Abstract
Canine melanoma is one of the most important diseases in small animal medicine. Protein phosphatase 2A (PP2A), a well conserved serine/threonine phosphatase, plays a critical role as a tumor suppressor. SET/I2PP2A is an endogenous inhibitor for PP2A, which directly binds to PP2A and suppresses its phosphatase activity. Elevated SET protein levels have been reported to exacerbate human tumor progression. The role of SET in canine melanoma, however, has not been understood. Here, we investigated the potential therapeutic role for SET inhibitors in canine melanoma. The expression of SET protein was observed in 6 canine melanoma cell lines. We used CMeC-1 cells (primary origin) and CMeC-2 cells (metastatic origin) to generate cell lines stably expressing SET-targeting shRNAs. Knockdown of SET expression in CMeC-2, but not in CMeC-1, leads to decreased cell proliferation, invasion and colony formation. Phosphorylation level of p70 S6 kinase was decreased by SET knockdown in CMeC-2, suggesting the involvement of mTOR (mammalian target of rapamycin)/p70 S6 kinase signaling. The SET inhibitors, OP449 and FTY720, more effectively killed CMeC-2 than CMeC-1. We observed PP2A activation in CMeC-2 treated with OP449 and FTY720. These results demonstrated the potential therapeutic application of SET inhibitors for canine melanoma.
Keywords: canine, melanoma, PP2A, SET
Melanoma accounts for 3% of all tumors and about 7% of malignant tumors in dogs, with the primary tumor sites being the oral cavity (56%), lips (23%), skin (11%) and digits (8%) [5, 11]. The malignancy of canine melanoma varies dependent on the site of the primary lesion, with oral melanoma as the most common malignancy in dogs [1]. Radiation therapy is applied either alone or as an adjuvant therapy after surgery, but only a limited number of facilities can apply this treatment [2]. Although the literature reported that some canine melanomas respond to carboplatin, the ratio of complete response is quite low [7]. Therefore, the identification of novel therapeutic targets is required to improve treatment outcomes.
Protein phosphatase 2A (PP2A) is a well conserved serine/threonine phosphatase and an important tumor suppressor [20]. PP2A inhibits the tumorigenic potential, such as disordered cell proliferation and cancer cell stemness, by suppressing tumor-promoting signals, such as ERK1/2, Akt/PKB and c-Myc [16, 20]. SET/I2PP2A is a potent physiological inhibitor of PP2A, and the expression of SET is increased in various human cancers including breast cancer, pancreatic cancer, colorectal cancer and chronic myeloid leukemia [8, 9, 14, 18]. In human melanoma, SET knockdown (KD) suppressed tumor growth [8]. We previously reported that canine cells express several isoforms of the SET protein and showed that canine SETα protein has approximately 94% homology with human SETα [3].
OP449 is a peptide antagonist of SET, which directly binds to SET, leading to dissociation of PP2A from the inhibitor complex and allowing recovery of PP2A activity [4]. OP449 has been reported to decrease the tumorigenic potential of human B-cell chronic lymphocytic leukemia and breast cancer cells, and induce apoptosis [4, 14]. Previously, we reported OP449 recovers PP2A activity and induces apoptosis in canine lymphoma cells [10]. FTY720 (Fingolimod) is a sphingosine analog used as an immunosuppressant in human multiple sclerosis patients. FTY720 is phosphorylated by sphingosine kinase 2, and phospho-FTY720 downregulates sphingosine-1-phosphatase receptor 1. The nonphosphorylated form of FTY720 has been reported to directly interact with SET and recover PP2A activity [17]. These reports suggested the efficacy of SET inhibitors for canine tumors, however, the role of SET in tumorigenic potential of canine melanoma has not been investigated. Here, we show that knockdown of SET expression in a canine melanoma cell line leads to decreased cell proliferation, invasion and colony formation. OP449 and FTY720 recovered PP2A activity and showed anti-tumor effects on canine melanoma. Our data demonstrate the potential clinical application of SET inhibitors for canine melanoma.
MATERIALS AND METHODS
Cell culture: Canine melanoma cell lines, CMGD2 and CMGD5, were kindly provided by Dr. Modiano (University of Minnesota). CMeC-1, CMeC-2, KMeC and LMeC were previously described [13]. All cells were grown in RPMI1640 containing 10% FBS and 1 × anti-biotic/anti-micotic (Life Techologies, Carlsbad, CA, U.S.A.).
Antibodies: Antibodies were obtained from the indicated supplier: anti-total PP2Ac (Merck Millipore, Billerica, MA, U.S.A.), p97/VCP (GeneTex, Irvine, CA, U.S.A.), anti-tubulin alpha (Thermo Scientific, Woburn, MA, U.S.A.), anti-phospho ERK p42/p44, anti-phospho-Ser473 Akt1 (Cell Signaling, Beverly, MA, U.S.A.), anti-β-catenin (BD Biosciences, San Jose, CA, U.S.A.), anti-c-Myc (Cell Signaling), anti-SET (Abcam, Cambridge, MA, U.S.A.), anti-phospho-Thr389 p70 S6 kinase (Cell Signaling) and anti-phospho-Ser536 p65 NFκB (Cell Signaling).
Plasmid construction and lentivirus production: The seYFP (Venus) in pLV-Venus [15] was replaced with mCherry (pLVmC). shRNA complementary DNA strands (19-mer sequences: non-target shRNA (shNT), 5′-CAACAAGATGAGAGCACCA-3′; SET targeting shRNA (shSET), 5′-GGATGAAGGTGAAGAAGAT-3′) with flanking sequences were annealed and ligated into the MluI/ClaI sites of pLVmC. To produce lentiviruses, 3 µg of pLVSIN, 2.3 µg of a packaging plasmid (psPAX2) and 1.3 µg of a coat-protein plasmid expressing vesicular stomatitis virus G protein (pMD2.G) were transfected into Lenti-X 293T cells cultured in 60-mm dishes using PEI Max (Polysciences Inc., Warrington, PA, U.S.A.) according to the manufacturer’s instruction. Viral supernatants were collected after 48 hr, and after filtering (0.22 µm), were added to cells for 8 hr.
Immunoblotting: Immunoblotting was performed as previously described [19]. Briefly, cells were lysed in a buffer containing 50 mM Tris-HCl (pH 8.0), 5 mM ethylenediaminetetraacetic acid (EDTA) (pH 8.0), 5 mM ethylene glycol tetraacetic acid (EGTA), 1% Triton X100, 1 mM Na3VO4, 20 mM sodium pyrophosphate and Roche’s complete protease inhibitor cocktail. The proteins were separated by SDS-PAGE and transferred onto PVDF membrane (Bio-Rad Laboratories, Hercules, CA, U.S.A.). Membranes were blocked with 0.5% skim milk and treated with primary antibodies. Immunoreactive bands were detected using ECL Western Blotting Detection System (GE Healthcare, Freiburg, Germany) and visualized using a LAS-3000 luminescent image analyzer (Fujifilm, Tokyo, Japan).
Analysis of cell proliferation: 5.0 × 103 cells were seeded on 24-well plates, and after 3 days, Cell Counting Kit-8 (CCK8, Dojindo, Kumamoto, Japan.) was used to analyze cell proliferation according to the manufacturer’s instruction.
Matrigel invasion assay: To examine cell invasion, invasion assay was performed by using transwell chambers (Costar, Cambridge, MA, U.S.A.). After the polycarbonate membranes with an 8 µm pore were coated with 2% matrigel (BD Biosciences, San Jose, CA, U.S.A.), 1.5 × 104 cells of CMeC-1 or 4 × 104 cells of CMeC-2 in 200 µl media were added on the membrane (upper chamber). 880 µl of medium with 10% FBS was added on the lower chamber and cultured for 24 hr. The membranes were fixed with methanol for 15 min and stained with Giemsa solution (Muto Pure Chemicals, Tokyo, Japan). After the membranes were washed with distilled water, non-invaded cells were wiped with cotton-swab. The number of invaded cells through the membranes was randomly counted in 3 fields (× 400) under a light microscope (Eclipse TE2000, Nikon, Tokyo, Japan).
Colony formation assay: CMeC-1 (5.0 × 102 cells) and CMeC-2 (1.0 × 103 cells) were seeded on 60-mm dishes. After 1 week, cells were fixed with 99.5% ethanol, colonies were stained with Giemsa solution, and the number of colonies was counted.
Analysis of effects of SET inhibitors: 5.0 × 104 cells were seeded on 12-well plates and treated with OP449 for 1 day or FTY720 for 2 days. Cells were detached with 100 µl of trypsin-EDTA and trypan blue solution was added in an equal volume. Surviving cells were counted by microscopy.
PP2A activity assay: PP2A activity assay was performed as previously described [10]. Briefly, cells were treated with OP449 (2.5 µM) or FTY720 (10 µM) for 2 hr and lysed in a buffer containing 50 mM MOPS (pH 7.4), 0.1% NP-40, 0.1 mM EGTA and Roche’s complete protease inhibitor cocktail (Roche, Indianapolis, IN, U.S.A.). Cell lysates were treated with or without type 2A phosphatase inhibitor okadaic acid (10 nM, LC Laboratories,Woburn, MA, U.S.A.), and phosphatase reaction was performed in a reaction buffer containing 50 mM MOPS (pH 7.4), 24 mM MgCl2, 2 mM MnCl2, 0.03% 2-mercaptoethanol, 2.9% glycerol and 0.2 mM phospho-peptides (K-R-pT-I-R-R, Millipore, Billerica, MA, U.S.A.) for 20 min at room temperature. Concentration of released phosphate was analyzed by Malachite Green Assay. PP2A phosphatase activity was calculated by subtracting phosphatase activity levels in the presence of 10 nM okadaic acid treated samples from the phosphatase activity levels in the absences of any inhibitors.
Statistical analysis: The results are expressed as means ± S.E. Student’s t test was used for comparison between two groups. Groups more than 3 were compared using one-way analysis of variance, after which Fisher LSD test was used. For all analyses, a probability value of P<0.05 was considered statistically significant.
RESULTS
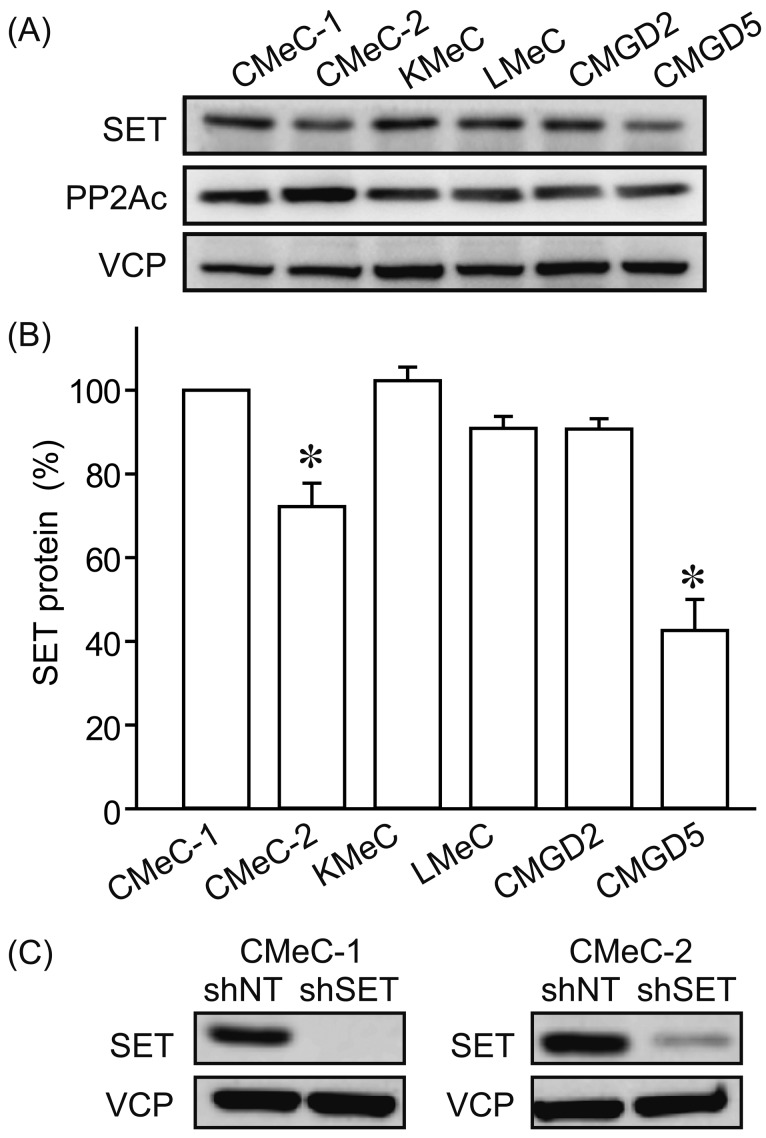
The protein expression levels of SET in 6 canine melanoma cell lines (CMeC-1, CMeC-2, KMeC, LMeC, CMGD2 and CMGD5) were examined by immunoblotting (Fig. 1A and 1B). We observed relatively low level of SET protein in CMeC-2 and CMGD5. CMeC-1 is the primary tumor cell lines from skin origin, and CMeC-2 is the lung metastasis origin from CMeC-1 xenotransplantation. CMeC-2 expresses about 30% less SET protein than CMeC-1. We used these cells to generate cell lines stably expressing non-target shRNA (shNT) and SET targeting shRNA (shSET). As shown in Fig. 1C, shSET effectively suppressed SET protein expression in both CMeC-1 and CMeC-2.
Fig. 1.
Protein levels of SET in various canine melanoma cell lines. (A, B) Protein levels of SET and PP2Ac in various canine melanoma cell lines were determined by immunoblotting. SET band densities were normalized to CMeC-1 as 100%. VCP (valosin containing protein) was used as a loading control. Representative picture (A) and quantitative data (B) from 3 independent experiments are shown. (C) Effects of non-target shRNA (shNT) and SET targeting shRNA (shSET) on SET protein levels were examined by immunoblotting. Representative pictures from 3 independent experiments are shown.
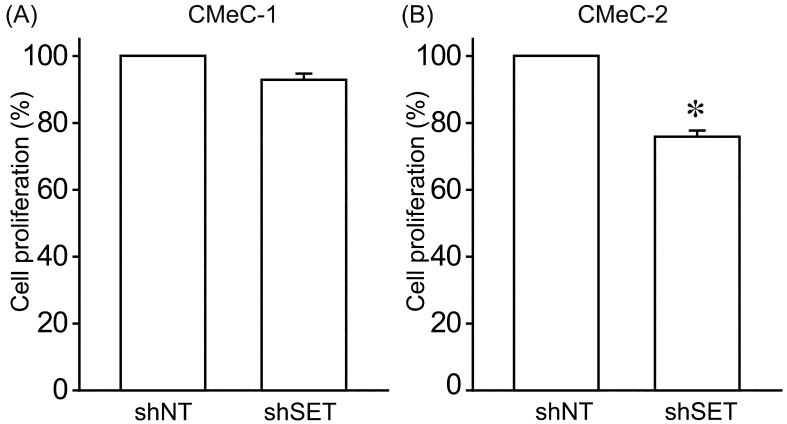
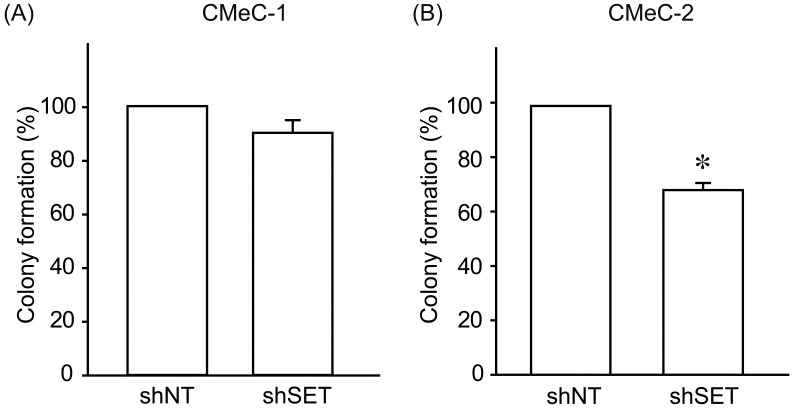
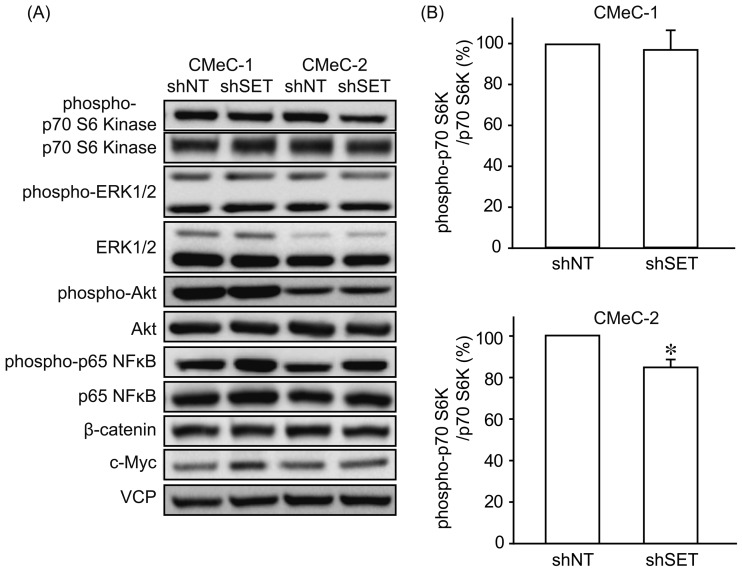
We examined the effects of SET knockdown (KD) on tumorigenic potential of canine melanoma cell lines. Interestingly, SET KD inhibited cell proliferation (Fig. 2) and invasion (Fig. 3) of CMeC-2 (low SET expression), but not CMeC-1 (high SET expression), suggesting SET plays an important role in the tumorigenic potential of CMeC-2, and higher protein level of SET is not correlated with the effects of SET KD. Consistent with these data, the ability to form colonies with CMeC-2, but not CMeC-1, was suppressed by SET KD (Fig. 4). Immunoblotting was performed to clarify the cell signaling affected by SET KD (Fig. 5). We observed Thr412 phosphorylation level of p70 S6 kinase (corresponding to human Thr389) was suppressed by SET KD in CMeC-2, suggesting the involvement of mTOR (mammalian target of rapamycin)/p70 S6K signaling. Phosphorylation levels of ERK1/2, Akt/PKB and p65 NFκB, and protein levels of β-catenin and c-Myc were not different between shNT and shSET, indicating these signaling pathways are not affected by SET KD.
Fig. 2.
SET knockdown suppresses cell proliferation of CMeC-2 cells. Cell proliferation of CMeC-1 (A) and CMeC-2 (B) expressing shNT and shSET was analyzed by Cell Counting Kit-8. Quantitative data from 2 independent experiments performed in duplicate are shown. *P<0.05 vs. shNT.
Fig. 3.
SET knockdown suppresses invasion of CMeC-2 cells. Invasion of CMeC-1 (A) and CMeC-2 (B) expressing shNT and shSET was analyzed by Matrigel invasion assay. Quantitative data from 2 independent experiments performed in duplicate are shown. *P<0.05 vs. shNT.
Fig. 4.
SET knockdown suppresses colony formation of CMeC-2 cells. Colony formation ability of CMeC-1 (A) and CMeC-2 (B) expressing shNT and shSET was analyzed by colony formation assay. Quantitative data from 2 independent experiments performed in duplicate are shown. *P<0.05 vs. shNT.
Fig. 5.
SET knockdown inhibits mTOR/p70 S6 kinase signaling. Immunoblotting was performed using indicated antibodies to analyze the effect of SET knockdown on intracellular signaling. Representative images (A) and quantitative data (B) for phospho-p70 S6 kinase from 3 independent experiments are shown. *P<0.05, NS: not significantly different.
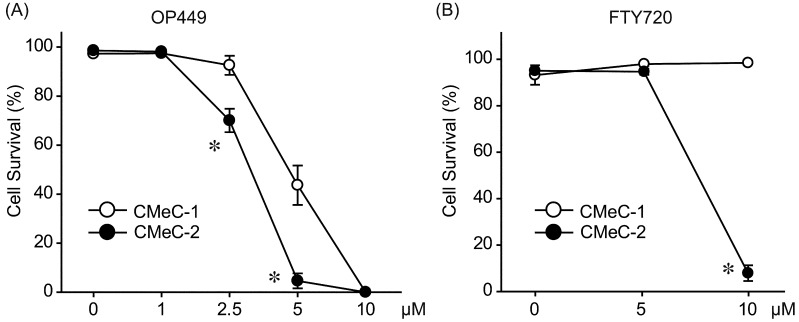
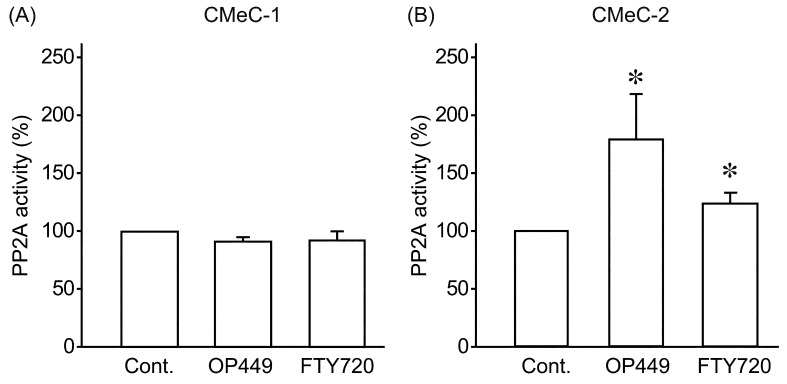
Next, we examined the anti-tumor effects of SET inhibitors, OP449 and FTY720, on canine melanoma cell lines. OP449 dose-dependently decreased cell survival rate for both CMeC-1 and CMeC-2 (Fig. 6A). OP449 had more potent effects on CMeC-2. Consistent with this, FTY720 effectively killed CMeC-2, but did not affect cell survival rate of CMeC-1 (Fig. 6B). We further examined whether SET inhibitors restored PP2A activity. Phosphatase activity in extracts of cells treated with or without 2.5 µM of OP449 or 10 µM of FTY720 for 2 hr was assayed with a relatively specific phosphopeptide (K-R-pT-I-R-R) as a substrate. As shown in Fig. 7, OP449 and FTY720 increased PP2A activity about 1.5 fold in CMeC-2, but did not change PP2A activity in CMeC-1. These data suggest that SET inhibitors effectively antagonize canine SET and exert anti-tumor effects.
Fig. 6.
SET inhibitors induce cell death in CMeC-2 cells. CMeC-1 and CMeC-2 were treated with indicated concentration of OP449 for 24 hr (A) or FTY720 for 48 hr (B). Cell viability was assessed by trypan blue exclusion assay. Quantitative data from 3–5 independent experiments are shown. *P<0.05 vs. CMeC-1.
Fig. 7.
SET inhibitors increase PP2A activity in CMeC-2 cells. CMeC-1 (A) and CMeC-2 (B) were treated with OP449 (2.5µM) or FTY720 (10 µM) for 2 hr, and PP2A activity was assayed. Quantitative data from 3–8 independent experiments are shown. *P<0.05 vs. control (Cont.).
DISCUSSION
In this study, we observed SET KD decreased tumorigenic potential of SET-low expressing CMeC-2, but not SET-high expressing CMeC-1. Consistent with this observation, SET inhibitors more effectively killed CMeC-2 melanoma cells. Although increased SET expression and its correlation with adverse prognosis were observed in various human tumors [6, 8], our unexpected results suggest that a higher level of SET expression does not always mean a higher contribution for tumorigenic potential. The molecular mechanism of this complexity remains unknown, but genetic mutations or differences in post-translational modifications on SET may contribute to this mechanism. Species differences may also play a role in this result, because dogs specifically express a carboxy-terminal truncated version of SET, which can associate with PP2A [22], but may not associate as well with other tumor suppressors.
We observed that SET KD decreased cell proliferation, invasion and colony formation of CMeC-2, indicating an important role of SET in the tumorigenic potential of CMeC-2. It has been reported that SET increases c-Myc protein levels [14], but we did not observe decreased c-Myc expression by SET KD in CMeC-2. ERK1/2, Akt/PKB, NFκB, Wnt/β-catenin and mTOR/p70 S6 kinase signaling are frequently upregulated in tumors, and PP2A has been reported to suppress these tumor promoting signals [16]. Among them, we observed decreased phosphorylation levels of p70 S6 kinase Thr412 (corresponding to human Thr389) by SET KD, which is the target of mTOR. Going further, it has been reported that PP2A directly associates with p70 S6 kinase, and knockdown of one of the regulatory subunits of PP2A enhances p70 S6 kinase Thr389 phosphorylation [12, 21]. These results suggest that SET KD restored PP2A activity which suppressed p70 S6 kinase phosphorylation, leading to decreased tumorigenic potential of canine melanoma cells.
We tested a potential therapeutic role for SET inhibitors, OP449 and FTY720, in canine melanoma. Previously, we reported that OP449 selectively induced apoptosis for SET high-expressing canine lymphoma cells [10]. However, in this study, both OP449 and FTY720 have more potent effects on SET low-expressing CMeC-2 cells. While protein levels of SET may be a biomarker for disease severity, these results do not support that SET levels predict the response of SET-targeting drugs in canine melanoma. Interestingly, effects of OP449 and FTY720 on CMeC-1 were different, i.e. 5 µM of OP449 killed almost 60% of CMeC-1 in 24 hr, but even treatment with 10 µM of FTY720 for 48 hr could not induce cell death, although it suppressed cell proliferation (data not shown). Because FTY720 is phosphorylated by sphingosine kinase 2, and phospho-FTY720 downregulates sphingosine-1-phosphatase receptor 1, a lack of potency, a lack of penetration into the cell or a PP2A independent mechanism may produce this inconsistency. Nonphosphorylated forms of FTY720, such as FTY720-Ome and FTY720-regioisomer, may provide effective tools to resolve this lack of effect.
Acknowledgments
This work was partly supported by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (Grant#25660227). The funding source had no role in the study design; collection, analysis or interpretation of data; in the writing of the manuscript; or the decision to submit the manuscript for publication.
REFERENCES
- 1.Bergman P. J.2007. Canine oral melanoma. Clin. Tech. Small Anim. Pract. 22: 55–60. doi: 10.1053/j.ctsap.2007.03.004 [DOI] [PubMed] [Google Scholar]
- 2.Blackwood L., Dobson J. M.1996. Radiotherapy of oral malignant melanomas in dogs. J. Am. Vet. Med. Assoc. 209: 98–102. [PubMed] [Google Scholar]
- 3.Bulmer J. N.1989. Decidual cellular responses. Curr. Opin. Immunol. 1: 1141–1147. doi: 10.1016/0952-7915(89)90006-X [DOI] [PubMed] [Google Scholar]
- 4.Christensen D. J., Chen Y., Oddo J., Matta K. M., Neil J., Davis E. D., Volkheimer A. D., Lanasa M. C., Friedman D. R., Goodman B. K., Gockerman J. P., Diehl L. F., de Castro C. M., Moore J. O., Vitek M. P., Weinberg J. B.2011. SET oncoprotein overexpression in B-cell chronic lymphocytic leukemia and non-Hodgkin lymphoma: a predictor of aggressive disease and a new treatment target. Blood 118: 4150–4158. doi: 10.1182/blood-2011-04-351072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cotchin E.1955. Melanotic tumours of dogs. J. Comp. Pathol. 65: 115–129. doi: 10.1016/S0368-1742(55)80011-2 [DOI] [PubMed] [Google Scholar]
- 6.Cristóbal I., Garcia-Orti L., Cirauqui C., Cortes-Lavaud X., García-Sánchez M. A., Calasanz M. J., Odero M. D.2012. Overexpression of SET is a recurrent event associated with poor outcome and contributes to protein phosphatase 2A inhibition in acute myeloid leukemia. Haematologica 97: 543–550. doi: 10.3324/haematol.2011.050542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dank G., Rassnick K. M., Sokolovsky Y., Garrett L. D., Post G. S., Kitchell B. E., Sellon R. K., Kleiter M., Northrup N., Segev G.2014. Use of adjuvant carboplatin for treatment of dogs with oral malignant melanoma following surgical excision. Vet. Comp. Oncol. 12: 78–84. doi: 10.1111/j.1476-5829.2012.00338.x [DOI] [PubMed] [Google Scholar]
- 8.Dong L., Zhu J., Wen X., Jiang T., Chen Y.2014. Involvement of SET in the Wnt signaling pathway and the development of human colorectal cancer. Oncol. Lett. 7: 1203–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farrell A. S., Allen-Petersen B., Daniel C. J., Wang X., Wang Z., Rodriguez S., Impey S., Oddo J., Vitek M. P., Lopez C., Christensen D. J., Sheppard B., Sears R. C.2014. Targeting inhibitors of the tumor suppressor PP2A for the treatment of pancreatic cancer. Mol. Cancer Res. 12: 924–939. doi: 10.1158/1541-7786.MCR-13-0542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujiwara N., Kawasaki H., Yabe R., Christensen D. J., Vitek M. P., Mizuno T., Sato K., Ohama T.2013. A potential therapeutic application of SET/I2PP2A inhibitor OP449 for canine T-cell lymphoma. J. Vet. Med. Sci. 75: 349–354. doi: 10.1292/jvms.12-0366 [DOI] [PubMed] [Google Scholar]
- 11.Goldschmidt M. H., Shofer F. S.1992. Skin Tumors of the Dog and Cat, 1st ed. Pergamon Press, Oxford. [Google Scholar]
- 12.Hahn K., Miranda M., Francis V. A., Vendrell J., Zorzano A., Teleman A. A.2010. PP2A regulatory subunit PP2A-B’ counteracts S6K phosphorylation. Cell Metab. 11: 438–444. doi: 10.1016/j.cmet.2010.03.015 [DOI] [PubMed] [Google Scholar]
- 13.Inoue K., Ohashi E., Kadosawa T., Hong S. H., Matsunaga S., Mochizuki M., Nishimura R., Sasaki N.2004. Establishment and characterization of four canine melanoma cell lines. J. Vet. Med. Sci. 66: 1437–1440. doi: 10.1292/jvms.66.1437 [DOI] [PubMed] [Google Scholar]
- 14.Janghorban M., Farrell A. S., Allen-Petersen B. L., Pelz C., Daniel C. J., Oddo J., Langer E. M., Christensen D. J., Sears R. C.2014. Targeting c-MYC by antagonizing PP2A inhibitors in breast cancer. Proc. Natl. Acad. Sci. U.S.A. 111: 9157–9162. doi: 10.1073/pnas.1317630111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCaffrey L. M., Macara I. G.2009. The Par3/aPKC interaction is essential for end bud remodeling and progenitor differentiation during mammary gland morphogenesis. Genes Dev. 23: 1450–1460. doi: 10.1101/gad.1795909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Millward T. A., Zolnierowicz S., Hemmings B. A.1999. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem. Sci. 24: 186–191. doi: 10.1016/S0968-0004(99)01375-4 [DOI] [PubMed] [Google Scholar]
- 17.Neviani P., Harb J. G., Oaks J. J., Santhanam R., Walker C. J., Ellis J. J., Ferenchak G., Dorrance A. M., Paisie C. A., Eiring A. M., Ma Y., Mao H. C., Zhang B., Wunderlich M., May P. C., Sun C., Saddoughi S. A., Bielawski J., Blum W., Klisovic R. B., Solt J. A., Byrd J. C., Volinia S., Cortes J., Huettner C. S., Koschmieder S., Holyoake T. L., Devine S., Caligiuri M. A., Croce C. M., Garzon R., Ogretmen B., Arlinghaus R. B., Chen C. S., Bittman R., Hokland P., Roy D. C., Milojkovic D., Apperley J., Goldman J. M., Reid A., Mulloy J. C., Bhatia R., Marcucci G., Perrotti D.2013. PP2A-activating drugs selectively eradicate TKI-resistant chronic myeloid leukemic stem cells. J. Clin. Invest. 123: 4144–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neviani P., Santhanam R., Trotta R., Notari M., Blaser B. W., Liu S., Mao H., Chang J. S., Galietta A., Uttam A., Roy D. C., Valtieri M., Bruner-Klisovic R., Caligiuri M. A., Bloomfield C. D., Marcucci G., Perrotti D.2005. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell 8: 355–368. doi: 10.1016/j.ccr.2005.10.015 [DOI] [PubMed] [Google Scholar]
- 19.Ohama T., Brautigan D. L.2010. Endotoxin conditioning induces VCP/p97-mediated and inducible nitric-oxide synthase-dependent Tyr284 nitration in protein phosphatase 2A. J. Biol. Chem. 285: 8711–8718. doi: 10.1074/jbc.M109.099788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perrotti D., Neviani P.2013. Protein phosphatase 2A: a target for anticancer therapy. Lancet Oncol. 14: e229–e238. doi: 10.1016/S1470-2045(12)70558-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson R. T., Desai B. N., Hardwick J. S., Schreiber S. L.1999. Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycinassociated protein. Proc. Natl. Acad. Sci. U.S.A. 96: 4438–4442. doi: 10.1073/pnas.96.8.4438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yabe R., Fujiwara N., Mizuno T., Usui T., Ohama T., Sato K.2014. Characterization of SET/I2PP2A isoforms in dogs. J. Vet. Med. Sci. 76: 1235–1240. doi: 10.1292/jvms.14-0209 [DOI] [PMC free article] [PubMed] [Google Scholar]