Abstract
Hypoxic conditions in various cancers are believed to relate with their malignancy, and hypoxia inducible factor-1α (HIF-1α) has been shown to be a major regulator of the response to low oxygen. In this study, we examined HIF-1α expression in canine lymphoma using cell lines and clinical samples and found that these cells expressed HIF-1α. Moreover, the HIF-1α inhibitors, echinomycin, YC-1 and 2-methoxyestradiol, suppressed the proliferation of canine lymphoma cell lines. In a xenograft model using NOD/scid mice, echinomycin treatment resulted in a dose-dependent regression of the tumor. Our results suggest that HIF-1α contributes to the proliferation and/or survival of canine lymphoma cells. Therefore, HIF-1α inhibitors may be potential agents to treat canine lymphoma.
Keywords: canine lymphoma, echinomycin, HIF inhibitor, hypoxia inducible factor, xenograft model
Hypoxia inducible factor (HIF) is a transcription factor that induces the expression of various genes to resist tissue hypoxia. HIF forms heterodimers, which consist of an alpha and beta subunit. There are three HIF alpha subunits (1α, 2α and 3α) and a single beta subunit (1β) [26]. The HIF-1 (1α and 1β) and HIF-2 (2α and 1β) proteins are constitutively expressed; however, under normoxic conditions, a specific proline residue in the HIF alpha subunit is hydroxylated by prolylhydroxylase 2 (PHD2). This allows the von Hippel-Lindau (VHL) protein to recognize it and recruit ubiquitin ligase [3, 11]. As a result, the HIF-1α and −2α proteins are degraded under well-oxygenated conditions. This degradation does not occur under hypoxic conditions, because PHD2 requires oxygen to combine the hydroxyl group to the HIF α subunit. Undegraded, the HIF-1α and −2α proteins are able to migrate to the nucleus and act as transcription factors via heterodimerization with HIF-1β [10], resulting in the expression of several genes, such as vascular endothelial growth factor (VEGF), glucose transporter-1 (GLUT1) and pyruvate dehydrogenase isozyme 1 (PDK1), which relates with angiogenesis and metabolism [6, 7].
Cancer tissue has hypoxic regions that express the HIF-1 protein, which causes resistance to chemotherapy, angiogenesis, increasing metastasis and maintenance of cancer stem cells [8, 12, 14]. Human hematopoietic malignancies, such as leukemia and lymphoma, also express high levels of HIF-1, the expression of which is considered a poor prognostic factor [2, 4, 33]. Recently, Wang et al. [32] reported that treatment with an HIF-1α inhibitor resulted in tumor regression in murine lymphoma, which is caused by an abrogation of the epilepsy, progressive myoclonus type 2A (Epm2a) gene, and also showed that HIF-1α knockdown with small hairpin RNA (shRNA) resulted in the growth suppression of lymphoma cells isolated from the transgenic mice.
Lymphoma is the most common hematopoietic malignancy in dogs. Generally, lymphoma patients are treated with multidrug chemotherapies. The remission rate and duration have been reported as 80% and more than 9 months, respectively [5, 13]. However, almost all lymphoma patients experience a recurrence and develop drug resistance. Therefore, a novel treatment is strongly desired.
Here, we examined whether HIF-1α contributes to tumorigenesis and/or the survival of canine lymphoma, and investigated whether HIF-1α inhibitors could suppress the proliferation of canine lymphoma cells in vitro and in vivo.
MATERIALS AND METHODS
Cells: Seven canine lymphoma cell lines; the CLBL-1 [24], GL-1 [20] and 17–71 [23] B cell lines; the CL-1 [19], CLC [29], CLGL90 [28] and Nody-1 [29] T cell lines; and a Raji human lymphoma cell line were used. CLBL-1, GL-1, CL-1, CLC, Nody-1 and Raji cell lines were cultured in RPMI1640 medium, and CLGL90 and 17–71 cell lines were cultured in Dulbecco’s Modified Eagle medium, which were supplemented 10% fetal bovine serum, penicillin (100 U/ml), streptomycin (100 µg/ml) and 2-mercaptoethanol (55 µM). Cells were incubated at 37°C in a humidified incubator containing 21% O2 and 5% CO2.
Peripheral blood mononuclear cells (PBMCs), which served as a control, were collected from healthy beagle dogs using Lymphoprep (Axis-Shield PoC AS, Oslo, Norway). Briefly, heparinized whole blood was centrifuged, and the buffy coat was suspended in PBS. The diluted cells were gently overlaid on Lymphoprep and then centrifuged at 800 × g for 30 min. The PBMCs layer was collected and diluted with PBS. The isolated PBMCs were overlaid on whipped fetal bovine serum in order to remove the contaminating platelets. After a centrifugation at 1,000 × g for 10 min, the purified PBMCs were obtained as the cell pellet and were washed with PBS.
Healthy dog tissue samples and clinical samples: Tissues were obtained from a healthy beagle, which was euthanized by anesthesia. Clinical canine lymphoma samples were collected from patients at the Yamaguchi University Animal Medical Center with a written informed consent from dog owners. Lymphoma cells were collected from swelled lymph nodes by fine needle aspiration using a 21–23 gauge needle and 5 ml disposable syringe. In patient 5, which was used for immunoblotting, lymphoma cells also existed in the peripheral blood; thus, blood was collected by the routine blood collection method.
Reagents: Echinomycin (Calbiochem, San Diego, CA, U.S.A.), YC-1 (Cayman Chemicals, Ann Arbor, MI, U.S.A.) and 2-methoxyestradiol (Merck Millipore, Billerica, MA, U.S.A.) were dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO, U.S.A.) and stored at −20°C until use.
Quantitative real-time PCR: Total RNA was isolated from each cell line, PBMCs, canine normal tissues and clinical samples (patients 1–4) using the ISOGEN II (Nippon Gene, Tokyo, Japan) and resolved with nuclease free water. One µg of total RNA was treated with DNase I using a Turbo DNA-free kit (Applied Biosystems, Carlsbad, CA, U.S.A.) and transcribed into cDNA with Superscript III (Invitrogen Life Technologies, Carlsbad, CA, U.S.A.) according to the manufacturer’s instructions. The single-stranded cDNA was subjected to real-time PCR amplification with a QuantiTect SYBR Green PCR kit (Qiagen, Valencia, CA, U.S.A.) according to the manufacturer’s protocol. Real-time PCR was performed with StepOne PCR system (Perkin-Elmer, Waltham, MA, U.S.A.). The data were analyzed using StepOne software v.2.2.2. The following primers were used: canine HIF-1α forward 5′-TGACGGTTCACTTTTTCAAGC-3′ and reverse 5′-TTGCTCCATTCCATTCTGTTC-3′; canine GLUT forward 5′-ACTGCCCTGGATGTCGTATC-3′ and reverse 5′-GGACCCTGGCTGAAGAGTTC-3′; and RPL32 forward 5′-TGGTTACAGGAGCAACAAGAAA-3′ and reverse 5′-GCACATCAGCAGCACTTCA-3′.
The relative expression levels of HIF-1α, GLUT1 and PDK1 were calculated as ΔCt from the difference between expression of the internal control, RPL32 [21], and 2-ΔCt were calculated to represent the expression levels of each gene. Mean cycle threshold (Ct) value less than 35 was included in this study. After the PCR, all products were analyzed by the melting curves confirming no other genes were amplified.
Immunoblotting: Cultured cells, clinical samples (case 1–5) and PBMCs were washed twice with ice cold PBS. Tissue samples were homogenized in liquid nitrogen before cell lysis. Samples were lysed with a lysis buffer [50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1% NP-40, 1% sodium lauryl sulfate, protease inhibitor cocktail (Nacalai Tesque, Kyoto, Japan), 1 mM Na3VO4 and 50 mM NaF] and then sonicated for 15 min (30 cycles of 10 sec on/20 sec off). Then, lysates were boiled at 100°C by heat block for 5 min. The cell suspension was centrifuged at 15,000 × g at 4°C for 15 min, and the supernatant was transferred into a new tube as the whole cell lysate. The amount of protein in the cell lysate was measured with a Micro BCA™ Protein Assay Reagent Kit (Thermo Fischer Scientific, Waltham, MA, U.S.A.).
The lysate was subjected to SDS-PAGE on a polyacrylamide gel containing 5.5‒13.2% acrylamide. After SDS-PAGE, the proteins were transferred to Immobilon® Membranes (Merck Millipore). The membrane was blocked with a blocking buffer (TBS-T; Tris-buffered saline with 0.05% Tween 20 and 5% skimmed milk or 5% bovine serum albumin) for 1 hr at room temperature and then incubated with a primary antibody overnight at 4°C. Rabbit polyclonal anti-HIF-1α (NB100-449) was purchased from Novus Biologicals (Littleton, CO, U.S.A.) and used at a 1:500 dilution [22]. Mouse monoclonal antibody for β-actin (AC-15) was purchased from Santa Cruz Biotechnology (Dallas, TX, U.S.A.) and used at a 1:2,000 dilution. Rabbit polyclonal anti-Lamin B1 was purchased from Abcam (Cambridge, U.K.) and used at a 1:1,000 dilution. The membranes were washed twice in TBS-T and then incubated with a secondary antibody for 1 hr at room temperature. An antibody for horseradish peroxidase-conjugated mouse IgG (1:4,000 dilution) and rabbit IgG (1:4,000 dilution) were from Thermo Fischer Scientific. Then, the chemiluminescence was detected by using Western Lightning® Plus-ECL (Perkin-Elmer) and LAS-3000 mini (FUJIFILM, Tokyo, Japan).
Cytotoxicity assay: Canine lymphoma cell lines were treated with echinomycin, YC-1 or 2-methoxyestradiol at various concentrations in 96-well plates. After 48-hr incubation at 37°C, 10 µl 5 mg/ml 3-(4,5-di-methylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) was added. After further 4 hr incubation, 100 µl MTT-lysis buffer [20% SDS and 40% N,N-dimethylformamide (Nacalai Tesque)] was added. After 1 hr, the absorbance was measured at 570 nm. Each experiment was performed in triplicate and independently repeated 3 times. The concentration of each drug that inhibited the cell growth by 50% (IC50) was calculated from the drug survival curves.
Flow cytometry: The CLBL-1 cells were treated with echinomycin or DMSO as the negative control, for 12 hr. Apoptosis was detected using the MEBCYTO® Apoptosis Kit (MBL, Nagoya, Japan) according to manufacturer’s protocol. The fluorescence intensity of 10,000 cells was measured with BD AccuriTM C6 Flowcytometer (BD Bioscience, San Jose, CA, U.S.A.). Data were analyzed using the FlowJoX v10 software (FLOWJO, LLC, Ashland, OR, U.S.A.).
Xenograft models: NOD/scid mice (CLEA Japan, Tokyo, Japan) were maintained under pathogen-free conditions, and studies were conducted in accordance with the Yamaguchi University Animal Care and Use Committee (approval number 220). CLBL-1 cells (5 × 106 cells in 50 µl PBS) were implanted subcutaneously into the right hind limb of 7- to 8-week-old female mice under general anesthesia. When the tumor volume reached 100 mm3, as calculated from tumor width and length, echinomycin or DMSO was injected intraperitoneally every other day 5 times. Tumor size was measured every other day. When the tumor size exceeded 4,500 mm3, the mouse was euthanized with diethyl ether anesthesia. Statistical analysis was performed using the Student’s t-test. A P value <0.05 was considered statistically significant.
RESULTS
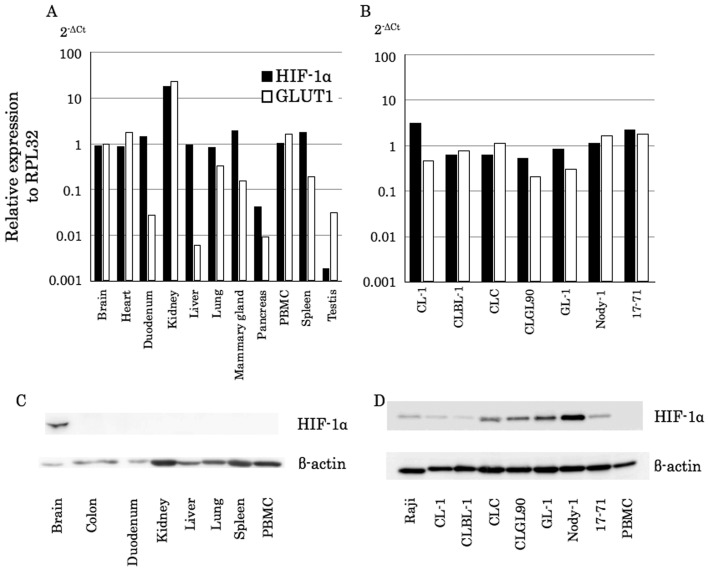
Canine HIF-1α gene and protein expressed in canine lymphoma cell lines under normoxic conditions: At first, we examined gene expression levels of canine HIF-1α (cHIF-1α) using normal tissue samples obtained from a healthy beagle dog and seven canine lymphoma cell lines incubated under normoxic conditions (21% O2), by real-time PCR. Although the expression levels varied in the tissues, the cHIF-1α gene was expressed in all canine tissues (Fig. 1A, black bars). On the other hand, one of the representative target genes of HIF-1α, GLUT1, was expressed without correlation to the expression pattern to HIF-1α (Fig. 1A, white bars). In contrast, all canine lymphoma cell lines expressed the cHIF-1α gene, and the expression levels of cHIF-1α were similar to those of the GLUT1 gene (Fig. 1B).
Fig. 1.
cHIF-1α mRNA and protein expression in canine normal tissues and lymphoma cell lines. (A and B) The cHIF-1α mRNA expression levels in healthy dog tissues (A) and canine lymphoma cell lines (B) were examined by real-time PCR. Expression levels of cHIF-1α and GLUT1 were calculated as ΔCt in comparison to the reference gene, RPL32. The 2-ΔCt values represent relative expression levels. (C and D) The cHIF-1α protein expression levels in healthy dog tissues (C) and the canine lymphoma cell lines (D) were examined by western blotting.
Next, we performed the immunoblotting for cHIF-1α to compare protein expression levels. While it has been reported that the adrenal, kidney, pancreas, spleen and tonsil tissues weakly express HIF-1α protein in human normal tissue samples [37], only brain tissue expressed cHIF-1α in dogs (Fig. 1C). On the other hand, all canine lymphoma cell lines demonstrated cHIF-1α expression (Fig. 1D).
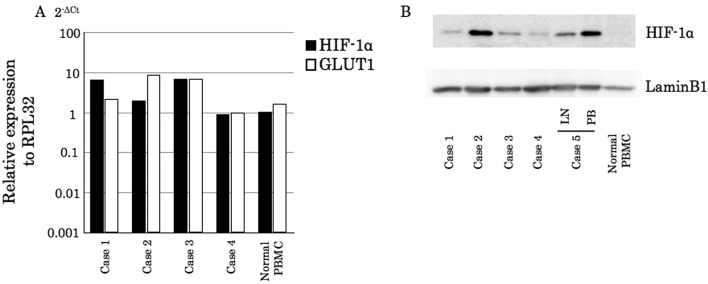
Canine lymphoma cells obtained from clinical cases also express cHIF-1α: To examine whether the protein expression of cHIF-1α is limited to the cell lines, we next investigated whether clinical samples obtained from canine lymphoma cases express the cHIF-1α gene and protein using real-time PCR and immunoblotting, respectively. The gene expression level was at a similar level in PBMCs isolated from the healthy dog (Fig. 2A). However, all tested lymphoma samples expressed the cHIF-1α protein (Fig. 2B). Therefore, the mRNA and cHIF-1α protein were expressed not only in the cell lines, but also in the primary cells obtained from the clinical cases.
Fig. 2.
Analysis of the cHIF-1α expression levels in clinical samples from canine lymphoma patients. (A) mRNA expression levels in canine lymphoma. The samples were obtained by fine needle aspiration from the affected lymph nodes. Expression levels of cHIF-1α and GLUT1 were calculated as ΔCt in comparison to the reference gene, RPL32. The 2-ΔCt values represent relative expression levels. (B) The cHIF-1α protein levels in canine lymphoma. In patient 5, the cancer cells were in multiple lymph nodes (LN) and the peripheral blood (PB). Normal PBMC, PBMCs obtained from a healthy beagle.
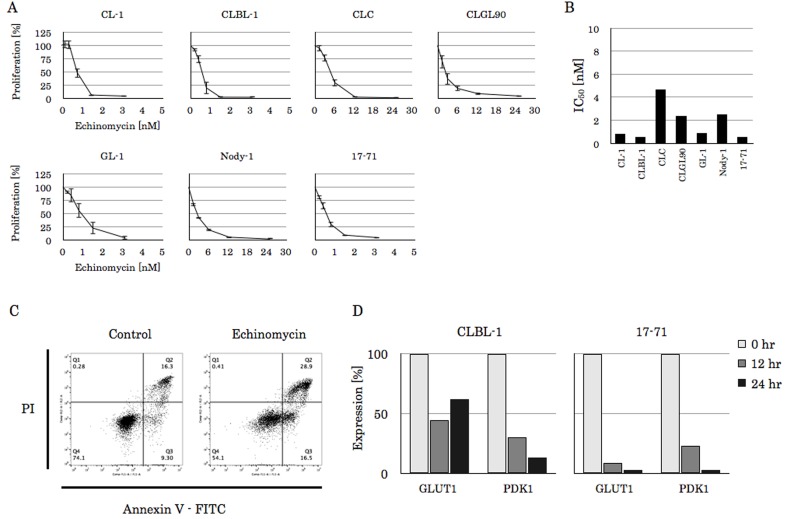
HIF-1α inhibitors showed a cytotoxic effect to canine lymphoma cell lines: To examine the effects of HIF-1α inhibition on canine lymphoma cells, the lymphoma cell lines were incubated with a HIF-1α inhibitor, echinomycin, and we performed the MTT assay to evaluate the proliferating capacity. Echinomycin treatment resulted in the inhibition of cell proliferation on all the lymphoma cell lines in a dose dependent manner (Fig. 3A). Notably, CL-1, CLBL-1, GL-1 and 17–71 cells, in which the IC50 were 0.80 nM, 0.54 nM, 0.88 nM and 0.52 nM, respectively, showed high sensitivity to echinomycin (Fig. 3B). When the Annexin V staining using a FACS analysis was performed, the Annexin V positive cells were 25.6% in the DMSO control and 45.4% in the echinomycin treated cells, respectively (Fig. 3C). Therefore, it is considered that this effect results from apoptosis. To confirm the inhibitory effect of echinomycin against HIF-1α, the expression of the downstream target genes, GLUT1 and PKD1, was examined by quantitative real-time PCR using the CLBL-1 and 17–71 cells, which showed lower IC50 to echinomycin than those of the others. As depicted in Fig. 3D, CLBL-1 and 17–71 cells incubated with 2 nM echinomycin for 12 to 24 hr exhibited suppressed GLUT1 and PDK1 gene expression.
Fig. 3.
The effect of echinomycin on canine lymphoma cell lines. (A) The proliferation rate of canine lymphoma cell lines in the absence or presence of echinomycin. Cell lines were incubated with each concentration of echinomycin for 48 hr at 37°C and subjected to MTT assay. The experiment was performed in triplicate, and data are the mean ± standard deviation (SD) of three independent experiments. (B) The echinomycin IC50 were calculated from (A). (C) The apoptosis rate in the presence of DMSO control or 2 nM echinomycin in CLBL-1 cell line. Cells were incubated with agents for 12 hr at 37°C and then stained with Annexin V-FITC. The fluorescence intensity was analyzed using flow cytometry. (D) The CLBL-1 and 17–71 cells were incubated with 2 nM echinomycin for 12 or 24 hr and subjected to real-time PCR to examine the expression levels of GLUT1 and PDK1, the downstream genes of HIF-1α. The relative expression levels were calculated as ΔCt from the difference between expression of the internal control, RPL32. Data are reported as expression rates compared to the initial point.
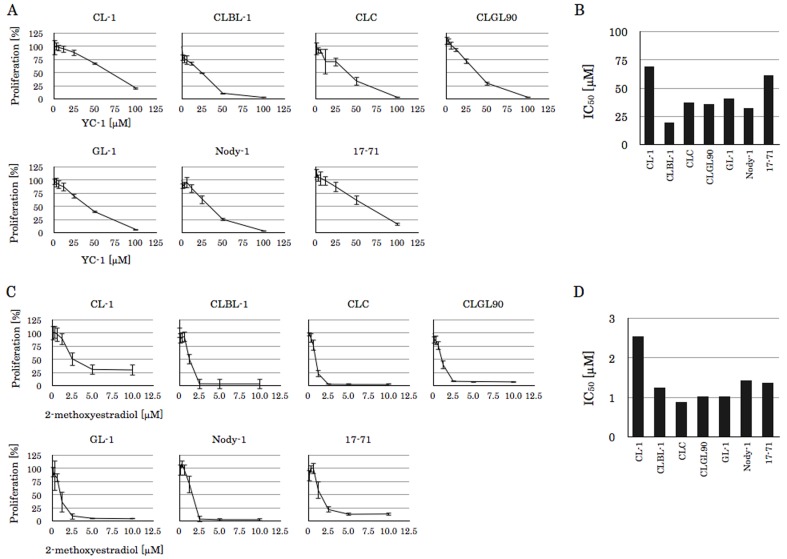
Other HIF-1α inhibitors, YC-1 and 2-methoxyestradiol, also inhibited lymphoma-cell proliferation; however, the sensitivity of this inhibition evaluated from the IC50 varied from the results of echinomycin treatment cells (Fig. 4). For example, 17–71 cells showed the lowest IC50 against echinomycin, but cell lines showing the lowest IC50 to YC-1 and 2-methoxyestradiol were CLBL-1 and CLC, respectively.
Fig. 4.
The effect of YC-1 and 2-methoxyestradiol on canine lymphoma cell lines. (A) The proliferation rate of canine lymphoma cell lines in the absence or presence of the agent. Cell lines were incubated with each concentration of YC-1 for 48 hr at 37°C and subjected to MTT assay, and data are the mean ± standard deviation (SD) of three independent experiments. (B) The YC-1 IC50 were calculated from (A). (C) The proliferation rate of canine lymphoma cell lines in the absence or presence of the agent. Cell lines were incubated with each concentration of 2-methoxyestradiol for 48 hr at 37°C and subjected to MTT assay, and data are the mean ± standard deviation (SD) of three independent experiments. (D) The 2-methoxyestradiol IC50 were calculated from (C).
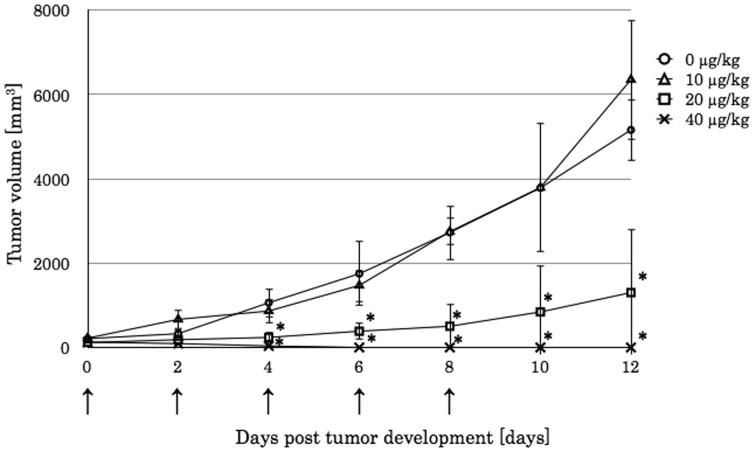
Engrafted lymphoma was regressed by echinomycin: Finally, we tested the effect of echinomycin in the mouse xenograft model using NOD/ShiJic-scidJcl mice, which lack not only functional B and T lymphocytes, but also have a lower activity of NK cells, complements and macrophages. The tumor volume reached 100 mm3 by days 8‒10 after the CLBL-1 cell line was inoculated. Compared to the DMSO control treatment, the echinomycin treatment did not affect the tumor at a dosage of 10 µg/kg for a total of 5 times. When the dosage was increased to 20 µg/kg 5 times every other day, the tumor volume of the xenografted canine lymphoma significantly decreased. Especially, five treatments of 40 µg/kg echinomycin resulted in complete tumor regression, after which the tumor was macroscopically undetectable (Fig. 5).
Fig. 5.
The engrafted CLBL-1 tumor size, which inoculated to NOD/scid mice. When the tumor size exceeded 100 mm3, it was considered as day 0. Any concentration of echinomycin or DMSO was injected intraperitoneally at days 0, 2, 4, 6 and 8 (arrow). The tumor size was measured every other day. Data represent the median and SD of each group (n=4). The * corresponds to a P<0.05 between the 20 or 40 µg/kg echinomycin treated groups and the untreated group.
DISCUSSION
In human medicine, reports that mention the importance of angiogenesis and metabolic changes in cancer metastasis or proliferation have increased [1, 15, 37]. Hypoxia is one of the most significant factors that promote angiogenesis or metabolic changes, and HIF-1 is a major regulator in the presence of low O2 levels [25]. However, there are few reports exploring the relationship between cancers and HIF in the veterinary field [17, 18, 22].
Canine lymphoma has a high recurrence rate, and thus, new treatments against this disease are desired. In this study, we showed that HIF-1α is expressed in the canine lymphoma cells, both in cell lines and clinical samples, and that its inhibition leads to suppression of cell proliferation both in vitro and in vivo.
The quantitative real-time PCR showed that the HIF-1α gene was expressed at a various levels in normal canine tissues, whereas the expression of HIF-1α in canine lymphoma cell lines was at similar levels (Fig. 1). However, HIF-1α is regulated by protein degradation, but not gene expression [26]; therefore, the analysis of protein levels is more important. Interestingly, all seven canine lymphoma cell lines included in this study expressed the HIF-1α protein at the 21% normoxic condition. These results suggest that canine lymphoma cells can use the HIF-1α protein, which induces tumor proliferation, survival and an anti-apoptotic effect like human cancer cells [31], even under normal oxygen concentrations through oxygen independent pathways. Previous studies using murine lymphoma models also supported our idea. In murine lymphoma, treatment with an HIF-1α inhibitor and HIF-1α knockdown resulted in the suppression of lymphoma cell proliferation in vitro and in vivo [32]. Furthermore, lymphocytes from HIF-1α transgenic mice exhibited prolonged survival duration and formed lymphoma [27].
As demonstrated in Fig. 2, all canine lymphoma clinical samples expressed HIF-1α, similar to the cell lines. The cHIF-1α expression data support the idea that HIF-1α has a role in cancer cell proliferation and/or survival in canine lymphoma. However, it is still unclear how canine HIF-1α is stabilized in canine lymphoma cells. In human cells, phosphorylation of the mammalian target of rapamycin (mTOR) and/or the p70 S6 kinase (S6K1) contributes to the oxygen independent stabilization of HIF-1 [9, 34]. Although we analyzed the phosphorylation of these 2 pathways and Akt by immunoblotting, the HIF-1α expression levels seem to be unrelated (data not shown).
The known drugs that have an inhibitory potential of HIF-1α are highly diverse, and there is no specific HIF-1α inhibitor [35]. Therefore, we used three HIF-1α inhibitors, echinomycin, YC-1 and 2-methoxyestradiol. These inhibitors have different mechanisms by which they inhibit HIF-1α transcriptional activity. It is reported that echinomycin binds to the hypoxia responsible element (HRE) on DNA [30] instead of HIF-1; moreover, YC-1 inhibits HIF-1 protein accumulation and promotes its degradation [36], and 2-methoxyestradiol inhibits HIF-1α translation and translocation into nucleus [16]. Here, we showed that these agents inhibited the proliferation of canine lymphoma cell lines in a dose-dependent manner (Figs. 3 and 4). These data suggest that the expression of HIF-1α in canine lymphoma cells is important for their proliferation and survival, and therefore, HIF-1α inhibitors may be potential agents for canine lymphoma treatment. In the Epm2a gene-targeted mouse lymphoma model, either echinomycin or HIF-1α shRNA suppressed the expression of the downstream genes of HIF-1α, such as GLUT1 and Hes1, and resulted in prolonged mouse survival [32]. Although we also found the HIF-1α inhibitors suppressed the proliferation of canine lymphoma cells, it is necessary to confirm the specificity of their HIF-1α inhibition in this experiment. However, because of a technical issue that genes are not easily introduced into canine lymphoma cells by retrovirus or lipofection, we could not successfully silence HIF-1α expression. When a new method that can induce expression of exogenous genes in canine lymphoma cells is developed, we will try the gene silencing of HIF-1α in the future.
We show that echinomycin has the same robust effect on canine lymphoma cells as in the mouse xenograft model (Fig. 5). Past studies using the Epm2a gene-targeted mice lymphoma and human leukemia cells reported that 5 injections of 10 µg/kg echinomycin could treat those tumors [32]. On the other hand, this dose had no effect on canine lymphoma in our models. Although the mice strain used in this study is different from the previous report, our result suggests that canine lymphoma cells have a higher resistance potential. Since the dose increment leads to tumor regression, we should administer echinomycin at a high dosage or in combination with other chemotherapy if using this drug in clinics.
In this study, we revealed that the HIF-1α protein is ubiquitously expressed in canine lymphoma cell lines and clinical samples. Because these cell lines were incubated at normoxic conditions, it was suggested that HIF-1α is stabilized on oxygen independent pathways. Moreover, three different types of HIF-1α inhibitors suppressed the cell proliferation of canine lymphoma cell lines. Whether this effect was specific to the HIF-1α inhibition is still unknown; it is necessary to perform gene silencing of the HIF-1α gene. However, we guess echinomycin, which showed an anti-lymphoma effect in vivo, can be used as an anti-cancer agent clinically, although its side effects to dogs should be explored before the use. This study is the first report to demonstrate a relationship between HIF-1α and canine lymphoma. Although additional research is needed, the HIF-1α inhibitors may be potential agents to treat the canine lymphoma.
Acknowledgments
We would like to acknowledge the technical expertise of the DNA Core Facility of the Center for Gene Research, Yamaguchi University. This study was supported by JSPS KAKENHI Grant Number 15K07744.
REFERENCES
- 1.Chen J.Q., Russo J.2012. Dysregulation of glucose transport, glycolysis, TCA cycle and glutaminolysis by oncogenes and tumor suppressors in cancer cells. Biochim. Biophys. Acta 1826: 370–384. [DOI] [PubMed] [Google Scholar]
- 2.Deeb G., Vaughan M. M., McInnis I., Ford L. A., Sait S. N. J., Starostik P., Wetzler M., Mashtare T., Wang E. S.2011. Hypoxia-inducible factor-1α protein expression is associated with poor survival in normal karyotype adult acute myeloid leukemia. Leuk. Res. 35: 579–584. doi: 10.1016/j.leukres.2010.10.020 [DOI] [PubMed] [Google Scholar]
- 3.Epstein A. C. R., Gleadle J. M., McNeill L. A., Hewitson K. S., O’Rourke J., Mole D. R., Mukherji M., Metzen E., Wilson M. I., Dhanda A., Tian Y. M., Masson N., Hamilton D. L., Jaakkola P., Barstead R., Hodgkin J., Maxwell P. H., Pugh C. W., Schofield C. J., Ratcliffe P. J.2001. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107: 43–54. doi: 10.1016/S0092-8674(01)00507-4 [DOI] [PubMed] [Google Scholar]
- 4.Evens A. M., Sehn L. H., Farinha P., Nelson B. P., Raji A., Lu Y., Brakman A., Parimi V., Winter J. N., Schumacker P. T., Gascoyne R. D., Gordon L. I.2010. Hypoxia-inducible factor-1 expression predicts superior survival in patients with diffuse large B-cell lymphoma treated with R-CHOP. J. Clin. Oncol. 28: 1017–1024. doi: 10.1200/JCO.2009.24.1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garrett L. D., Thamm D. H., Chun R., Dudley R., Vail D. M.2002. Evaluation of a 6-month chemotherapy protocol with no maintenance therapy for dogs with lymphoma. J. Vet. Intern. Med. 16: 704–709. doi: 10.1111/j.1939-1676.2002.tb02411.x [DOI] [PubMed] [Google Scholar]
- 6.Gleadle J. M., Ratcliffe P. J.1997. Induction of hypoxia-inducible factor-1, erythropoietin, vascular endothelial growth factor, and glucose transporter-1 by hypoxia: evidence against a regulatory role for Src kinase. Blood 89: 503–509. [PubMed] [Google Scholar]
- 7.Hickey M. M., Simon M. C.2006. Regulation of angiogenesis by hypoxia and hypoxia-inducible factors. Curr. Top. Dev. Biol. 76: 217–257. [DOI] [PubMed] [Google Scholar]
- 8.Huang D., Li C., Zhang H.2014. Hypoxia and cancer cell metabolism. Acta Biochim. Biophys. Sin. (Shanghai) 46: 214–219. doi: 10.1093/abbs/gmt148 [DOI] [PubMed] [Google Scholar]
- 9.Jiang B. H., Jiang G., Zheng J. Z., Lu Z., Hunter T., Vogt P. K.2001. Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ. 12: 363–369. [PubMed] [Google Scholar]
- 10.Jiang B.H., Rue E., Wang G. L., Roe R., Semenza G. L.1996. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J. Biol. Chem. 271: 17771–17778. doi: 10.1074/jbc.271.30.17771 [DOI] [PubMed] [Google Scholar]
- 11.Kaelin W. G., Ratcliffe P. J.2008. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30: 393–402. doi: 10.1016/j.molcel.2008.04.009 [DOI] [PubMed] [Google Scholar]
- 12.Keith B., Simon M. C.2007. Hypoxia-inducible factors, stem cells, and cancer. Cell 129: 465–472. doi: 10.1016/j.cell.2007.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keller E. T., MacEwen E. G., Rosenthal R. C., Helfand S. C., Fox L. E.1993. Evaluation of prognostic factors and sequential combination chemotherapy with doxorubicin for canine lymphoma. J. Vet. Intern. Med. 7: 289–295. doi: 10.1111/j.1939-1676.1993.tb01021.x [DOI] [PubMed] [Google Scholar]
- 14.Liao D., Corle C., Seagroves T. N., Johnson R. S.2007. Hypoxia-inducible factor-1alpha is a key regulator of metastasis in a transgenic model of cancer initiation and progression. Cancer Res. 67: 563–572. doi: 10.1158/0008-5472.CAN-06-2701 [DOI] [PubMed] [Google Scholar]
- 15.Liao D., Johnson R. S.2007. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 26: 281–290. doi: 10.1007/s10555-007-9066-y [DOI] [PubMed] [Google Scholar]
- 16.Mabjeesh N. J., Escuin D., LaVallee T. M., Pribluda V. S., Swartz G. M., Johnson M. S., Willard M. T., Zhong H., Simons J. W., Giannakakou P.2003. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell 3: 363–375. doi: 10.1016/S1535-6108(03)00077-1 [DOI] [PubMed] [Google Scholar]
- 17.Madej J. A., Madej J. P., Dziegiel P., Pula B., Nowak M.2013. Expression of hypoxia-inducible factor-1α and vascular density in mammary adenomas and adenocarcinomas in bitches. Acta Vet. Scand. 55: 73. doi: 10.1186/1751-0147-55-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mees G., Vangestel C., Dierckx R., Loomans S., Van Damme N., Peremans K., De Rooster H., Van Goethem B., Pauwels P., Ducatelle R., Van de Wiele C.2011. Metabolic correlates of tumour hypoxia in malignant canine mammary carcinoma. Res. Vet. Sci. 91: e125–e128. doi: 10.1016/j.rvsc.2011.01.014 [DOI] [PubMed] [Google Scholar]
- 19.Momoi Y., Okai Y., Watari T., Goitsuka R., Tsujimoto H., Hasegawa A.1997. Establishment and characterization of a canine T-lymphoblastoid cell line derived from malignant lymphoma. Vet. Immunol. Immunopathol. 59: 11–20. doi: 10.1016/S0165-2427(97)00053-6 [DOI] [PubMed] [Google Scholar]
- 20.Nakaichi M., Taura Y., Kanki M., Mamba K., Momoi Y., Tsujimoto H., Nakama S.1996. Establishment and characterization of a new canine B-cell leukemia cell line. J. Vet. Med. Sci. 58: 469–471. doi: 10.1292/jvms.58.469 [DOI] [PubMed] [Google Scholar]
- 21.Peters I. R., Peeters D., Helps C. R., Day M. J.2007. Development and application of multiple internal reference (housekeeper) gene assays for accurate normalisation of canine gene expression studies. Vet. Immunol. Immunopathol. 117: 55–66. doi: 10.1016/j.vetimm.2007.01.011 [DOI] [PubMed] [Google Scholar]
- 22.Petty J. C., Lana S. E., Thamm D. H., Charles J. B., Bachand A. M., Bush J. M., Ehrhart E. J.2008. Glucose transporter 1 expression in canine osteosarcoma. Vet. Comp. Oncol. 6: 133–140. doi: 10.1111/j.1476-5829.2007.00155.x [DOI] [PubMed] [Google Scholar]
- 23.Rosales C., Jeglum K. A., Obrocka M., Steplewski Z.1988. Cytolytic activity of murine anti-dog lymphoma monoclonal antibodies with canine effector cells and complement. Cell. Immunol. 115: 420–428. doi: 10.1016/0008-8749(88)90194-3 [DOI] [PubMed] [Google Scholar]
- 24.Rütgen B. C., Hammer S. E., Gerner W., Christian M., de Arespacochaga A. G., Willmann M., Kleiter M., Schwendenwein I., Saalmüller A.2010. Establishment and characterization of a novel canine B-cell line derived from a spontaneously occurring diffuse large cell lymphoma. Leuk. Res. 34: 932–938. doi: 10.1016/j.leukres.2010.01.021 [DOI] [PubMed] [Google Scholar]
- 25.Semenza G. L.1998. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr. Opin. Genet. Dev. 8: 588–594. doi: 10.1016/S0959-437X(98)80016-6 [DOI] [PubMed] [Google Scholar]
- 26.Semenza G. L.1999. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu. Rev. Cell Dev. Biol. 15: 551–578. doi: 10.1146/annurev.cellbio.15.1.551 [DOI] [PubMed] [Google Scholar]
- 27.Sueoka E., Sueoka-Aragane N., Sato A., Ide M., Nakamura H., Sotomaru Y., Taya C., Yonekawa H., Kitagawa T., Kubota Y., Kimura S., Nakachi K., Tanimoto K.2013. Development of lymphoproliferative diseases by hypoxia inducible factor-1alpha is associated with prolonged lymphocyte survival. PLoS ONE 8: e57833. doi: 10.1371/journal.pone.0057833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suter S. E., Chein M. B., von Messling V., Yip B., Cattaneo R., Vernau W., Madewell B. R., London C. A.2005. In vitro canine distemper virus infection of canine lymphoid cells: a prelude to oncolytic therapy for lymphoma. Clin. Cancer Res. 11: 1579–1587. doi: 10.1158/1078-0432.CCR-04-1944 [DOI] [PubMed] [Google Scholar]
- 29.Umeki S., Ema Y., Suzuki R., Kubo M., Hayashi T., Okamura Y., Yamazaki J., Tsujimoto H., Tani K., Hiraoka H., Okuda M., Mizuno T.2013. Establishment of five canine lymphoma cell lines and tumor formation in a xenotransplantation model. J. Vet. Med. Sci. 75: 467–474. doi: 10.1292/jvms.12-0448 [DOI] [PubMed] [Google Scholar]
- 30.Van Dyke M. M., Dervan P. B.1984. Echinomycin binding sites on DNA. Science 225: 1122–1127. doi: 10.1126/science.6089341 [DOI] [PubMed] [Google Scholar]
- 31.Vaupel P., Mayer A.2007. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 26: 225–239. doi: 10.1007/s10555-007-9055-1 [DOI] [PubMed] [Google Scholar]
- 32.Wang Y., Liu Y., Malek S. N., Zheng P., Liu Y.2011. Targeting HIF1α eliminates cancer stem cells in hematological malignancies. Cell Stem Cell 8: 399–411. doi: 10.1016/j.stem.2011.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wellmann S., Guschmann M., Griethe W., Eckert C., Stackelberg A., Lottaz C., Moderegger E., Einsiedel H. G., Eckardt K.U., Henze G., Seeger K.2004. Activation of the HIF pathway in childhood ALL, prognostic implications of VEGF. Leukemia 18: 926–933. doi: 10.1038/sj.leu.2403332 [DOI] [PubMed] [Google Scholar]
- 34.Wouters B. G., Koritzinsky M.2008. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat. Rev. Cancer 8: 851–864. doi: 10.1038/nrc2501 [DOI] [PubMed] [Google Scholar]
- 35.Xia Y., Choi H.K., Lee K.2012. Recent advances in hypoxia-inducible factor (HIF)-1 inhibitors. Eur. J. Med. Chem. 49: 24–40. doi: 10.1016/j.ejmech.2012.01.033 [DOI] [PubMed] [Google Scholar]
- 36.Yeo E.J., Chun Y.S., Park J.W.2004. New anticancer strategies targeting HIF-1. Biochem. Pharmacol. 68: 1061–1069. doi: 10.1016/j.bcp.2004.02.040 [DOI] [PubMed] [Google Scholar]
- 37.Zhong H., De Marzo A. M., Laughner E., Lim M., Hilton D. A., Zagzag D., Buechler P., Isaacs W. B., Semenza G. L., Simons J. W.1999. Overexpression of hypoxia-inducible factor 1 alpha in common human cancers and their metastases. Cancer Res. 59: 5830–5835. [PubMed] [Google Scholar]