Abstract
Dermanyssus gallinae, the poultry red mite, is an obligatory blood-sucking ectoparasite. The genetic diversity of D. gallinae has been examined in some countries, but so far not in Asian countries. Here, we sequenced a part of the mitochondrial cytochrome oxidase subunit I (COI) and16S rRNA genes and nuclear internal transcribed spacers (ITS) region in 239 mite samples collected from 40 prefectures throughout Japan. The COI and 16S rRNA nucleotide sequences were classified into 28 and 26 haplotypes, respectively. In phylogenetic trees, the haplotypes clustered into 2 haplogroups corresponding to haplogroups A and B, which were previously reported. Haplogroups A and B were further subdivided into sub-haplogroups AJ1 and AJ2, and BJ1 and BJ2, respectively. In both trees, the sequences of haplotypes in AJ1 and BJ2 were relatively distant from those reported in other countries, while some sequences in AJ2 and BJ1 were identical to those in Europe. In addition, the ITS sequences were classified into two sequences, and both sequences were closely related to the sequences found in European countries. These findings indicate a possibility of international oversea transmission of D. gallinae.
Keywords: 16S rRNA gene, COI gene, ITS region, Japan, phylogenetic diversity, poultry red mite
Dermanyssus gallinae (De Geer, 1778), order Mesostigmata, Acari, also known as the poultry red mite, is an obligatory blood-sucking parasite of both domestic and wild birds [5, 11]. In poultry farms, it causes irritation, loss of weight, reduction in egg production, anemia and death, leading to economic loss in the poultry industry [3].
D. gallinae is distributed throughout the world [10], and their genetic diversity based on nucleotide sequences has been examined in Europe, the United States, Brazil and Australia. The genes sequenced include mitochondrial cytochrome oxidase subunit I (COI) gene [7, 8, 13] and 16S rRNA [12, 13], and the nuclear internal transcribed spacers (ITS) region [2, 6, 8, 9, 13]. These molecular epidemiological studies revealed intra- and international migrations of the mite in these countries. However, such phylogenetic studies have not yet been conducted in Asian countries including Japan. In this study, we analyzed the COI, 16S rRNA and ITS sequences of mite samples collected throughout Japan and compared them with sequences obtained in other countries to reveal the genetic diversity of D. gallinae.
MATERIALS AND METHODS
Dermanyssus gallinae: A total of 239 samples of D. gallinae were collected from 226 chicken farms from 2005 to 2012 in 40 prefectures in Japan. All samples were fixed in 99.5% ethanol and stored at room temperature until use.
DNA extraction: DNA was prepared from the whole body of each individual adult mite obtained from each sample. The mite was homogenized with zirconia beads using TissueLyser II (Qiagen Inc., Chatsworth, CA, U.S.A.) in 20 µl of buffer 1 provided by Ten Minute DNA Release Kit-1 (Jacksun Easy Biotech Inc., Bronx, NY, U.S.A.), and DNA samples were prepared according to the manufacturer’s instructions. The DNAs were stored at −30°C until use.
Polymerase chain reaction (PCR): A part of nucleotide sequence of mitochondrial COI gene was amplified by using the primers forward CO1Fyuw114 (5′-AGATCTTTAATTGAAGGGGG-3′) and reverse CO1Ryuw114 (5′- AAGATCAAAGAATCGGTGG-3′) designed in this study. The amplified fragment corresponded to nucleotide position from 61 to 742 of COI gene, accession number AM921853. A part of nucleotide sequence of 16S rRNA gene was amplified by using pair of primers forward Rh16S (5′- GCTCAATGATTTTTTAAATTGCTG-3′) and reverse Rh16S (5′- CCGGTCTGAACTCAGATCATG-3′) [4]. The expected length of PCR product was 444-bp, corresponding to nucleotide position from 3 to 446 of 16S rRNA gene, accession number L34326. A part of nucleotide sequence of ITS region including ITS1, 5.8S rRNA and ITS2 sequences was amplified by using the primers forward ITS-Fyuw125 (5′-AGTCGTAACAAGGTTTCCG-3′) and reverse ITS-Ryuw125 (5′-TCCTCCGCTTATTGATATGC-3′) designed in this study. The expected length of PCR product was 561-bp, corresponding to nucleotide position from 97 to 657 of ITS region, accession number GQ129212.
PCR was performed in a volume of 25 µl containing 12.5 µl of Hot start GoTaq (Promega, Fitchburg, WI, U.S.A.), 1.0 µl mixture of forward and reverse primers (final concentration was 0.5 µM each) and 2 µl of DNA template (for amplification of COI and ITS) or 1 µl (for amplification of 16S rRNA gene). The PCR for COI gene was conducted in one cycle of 95°C for 2 min, 40 cycles of 95°C for 20 sec, 55°C for 30 sec, 72°C for 1 min and a final cycle of 72°C for 4 min. The PCR for 16S rRNA was performed in accordance with the previous study [4]. PCR for ITS was conducted in one denaturation step at 95°C for 4 min and followed by 40 cycles of 95°C for 30 sec, 56°C for 30 sec and 72°C for 45 sec. The final cycle was 72°C in 7 min. After the amplification, reaction mixtures were subjected to electrophoresis in 1.2% (w/v) agarose gels and stained with 0.1% Gel Red (Biotium, Inc., Hayward, CA, U.S.A.). The obtained PCR amplicons were excised and purified by using MinElute Gel Extraction Kit (Qiagen, Hilden, Germany) for the nucleotide sequence analysis.
Nucleotide sequencing: Nucleotide sequences were determined using Applied Biosystems 3130 Genetic Analyzer (Applied Biosystems, Carlsbad, CA, U.S.A.) with the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) according to the manufacturer’s instructions. Nucleotide sequences were assembled and manually edited by using CodonCode Aligner 3.6.1 (CodonCode, Dedham, MA, U.S.A.). After elimination of primer sequences from the obtained sequences, the resultant sequences were analyzed as described below.
Sequence analysis: The determined sequences of COI and 16S rRNA genes were assigned to their haplotypes. Mites with identical sequences were designated as one haplotype.
For the phylogenetic analysis of COI sequences obtained in this study, 476-bp covered from the position from 121 to 596 of the sequence accession number AM921853 were used, because this sequence region is most commonly reported in the database. In this study, we also used 926 COI sequences, detected in European countries, U.S.A., Brazil and Australia, obtained from the database. In the case of 16S rRNA sequence, 299-bp corresponding to the sequences of D. gallinae (accession number L34326) at the position from 103 to 401 were used for the phylogenetic analysis to reveal the phylogenetic relationship in the world, because this is the region most frequently reported. In this study, we also used 17 16S rRNA sequences detected in European countries, obtained from the database.
The nucleotide sequences were aligned using ClustalX with default settings [1]. Subsequently, the phylogenetic trees were constructed by the Neighbor-Joining (NJ) method [14] with the Kimura two-parameter option. A bootstrap analysis was done with 1,000 replicates.
RESULTS
Nucleotide sequence analysis of COI gene: The nucleotide sequences of 643-bp of COI gene of 101 mite samples obtained in Japan (accession numbers LC029457-LC029557) were classified into 28 haplotypes (Table 1).
Table 1. Number of D. galliinae samples classified into each haplogrop, sub-haplogroup and haplotype based on the nucleotide sequences of COI and 16S rRNA, and sequence type of ITS.
| COI | 16S rRNA Haplotypec) | ITS | Total | ||||
|---|---|---|---|---|---|---|---|
| Haplogroupa) | Sub-haplogroup | Haplotypeb) | Type I | Type II | ND | ||
| A | AJ1 | AJ1.1 | AJ1.1 | 5 | 0 | 19 | 24 |
| AJ1.6 | 0 | 0 | 2 | 2 | |||
| AJ1.10 | 1 | 0 | 0 | 1 | |||
| AJ1.11 | 0 | 0 | 2 | 2 | |||
| AJ1.12 | 0 | 0 | 1 | 1 | |||
| AJ1.2 | AJ1.1 | 1 | 0 | 0 | 1 | ||
| AJ1.3 | AJ1.1 | 1 | 0 | 0 | 1 | ||
| AJ1.4 | AJ1.1 | 0 | 0 | 1 | 1 | ||
| AJ1.5 | AJ1.1 | 0 | 0 | 1 | 1 | ||
| AJ1.6 | AJ1.1 | 0 | 0 | 2 | 2 | ||
| AJ1.7 | AJ1.3 | 0 | 1 | 0 | 1 | ||
| AJ1.8 | AJ1.1 | 1 | 0 | 0 | 1 | ||
| AJ1.9 | AJ1.1 | 1 | 0 | 12 | 13 | ||
| AJ1.5 | 0 | 0 | 1 | 1 | |||
| AJ1.10 | AJ1.1 | 0 | 0 | 1 | 1 | ||
| AJ1.11 | AJ1.1 | 1 | 0 | 1 | 2 | ||
| AJ1.12 | AJ1.1 | 0 | 0 | 1 | 1 | ||
| AJ1.13 | AJ1.1 | 0 | 0 | 1 | 1 | ||
| AJ1.14 | AJ1.6 | 0 | 1 | 1 | 2 | ||
| AJ2 | AJ2.1 | AJ2.1 | 7 | 0 | 3 | 10 | |
| AJ2.2 | 0 | 0 | 1 | 1 | |||
| AJ2.2 | AJ2.4 | 0 | 1 | 0 | 1 | ||
| B | BJ1 | BJ1.1 | BJ1.1 | 0 | 0 | 1 | 1 |
| BJ1.3 | 1 | 0 | 0 | 1 | |||
| BJ1.2 | BJ1.1 | 1 | 1 | 0 | 2 | ||
| BJ1.3 | BJ1.1 | 0 | 1 | 0 | 1 | ||
| BJ1.4 | BJ1.1 | 0 | 0 | 1 | 1 | ||
| BJ1.5 | BJ1.1 | 0 | 0 | 1 | 1 | ||
| BJ1.6 | BJ1.1 | 0 | 0 | 1 | 1 | ||
| BJ2 | BJ2.1 | BJ2.1 | 4 | 0 | 10 | 14 | |
| BJ2.3 | 0 | 0 | 1 | 1 | |||
| BJ2.5 | 0 | 1 | 0 | 1 | |||
| BJ2.2 | BJ2.1 | 0 | 0 | 1 | 1 | ||
| BJ2.3 | BJ2.1 | 0 | 0 | 1 | 1 | ||
| BJ2.4 | BJ2.6 | 0 | 1 | 0 | 1 | ||
| BJ2.5 | BJ2.1 | 0 | 0 | 1 | 1 | ||
| BJ2.6 | BJ2.1 | 0 | 0 | 2 | 2 | ||
| ND | ND | ND | AJ1.1 | 0 | 0 | 81 | 81 |
| AJ1.2 | 0 | 0 | 2 | 2 | |||
| AJ1.4 | 0 | 0 | 1 | 1 | |||
| AJ1.6 | 0 | 0 | 1 | 1 | |||
| AJ1.7 | 0 | 0 | 1 | 1 | |||
| AJ1.8 | 0 | 0 | 1 | 1 | |||
| AJ1.9 | 0 | 0 | 1 | 1 | |||
| AJ1.11 | 0 | 0 | 3 | 3 | |||
| AJ2.1 | 0 | 0 | 2 | 2 | |||
| AJ2.3 | 0 | 0 | 1 | 1 | |||
| AJ2.5 | 0 | 0 | 1 | 1 | |||
| BJ1.1 | 0 | 0 | 19 | 19 | |||
| BJ1.2 | 0 | 0 | 2 | 2 | |||
| BJ2.1 | 1 | 1 | 18 | 20 | |||
| BJ2.2 | 0 | 0 | 1 | 1 | |||
| BJ2.4 | 0 | 0 | 1 | 1 | |||
| Total | 28 haplotypes | 26 haplotypes | 25 | 8 | 206 | 239 | |
ND: Not determined. a) Haplogroups reported by Oines and Brannstrom [8]. b) Haplotypes were determined based on the nucleotide sequences of 643-bp of COI gene. c) Haplotypes were determined based on the nucleotide sequences of 397-bp of 16S rRNA.
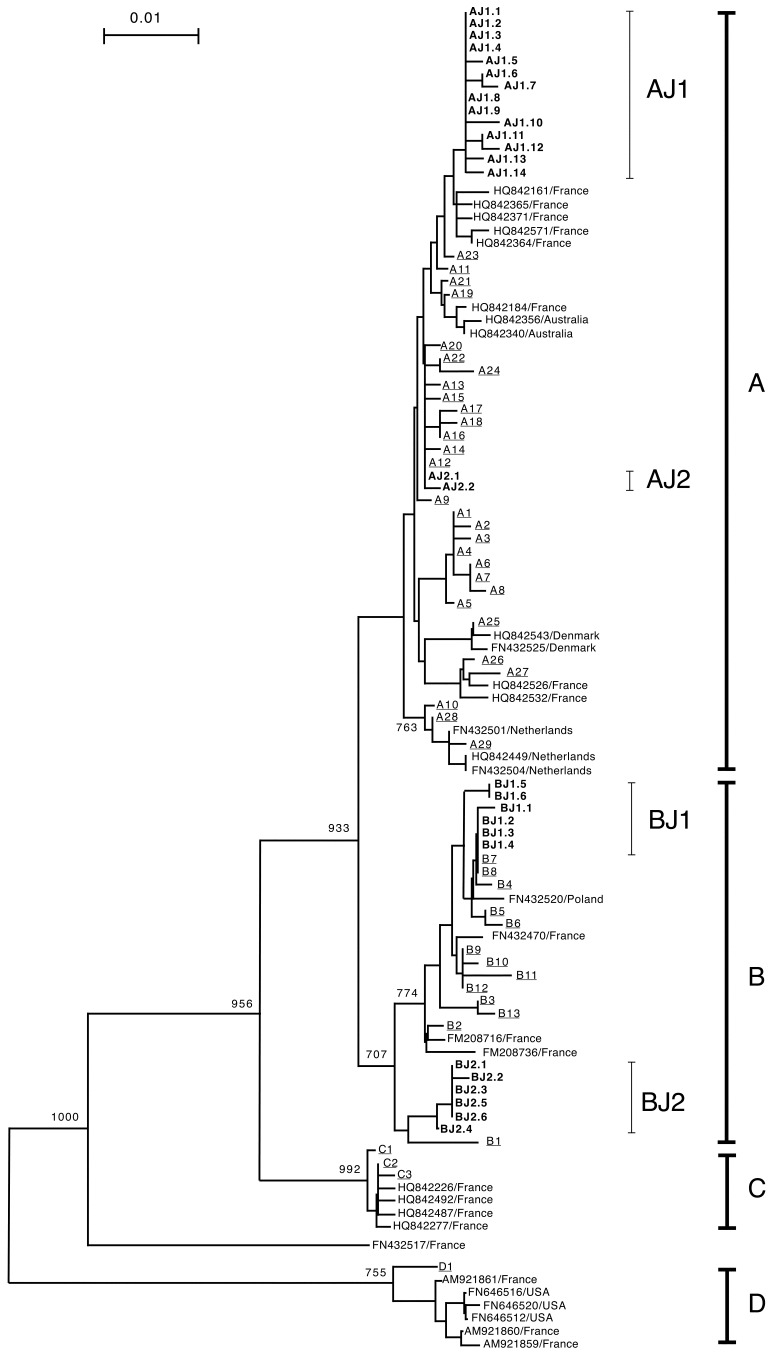
In a phylogenetic tree based on the 476-bp COI nucleotide sequence (Fig. 1), mites from Japan and other countries clustered into 4 haplogroups A, B, C and D as previously reported [8]. All 28 haplotypes found in Japan clustered in either haplogroup A or B. These haplotypes diverged into two sub-lineages designated as sub-haplogroups AJ1 and AJ2, and BJ1 and BJ2, respectively. AJ1 consisted of 14 haplotypes (AJ1.1-AJ1.14), AJ2 consisted of two haplotypes (AJ2.1 and AJ2.2), BJ1 consisted of six haplotypes (BJ1.1-BJ1.6) and BJ2 consisted of six haplotypes (BJ2.1-BJ2.6). Sample numbers classified into each haplotype are shown in Table 1.
Fig. 1.
Phylogenetic tree constructed with nucleotide sequences of COI gene using Neighbor-Joining method. The tree was constructed with 476-bp of COI gene sequences of D. gallinae in Japan determined in this study and those of other countries obtained from the database. Haplotypes designated based on the 643-bp of COI gene of 101 mite samples collected in Japan are shown in boldface. The haplotypes previously reported [8] in other countries are underlined. Other sequences obtained from the database are shown with the accession number and the country where the mites were detected. Bootstrap values more than 700 are shown. The haplotypes, A, B, C and D, previously reported [8] are indicated with vertical thick lines. The haplotypes found in Japan clustered in haplogroups A and B. The haplotypes found in Japan in each haplogroup A and B diverged into two clades designated as sub-haplogroups AJ1 and AJ2, and BJ1 and BJ2, respectively. These sub-haplogroups are indicated with thin vertical lines. Sub-haplogroups AJ2 and BJ1 are closely related to the haplotypes found in European countries. However, the haplotypes in sub-haplogroups AJ1 and BJ2 are relatively distant from the haplotypes found in other countries.
The sequences of 14 and 6 haplotypes in AJ1 and BJ2, respectively, were found only in Japan. Phylogenetic analysis showed that the sequences of haplotypes in AJ1 and BJ2 were relatively distant from the sequences detected in other countries. On the other hand, the sequences of haplotypes in AJ2 and BJ1 were closely related to the sequences previously detected in several European countries (Fig. 1). The 476-bp sequence of haplotype AJ2.1 was identical to the sequence of A12 haplotype detected in Norway [8]. In addition, the 6 haplotypes in BJ1 were closely related to 11 reported haplotypes, B3 to B13 of haplogroup B [8], and the 476-bp sequences of haplotypes BJ1.2, BJ1.3 and BJ1.4 were identical to the sequence of haplotype B7 distributed in Sweden, Netherlands and Poland, and B8 distributed in Scotland [8].
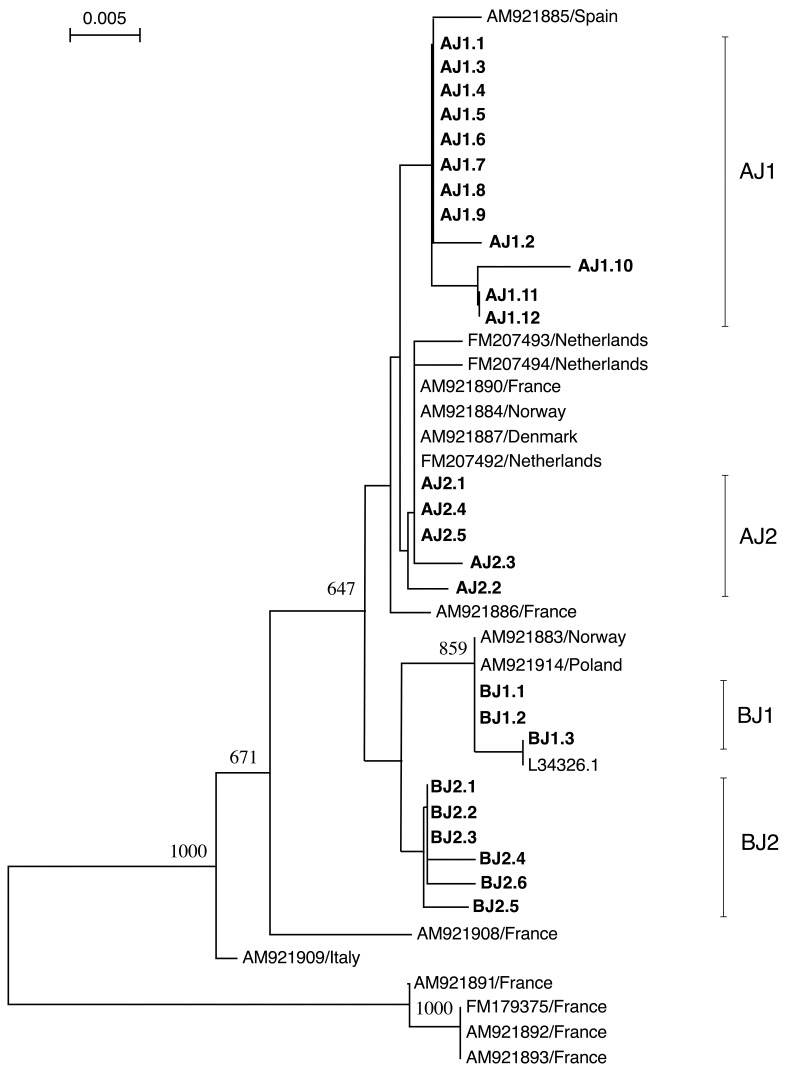
Nucleotide sequence analysis of 16S rRNA: The 397-bp 16S rRNA sequences of the 239 samples (accession numbers LC029560-LC029798) were determined and were classified into 26 haplotypes (Table 1). In a phylogenetic tree constructed with the 299-bp of the 397-bp sequence, which was most commonly deposited in the database, these haplotypes diverged into two lineages, each having two clades (Fig. 2).
Fig. 2.
Phylogenetic tree constructed with the nucleotide sequences of 16S rRNA gene using Neighbor-Joining method. The tree was constructed with 299-bp of 16S rRNA gene sequences of D. gallinae in Japan determined in this study and those of other countries obtained from the database. Haplotypes found in Japan were designated based on the 397-bp of 16S rRNA gene of D. gallinae obtained from 239 samples and are shown in boldface in the tree. Other sequences obtained from the database are shown with the accession number and the country where the mites were detected. Bootstrap values more than 600 are shown. The haplotypes found in Japan clustered in two haplogroups. Subsequently, the haplotypes in each haplogroup diverged into two lineages designated as sub-haplogroups, AJ1 and AJ2, and BJ1 and BJ2, respectively. These sub-haplogroups are indicated with the thin vertical lines. Sub-haplogroups AJ2 and BJ1 are closely related to the sequences found in European countries. However, the sub-haplogroups AJ1 and BJ2 are relatively distant from the haplotypes found in other countries.
The phylogenetic relationships of 101 mite samples, which were used for both 16S rRNA and COI analyses, in the 16S rRNA tree were consistent with the relationships among haplogroups and sub-haplogroups found in the phylogenetic tree constructed with the COI sequences. Because the same phylogenetic relationships were found in both trees, the 2 lineages and 4 clades observed in the 16S rRNA phylogenetic tree were designated as haplogroups A and B, and sub-haplogroups AJ1 and AJ2, and BJ1 and BJ2, respectively (Fig. 2), according to the COI tree.
AJ1 in this tree consisted of 12 haplotypes (AJ1.1 to AJ1.12), and AJ2 consisted of 5 haplotypes (AJ2.1 to AJ2.5). BJ1 consisted of 3 haplotypes (BJ1.1 to BJ1.3), and BJ2 consisted of 6 haplotypes (BJ2.1 to BJ2.6). Of the 239 sequences examined, 130 sequences belonged to the haplotype AJ1.1 of 16S rRNA. This haplotype was the most frequently found in Japan. In the B haplogroup, BJ2.1with 39 sequences and BJ1.1 with 26 sequences were the most common haplotypes.
The haplotypes in AJ2 and BJ1 clustered with the sequences detected in European countries, respectively (Fig. 2). The 299-bp sequences of haplotypes AJ2.1, AJ2.4 and AJ2.5 were identical to the sequences detected in France (accession No. AM921890), Norway (accession No. AM921884), Denmark (accession No. AM921887) and Netherlands (accession No. FM207492). The sequences of haplotypes BJ1.1 and BJ1.2 were identical to the sequences detected in Norway (accession No. AM921883) and Poland (accession No. AM921914). On the other hand, all haplotypes in sub-haplogroup BJ2 were relatively distant from the sequences reported in European countries. In the case of the haplotypes in sub-haplogroup AJ1, one sequence obtained from Spain (accession No. AM921885) clustered with AJ1 sub-haplogroup. However, no sequences found in Japan were identical to this sequence.
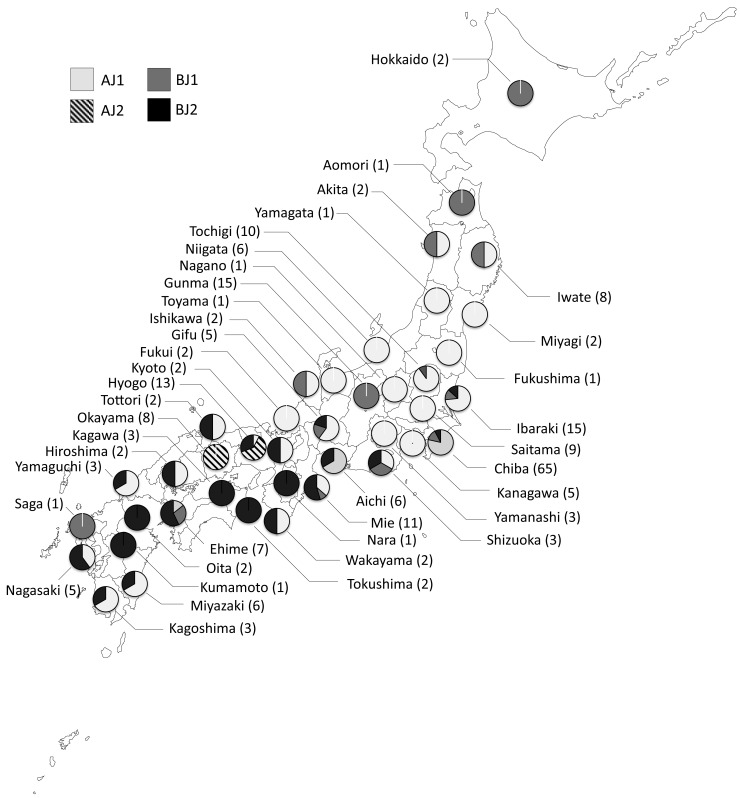
Distribution of the sub-haplogroups of the mite in Japan: Geographical distribution of sub-haplogroups determined based on COI and 16S rRNA sequences was examined (Fig. 3). Based on the 16S rRNA sequences, sub-haplogroup AJ1 was distributed all over Japan (30/40 studied prefectures), while mites in AJ2 were found only in 2 marginal prefectures, Hyogo and Okayama. Mites in BJ1 were found in 14 prefectures located through Japan. Although the number is relatively restricted, these prefectures were located all over Japan, from the most northerly prefecture (Hokkaido) to the most southerly prefectures (Saga). Mites in BJ2 were detected over an area on the west side from Chiba prefecture in Japan (21/40 studied prefectures).
Fig. 3.
Geographical distribution of sub-haplogroups of D. gallinae in Japan based on 16S rRNA sequences. Proportions of 4 sub-haplotypes, AJ1, AJ2, BJ1 and BJ2, found in 40 prefectures are shown in pie charts on map. The prefecture names are shown with sample numbers in brackets. The sub-haplogroups AJ1 and BJ1 were found all over Japan. AJ2 was found only in two adjacent prefectures, Hyogo and Okayama. BJ2 was mainly distributed in the western area of Chiba prefecture.
Nucleotide sequence analysis of the ITS region: Alignment of the 522-bp of ITS region of 33 samples (accession numbers LC034921-LC034953) revealed that there are 2 sequence types that differ in 2 positions corresponding to 199 and 516 of the reported ITS sequence (accession number GQ129212). Positions 199 and 516 are located in the ITS1 and ITS2 regions, respectively. Of the 33 samples, 25 samples of both positions at 199 and 516 were C (type I), and the other 8 samples were T and A (type II), respectively (Table 1). The type II sequences obtained from the 8 samples were identical to the sequence of ITS region of the mites collected in Sweden (accession number GQ12912), Denmark (accession number AM903303) and Norway (accession number AM931072). On the other hand, none of the sequences were identical to the type I sequence. However, 2 sequences, accession numbers AM903308 (from Italy) and AM930889 (from France), were identical, except for one N (indicating any nucleotide) in each sequence at a different position.
All mite samples which showed the same ITS sequence were classified into the same haplotype, except for four samples (Table 1). The ITS sequences of 2 samples collected in Hokkaido (type I) and Iwate (type II) prefectures are different, but their haplotypes of COI (BJ1.2) and 16S rRNA (BJ1.1) are the same. Similarly, the ITS sequences of 2 other samples collected in Chiba prefecture are different, but their 16S rRNA haplotype is BJ2.1 (COI haplotype was not determined).
DISCUSSION
In this study, D. gallinae were harvested from 40 prefectures all over Japan, and parts of their COI gene and 16S rRNA sequences were determined. The sequence analyses revealed that the haplotypes in AJ2 and BJ1 sub-haplogroups in both the COI and 16S rRNA trees were closely related to the sequences of haplotypes found in Europe. Although it is unclear which countries were the origins of these haplotypes, the findings indicate that the mites of these haplotypes distributed in Japan are genetically related to the mites in European countries. In the case of the haplotypes in the AJ2 sub-haplogroup, the distribution in Japan was restricted to two adjacent prefectures. Because the mites can be easily spread with the transportation of infested chickens or related materials among poultry farms, such a restricted distribution implies that the haplotypes in AJ2 were recently introduced to Japan.
The sequences of the haplotypes in the AJ1 sub-haplogroup were different from all of the other sequences and phylogenetically distant from the mites detected in other countries. In addition, the haplotypes in the AJ1 sub-haplogroup were found most frequently and were most widely distributed in Japan. These findings imply that mites with haplotypes in the AJ1 sub-haplogroup are indigenous and may have independently evolved in Japan. In the case of the haplotypes in the BJ2 sub-haplogroup, their sequences were also different from sequences reported in other countries. However, their distribution, in contrast to the distribution of AJ1, was restricted to western Japan. Because the mites can be easily spread as mentioned above, it seems more likely that the haplotypes in BJ2 are not indigenous but were relatively recently introduced to western Japan from other countries where COI or 16S rRNA sequences of mites have not yet been determined. Of the haplotypes in the BJ2 sub-haplogroup, BJ2.1 haplotypes of COI and 16S rRNA were most frequently detected. This finding implies that the haplotypes of the BJ2 sub-haplogroup found in this study might have diverged from the BJ2.1 haplotype in Japan.
Most of the mite samples that had the same ITS sequences were classified into the same haplotypes. However, the ITS sequences of some samples were different from each other, but their haplotypes were the same. Because both COI and 16S rRNA are mitochondrial genes and the ITS region is a nuclear gene, these sequences are useful for revealing hybridization histories between different lineages [10]. The incongruence between the haplotype based on the mitochondrial gene sequence and the sequence of nuclear genes found in this study suggests that a hybridization event occurred between different haplotypes of the mites.
This study revealed genetic diversities of D. gallinae distributed in Japan. In addition, phylogenetic relationships among the mites in Japan and other countries indicate the possibility of oversea transmission of the mites. However, it should be noted that nucleotide sequences of D. gallinae have been determined for only a few areas despite their worldwide distribution. For a comprehensive understanding of intra- and international migrations of D. gallinae, further sequence analyses of the mites collected from all over the world are needed.
Acknowledgments
This work was supported by JSPS, KAKENHI Grant Number 23580423. The authors also thank the Ministry of Education, Sports, Science and Technology, Japan (MEXT) for the doctoral scholarship (CTTH).
REFERENCES
- 1.Aiyar A.2000. The use of CLUSTAL W and CLUSTAL X for multiple sequence alignment. Methods Mol. Biol. 132: 221–241. [DOI] [PubMed] [Google Scholar]
- 2.Brännström S., Morrison D. A., Mattsson J. G., Chirico J.2008. Genetic differences in internal transcribed spacer 1 between Dermanyssus gallinae from wild birds and domestic chickens. Med. Vet. Entomol. 22: 152–155. doi: 10.1111/j.1365-2915.2008.00722.x [DOI] [PubMed] [Google Scholar]
- 3.Chauve C.1998. The poultry red mite Dermanyssus gallinae (De Geer, 1778): current situation and future prospects for control. Vet. Parasitol. 79: 239–245. doi: 10.1016/S0304-4017(98)00167-8 [DOI] [PubMed] [Google Scholar]
- 4.De Rojas M., Mora M. D., Ubeda J. M., Cutillas C., Navajas M., Guevara D. C.2001. Phylogenetic relationships in rhinonyssid mites (Acari: Rhinonyssidae) based on mitochondrial 16S rDNA sequences. Exp. Appl. Acarol. 25: 957–967. doi: 10.1023/A:1020651214274 [DOI] [PubMed] [Google Scholar]
- 5.Gray R. M, Barry M. O.2009. Mites (Acari). pp. 433–492. In: Medical and Veterinary Entomology, 2nd ed. (GARY, R. and LANCE, A. eds.), Academic Press, London. [Google Scholar]
- 6.Marangi M., Cantacessi C., Sparagano O. A., Camarda A., Giangaspero A.2014. Molecular characterization and phylogenetic inferences of Dermanyssus gallinae isolates in Italy within an European framework. Med. Vet. Entomol. 28: 447–452. doi: 10.1111/mve.12050 [DOI] [PubMed] [Google Scholar]
- 7.Marangi M., de Luna C. J., Cafiero M. A., Camarda A., le Bouquin S., Huonnic D., Giangaspero A., Sparagano O. A.2009. Phylogenetic relationship between Dermanyssus gallinae populations in European countries based on mitochondrial COI gene sequences. Exp. Appl. Acarol. 48: 143–155. doi: 10.1007/s10493-009-9237-3 [DOI] [PubMed] [Google Scholar]
- 8.Oines O., Brannstrom S.2011. Molecular investigations of cytochrome c oxidase subunit I (COI) and the internal transcribed spacer (ITS) in the poultry red mite, Dermanyssus gallinae, in northern Europe and implications for its transmission between laying poultry farms. Med. Vet. Entomol. 25: 402–412. doi: 10.1111/j.1365-2915.2011.00958.x [DOI] [PubMed] [Google Scholar]
- 9.Potenza L., Cafiero M. A., Camarda A., La Salandra G., Cucchiarini L., Dacha M.2009. Characterization of Dermanyssus gallinae (Acarina: Dermanissydae) by sequence analysis of the ribosomal internal transcribed spacer regions. Vet. Res. Commun. 33: 611–618. doi: 10.1007/s11259-009-9210-y [DOI] [PubMed] [Google Scholar]
- 10.Roy L., Buronfosse T.2011. Using mitochondrial and nuclear sequence data for disentangling population structure in complex pest species: a case study with Dermanyssus gallinae. PLoS ONE 6: e22305. doi: 10.1371/journal.pone.0022305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy L., Chauve C. M.2007. Historical review of the genus Dermanyssus Duges, 1834 (Acari: Mesostigmata: Dermanyssidae). Parasite 14: 87–100. doi: 10.1051/parasite/2007142087 [DOI] [PubMed] [Google Scholar]
- 12.Roy L., Dowling A. P., Chauve C. M., Buronfosse T.2009. Delimiting species boundaries within Dermanyssus Duges, 1834 (Acari:Dermanyssidae) using a total evidence approach. Mol. Phylogenet. Evol. 50: 446–470. doi: 10.1016/j.ympev.2008.11.012 [DOI] [PubMed] [Google Scholar]
- 13.Roy L., Dowling A. P., Chauve C. M., Buronfosse T.2010. Diversity of phylogenetic information according to the locus and the taxonomic level: an example from a parasitic mesostigmatid mite genus. Int. J. Mol. Sci. 11: 1704–1734. doi: 10.3390/ijms11041704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saitou N., Nei M.1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4: 406–425. [DOI] [PubMed] [Google Scholar]