Abstract
Inhibins, as members of the transforming growth factor beta (TGF-β) superfamily, downregulate the synthesis and secretion of follicle-stimulating hormone (FSH) in an endocrine manner. The role of inhibin/betaglycan in the ovary regulation recently gained attention. To date, no data exist on the function of inhibin α subunit and betaglycan in cystic follicles. In this study, the expressions of inhibin α subunit and betaglycan in cystic follicles were investigated using immunohistochemistry, real-time PCR and Western blot analysis. Both inhibin α subunit and betaglycan immunoreactivities were mainly localized in the granulosa cells of follicles. Expression of inhibin α subunit and betaglycan was inferior in cystic follicles compared with that in normal large follicles. However, the result of enzyme-linked immunosorbent assay showed no significant difference in the decreasing in concentration of inhibin α subunit in cystic follicular fluid compared with the control (P>0.05). In this study, we explored the effects of FSH on betaglycan expression in granulosa cells in vitro. As expected, a significant increase in the expressions of betaglycan mRNA and protein in granulosa cells was observed in response to exogenous FSH (30 ng/ml) (P<0.05) compared with the control. Consequently, this study provides evidence that the expressions of inhibin α subunit and betaglycan are inferior in cystic follicles, and this may be caused by the decrease in FSH in the presence of a cystic follicle.
Keywords: betaglycan, FSH, inhibin α subunit, porcine cystic follicles
Cystic ovarian disease (COD) is a significant cause of infertility in female domestic animals. The most common type of COD is the functional cyst, which falls into 2 categories: follicular cysts and corpus luteum cysts. Although the majority of porcine ovarian follicular cysts regress spontaneously and are clinically unapparent, they can affect farrowing rates and litter size [29].
Inhibins are a family of growth factors, with α subunit as the functional center. Inhibin α subunit joins either the beta A or beta B subunit to form inhibin A and B, respectively. Inhibin is produced in the ovaries and testis. The major role of inhibin is inhibition of production of follicle-stimulating hormone (FSH) and gonadotropin-releasing hormone (GnRH) from the pituitary gland and hypothalamus, respectively [28]; inhibin also affects follicle development. Recently, our results showed that inhibin not only acts in an endocrine manner as mentioned above but also acts in an autocrine or paracrine manner in the development of ovarian follicles [30]. Inhibin A in theca cells can increase the expression of 3-beta-hydroxysteroid dehydrogenase (3β-HSD) [10], and 3β-HSD expression increased in granulosa cell cystic follicles compared with in normal large follicles [24]. Although the expression of 3β-HSD increased in CF compared with that in the normal large follicles, the distribution of 3β-HSD in follicles was different. The frequencies of 3β-HSD-positive granulosa cells in cystic follicles were significantly higher than those in the healthy follicles. However, the frequencies of 3β-HSD-positive theca cells in CF were decreased [5]. The different levels and distributions of 3β-HSD in the granulosa and theca interna layers between cystic and normal follicles may be one of the reasons why follicles fail to ovulate.
Recent studies suggest that betaglycan, known as transforming growth factor beta receptor III, is expressed in male and female reproductive tracts, and inhibin/betaglycan can potentially play an important role in local autocrine and paracrine regulation in the ovary [1, 17]. However, no detailed data are available concerning the expression of inhibin and betaglycan in normal follicles and cystic follicles, and the contributions of the local actions of inhibin/betaglycan in the ovary to regulation of the cystic processes of follicles are unclear. Recent studies show that betaglycan expression in rat granulosa cells is regulated by FSH [2], and the FSH concentration in serum was shown to decrease if follicular cysts are present in the porcine ovary [24]. Therefore, the possible role of the decrease in FSH in the occurrence of follicular cysts is worth studying.
The aim of this study was to investigate the expression of inhibin α and betaglycan in normal and cystic follicles and explore the effects of FSH on the expression of betaglycan in porcine granulosa cells. A study of the effect of the inhibin/betaglycan system on regulation of the follicular cystic process will lead to better understanding of and therapies for follicular cysts.
MATERIALS AND METHODS
Animals and sample preparation: Porcine ovaries with or without cystic follicles were obtained from a slaughterhouse and collected into a bottle filled with 37°C saline and transported to the laboratory within 20 min. In this study, follicles exceeding 21 mm in diameter were regarded as follicular cysts [15], and follicles between 8 mm to 10 mm were regarded as normal large follicles. For each group, ovarian samples were collected from five animals (n=5).
Immunohistochemistry: Formalin-fixed ovaries were dehydrated in a graded series of ethanol, embedded in paraffin wax and sectioned into 4-µm sections. After deparaffinization with xylene and rehydration in graded ethanol, the tissue sections were subjected to antigen retrieval by autoclaving in 0.01 M sodium citrate buffer (pH 6.0) at 121°C for 10 min. After washing in PBS, the sections were incubated with 3% BSA/PBS for 30 min to block nonspecific immunoglobulin binding. Then, the sections were incubated overnight at 4°C with rabbit anti-mouse polyclonal antibodies directed against inhibin α or betaglycan (Bioss, Beijing, P.R. China) (diluted 1:200), respectively, and rinsed in PBST for 5 min three times. After incubation with specific antibodies, sections were washed and treated with biotinylated goat anti-rabbit IgG/HRP (Bioss) for 15 min at 37°C. These sections were subsequently stained with diaminobenzidine (DAB, Maixin Biotechnology Development Co., Fuzhou, Fujian, P.R. China) at room temperature until the desired color development was achieved. A brown color indicated positive staining.
Enzyme-linked immunosorbent assay (ELISA): Follicular fluid was obtained from normal large follicles and cystic follicles by centrifugation of samples for 10 min at 1,500 × g. The supernatants were collected and frozen in tubes at −80°C until used. The concentration of inhibin α subunit in the supernatant of follicular fluid was analyzed using ELISA according to the protocol suggested by the manufacturer (CUSABIO Biotech, Hubei, P.R. China).
Cell culture and treatment: Porcine granulosa cells were isolated as previously described [27]. Follicular aspirates from 3–6 mm follicles were centrifuged at 250 × g for 6 min, and cell aggregates were washed three times with Hank’s Balanced Salt Solution (HBSS; centrifuged at 250 × g for 6 min). The viability of granulosa cells was examined by staining with trypan blue dye (over 70%) before cell culture. Granulosa cells suspended in DMEM/F12 (Invitrogen New Zealand limited, Auckland, New Zealand) containing 10% fetal calf serum (Invitrogen Life Technologies Corporation, Carlsbad, CA, U.S.A.) were seeded in 6-well plates and preincubated for 48 hr at 37°C in a humidified 5% CO2 incubator. After preincubation, the medium was changed, and the granulosa cells were cultured with 30 ng/ml FSH (Ningbo Sansheng Pharmaceutical, Ningbo, P.R. China) in 2 ml DMEM/F12 supplemented with 2 mM GlutaMAX™-1 (Invitrogen New Zealand limited), 20 µl Insulin-Transferrin-Selenium-Supplement (100 ×) (Invitrogen Life Technologies Corporation), 100 IU/ml penicillin and 0.1 mg/ml streptomycin. The granulosa cells were then incubated at 38.5°C in a humidified atmosphere with 5% CO2. After treatment for 0, 24 or 48 hr, the culture medium was discarded, and the cells were rinsed with cold PBS. To prepare cell lysates for quantitative real-time PCR or Western blot analysis of betaglycan, granulosa cells were lysed in 300 µl TRIzol reagent (Invitrogen Life Technologies Corporation) or 100 µl cell lysis buffer (Beyotime Institute of Biotechnology, Jiangsu, P.R. China), respectively. Cell lysates were stored at −80°C. Total RNA and protein were isolated within 6 hr.
RNA isolation and reverse transcription: Upon arrival at the laboratory, the granulosa cells were scraped from the follicular walls of porcine ovaries with or without cystic follicles [23]. After washing twice with PBS, total RNA from about 0.5 × 106 granulosa cells was extracted using 1 ml TRIzol reagent (Invitrogen, Life Technologies Corporation) according to the manufacturer’s instructions. The RNA concentration of each sample was measured using a NanoDrop 2000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, U.S.A.). The ratio of absorbance at the wavelength of 280 and 260 nm was between 1.8 and 2.0. Reverse transcription of RNA was performed with a commercial kit (Promega Corporation., Madison, WI, U.S.A.), and cDNA was stored in −80°C until use.
Quantitative real-time PCR analysis: The specific primers used for amplifying gene-encoding inhibin A (INHA), betaglycan and beta-actin (β-actin) are shown in Table 1. The quantitative PCR reactions were performed with an Eppendorf Mastercycler ep realplex real-time PCR system using FastStart Universal SYBR Green Master (ROX). Amplification reactions were performed in a mixture with a final volume of 25 µl containing 25 ng cDNA (2.5 µl), 12.5 µl ROX, 0.75 µl forward primer (10 µM), 0.75 µl reverse primer (10 µM) and 8.5 µl nuclease-free water. The cycling conditions for INHA and betaglycan were 95°C for 5 min for denaturing, followed by 40 cycles of 94°C for 15 sec, 59°C for 15 sec and 72°C for 20 sec. Results were normalized against the expression of the internal housekeeping gene β-actin. Results of real-time PCR were analyzed using the 2−ΔΔCT method [12] to compare the relative transcription levels of the target genes in each sample.
Table 1. Primers used for quantitative PCR for the detection of INHA, betaglycan and β-actin.
| Gene | Primer | Sequence (5′-3′) | PCR fragment size (bp) | Reference sequence |
|---|---|---|---|---|
| INHA | Forward | CCAGGCCATCCTTTTCCCGGCTA | 180 | DQ_356013 |
| Reverse | CCTGTCTGTCCAGTCCCGTGT | |||
| betaglycan | Forward | CTCGAACCCCTACAGTGCTT | 298 | NM_214272.1 |
| Reverse | ATGTTACTGGACTGTAGCCAT | |||
| β-actin | Forward | CTCCCTGGATGAAGAGCTACGAG | 157 | DQ452569.1 |
| Reverse | TCGCACTTCATGATGGAGTTGA |
Western blot analysis: About 1 × 106 granulosa cells scraped from follicular walls were lysed in 200 µl cell lysis buffer supplemented with 1 mM PMSF (Beyotime Institute of Biotechnology, Jiangsu, P.R. China). After centrifugation at 13,000 × rpm at 4°C for 5 min, the supernatant was collected, and the concentration of protein was determined using bicinchoninic acid (BCA) protein assay kits (Beyotime Institute of Biotechnology). Normalized 30 µg proteins from each sample were separated by 12% SDS-PAGE and subsequently transferred onto PVDF membranes (EMD Millipore, Billerica, MA, U.S.A.) at 80 V for 1.5 hr (Bio-Rad wet transfer system). After 2 hr of blocking with TBST containing 5% nonfat milk, the membranes were incubated with rabbit anti-mouse polyclonal antibodies specific to inhibin α, betaglycan (Bioss), and β-actin (Boster Inc., Wuhan, P.R. China) (diluted 1:200 in TBST) at 4°C overnight. The membranes were washed with TBST (3 × 5 min) and incubated for 1 hr with HRP-conjugated goat anti-rabbit secondary antibody (diluted 1:1,000 in TBST). They were then washed several times with TBST, and blots were visualized with a BeyoECL Plus kit (Beyotime Institute of Biotechnology) in accordance with the manufacturer’s protocols. All blots were exposed to the X-ray film for 30 sec. Each experiment was performed three times.
Statistical analysis: Immunohistochemistry, Western blot analysis, quantitative real-time PCR and FSH treatment experiments were repeated three times. All data are presented as means and standard errors. Statistical analysis was performed using one-way ANOVA (as implemented in the SPSS 13.0. software) followed by Dunnett’s multiple range test. Differences with a probability of P<0.05 were considered significant.
RESULTS
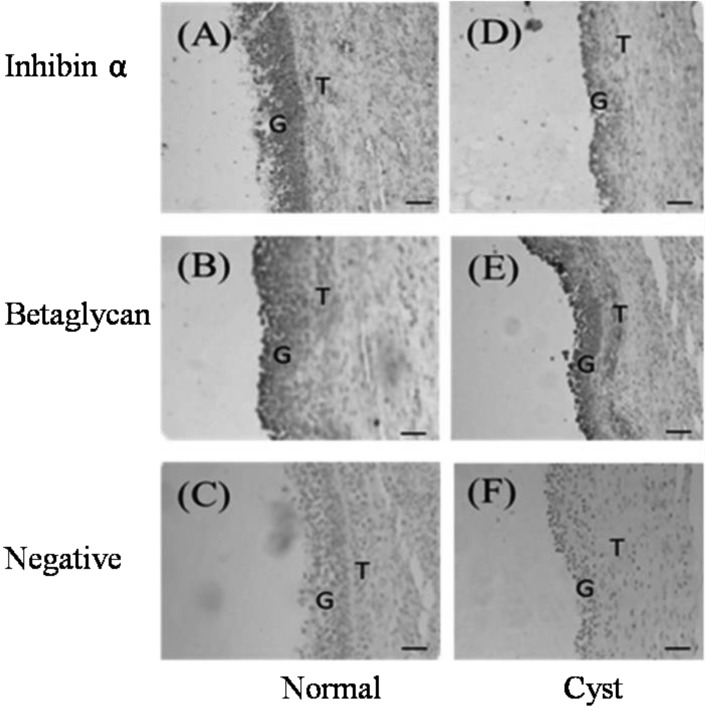
Localization of inhibin α and betaglycan: The localization of inhibin α and betaglycan proteins in both normal large and cystic follicles was investigated by immunohistochemistry with rabbit anti-mouse inhibin α and betaglycan polyclonal antibodies. Betaglycan was detected in granulosa and theca cells in both normal and cystic follicles, but the immunoreactivity of inhibin α subunit was detected in granulosa cells only (Fig. 1). No immunostaining was observed in negative controls in which the antibody was replaced with normal goat serum (Fig. 1).
Fig. 1.
Immunohistochemical localization of inhibin α and betaglycan in porcine follicles (original magnification × 100). Brown indicates the presence of the specified protein. (A–C) Normal large follicles: anti-inhibin α IgG (A), anti-betaglycan IgG (B) and negative control (C). (D–F) Cystic follicles: anti-inhibin α IgG (D), anti-betaglycan IgG (E) and negative control (F). G indicates granulosa cells, and T indicates theca cells.
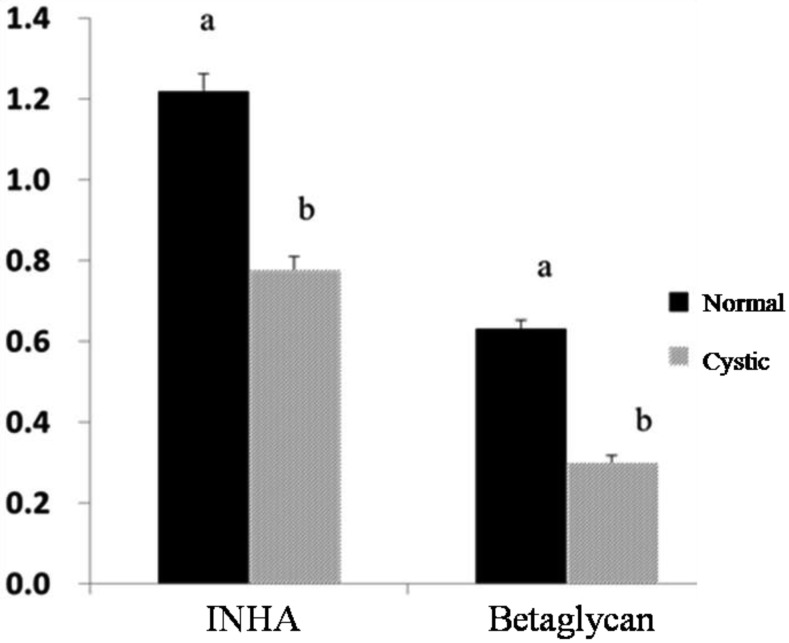
Quantification of INHA and betaglycan mRNAs: The quantitative real-time PCR analysis of INHA and betaglycan mRNAs in normal and cystic follicles is presented in Fig. 2. The expressions of INHA and betaglycan mRNAs in granulosa cells from normal large follicles were significantly higher than those in cystic follicles (P<0.05) (Fig. 2).
Fig. 2.
Analysis of INHA and betaglycan mRNA in cystic follicles (grey) and normal large follicles (dark). The relative mRNA levels of INHA and betaglycan presented in the figure were corrected to the level of β-actin gene mRNA. Bars indicate the mean ± SEM inhibin α and betaglycan intensities relative to β-actin protein. Different letters above bars indicate statistically significant differences (P<0.05).
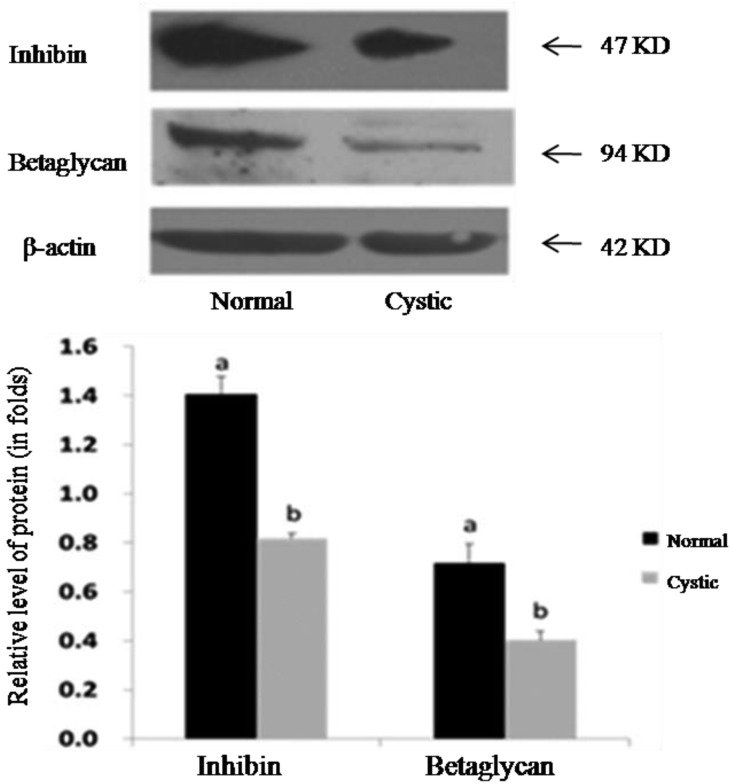
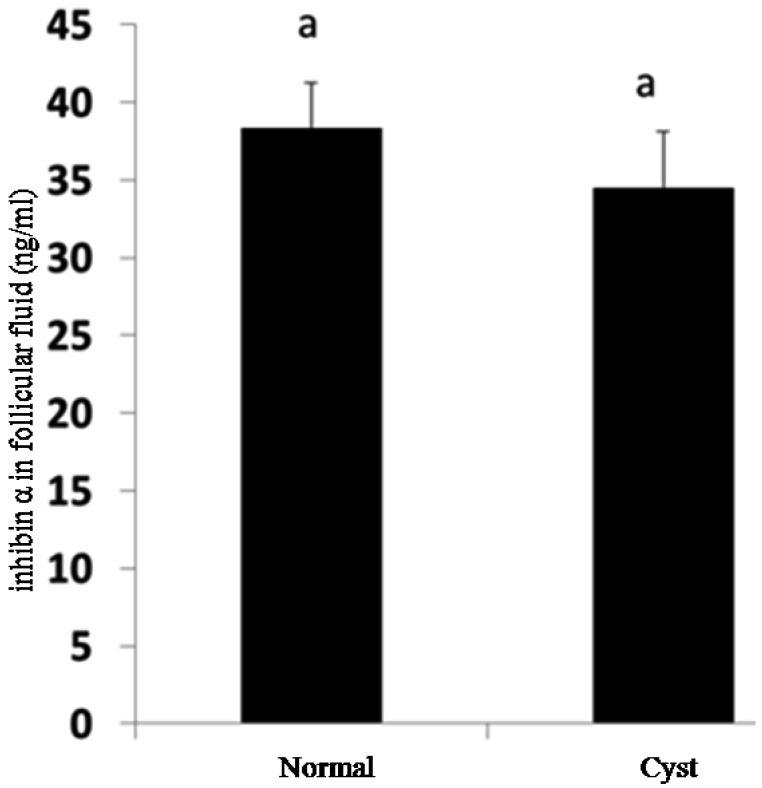
Detection of inhibin α and betaglycan proteins: Inhibin α and betaglycan proteins in granulosa cells from normal and cystic follicles were evaluated using Western blot analysis. The results show that a significant decrease in inhibin α was observed in granulosa cells from samples with cystic follicles compared with those from samples with normal large follicles (P<0.05) (Fig. 3). The concentration of inhibin α subunit in follicular fluid from normal large follicles and cystic follicles was measured using ELISA analysis. Although the level of inhibin α decreased in cystic follicles compared with that in normal large follicles, no significant difference was observed (P>0.05) (Fig. 4).
Fig. 3.
Detection of INHA and betaglycan in cystic follicles and normal large follicles. (A) Representative photographs of Western blotting for inhibin α, betaglycan and β-actin (as an internal control). (B) Western blotting analysis showing inhibin α and betaglycan compared with β-actin protein. Bars indicate the mean±SEM inhibin α and betaglycan intensities relative to β-actin protein. Different letters above bars indicate statistically significant differences (P<0.05).
Fig. 4.
Concentration of INHA in follicular fluid measured by ELISA. There was no significant difference between samples from cystic follicles and normal large follicles (P>0.05). Different letters above bars indicate statistically significant differences (P<0.05).
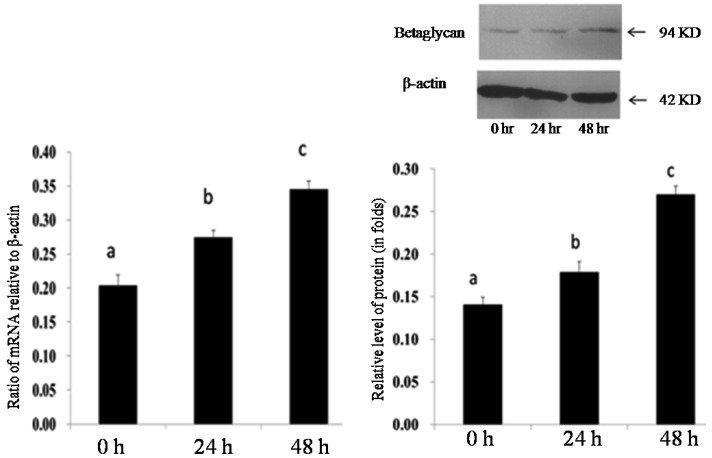
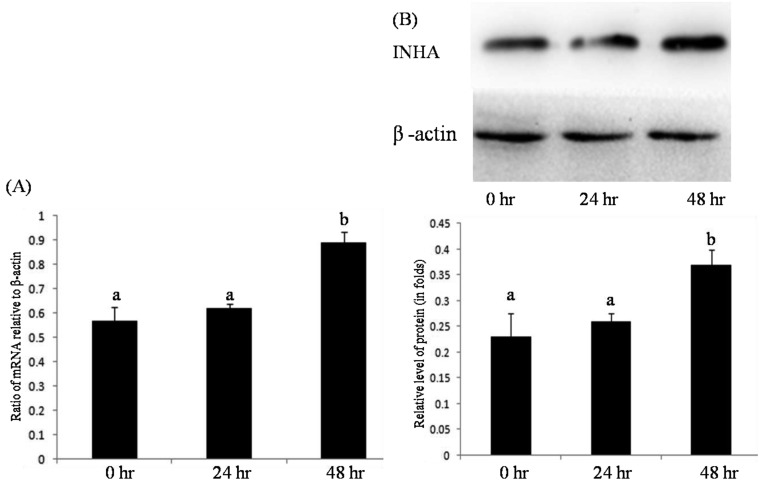
Effect of FSH on expression of INHA and betaglycan in granulosa cells: The effects of FSH on the expression of INHA and betaglycan mRNA and on protein in cultured granulosa cells were investigated using quantitative real-time PCR and Western blot analysis, respectively. The results indicated that the levels of both mRNA and protein of INHA and betaglycan in granulosa cells treated with FSH (30 ng/ml) were significantly higher than those in the control (P<0.05) (Figs. 5 and 6).
Fig. 5.
Effect of FSH on expression of betaglycan in granulosa cells in vitro. Relative folds of betaglycan mRNA (A) and protein (B) in cultured granulosa cells after 0 hr, 24 hr and 48 hr treatments with 30 ng/ml FSH. Bars indicate the mean ± SEM. Different letters above bars indicate statistically significant differences (P<0.05).
Fig. 6.
Effect of FSH on expression of INHA in granulosa cells in vitro. Relative folds of inhibin α mRNA (A) and protein (B) in cultured granulosa cells after 0 hr, 24 hr and 48 hr treatments with 30 ng/ml FSH. Bars indicate the mean ± SEM. Different letters above bars indicate statistically significant differences (P<0.05).
DISCUSSION
Follicular cyst is a common ovarian disease characterized by the presence of large ovarian follicular structures in the absence of a corpus luteum and ovarian cyclicity [20]. However, the pathogenesis of porcine follicular cysts is still unclear. Endocrine, autocrine and paracrine factors are involved in the regulation of follicular development and ovulation: reproductive hormones secreted by the hypothalamus-pituitary-ovarian axis are involved in endocrine regulation, and growth factors are involved in autocrine and paracrine regulation [18]. The precise cooperation of hormones and growth factors results in normal function of follicle development, follicular maturation and ovulation. Disruption of regulation can lead to follicular development disorder [13]. Follicular cysts in pigs may be caused by lack of an LH surge [8]. The ovary, which is a target organ for many hormones and growth factors, plays a crucial role in maintenance of the hypothalamus-pituitary-ovary axis and the endocrine system. Blockage of the LH surge and growth factors secreted by the ovary contribute significantly to the regulation of follicular cyst onset [20, 21].
Previous studies show that significant differences exist in the apoptosis and proliferation of follicular cells between normal and cystic porcine follicles [26]. These findings indicate that abnormal expression of apoptosis-related or anti-apoptotic factors may be responsible for the occurrence and persistence of porcine cystic follicles.
The TGF-β superfamily consists of a large number of structurally related polypeptides [14], which include TGFβs, growth and differentiation factors, bone morphogenetic proteins, activins and inhibins [19]. Inhibin produced by the gonads is first isolated from follicular fluid [9], which can regulate the development of follicles by preventing the production of GnRH and FSH in the hypothalamus and pituitary gland [28].
Betaglycan, the receptor of inhibin, is expressed in female reproductive tracts [1, 3, 17], indicating that inhibin can regulate the development of reproductive tissues through an autocrine or paracrine pattern [7]. Therefore, studying the function of inhibins within the ovary is essential in revealing the mechanism of follicular cysts. This study describes for the first time the expression of inhibin α subunit and betaglycan in normal large and cystic follicles. We demonstrated that betaglycan proteins were localized in porcine granulosa and thecal cells (Fig. 1), which is similar to the rat ovary [2].
3β-HSD is essential for the biosynthesis of mineralcorticoid, glucocorticoid and reproductive steroid hormones [6] and the expression of 3β-HSD is increased in cystic follicles compared with that in normal large follicles [24]. Moreover, the expression of 3β-HSD mRNA is decreased by activin, but inhibin A can significantly increase the expression of 3β-HSD in ovarian thecal cells [10]. 3β-HSD is also a key molecule in the synthesis of estrogen, which is important for LH-induced ovulation by increasing expression of the LH receptor in the ovary [4]. However, the frequency of 3β-HSD-positive theca cells is decreased in cystic follicles [5]. According to available data, we hypothesize that downregulation of the inhibin α subunit in cystic follicles could be associated with a decreased level of 3β-HSD, leading to the formation of follicular cysts.
In this study, decreased expression of inhibin α subunit and betaglycan was found in cystic follicles compared with large follicles (Figs. 2 and 3). Inhibin is mainly produced in the granulosa cells of ovarian follicles, so a decreased expression of inhibin α subunit and betaglycan in cystic follicles may be associated with the persistence of cystic follicles. However, the results of ELISA show that although the concentration of inhibin α subunit in cystic follicular fluid decreased, no significant difference was found in comparison with the control (Fig. 4). The reason for this phenomenon may be the large volume of follicular fluid that diluted the concentration of inhibin α subunit. In our previous study, apoptosis of granulosa cells increased in cystic follicles [25], which might lead to a decrease in inhibin A and betaglycan synthesis.
Moreover, an increasing number of studies show that inhibin inhibits the secretion of FSH in an autocrine manner and plays an important role in ovary by autocrine and paracrine manners through binding to betaglycan [22]. Previous studies suggest that the expression of betaglycan in granulosa cells can be upregulated by FSH in vitro [11, 16], and a lower level of FSH in serum is found when a follicular cyst is present in the ovary [24]. In the present study, the mRNA and protein of both inhibin A and betaglycan significantly increased in porcine granulosa cells treated with exogenous FSH in a time-dependent manner (Figs. 5 and 6). We assume that the decrease in betaglycan expression may be due to the decrease in FSH secreted by gonadotrophs of the anterior pituitary gland, which can affect the role of inhibin A in the development of follicles.
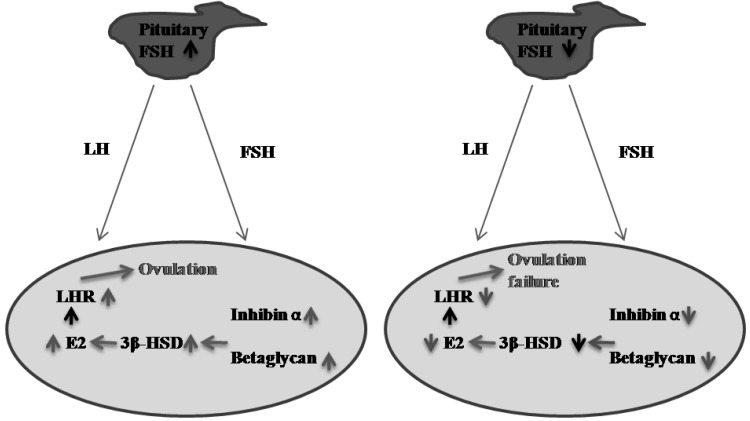
In conclusion, we demonstrated that inhibin α subunit and betaglycan are downregulated in cystic follicles and that betaglycan expression in granulosa cells is regulated by FSH. These findings may help us to understand the role of the inhibin A/betaglycan system in the ovary and may provide novel insights into the mechanisms of ovarian follicle cysts (Fig. 7).
Fig. 7.
Hypothesis concerning cystic follicle (CF) formation in the pig. Ovarian development and ovulation are mainly regulated by gonadotrophins via an endocrine pathway. Firstly, FSH stimulates expression of inhibin α and betaglycan in granulosa cells in the ovary, which increases the synthesis of estrogen through upregulation of 3β-HSD. Estrogen leads to LH-induced ovulation through increased expression of LH receptor (A). However, if synthesis of FSH is insufficient in the pituitary, ovulation could fail, and cystic follicles could form due to decreased expression of inhibin α and betaglycan (B).
Acknowledgments
This study was supported by the National Key Foundation Research and Development Program (“973” program) of China (No. 2011CB101003), Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, No. IRT1248), National Natural Science Foundation of China (31372308) and Science & Technology Pillar Program of Liaoning Province, P.R. China (No. 2013022054).
REFERENCES
- 1.Bilandzic M., Chu S., Farnworth P. G., Harrison C., Nicholls P., Wang Y. R., Escalona M., Fuller P. J., Findlay J. K., Stenvers K. L.2009. Loss of betaglycan contributes to the malignant properties of human granulosa tumor cells. Mol. Endocrinol. 23: 539–548. doi: 10.1210/me.2008-0300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drummond A. E., Le M. T., Ehier J. F., Dyson M.2002. Expression and localization of activin receptors. Endocrinology 143: 1423–1433. [DOI] [PubMed] [Google Scholar]
- 3.Findlay J. K., Drummond A. E., Dyson M. L., Baillie A. J., Robertson D. M., Ethier J. F.2002. Recruitment and development of the follicle; the roles of the transforming growth factor-beta superfamily. Mol. Cell. Endocrinol. 191: 35–43. doi: 10.1016/S0303-7207(02)00053-9 [DOI] [PubMed] [Google Scholar]
- 4.Ikeda S., Nakamura K., Kogure K., Omori Y., Yamashita S., Kubota K., Mizutani T., Miyamoto K., Minegishi T.2008. Effect of estrogen on the expression of luteinizing hormone-human chorionic gonadotropin receptor messenger ribonucleic acid in cultured rat granulosa cells. Endocrinology 149: 1524–1533. doi: 10.1210/en.2007-1163 [DOI] [PubMed] [Google Scholar]
- 5.Isobe N., Nakao T., Yoshimura Y.2003. Immunohistochemical localization of 3beta-hydroxysteroid dehydrogenase in the granulosa and theca interna layers of bovine cystic follicles. J. Reprod. Dev. 49: 227–233. doi: 10.1262/jrd.49.227 [DOI] [PubMed] [Google Scholar]
- 6.Johnson M., Everitt B.1997. Essential Reproduction, 4th ed. Blackwell Scientific Publications, Oxford. [Google Scholar]
- 7.Juengel J. L., McNatty K. P.2005. The role of proteins of the transforming growth factor-beta superfamily in the intraovarian regulation of follicular development. Hum. Reprod. Update 11: 143–160. [DOI] [PubMed] [Google Scholar]
- 8.Knox R. V., Vatzias G., Naber C. H., Zimmerman D. R.2003. Plasma gonadotropins and ovarian hormones during the estrous cycle in high compared to low ovulation rate gilts. J. Anim. Sci. 81: 249–260. [DOI] [PubMed] [Google Scholar]
- 9.Lee V. W., McMaster J., Quigg R., Leversha L.1982. Ovarian and circulating inhibin levels in immature female rats treated with gonadotropin and after castration. Endocrinology 111: 1849–1854. doi: 10.1210/endo-111-6-1849 [DOI] [PubMed] [Google Scholar]
- 10.Young J. M., McNeilly A. S.2012. Inhibin removes the inhibitory effects of activin on steroid enzyme expression and androgen production by normal ovarian thecal cells. J. Mol. Endocrinol. 48: 49–60. doi: 10.1530/JME-11-0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J., Kuulasmaa T., Kosma V. M., Bützow R., Vänttinen T., Hydén-Granskog C., Voutilainen R.2003. Expression of betaglycan, an inhibin coreceptor, in normal human ovaries and ovarian sex cord-stromal tumors and its regulation in cultured human granulosa-luteal cells. J. Clin. Endocrinol. Metab. 88: 5002–5008. doi: 10.1210/jc.2003-030704 [DOI] [PubMed] [Google Scholar]
- 12.Livak K. J., Schmittgen T. D.2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) method. Methods 25: 402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Diaz M. C., Bosu T. K.1992. A review and an update of cystic ovarian degerneration in ruminants. Theriogenology 37: 1163–1183. doi: 10.1016/0093-691X(92)90173-O [DOI] [Google Scholar]
- 14.Massagué J.1998. TGF-beta signal transduction. Annu. Rev. Biochem. 67: 753–791. doi: 10.1146/annurev.biochem.67.1.753 [DOI] [PubMed] [Google Scholar]
- 15.McGaughey R. W.1975. A comparison of the fluids from small and large ovarian follicles of the pig. Biol. Reprod. 13: 147–153. doi: 10.1095/biolreprod13.2.147 [DOI] [PubMed] [Google Scholar]
- 16.Omori Y., Nakamura K., Yamashita S., Matsuda H., Mizutani T., Miyamoto K., Minegishi T.2005. Effect of follicle-stimulating hormone and estrogen on the expression of betaglycan messenger ribonucleic acid levels in cultured rat granulosa cells. Endocrinology 146: 3379–3386. doi: 10.1210/en.2004-1665 [DOI] [PubMed] [Google Scholar]
- 17.Sarraj M. A., Chua H. K., Umbers A., Loveland K. L., Findlay J. K., Stenvers K. L.2007. Differential expression of TGFBR3 (betaglycan) in mouse ovary and testis during gonadogenesis. Growth Factors 25: 334–345. doi: 10.1080/08977190701833619 [DOI] [PubMed] [Google Scholar]
- 18.Schams D., Berisha B.2002. Steroids as local regulators of ovarian activity in domestic animals. Domest. Anim. Endocrinol. 23: 53–65. doi: 10.1016/S0739-7240(02)00145-5 [DOI] [PubMed] [Google Scholar]
- 19.Shi Y., Massague J.2003. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113: 685–700. doi: 10.1016/S0092-8674(03)00432-X [DOI] [PubMed] [Google Scholar]
- 20.Silvia W. J., Halter T. B., Nugent A. M., Laranja da Fonseca L. F.2002. Ovarian follicular cysts in dairy cows: an abnormality in folliculogenesis. Domest. Anim. Endocrinol. 23: 167–177. doi: 10.1016/S0739-7240(02)00154-6 [DOI] [PubMed] [Google Scholar]
- 21.Sirois J., Fortune J. E.1990. Lengthening the bovine estrous cycle with low levels of exogenous progesterone: a model for studying ovarian follicular dominance. Endocrinology 127: 916–925. doi: 10.1210/endo-127-2-916 [DOI] [PubMed] [Google Scholar]
- 22.Stenvers K. L., Findlay J. K.2010. Inhibins: from reproductive hormones to tumor suppressors. Trends Endocrinol. Metab. 21: 174–180. doi: 10.1016/j.tem.2009.11.009 [DOI] [PubMed] [Google Scholar]
- 23.Stoklosowa S., Bahr J., Gregoraszczuk E. L.1978. Some morphological and functional characteristics of the porcine theca interna in tissue culture. Biol. Reprod. 19: 712–719. doi: 10.1095/biolreprod19.4.712 [DOI] [PubMed] [Google Scholar]
- 24.Sun Y. L.2011. Study of Mechanism on Porcine Follicular Cysts (D). Jilin University, Changchun. [Google Scholar]
- 25.Sun Y. L., Zhang J., Ping Z. G., Fan L. N., Wang C. Q., Zheng L. W., Zhou X.2011. Expression of 3β- hydroxysteroid dehydrogenase (3β-HSD) in normal and cystic follicles in sows. Afr. J. Biotechnol. 10: 6184–6189. [Google Scholar]
- 26.Sun Y. L., Zhang J., Ping Z. G., Wang C. Q., Sun Y. F., Chen L., Li X. Y., Li C. J., Zhu X. L., Liu Z., Zhang W., Zhou X.2012. Relationship between apoptosis and proliferation in granulosa and theca cells of cystic follicles in sows. Reprod. Domest. Anim. 47: 601–608. doi: 10.1111/j.1439-0531.2011.01929.x [DOI] [PubMed] [Google Scholar]
- 27.Tiemann U., Tomek W., Schneider F., Vanselow J.2003. Effects of the mycotoxins and 1zearalenol on regulation of progesterone synthesis in cultured granulosa cells from porcine ovaries. Reprod. Toxicol. 17: 673–681. doi: 10.1016/j.reprotox.2003.07.001 [DOI] [PubMed] [Google Scholar]
- 28.van Zonneveld P., Scheffer G., Broekmans F., Blankenstein M., de Jong F., Looman C., Habbema J., te Velde E.2003. Do cycle disturbances explain the age-related decline of female fertility Cycle characteristics of women aged over 40 years compared with a reference population of young women. Hum. Reprod. 18: 495–501. doi: 10.1093/humrep/deg138 [DOI] [PubMed] [Google Scholar]
- 29.Waberski D., Kunz-Schmidt A., Borchardt Neto G., Richter L., Weitze K. F.2000. Real-time ultrasound diagnosis of ovulation and ovarian cysts in sows and its impact on artificial insemination efficiency. J. Anim. Sci. 77: 1–8. [Google Scholar]
- 30.Wang C. Q., Li C. J., Zhou X.2012. Effect of inhibin A on the expression of Brain-derived neurophic factor (BDNF) in porcine granulosa cells and the expansion of cumulus oocyte complexes (COCs) in vitro. Reprod. Domest. Anim. 47 (Suppl. 4: 533. [Google Scholar]