003. Influence of maternal immunity on antibody and T-cell response in mice. Vaccine 21: 2022–2031. doi: 10.1016/S0264-410X(02)00776-4 [DOI] [PubMed] [Google Scholar]
- 6.Bouma A.1998. The influence of maternal immunity on the development of the in vitro lymphocyte proliferation response against pseudorabies virus in pigs. Res. Vet. Sci. 64: 167–171. doi: 10.1016/S0034-5288(98)90014-5 [DOI] [PubMed] [Google Scholar]
- 7.Brüggemann M., Rajewskyk K.1982. Regulation of the antibody response against hapten-coupled erythrocytes by monoclonal anti-hapten antibodies of various isotypes. Cell. Immunol. 71: 365–373. doi: 10.1016/0008-8749(82)90270-2 [DOI] [PubMed] [Google Scholar]
- 8.Chan P. L., Sinclair N. R.1973. Regulation of the immune response. VI. Inability of F(ab’) 2 antibody to terminate established immune responses and its ability to interfere with IgG antibody-mediated immunosuppression. Immunology 24: 289–301. [PMC free article] [PubMed] [Google Scholar]
- 9.Crow A. R., Freedman J., Hannach B., Lazarus A. H.2000. Monoclonal antibody-mediated inhibition of the human HLA alloimmune response to platelet transfusion is antigen specific and independent of Fcgamma receptor-mediated immune suppression. Br. J. Haematol. 110: 481–487. doi: 10.1046/j.1365-2141.2000.02179.x [DOI] [PubMed] [Google Scholar]
- 10.D’Ambrosio D., Hippen K. L., Minskoff S. A., Mellman I., Pani G., Siminovitch K. A., Cambier J. C.1995. Recruitment and activation of PTP1C in negative regulation of antigen receptor signaling by FcγRIIB1. Science 268: 293–297. doi: 10.1126/science.7716523 [DOI] [PubMed] [Google Scholar]
- 11.Darbyshire J. H., Peters R. W.1985. Humoral antibody response and assessment of protection following primary vaccination of chicks with maternally derived antibody against avian infectious bronchitis virus. Res. Vet. Sci. 38: 14–21. [PubMed] [Google Scholar]
- 12.De Vriese J., Steensels M., Palya V., Gardin Y., Moore Dorsey K., Lambrecht B., Van Borm S., van den Berg T.2010. Passive protection afforded by maternally-derived antibodies in chickens and antibodies’ interference with the protection elicited by avian influenza-inactivated vaccines in progeny. Avian Dis. 54: 246–252. doi: 10.1637/8908-043009-Reg.1 [DOI] [PubMed] [Google Scholar]
- 13.Elazab M. F. A., Fukushima Y., Fugita Y., Horiuchi H., Matsuda H., Furusawa S.2010. Induction of immune suppression in the chick by an optimal dose of an immunizing antigen in presence of its specific maternal antibody. J. Vet. Med. Sci. 72: 257–262. doi: 10.1292/jvms.09-0298 [DOI] [PubMed] [Google Scholar]
- 14.Elazab M. F. A., Fukushima Y., Horiuchi H., Matsuda H., Furusawa S.2009. Prolonged suppression of chick humoral immune response by antigen specific maternal antibody. J. Vet. Med. Sci. 71: 417–424. doi: 10.1292/jvms.71.417 [DOI] [PubMed] [Google Scholar]
- 15.Ellis J., West K., Cortese V., Konoby C., Weigel D.2001. Effect of maternal antibodies on induction and persistence of vaccine-induced immune responses against bovine viral diarrhea virus type II in young calves. J. Am. Vet. Med. Assoc. 219: 351–356. doi: 10.2460/javma.2001.219.351 [DOI] [PubMed] [Google Scholar]
- 16.Endsley J. J., Roth J. A., Ridpath J., Neill J.2003. Maternal antibody blocks humoral but not T cell responses to BVDV. Biologicals 31: 123–125. doi: 10.1016/S1045-1056(03)00027-7 [DOI] [PubMed] [Google Scholar]
- 17.Enriquez-Rincon F., Klaus G. G.1984. Differing effects of monoclonal anti-hapten antibodies on humoral responses to soluble or particulate antigens. Immunology 52: 129–136. [PMC free article] [PubMed] [Google Scholar]
- 18.Faulkner O. B., Estevez C., Yu Q., Suarez D. L.2013. Passive antibody transfer in chickens to model maternal antibody after avian influenza vaccination. Vet. Immunol. Immunopathol. 152: 341–347. doi: 10.1016/j.vetimm.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 19.Furusawa S., Okitsu-Negishi S., Yoshino K., Mizoguchi M., Noguchi Y.1987. Anti-idiotypic antibody as a mirror image of the paratope of the original antibody. Int. Arch. Allergy Appl. Immunol. 84: 263–270. doi: 10.1159/000234433 [DOI] [PubMed] [Google Scholar]
- 20.Gans H., DeHovitz R., Forghani B., Beeler J., Maldonado Y., Arvin A. M.2003. Measles and mumps vaccination as a model to investigate the developing immune system: passive and active immunity during the first year of life. Vaccine 21: 3398–3405. doi: 10.1016/S0264-410X(03)00341-4 [DOI] [PubMed] [Google Scholar]
- 21.Gans H. A., Maldonado Y., Yasukawa L. L., Beeler J., Audet S., Rinki M. M., DeHovitz R., Arvin A. M.1999. IL-12, IFN-gamma, and T cell proliferation to measles in immunized infants. J. Immunol. 162: 5569–5575. [PubMed] [Google Scholar]
- 22.Good A. H., Wofsy L., Henry C., Kimura J.1980. Preparation of hapten-modified protein antigens. pp. 343–350 In: Selected Methods in Cellular Immunology (Mishel, B. B. and Shiigi, S. M. eds.), Freeman, San Francisco. [Google Scholar]
- 23.Harte P. G., Playfair J. H.1983. Failure of malaria vaccination in mice born to immune mothers. II. Induction of specific suppressor cells by maternal IgG. Clin. Exp. Immunol. 51: 157–164. [PMC free article] [PubMed] [Google Scholar]
- 24.Heyman B.1999. Antibody feedback suppression: towards a unifying concept? Immunol. Lett. 68: 41–45. doi: 10.1016/S0165-2478(99)00028-0 [DOI] [PubMed] [Google Scholar]
- 25.Heyman B.2000. Regulation of antibody responses via antibodies, complement, and Fc receptors. Annu. Rev. Immunol. 18: 709–737. doi: 10.1146/annurev.immunol.18.1.709 [DOI] [PubMed] [Google Scholar]
- 26.Heyman B., Wigzell H.1984. Immunoregulation by monoclonal sheep erythrocyte-specific IgG antibodies: suppression is correlated to level of antigen binding not to isotype. J. Immunol. 132: 1136–1143. [PubMed] [Google Scholar]
- 27.Hodgins D. C., Kang S. Y., Arriba L., Parreno V., Ward L. A., Yuan L., To T., Saif L. J.1999. Effects of maternal antibodies on protection and development of antibody responses to human rotavirus in gnotobiotic pigs. J. Virol. 73: 186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ingrao F., Rauw F., Lambrecht B., van den Berg T.2013. Infectious bursal disease: a complex host-pathogen interaction. Dev. Comp. Immunol. 41: 429–438. doi: 10.1016/j.dci.2013.03.017 [DOI] [PubMed] [Google Scholar]
- 29.Jeurissen S. H. M., Boonstra-Blom A. G., Cornelissen J. B. W. J., Dijkstra G.2000. Effect of antigen-specific maternal immunity on the induction of responses to the same antigen in the offspring. pp. 83–89. In: Proceedings of the 6th Avian Immunology Research Group Meeting, American Association of Avian Pathologists (Schat, K.A. ed.), Pennsylvania.
- 30.Kalergis A. M., Ravetch J. V.2002. Inducing tumor immunity through the selective engagement of activating Fc receptors on dendritic cells. J. Exp. Med. 195: 1653–1659. doi: 10.1084/jem.20020338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karlsson M. C., Getahun A., Heyman B.2001. Fc gamma RIIB in IgG-mediated suppression of antibody responses: different impact in vivo and in vitro. J. Immunol. 167: 5558–5564. doi: 10.4049/jimmunol.167.10.5558 [DOI] [PubMed] [Google Scholar]
- 32.Karlsson M. C., Wernersson S., Diaz D. S. T., Gustavsson S., Heyman B.1999. Efficient IgG-mediated suppression of primary antibody responses in Fc gamma receptor-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 96: 2244–2249. doi: 10.1073/pnas.96.5.2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim D., Huey D., Oglesbee M., Niewiesk S.2011. Insights into the regulatory mechanism controlling the inhibition of vaccine-induced seroconversion by maternal antibodies. Blood 117: 6143–6151. doi: 10.1182/blood-2010-11-320317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J. K., Kayali G., Walker D., Forrest H. L., Ellebedy A. H., Griffin Y. S., Rubrum A., Bahgat M. M., Kutkat M. A., Ali M. A., Aldridge J. R., Negovetich N. J., Krauss S., Webby R. J., Webster R. G.2010. Puzzling inefficiency of H5N1 influenza vaccines in Egyptian poultry. Proc. Natl. Acad. Sci. U.S.A. 107: 11044–11049. doi: 10.1073/pnas.1006419107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maas R., Rosema S., van Zoelen D., Venema S.2011. Maternal immunity against avian influenza H5N1 in chickens: limited protection and interference with vaccine efficacy. Avian Pathol. 40: 87–92. doi: 10.1080/03079457.2010.541226 [DOI] [PubMed] [Google Scholar]
- 36.Manca F., Fenoglio D., Li Pira G., Kunkl A., Celada F.1991. Effect of antigen/antibody ratio on macrophage uptake, processing, and presentation to T cells of antigen complexed with polyclonal antibodies. J. Exp. Med. 173: 37–48. doi: 10.1084/jem.173.1.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morein B., Abusugra I., Blomqvist G.2002. Immunity in neonates. Vet. Immunol. Immunopathol. 87: 207–213. doi: 10.1016/S0165-2427(02)00078-8 [DOI] [PubMed] [Google Scholar]
- 38.Pertmer T. M., Oran A. E., Moser J. M., Madorin C. A., Robinson H. L.2000. DNA vaccines for influenza virus: differential effects of maternal antibody on immune responses to hemagglutinin and nucleoprotein. J. Virol. 74: 7787–7793. doi: 10.1128/JVI.74.17.7787-7793.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Platt R., Widel P. W., Kesl L. D., Roth J. A.2009. Comparison of the humoral and cellular immune responses to a pentavalent modified live virus vaccine in three age groups of calves with maternal antibodies, before and after BVDV type 2 challenge. Vaccine 27: 4508–4519. doi: 10.1016/j.vaccine.2009.05.012 [DOI] [PubMed] [Google Scholar]
- 40.Polewicz M., Gracia A., Buchanan R., Strom S., Halperin S. A., Potter A. A., Babiuk L. A., Gerdts V.2011. Influence of maternal antibodies on active pertussis toxoid immunization of neonatal mice and piglets. Vaccine 29: 7718–7726. doi: 10.1016/j.vaccine.2011.07.135 [DOI] [PubMed] [Google Scholar]
- 41.Premenko-Lanier M., Hodge G., Rota P., Tamin A., Bellini W., McChesney M.2006. Maternal antibody inhibits both cellular and humeral immunity in response to measles vaccination at birth. Virology 350: 429–432. doi: 10.1016/j.virol.2006.02.029 [DOI] [PubMed] [Google Scholar]
- 42.Rauw F., Gardin Y., Palya V., vanBorm S., Gonze M., Lemaire S., van denBerg T., Lambrecht B.2009. Humoral, cell-mediated and mucosal immunity induced by oculo-nasal vaccination of one-day-old SPF and conventional layer chicks with two different live Newcastle disease vaccines. Vaccine 27: 3631–3642. doi: 10.1016/j.vaccine.2009.03.068 [DOI] [PubMed] [Google Scholar]
- 43.Sharma J. M.1999. Introduction to poultry vaccines and immunity. Adv. Vet. Med. 41: 481–494. [DOI] [PubMed] [Google Scholar]
- 44.Siegrist C. A.2003. Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine 21: 3406–3412. doi: 10.1016/S0264-410X(03)00342-6 [DOI] [PubMed] [Google Scholar]
- 45.Siegrist C. A.2001. Neonatal and early life vaccinology. Vaccine 19: 3331–3346. doi: 10.1016/S0264-410X(01)00028-7 [DOI] [PubMed] [Google Scholar]
- 46.Siegrist C. A.2007. The challenges of vaccine responses in early life: selected examples. J. Comp. Pathol. 137: S4–S9. doi: 10.1016/j.jcpa.2007.04.004 [DOI] [PubMed] [Google Scholar]
- 47.Siegrist C. A., Barrios C., Martinez X., Brandt C., Berney M., Córdova M., Kovarik J., Lambert P. H.1998. Influence of maternal antibodies on vaccine responses: inhibition of antibody but not T cell responses allows successful early prime-boost strategies in mice. Eur. J. Immunol. 28: 4138–4148. doi: [DOI] [PubMed] [Google Scholar]
- 48.Siegrist C. A., Córdova M., Brandt C., Barrios C., Berney M., Tougne C., Kovarik J., Lambert P. H.1998. Determinants of infant responses to vaccines in presence of maternal antibodies. Vaccine 16: 1409–1414. doi: 10.1016/S0264-410X(98)00100-5 [DOI] [PubMed] [Google Scholar]
- 49.Song C. H., Calandra G. B., Palmer C. J., Miller A., Sercarz E. E., Keller M. A.1990. Inhibition of offspring response to HEL-CFA by administration of anti-HEL MAB to the mother is not related to the predominant idiotype, IdXE, or specificity of the MAB. Cell. Immunol. 131: 311–324. doi: 10.1016/0008-8749(90)90257-R [DOI] [PubMed] [Google Scholar]
- 50.Stone H. D., Brugh M., Xie Z.1992. Simulation of maternal immunity by inoculation of immune yolk preparations into the yolk sac of 1-day-old chickens. Avian Dis. 36: 1048–1051. doi: 10.2307/1591572 [DOI] [PubMed] [Google Scholar]
- 51.van Eck J. H., van Wiltenburg N., Jaspers D.1991. An Ulster 2C strain-derived Newcastle disease vaccine: efficacy and excretion in maternally immune chickens. Avian Pathol. 20: 481–495. doi: 10.1080/03079459108418786 [DOI] [PubMed] [Google Scholar]
- 52.Van Savage J., Decker M. D., Edwards K. M., Sell S. H., Karzon D. T.1990. Natural history of pertussis antibody in the infant and effect on vaccine response. J. Infect. Dis. 161: 487–492. doi: 10.1093/infdis/161.3.487 [DOI] [PubMed] [Google Scholar]
- 53.Xiang Z. Q., Ertl H. C. J.1992. Transfer of maternal antibodies results in inhibition of specific immune responses in the offspring. Virus Res. 24: 297–314. doi: 10.1016/0168-1702(92)90125-S [DOI] [PubMed] [Google Scholar]
- 54.Yasuda M., Furusawa S., Satoh A., Taura Y., Nakama S., Yoshihara K., Hirota Y.1995. Effects of 6 hydroxydopamine on the antibody response to the hapten Dinitrophenyl in the chicken. J. Vet. Med. Sci. 57: 1073–1075. doi: 10.1292/jvms.57.1073 [DOI] [PubMed] [Google Scholar]