Abstract
In 2007–2008, a canine distemper virus (CDV) epidemic occurred among wild animals in Wakayama Prefecture, Japan, and many mammals, including the wild boar and deer, were infected. In this study, CDV prevalence among wild animals was surveyed before and after the epidemic. At first, an enzyme-linked immunosorbent assay (ELISA) with horseradish peroxidase-conjugated protein A/G was established to detect CDV antibodies in many mammalian species. This established ELISA was available for testing dogs, raccoons and raccoon dogs as well as virus-neutralization test. Next, a serological survey of wild mammalians was conducted, and it was indicated that many wild mammalians, particularly raccoons, were infected with CDV during the epidemic, but few were infected before and after the epidemic. On the other hand, many raccoon dogs died during the epidemic, but CDV remained prevalent in the remaining population, and a small epidemic occurred in raccoon dogs in 2012–2013. These results indicated that the epidemic of 2007–2008 may have been intensified by transmission to raccoons.
Keywords: canine distemper virus, raccoon, raccoon dog
Canine distemper virus (CDV) is an enveloped, negative-sense, single-stranded RNA virus belonging to the genus Morbillivirus, family Paramyxoviridae, order Mononegavirales [10] and causes lethal disease, including pyrexia, anorexia, nasal discharge, diarrhea, lymphopenia and encephalitis [1].
CDV traditionally causes potentially lethal disease among members of Canidae, Mustelidae and Procyonidae. CDV has been recently recognized as a cause of morbidity and mortality in many mammalian species, such as lions (Panthera leo) in Tanzania’s Serengeti National Park in 1994 [21]; lions, tigers (Panthera tigris), leopards (Panthera pardus) and a jaguar (Panthera onca) in North American zoos in 1991–1992 [2]; one Siberian tiger (Panthera tigris altaica) in Pokrovka, Russia, in 2004 [19]; lynx (Lynx canadensis) and bobcats (Lynx rufus) in eastern Canada [5]; binturongs (Arctictis binturong) [3, 8]; Iberian lynxes (Lynx pardinus) [11]; Amur tigers (Panthera tigris altaica) [25]; collared peccary (Tayassu tajacu) [16]; a wild black bear (Ursus americana) [4]; Japanese monkey (Macaca fuscata) [28]; hand-feeding Rhesus monkeys (Macaca mulatta) [18, 26]; and cynomolgus monkeys (Macaca fascicularis) [24]. We also reported an outbreak of CDV in tigers (Panthera tigris) and a risk of CDV transmission from wild animals to zoo animals, including large felids and endangered species [14]. Therefore, epidemic of CDV in wild animals is considered to be a serious problem in domestic dogs, wild animals and endangered species.
In 2007, a large epidemic of CDV infection occurred among wild mammalians in and around Tanabe city in Wakayama Prefecture [9]. Seven dead raccoon dogs and one dead weasel were confirmed to be infected with CDV by virus isolation, immunostaining with an anti-CDV polyclonal antibody and a commercially available CDV antigen-detection kit. A serological survey using a virus-neutralization (VN) test showed that many wild animals, including the wild boar and sika deer, around the epidemic site were positive for the CDV antibody. In particular, 52 out of 104 (50%) captured healthy raccoons were seropositive. Therefore, it was speculated that raccoons seemed to be the reservoir for CDV and transmit CDV to other wild animals. Subsequent to that study, we had an opportunity to test pre-epidemic blood samples collected from raccoons in this area. However, the blood was hemolytic and not suitable for VN testing. Therefore, another method to detect the CDV antibody from many mammalian species in addition to the VN test was required.
In this study, we established an enzyme-linked immunosorbent assay (ELISA) to detect antibodies from many mammalian species. ELISA was then implemented in a sero-survey of CDV in wild animal species in Wakayama Prefecture before and after the epidemic.
MATERIALS AND METHODS
Cells: A72/cSLAM and CRFK/cSLAM cells expressing the canine signaling lymphocyte activation molecule (SLAM) [15] were grown in Dulbecco’s modified Eagle’s medium (DMEM: Life Technologies, Carlsbad, CA, U.S.A.) containing 10% fetal calf serum (FCS), 100 U/ml penicillin and 100 µg/ml streptomycin (Life Technologies). The cells were maintained in a humidified 5% CO2 incubator at 37°C.
Virus: CDV KDK-1 strain (genotype Asia-1) was isolated from a diseased dog in Kagoshima, Japan, in 1991 using Vero cells [13] and propagated in A72/cSLAM cells after six passages in Vero cells.
ELISA
Preparation of antigens: ELISA antigen was prepared; KDK-1- or mock-infected Vero cells were lysed with lysis buffer, TSA (2 mM Tris-HCl pH 8.0, 140 mM sodium chloride and 0.025% sodium azide) containing 1% Triton X-100 and 0.5% sodium deoxycholate at 4°C for 1 hr and then centrifuged at 20,630 × g for 30 min. The supernatants were collected and kept at −80°C until use as ELISA antigens.
ELISA using anti-dog IgG or IgM antibody as a secondary antibody: Antigens were diluted to 5 µg/ml with an adsorption buffer (0.05 M carbonate–bicarbonate buffer, pH 9.6), and 100 µl was added per well into 96-well microplates (Maxisorp; Nunc, Roskilde, Denmark). After incubation at 37°C for 2 hr, plates were placed at 4°C overnight. The wells were washed three times with phosphate-buffered saline (PBS) and then incubated with 100 µl per well of 0.1% bovine serum albumin (BSA, Fraction V; Sigma, St. Louis, MO, U.S.A.) in PBS at 37°C for 30 min. After three washes with PBS containing 0.05% Tween20 (PBS-T), sera diluted with dilution buffer and PBS-T containing 10% FCS were added to duplicate wells, and plates were incubated at 37°C for 30 min. Next, wells were washed 3 times with PBS-T and incubated with 100 µl per well of peroxidase-conjugated sheep anti-dog IgG or goat anti-dog IgM antibody (Bethyl, Montgomery, TX, U.S.A.) diluted with dilution buffer at 37°C for 30 min. Following washing three times with PBS-T, 100 µl of horseradish peroxidase substrate kit (Bio-Rad, Hercules, CA, U.S.A.) was added to each well. After incubation at room temperature for 30 min, the enzymatic reaction was stopped by adding 100 µl of 2% oxalic acid to each well. The absorbance was measured by a spectrophotometer (Bio-Rad), with a 415-nm filter. All results were subtracted by the value of control wells containing extract from mock-infected cells.
ELISA using protein A/G as a secondary antibody: The antigen was diluted to 5 µg/ml with an adsorption buffer, and 100 µl was added per well into 96-well microplates. After incubation at 37°C for 2 hr, plates were placed at 4°C overnight. The wells were washed 3 times with PBS and then incubated with 100 µl per well of 1% Block Ace (Dainippon Pharmaceutical, Osaka, Japan) in PBS at 37°C for 30 min. Test sera were diluted with PBS-T containing 0.4% Block Ace. After three washes with PBS-T, 100 µl of diluted sera were added to duplicate wells, and plates were incubated at 37°C for 30 min. Next, the wells were washed three times with PBS-T and incubated with 100 µl per well of Peroxidase Conjugated Purified Recomb® Protein A/G (Thermo Fischer Scientific, Rockford, IL, U.S.A.) diluted with PBS-T containing 0.4% Block Ace at 37°C for 30 min. Following three washes with PBS-T, 100 µl of horseradish peroxidase substrate kit (Bio-Rad) was added to each well. After incubation at room temperature for 30 min, the enzymatic reaction was stopped by adding 100 µl of 2% oxalic acid to each well. The absorbance was measured by a spectrophotometer (Bio-Rad), with a 415-nm filter. All results were subtracted by the value of control wells containing extract from mock-infected cells.
Sera from dogs experimentally infected with CDV: Three dogs (beagle, female, 3 months old; NARC, Japan) were orally, intranasally and ocularly inoculated with 10 ml of viral solution containing 106 plaque-forming units (PFU) of CDV KochiO1A. CDV KochiO1A was isolated from a dead masked palm civet from Kochi Prefecture, Japan, in 2008. Blood samples were collected from the cephalic vein under anesthesia with ketamine on days 0, 3, 6, 9, 12, 15, 21 and 27 post challenge.
VN test: VN test to KDK-1 was performed by 75% plaque-reduction neutralization test (PRNT75) using our established cell line, CRFK/cSLAM [15]. To determine VN titers, sera were diluted to 1:5 and then serially diluted 2-fold with DMEM containing 2% FCS. Diluted sera were mixed with equal volumes of virus solution containing 100 PFU of KDK-1, followed by incubation at 37°C for 1 hr. Then, 50 µl of mixtures were added to each well of 24-well plates (Sumilon, Tokyo, Japan) containing subconfluent CRFK/cSLAM, and the plate was incubated at 37°C for 1 hr, washed twice with DMEM without FCS and overlaid with DMEM containing 0.8% agarose and 7% FCS. Then, plates were incubated at 37°C in 5% CO2 for 3–4 days. Cells were fixed with 5% buffered formaldehyde for 1 hr, and agarose layers were removed. After staining with crystal violet, plaques were counted. VN titer was expressed as the highest dilution of serum that reduced plaques by more than 75% in comparison with that of control wells without serum.
Animal samples: Between 2006 and 2012, a total of 1,686 serum samples were collected from wild animals captured in Wakayama Prefecture, under permission from the governor. Wild animals included 1,261 raccoons (Procyon lotor), 317 raccoon dogs (Nyctereutes procyonoides), 23 badgers (Meles meles), one weasel (Mustela itatsi), one Japanese marten (Martes melampus), three Korean yellow weasel (Mustela sibirica), one red fox (Vulpes vulpes), six Sika deer (Cervus nippon), 72 wild boars (Sus scrofa) and one alley cat (Felis silvestris catus). All serum samples were heat inactivated at 56°C for 30 min and stored at −20°C until use. Tissue swabs and fecal samples were collected from two raccoon dogs and one fox for virus isolation.
Virus isolation: Swabs and fecal samples were dissolved in 2 ml DMEM containing antibiotics, 200 U/ml penicillin and 200 µg/ml streptomycin, and then centrifuged at 2,000 × g for 15 min at 4°C. Tissue samples from dead animals were homogenized in a nine-fold volume of DMEM containing antibiotics and then centrifuged at 2,000 × g for 15 min at 4°C. The supernatants were passed through 0.45 µm filters (Millipore, Bedford, MA, U.S.A.) and then inoculated onto A72/cSLAM cells. The cells were incubated until a cytopathic effect (CPE) was observed.
Sequence analysis of hemagglutinin (H) gene: RNA was extracted from virus-infected cells or tissue samples using QIAGEN RNeasy Mini kit (QIAGEN, Hilden, Germany) or QIAGEN Viral RNA Mini Kit (QIAGEN), respectively, and reverse-transcription (RT) was performed with random 9-mer primer using TaKaRa RNA LA PCRTM kit (AMV) Ver.1.1 (Takara, Otsu, Japan) at 30°C for 10 min, 42°C for 30 min and 70°C for 15 min. H gene was amplified using primers, CDV-HR (5′-AGA TGG ACC TCA GGG TAT AG-3′) and CDV-HF (5′-AAC TTA GGG CTC AGG TAG TC-3′) [6], at 94°C for 2 min and 30 cycles consisting of denaturation at 94°C for 30 sec, annealing at 60°C for 30 sec and extension at 72°C for 3 min, followed by a final extension at 72°C for 15 min. The amplified products were purified using QIAquick PCR Purification kit (QIAGEN), and nucleotide sequences were directly determined using 3130 genetic analyzer (Applied Biosystem, Carlsbad, CA, U.S.A.). Primers used to sequence the H gene were described previously [9]. The nucleotide sequences were deposited in the DNA Data Bank of Japan (DDBJ, Table 4).
Table 4. Viruses isolated or detected from wild animals in Wakayama Prefecture.
| Strain | Animal species | Date of death | Sex | Weight (kg) | Virus isolation | Accession No. |
|---|---|---|---|---|---|---|
| (Month/Day/Year) | ||||||
| W729B/RD/070416a) | Raccoon dog | 4/16/2007 | ♂ | 2.4 | + | AB605891 |
| W812B/RD/080131a) | Raccoon dog | 1/31/2008 | ♀ | 2.4 | + | AB605890 |
| Wakayama/Fox/101125 | Fox | 11/24/2010 | ♀ | 5 | – | LC007974 |
| Wakayama/RD/110407 | Raccoon dog | 4/6/2011 | ♂ | 3.1 | – | LC007975 |
| Wakayama/RD/120927 | Raccoon dog | 9/26/2012 | ♂ | 3 | + | LC007976 |
a) These viruses were previously reported (Kameo et al. [9]).
Homology search and phylogenetic analysis: Homologies among strains were analyzed using the GENETYX® Ver.8 (GENETYX corporation, Tokyo, Japan), and phylogenetic trees were constructed by the neighbor-joining method [23] using MEGA5.0 software [27] on the basis of the amino acid pairwise distance.
RESULTS
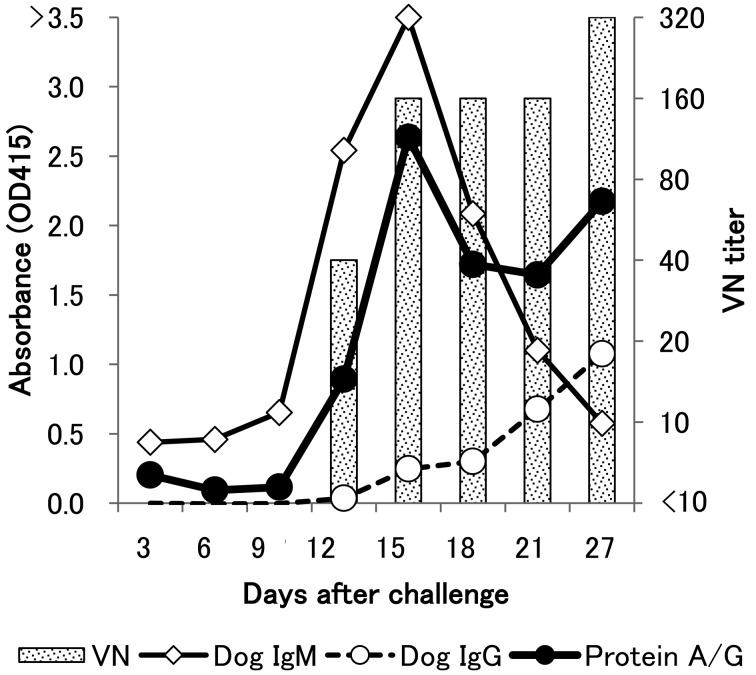
Although the VN test has been the standard assay to detect the CDV-specific antibodies in serum, it requires a large volume of serum, a special facility (biosafety level-2) and a high level of technical skill. In contrast, ELISA does not use live CDV and requires only a small amount of sample, and the procedure is simple. Therefore, to detect antibodies against CDV in many mammalians, we established a new ELISA using peroxidase-conjugated protein A/G (Thermo scientific, Waltham, MA, U.S.A.) as a second antibody and Block Ace (Dainippon Pharmaceutical, Osaka, Japan) as antibody blocking and dilution agent. At first, an ELISA using protein A/G was performed with sera collected from dogs experimentally infected with CDV. ELISA using protein A/G could detect CDV antibodies post infection. This study showed that ELISA and VN titer results were almost similar (Fig. 1). ELISA using either anti-IgG or anti-IgM antibody showed different results; IgG antibody gradually increased from day 12–27, and IgM antibody peaked on day 15 after infection. On the other hand, the anti-CDV antibody detected by protein A/G peaked on day 15 and then increased from day 21–27. A mixture of both IgG- and IgM-antibodies was detected by protein A/G.
Fig. 1.
Comparison between O.D. values in ELISA and titers of virus-neutralizing antibody in dogs. Three dogs (Nos.1–3) were experimentally infected with CDV KochiO1A, and sera were sequentially collected. This is representative data from Dog No.1. Dog sera were diluted to 1:100, and anti-dog IgG (open circle), IgM (open square) and protein A/G (black circle) were used as secondary antibodies. For the VN test, sera were two-fold diluted, and PRNT75 was performed (vertical bar).
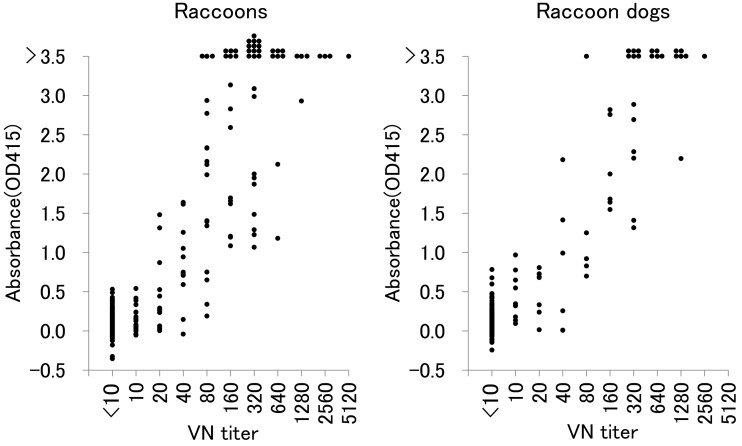
To confirm whether ELISA using protein A/G is reliable for many mammalian species, results were compared with those obtained by the VN test. In ELISA, sera were diluted to 1:100. The results of ELISA were correlated with those of the VN tests in raccoons and raccoon dogs (Fig. 2). In 660 raccoons, which were CDV negative by the VN test, average and standard deviation (S.D.) of OD values were 0.051 and 0.086, respectively. In 268 raccoon dogs, which were CDV negative by the VN test, average and S.D. of OD values were 0.097 and 0.131, respectively. To determine the cut-off value, average + 3 × S.D. was applied, and the cut-off values for ELISA were 0.309 and 0.491 for raccoons and raccoon dogs, respectively. In comparison with the result of VN test, specificity and sensitivity of this ELISA for raccoons were 98.3% and 73.9%, respectively, and those for raccoon dogs were 98.9% and 79.3%, respectively.
Fig. 2.
Comparison between O.D. values in ELISA and titers of virus-neutralizing antibody in raccoons and raccoon dogs. 803 and 326 sera of raccoons (A) and raccoon dogs (B), respectively, were analyzed by ELISA and PRNT75.
In a serological survey of CDV infection in raccoons, raccoon dogs and other wild animals, cut-off values of ELISA were tentatively determined to be 0.309, 0.491 and 0.5, respectively. The results indicated that 10.3% of raccoons, 13.2% of raccoon dogs, 18% of wild boars, 9% of badgers, one marten, one Siberian weasel and 2 Sika deer were sero-positive for CDV (Tables 1, 2, 3). In this study, stocks of hemolytic sera collected from raccoons in 2006 were not suitable for the VN test. ELISA could only detect the CDV-specific antibody from 2 raccoons (2.7%) from the 2006 sample set (Table 1). To examine whether these old hemolytic sera were suitable for ELISA, further ELISAs using Japanese encephalitis virus (JEV) antigen were performed. The results obtained indicated that many of the sera samples were positive for the JEV antibody (data not shown).
Table 1. Seroprevalence of CDV infection in raccoons.
| Places | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | Total | |
|---|---|---|---|---|---|---|---|---|---|
| Tanabe | No. of examined animals | 69 | 24 | 74 | 129 | 108 | 70 | 98 | 572 |
| % of positive animals | 1 | 63 | 28 | 7.8 | 4.6 | 11 | 5 | 11.4 | |
| Ryujin | No. of examined animals | 0 | 0 | 0 | 2 | 4 | 4 | 4 | 14 |
| % of positive animals | - | - | - | 50 | 0 | 0 | 50 | 21 | |
| Other towns | No. of examined animals | 6 | 11 | 58 | 130 | 140 | 164 | 166 | 675 |
| % of positive animals | 17 | 18 | 24 | 14.6 | 5.0 | 4.9 | 6.6 | 9.2 | |
| Total | No. of examined animals | 75 | 35 | 132 | 261 | 252 | 238 | 268 | 1261 |
| % of positive animals | 3 | 49 | 26.5 | 11.5 | 4.8 | 6.7 | 6.7 | 10.3 | |
Table 2. Seroprevalence of CDV infection in raccoon dogs.
| Places | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | Total | |
|---|---|---|---|---|---|---|---|---|
| Tanabe | No. of examined animals | 1 | 4 | 14 | 16 | 22 | 62 | 119 |
| % of positive animals | 0 | 50 | 0 | 6 | 9 | 31 | 20.2 | |
| Ryujin | No. of examined animals | 0 | 0 | 2 | 31 | 9 | 10 | 52 |
| % of positive animals | - | - | 0 | 13 | 11 | 70 | 24 | |
| Other towns | No. of examined animals | 2 | 1 | 22 | 62 | 14 | 45 | 146 |
| % of positive animals | 0 | 0 | 5 | 6 | 7 | 0 | 4.1 | |
| Total | No. of examined | 3 | 5 | 38 | 109 | 45 | 117 | 317 |
| % of positive animals | 0 | 40 | 3 | 8.3 | 9 | 22.2 | 13.2 | |
Table 3. Seroprevalence of CDV infection in other wild animals.
| Animals | 2007–2008 | 2009–2012 | Total | |||
|---|---|---|---|---|---|---|
| No. of examined animals | % of CDV-positive animals | No. of examined animals | % of CDV-positive animals | No. of examined animals | % of CDV-positive animals | |
| Badger(Meles meles) | 2 | 50 | 21 | 5 | 23 | 9 |
| Weasel(Mustela itatsi) | 1 | 0 | 0 | - | 1 | 0 |
| Marten(Martes melampus) | 1 | 100 | 0 | - | 1 | 100 |
| Siberian weasel(Mustela sibirica coreana) | 1 | 100 | 2 | 0 | 3 | 33 |
| Fox(Vulpes vulpes) | 1 | 0 | 0 | - | 1 | 0 |
| Sika deer(Cervus nippon) | 5 | 40 | 1 | 0 | 6 | 33 |
| Wild boar(Sus scrofa) | 41 | 27 | 31 | 6 | 72 | 18 |
| Alley cat(Felis silvestris catus) | 0 | - | 1 | 0 | 1 | 0 |
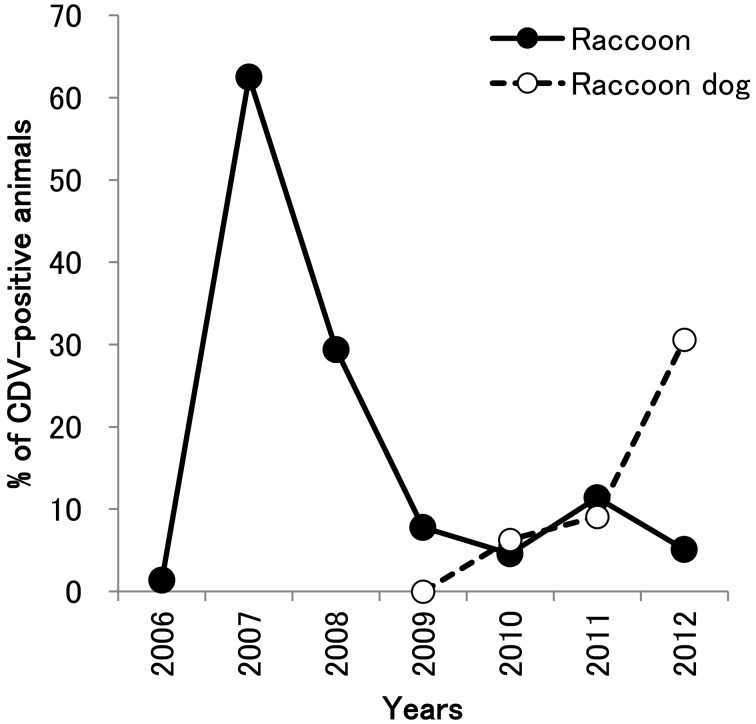
We previously reported the CDV epidemic among wild mammalians around Tanabe city in Wakayama Prefecture in 2007–2008 using the VN test [9]. In this study, we detected the anti-CDV antibodies by ELISA using protein A/G as described above (Tables 1 and 2, Fig. 3). In 2006, most raccoons did not possess the anti-CDV antibody (1.4%), but 62.5% of raccoons possessed the anti-CDV antibody in 2007 during the CDV epidemic. After the epidemic, 28.4% in 2008, 7.8% in 2009, 4.6% in 2010, 11.4% in 2011 and 5.1% in 2012 were CDV positive (Table 1, Fig. 3). The age of raccoons (calculated by the rings of teeth) indicated that many CDV-positive raccoons captured between 2009 and 2012 were born before the epidemic (date not shown).
Fig. 3.
Change in seroprevalence of CDV infection among raccoons and raccoon dogs in Tanabe city. The positive rate of CDV infection in raccoons (solid circle) and raccoon dogs (open circle) is plotted.
On the other hand, 0%, 6.3% and 9.1% of raccoon dogs in Tanabe city had anti-CDV antibodies in 2009, 2010 and 2011, respectively (Table 2). However, in 2012, 19 raccoon dogs (30.6%) were infected with CDV (Fig. 3), and the virus was also isolated from one diseased raccoon dog in Tanabe city (Table 4).
Tanabe city is surrounded by several other towns, including Ryujin. Before the CDV epidemic, one raccoon (16.7%) was CDV positive, but the number of seropositives was less than 10% after 2010. In raccoon dogs in Ryujin, the seroprevalence of CDV infection was approximately 10% during 2009–2011, although seven raccoon dogs (70%) became positive for CDV infection in 2012 (Table 2).
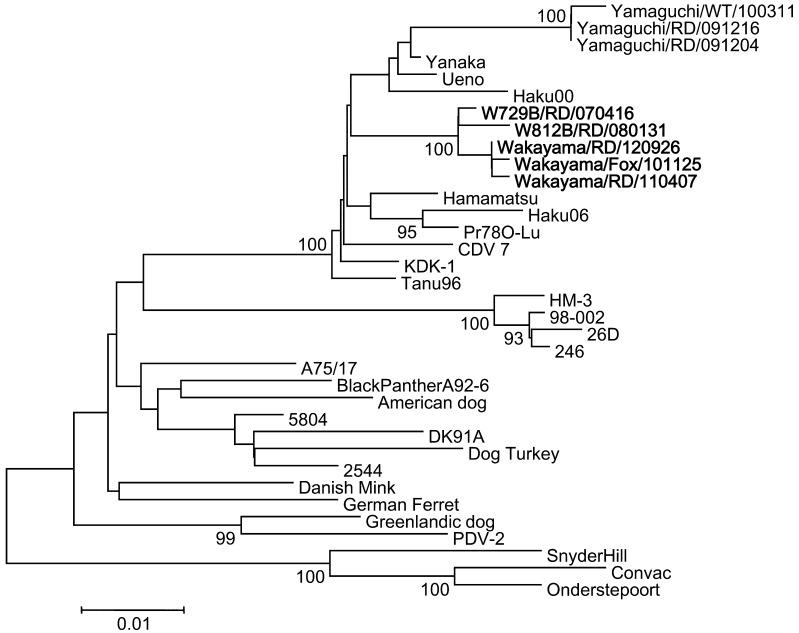
Two CDV genomes were detected by RT-PCR, one from a dead fox and the other from a dead raccoon dog, in 2010 and 2011, respectively (Table 4). Furthermore, one CDV virus was isolated from a dead raccoon dog in 2012 (Table 4). Phylogenetic analysis of the predicted H proteins was performed to elucidate the evolutionary relationships among CDV. We report that CDV in this area formed one cluster independent of animal species (Fig. 4).
Fig. 4.
Evolutionary relationship of CDV isolates inferred using the Neighbor-Joining method. Phylogenetic analysis was performed using a total of 607 amino acid positions with the MEGA5 program. Accession numbers of the sequences are BAK79007 (Yamaguchi/RD/091204), Yamaguchi/RD/091216), BAK79006 (Yamaguchi/WT/100311), BAA19586 (Yanaka), BAA19584 (Ueno), BAA19585 (Hamamatsu), BAG41887 (Pr780-Lu), BAA84209 (KDK-1), BAA33740 (Tanu96), BAB39167 (HM-3), BAA84208 (98-002), BAB39166 (26D), AAD49703 (A75/17), CAA90879 (Black panther A92-6), CAA87691 (American dog), CAA59359 (5804/Han90), AAM11476 (Dog Turkey), AAQ05829 (DK91A), CAB01252 (2544), CAA87688 (Danish mink), CAA59358 (German ferret), CAA87689 (Greenlandic dog), CAA59357 (PDV-2), AAG15490 (Snyder Hill), CAA84626 (Convac) and AAK54669 (Onderstepoort). Nucleotide sequences of Haku00 and Haku06 were reported by Hirama et al. (2004). Scale bar indicates the number of amino acid substitutions per site.
DISCUSSION
In this study, an ELISA using horseradish peroxidase-conjugated protein A/G was developed to detect the CDV antibody in various mammalian species. Comparing the VN test and ELISA, it was confirmed that the established ELISA was available for at least dogs, raccoons and raccoon dogs. Therefore, this method was applied for sero-surveillance in various animals.
We obtained 75 hemolytic blood samples that were collected from raccoons in 2006 and stored at −20°C. Because these hemolytic blood samples were not available for the VN test, our developed ELISA was used. The results indicated that 2 raccoons (2.7%) were CDV positive. To examine whether antibodies in the hemolytic blood samples were damaged, ELISA for JEV was also performed, because many raccoons in this area were seropositive for JEV [17]. The results showed that many raccoons were positive for JEV, indicating that antibodies in these hemolytic blood samples function well enough for ELISA.
The CDV epidemic among wild mammalians in and around Tanabe city in Wakayama Prefecture in 2007–2008 resulted in a widespread CDV infection of many species, particularly raccoons [9]. In this study, the seroprevalence of CDV infection before and after the CDV epidemic was examined. In Tanabe city, 62.5% and 28.4% of raccoons became positive in 2007 and 2008, respectively, but less than 10% of raccoons were positive for CDV in 2006 and after 2009. In addition, the age of raccoons (calculated by the rings of teeth) suggested that CDV-positive raccoons after 2009 were born before or during the CDV epidemic. These results indicated that most raccoons were infected with CDV during this epidemic and probably not after this epidemic. Since raccoons are considered as a natural reservoir of CDV in the U.S.A. [7, 12, 20, 22], raccoons may also spread CDV to other animals in Japan.
In 2007–2008, very few raccoon dogs were captured in Wakayama Prefecture, suggesting that CDV may have spread among raccoon dogs and killed many of them because of their high sensitivity to CDV. Many dead raccoon dogs were found during this epidemic [9]. Although the prevalence of CDV-positive raccoon dogs was less than 10% after the epidemic, 22.2% of raccoon dogs became positive for CDV in 2012. In particular, 70.0% and 30.6% of raccoon dogs were positive for CDV in Ryujin and Tanabe cities, respectively. In addition, CDV was detected in two raccoon dogs in 2011 and 2012. In particular, CDV was isolated from a dead raccoon dog in Tanabe city. These results indicated that CDV was maintained among a population of raccoon dogs, and a small epidemic without transmission to raccoons occurred in Tanabe and Ryujin cities in 2012.
In this study, it seems likely that raccoon dogs play a crucial role in maintenance of CDV in this area, and the epidemic in 2007 was enhanced by transmission of CDV to raccoons, resulting in many wild animals, including the Sika deer and wild boar, being infected with CDV. Raccoons were introduced from North America, and the population is rapidly increasing in Japan. These introduced species change the etiology of infectious diseases, including CDV.
Acknowledgments
Sera were collected from wild animals under the support of hunters and officers in Wakayama Prefecture. This work was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 24380166).
REFERENCES
- 1.Appel M. J.1969. Pathogenesis of canine distemper. Am. J. Vet. Res. 30: 1167–1182. [PubMed] [Google Scholar]
- 2.Appel M. J., Yates R. A., Foley G. L., Bernstein J. J., Santinelli S., Spelman L. H., Miller L. D., Arp L. H., Anderson M., Barr M.1994. Canine distemper epizootic in lions, tigers, and leopards in North America. J. Vet. Diagn. Invest. 6: 277–288. doi: 10.1177/104063879400600301 [DOI] [PubMed] [Google Scholar]
- 3.Chandra A. M., Ginn P. E., Terrell S. P., Ferguson B., Adjiri-Awere A., Dennis P., Homer B. L.2000. Canine distemper virus infection in binturongs (Arctictis binturong). J. Vet. Diagn. Invest. 12: 88–91. doi: 10.1177/104063870001200120 [DOI] [PubMed] [Google Scholar]
- 4.Cottrell W. O., Keel M. K., Brooks J. W., Mead D. G., Phillips J. E.2013. First report of clinical disease associated with canine distemper virus infection in a wild black bear (Ursus americana). J. Wildl. Dis. 49: 1024–1027. doi: 10.7589/2013-02-027 [DOI] [PubMed] [Google Scholar]
- 5.Daoust P. Y., McBurney S. R., Godson D. L., van de Bildt M. W., Osterhaus A. D.2009. Canine-distemper virus-associated encephalitis in free-living lynx (Lynx canadensis) and bobcats (Lynx rufus) of eastern Canada. J. Wildl. Dis. 45: 611–624. doi: 10.7589/0090-3558-45.3.611 [DOI] [PubMed] [Google Scholar]
- 6.Demeter Z., Lakatos B., Palade E. A., Kozma T., Forga’ch P., Rusvai M.2007. Genetic diversity of Hungarian canine distemper virus strains. Vet. Microbiol. 122: 258–269. doi: 10.1016/j.vetmic.2007.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoff G. L., Bigler W. J., Proctor S. J., Stallings L. P.1974. Epizootic of canine distemper virus infection among urban raccoons and gray foxes. J. Wildl. Dis. 10: 423–428. doi: 10.7589/0090-3558-10.4.423 [DOI] [PubMed] [Google Scholar]
- 8.Hur K., Bae J. S., Choi J. H., Kim J. H., Kwon S. W., Lee K. W., Kim D. Y.1999. Canine distemper virus infection in binturongs (Arctictis binturong). J. Comp. Pathol. 121: 295–299. doi: 10.1053/jcpa.1999.0322 [DOI] [PubMed] [Google Scholar]
- 9.Kameo Y., Nagao Y., Nishio Y., Shimoda H., Nakano H., Suzuki K., Une Y., Sato H., Shimojima M., Maeda K.2012. Epizootic canine distemper virus infection among wild mammals. Vet. Microbiol. 154: 222–229. doi: 10.1016/j.vetmic.2011.07.006 [DOI] [PubMed] [Google Scholar]
- 10.Lamb R. A., Kolakofsky D.2001. Paramyxoviridae: the viruses and their replication. pp. 1305–1443. In: Fields of Virology, 4th ed. (Knipe, D. M. and Howley, P. M. eds.), Lippincott Williams & Wilkins, Philadelphia. [Google Scholar]
- 11.Meli M. L., Simmler P., Cattori V., Martínez F., Vargas A., Palomares F., López-Bao J. V., Simón M. A., López G., León-Vizcaino L., Hofmann-Lehmann R., Lutz H.2010. Importance of canine distemper virus (CDV) infection in free-ranging Iberian lynxes (Lynx pardinus). Vet. Microbiol. 146: 132–137. doi: 10.1016/j.vetmic.2010.04.024 [DOI] [PubMed] [Google Scholar]
- 12.Mitchell M. A., Hungeford L. L., Nixon C., Esker T., Sullivan J., Koerkenmeier R., Dubey J. P.1999. Serologic survey for selected infectious disease agents in raccoons from Illinois. J. Wildl. Dis. 35: 347–355. doi: 10.7589/0090-3558-35.2.347 [DOI] [PubMed] [Google Scholar]
- 13.Mochizuki M., Hashimoto M., Hagiwara S., Yoshida Y., Ishiguro S.1999. Genotypes of canine distemper virus determined by analysis of the hemagglutinin genes of recent isolates from dogs in Japan. J. Clin. Microbiol. 37: 2936–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagao Y., Nishio Y., Shiomoda H., Tamaru S., Shimojima M., Goto M., Une Y., Sato A., Ikebe Y., Maeda K.2012. An outbreak of canine distemper virus in tigers (Panthera tigris): possible transmission from wild animals to zoo animals. J. Vet. Med. Sci. 74: 699–705. doi: 10.1292/jvms.11-0509 [DOI] [PubMed] [Google Scholar]
- 15.Nakano H., Kameo Y., Andoh K., Ohno Y., Mochizuki M., Maeda K.2009. Establishment of canine and feline cells expressing canine signaling lymphocyte activation molecule for canine distemper virus study. Vet. Microbiol. 133: 179–183. doi: 10.1016/j.vetmic.2008.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noon T. H., Heffelfinger J. R., Olding R. J., Wesche S. L., Reggiardo C.2003. Serologic survey for antibodies to canine distemper virus in collared peccary (Tayassu tajacu) populations in Arizona. J. Wildl. Dis. 39: 221–223. doi: 10.7589/0090-3558-39.1.221 [DOI] [PubMed] [Google Scholar]
- 17.Ohno Y., Sato H., Suzuki K., Yokoyama M., Uni S., Shibasaki T., Sashika M., Inokuma H., Kai K., Maeda K.2009. Detection of antibodies against Japanese encephalitis virus in raccoons, raccoon dogs and wild boars in Japan. J. Vet. Med. Sci. 71: 1035–1039. doi: 10.1292/jvms.71.1035 [DOI] [PubMed] [Google Scholar]
- 18.Qiu W., Zheng Y., Zhang S., Fan Q., Liu H., Zhang F., Wang W., Liao G., Hu R.2011. Canine distemper outbreak in rhesus monkeys, China. Emerg. Infect. Dis. 17: 1541–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quigley K. S., Evermann J. F., Leathers C. W., Armstrong D. L., Goodrich J., Duncan N. M., Miquelle D. G.2010. Morbillivirus infection on a wild Siberian tiger in the Russian Far East. J. Wildl. Dis. 46: 1252–1256. doi: 10.7589/0090-3558-46.4.1252 [DOI] [PubMed] [Google Scholar]
- 20.Raizman E. A., Dharmarajan G., Beasley J. C., Wu C. C., Pogranichniy R. M., Rhodes O. E., Jr2009. Serologic survey for selected infectious diseases in raccoons (Procyon lotor) in Indiana, USA. J. Wildl. Dis. 45: 531–536. doi: 10.7589/0090-3558-45.2.531 [DOI] [PubMed] [Google Scholar]
- 21.Roelke-Parker M. E., Munson L., Packer C., Kock R., Cleaveland S., Carpenter M., O’Brien S. J., Pospischil A., Hofmann-Lehmann R., Lutz H.1996. A canine distemper virus epidemic in Serengeti lions (Panthera leo). Nature 379: 441–445. doi: 10.1038/379441a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roscoe D. E.1993. Epizootiology of canine distemper in New Jersey raccoons. J. Wildl. Dis. 29: 390–395. doi: 10.7589/0090-3558-29.3.390 [DOI] [PubMed] [Google Scholar]
- 23.Saitou N., Nei M.1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4: 406–425. [DOI] [PubMed] [Google Scholar]
- 24.Sakai K., Nagata N., Ami Y., Seki F., Suzaki Y., Iwata-Yoshikawa N., Suzuki T., Fukushi S., Mizutani T., Yoshikawa T., Otsuki N., Kurane I., Komase K., Yamaguchi R., Hasegawa H., Saijo M., Takeda M., Morikawa S.2013. Lethal canine distemper virus outbreak in cynomolgus monkeys in Japan in 2008. J. Virol. 87: 1105–1114. doi: 10.1128/JVI.02419-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seimon T. A., Miquelle D. G., Chang T. Y., Newton A. L., Korotkova I., Ivanchuk G., Lyubchenko E., Tupikov A., Slabe E., McAloose D.2013. Canine distemper virus: an emerging disease in wild endangered Amur tigers (Panthera tigris altaica). MBio 4: e00410–e00413. doi: 10.1128/mBio.00410-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Z., Li A., Ye H., Shi Y., Hu Z., Zeng L.2010. Natural infection with canine distemper virus in hand-feeding Rhesus monkeys in China. Vet. Microbiol. 141: 374–378. doi: 10.1016/j.vetmic.2009.09.024 [DOI] [PubMed] [Google Scholar]
- 27.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S.2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28: 2731–2739. doi: 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshikawa Y., Ochikubo F., Matsubara Y., Tsuruoka H., Ishii M., Shirota K., Nomura Y., Sugiyama M., Yamanouchi K.1989. Natural infection with canine distemper virus in a Japanese monkey (Macaca fuscata). Vet. Microbiol. 20: 193–205. doi: 10.1016/0378-1135(89)90043-6 [DOI] [PubMed] [Google Scholar]