Abstract
A rock pigeon (Columba livia) caught in Akihabara, Tokyo, showed neurological symptoms, such as head tilt and circling. Pathological examinations revealed abundant Sarcocystic cysts in the skeletal muscle and myocardium with mild myositis, and numerous schizonts and sarcocysts with severe multifocal granulomatous T-lymphocytic infiltration in the central nervous system. A Sarcocystis calchasi-specific gene was detected in the muscle and brain. This case indicates S. calchasi was distributed in Japan and caused severe encephalitis to rock pigeons.
Keywords: encephalitis, rock pigeon, Sarcocystis calchasi, semi-nested PCR
Sarcocystis calchasi (S. calchasi) is a member of the Apicomplexa family and is the causative agent of pigeon protozoal encephalitis (PPE), reported in Germany in 2009 [13] and the United States in 2011 [21]. A previous study has reported the two-host life cycle of these parasites: the rock pigeon (Columba livia) as an intermediate host, and the northern goshawk (Accipiter g. genitalis) and European sparrowhawks (Accipiter nisus) as the definitive host [10, 12, 16]. Additionally, a recent study has reported S. calchasi caused encephalitis not only to rock pigeons but also to multiple psittacine species [19] and cockatiels [14]. S. calchasi induces severe biphasic disease in the rock pigeon, with polyuria, diarrhea and subsequent central nervous system (CNS) signs, such as torticollis, trembling and ataxia [11]. The histopathological lesions of PPE caused by S. calchasi consist of multifocal infiltration of mononuclear cells with prominent perivascular cuffing [9,10,11,12,13,14,15, 21]. The present report describes pathological features of S. calchasi infection in a rock pigeon and indicates that S. calchasi which causes severe encephalitis to rock pigeons as well as Newcastle disease [7, 20], pigeon herpes virus infection [4, 6] and highly pathogenic avian influenza (HPAI) [2] is distributed in Japan.
An adult rock pigeon was caught due to emaciation in Akihabara, Tokyo, and presented to a private veterinary clinic. The bird exhibited dark green diarrhea feces and CNS signs including torticollis and ataxia, and was subsequently euthanized due to poor prognosis and animal care concerns. At necropsy, there were no significant gross lesions in the visceral organs other than the numerous nematodes found in the small intestines. Tissue samples from the skeletal muscles (pectoral and thigh muscles), heart, trachea, lung, liver, spleen, proventriculus, gizzard, pancreas, small intestines, large intestines, brain and spinal cord from the first cervical vertebra to the synsacrum were fixed in 10% neutral buffered formalin. The tissues were routinely embedded in paraffin, sectioned at 4 µm and stained with hematoxylin and eosin (HE). Immunohistochemistry was performed to characterize the inflammatory cells in the brain lesions using rabbit polyclonal antibodies against CD3 [5, 15], Pax-5 [15], Iba-1 [3], GFAP [1, 5] and mouse monoclonal antibody against NeuN (clone A60) [18]. Primary antibodies used for immunohistochemistry are listed in Table 1. Tissue sections for the detection of CD3 were autoclaved in Dako Target Retrieval Solution, High pH (Dako, Kyoto, Japan) at 121°C for 10 min for antigen retrieval. The sections of Pax-5, Iba-1 and NeuN were autoclaved in 10 mM citrate buffer, pH 6.0 at 121°C for 10 min for antigen retrieval. Blocking of non-specific reactions was conducted by treatment with 10% hydrogen peroxide (H2O2) and methanol at room temperature for 5 min and then with 8% skimmed milk in Tris-buffered saline (TBS). The primary antibodies were applied at 4°C overnight. After rinsing with TBS, the labeling results were visualized using the EnVision+ System (Dako).
Table 1. Primary antibodies used in the present study.
| Antibody | Dilution | Antigen retrieval | Source |
|---|---|---|---|
| Rabbit polyclonal anti-CD3 | Ready to use | Autoclave (pH9) | Dako, Kyoto, Japan |
| Rabbit polyclonal anti-Pax5 | Ready to use | Autoclave (pH6) | Thermo scientific, Fremont, CA, U.S.A. |
| Rabbit polyclonal anti-Iba-1 | 1:500 | Autoclave (pH6) | Wako, Osaka, Japan |
| Rabbit polyclonal anti-GFAP (Glial fibrillary acidic protein) | 1:500 | None | Dako, Kyoto, Japan |
| Mouse monoclonal anti-NeuN (Neuronal Nuclei) | 1:100 | Autoclave (pH6) | Millipore, Darmstadt, Germany |
Histological examination revealed multifocal inflammation in both sides of the cerebrum and brain stem (Fig. 1). In the lesions, infiltration of mononuclear cells was observed with prominent perivascular cuffing and meningitis (Fig. 2). The immunohistochemistry results revealed that T-lymphocytes (CD3-positive cells) were the main inflammatory cells that had infiltrated the brain lesions (Fig. 3), and B-lymphocytes (Pax-5-positive cells) were rarely observed (Fig. 4). Diffuse and perivascular infiltration of macrophages/ microglia (Iba-1-positive cells) was also observed in the brain lesion (Fig. 5). Similar inflammatory lesions were detected throughout the spinal cord examined. No significant reactive astrocystes (GFAP-positive cells) were observed. Numerous schizonts and immature sarcocysts were found in inflammatory lesions in the brain and spinal cord, but some were also distributed in intact areas. The number of schizonts and immature sarcocysts detected in the brain and spinal cord was as follows: 68 schizonts and 42 immature sarcocysts in a cross section of the cerebrum; 10 schizonts and 9 immature sarcocysts in a cross section of the cerebellum; 8 schizonts and 2 immature sarcocysts in a cross section of the brain stem; and 9 schizonts and 2 immature sarcocysts in a cross section of the spinal cord. Some immature sarcocysts included numerous round metrocytes (Fig. 6). The schizonts contained many merozoites arranged radially around a residual body (Fig. 7). Sarcocysts and schizonts were mainly detected in the neuropil, and some schizonts were detected in the neuronal cell bodies (NeuN-positive cells) (Fig. 8). Neither bacterial nor fungal organisms were detected in the CNS.
Fig. 1.
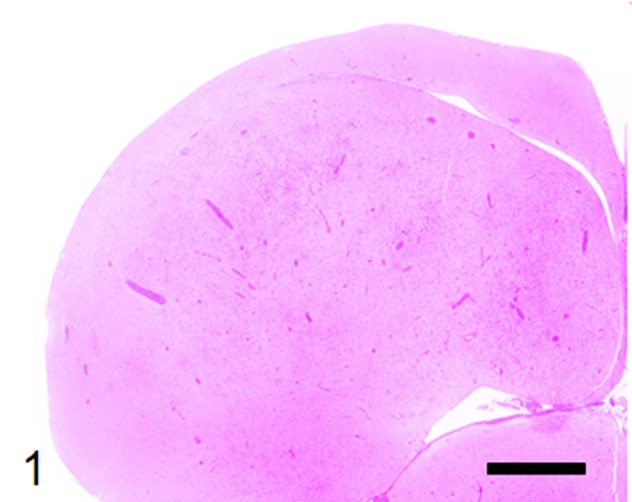
Multifocal severe inflammatory lesions in both sides of the cerebrum. HE stain, bar: 2 mm.
Fig. 2.
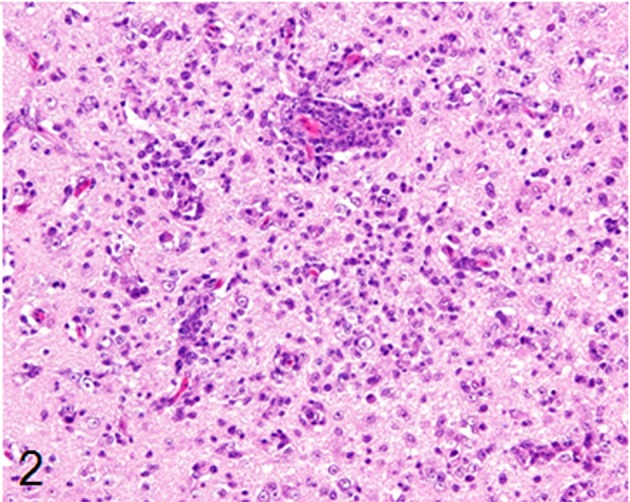
Infiltration of mononuclear cells, such as lymphocytes and macrophages, with prominent perivascular cuffing in the brain lesions. HE stain, bar: 50 µm.
Fig. 3.
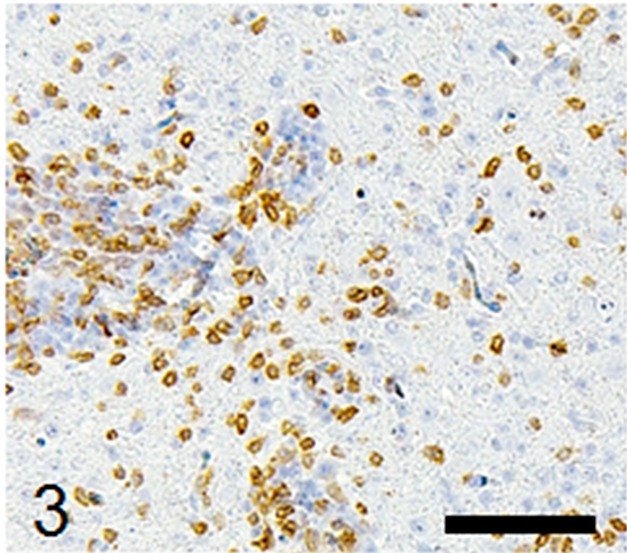
Infiltrating mononuclear cells in the brain lesions are mainly CD3-positive T-lymphocytes. Immunohistochemistry, Mayer’s hematoxylin counterstain, bar: 50 µm.
Fig. 4.
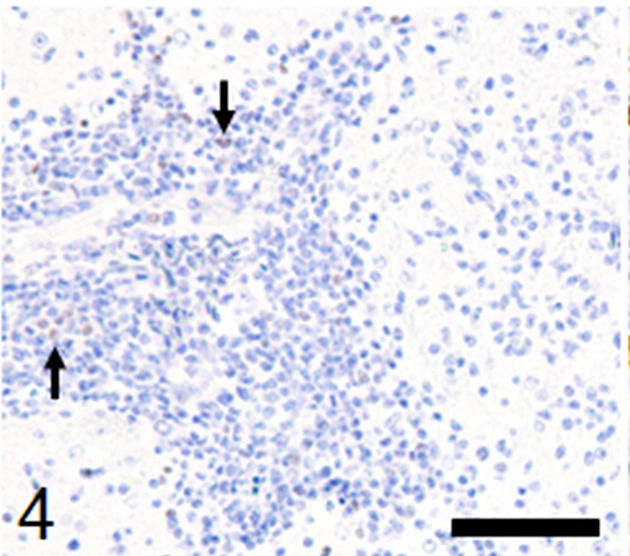
Only few Pax-5-positive B-lymphocytes infiltrate around the vessel (arrows). Immunohistochemistry, Mayer’s hematoxylin counterstain, bar: 50 µm.
Fig. 5.
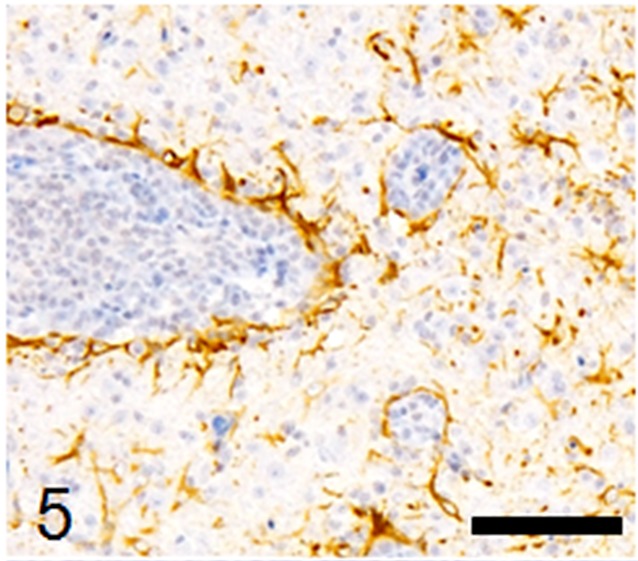
Proliferation of Iba-1-positive microglia around blood vessels and in the neuropil. Immunohistochemistry, Mayer’s hematoxylin counterstain, bar: 50 µm.
Fig. 6.
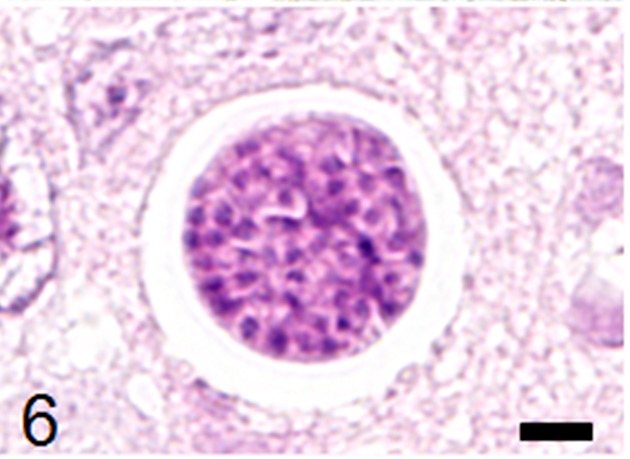
An immature sarcocyst in the brain containing numerous round-shaped metrocytes. HE stain, bar: 5 µm.
Fig. 7.
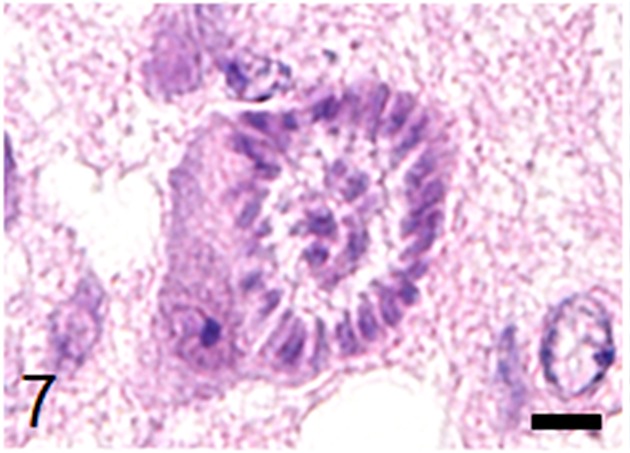
A mature schizont in a nerve cell containing many merozoites arranged radially around a residual body. Nissl bodies are pushed to the rim of the cytoplasm. HE stain, bar: 5 µm.
Fig. 8.
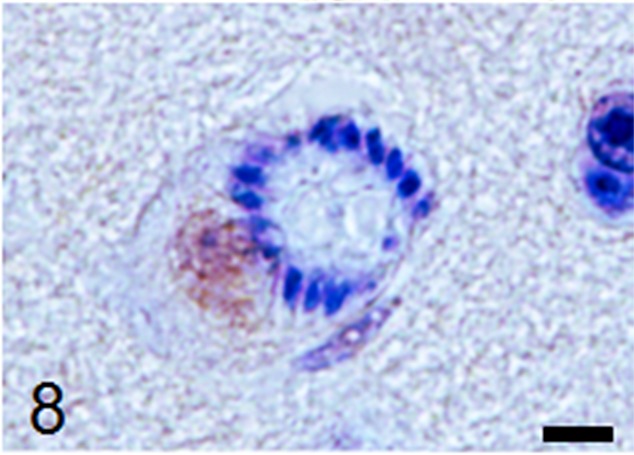
A NeuN-positive neuronal cell includes a schizont. Immunohistochemistry, Mayer’s counterstain, bar: 5 µm.
Numerous sarcocysts were detected in the pectoral and thigh muscles, and myocardium with mild myositis. The sizes of the sarcocysts in the pectral muscles were widths of 15 to 60 µm and lengths of 500 to 700 µm (Fig. 9), larger than that in the myocardium. The sarcocysts contained numerous metrocytes and bradyzoites separated by thin wall structures with hollows (Fig. 10). In the liver, portal infiltration of lymphocytes, plasma cells and macrophages was observed. No parasitic organisms were detected in the hepatic lesions. No significant histological lesions were observed in other organs.
Fig. 9.
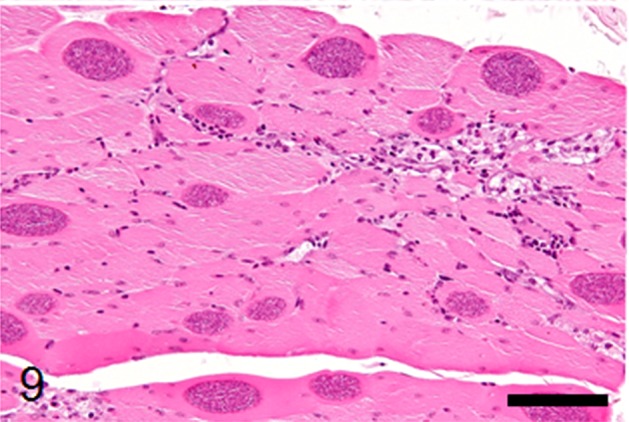
Numerous protozoal sarcocysts in the pectoral muscle with mild myositis. HE stain, bar: 100 µm.
Fig. 10.
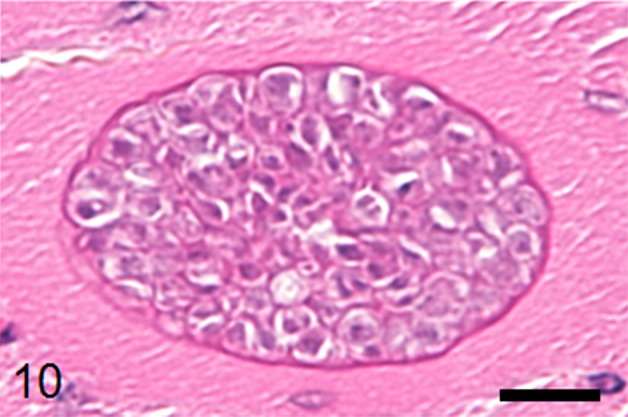
A sarcocyst in the pectoral muscle with an outer eosinophilic wall includes numerous metrocytes and bradyzoites separated by thin wall structures with hollows. HE stain, bar: 10 µm.
DNA was extracted from formalin-fixed and paraffin-embedded specimens of the thigh muscle and brain using the QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany). S.calchasi-specific primers (Sca1, Sca2 and SNca3) for the highly variable internal transcribed spacer (ITS) region 1 were used for semi-nested polymerase chain reaction (PCR) [16, 17]. A primer pair of Sca1/Sca2 was used for the initial amplification, and Sca1/SNca3 was used for the subsequent amplification. The PCR mixtures were prepared with the Takara Ex Taq Hot Start version kit (Takara, Otsu, Japan) according to the manufacturer’s direction. The thermal profile used was as follows; 2 min at 94°C, 10 sec at 98°C, 30 sec at 55°C, 30 cycles of 1min at 72°C and 10 min at 72°C. Semi-nested PCR amplicons were purified using the QIAquick Gel Extraction Kit (Qiagen) and sequenced using the same forward and reverse primers. The sequences were identified by BLAST analysis.
PCR using S. calchasi-specific primers revealed amplicons of the predicted size isolated from the skeletal muscle and brain samples. The 104 bp of partial nucleotide sequence in the ITS1 region was 99% homologous to that of S. calchasi(GeneBank accession no. FJ232948).
PPE caused by Sarcocystis calchasi is characterized by multifocal granulomatous lymphoplasmacytic infiltration with prominent perivascular cuffing and meningitis in the brain [9,10,11,12,13,14,15, 21]. The present immunohistochemical examinations demonstrated that multifocal and perivascular brain lesions were mainly composed of T-lymphocytes. Proliferation and activation of microglia were also observed in severe inflammatory lesions. These features in the present case were consistent with those in previous PPE cases [15].
Experimentally infected pigeons with S. calchasi had been shown to undergo a biphasic disease course [11]. In the acute phase, schizonts were found in various organs, prominently in the liver and spleen, and rarely in the brain [11, 15]. However, in the chronic phase, abundant sarcocysts were found in the skeletal muscle and only a few schizonts in the brain [9, 11, 13, 21]. These findings are consistent with those in naturally infected pigeons [13, 14, 21]. Recent studies have reported that a few immature sarcocysts were detected in the brain, even during the chronic phase, indicating their role as a continuous trigger of PPE [9, 15]. In the present case, numerous schizonts and immature sarcocysts were colocalized in the brain. One possible explanation of these two parasitic stages found concurrently in the present case is re-infection of S.calchasi; a bird in the chronic phase of S. calchasi-infection may be re-infected and concurrently develop lesions of the acute phase again.
PPE caused by S. calchasi exhibits CNS signs that are similar to Newcastle disease [7, 20], pigeon herpes virus infections [3, 6] and highly pathogenic avian influenza (HPAI) [2]. However, PPE in the present case can be histopathologically differentiated by the absence of visceral lesions, such as hepatic necrosis (HPAI and pigeon herpes virus), nephritis and hemorrhagic necrosis in the pancreas (Newcastle disease). Moreover, the dominant inflammatory cells in the cerebrum characterized in the present study (macrophages and T-lymphocytes) were different from that of Newcastle disease, pigeon herpes virus infection and HPAI. In addition, the definitive diagnosis was made by S. calchasi-specific semi-nested PCR. An incident of possible Sarcocystis infection in captive Ringneck Doves (Streptopelia risoria) at a zoo in Japan had been previously reported [8]. The present study confirmed that S. calchasi infection is occurring in wild rock pigeon in Japan as well as Europe and the United States. The Northern goshawks, the definitive host of S. calchasi, share their territory with rock pigeons in Japan, except for in the northern regions [21]. Therefore, besides Newcastle disease, pigeon herpes virus infection and HPAI, S. calchasi infection should be considered one of the differential diagnoses with pigeons exhibiting neurological signs in the area.
REFERENCES
- 1.Bras I. D., Gemensky-Metzler A. J., Kusewitt D. F., Colitz C. M., Wilkie D. A.2005. Immunohistochemical characterization of a malignant intraocular teratoid medulloepithelioma in a cockatiel. Vet. Ophthalmol. 8: 59–65. doi: 10.1111/j.1463-5224.2005.04043.x [DOI] [PubMed] [Google Scholar]
- 2.Brown J. D., Stallknecht D. E., Berghaus R. D., Swayne D. E.2009. Infectious and lethal doses of H5N1 highly pathogenic avian influenza virus for house sparrows (Passer domesticus) and rock pigeons (Columbia livia). J. Vet. Diagn. Invest. 21: 437–445. doi: 10.1177/104063870902100404 [DOI] [PubMed] [Google Scholar]
- 3.Cuadros M. A., Santos A. M., Martin-Oliva D., Calvente R., Tassi M., Marin-Teva J. L., Navascues J.2006. Specific immunolabeling of brain macrophages and microglial cells in the developing and mature chick central nervous system. J. Histochem. Cytochem. 54: 727–738. doi: 10.1369/jhc.5A6832.2006 [DOI] [PubMed] [Google Scholar]
- 4.Ducherty D. E.1999. Miscellaneous herpesviruses of birds. pp. 157–161. In: Field Manual of Wildlife Diseases: General Field Procedures and Diseases of Birds (Friend, M. and Franson, J. C. eds.), U. S. Geological Survey, Reston. [Google Scholar]
- 5.Kommers G. D., King D. J., Seal B. S., Carmichael K. P., Brown C. C.2002. Pathogenesis of six pigeon-origin isolates of Newcastle disease virus for domestic chickens. Vet. Pathol. 39: 353–362. doi: 10.1354/vp.39-3-353 [DOI] [PubMed] [Google Scholar]
- 6.Kunkle R. A., Duhamel G. E.1991. An outbreak of herpesvirus infection in a flock of ringed turtle doves (Streptopelia risoria). J. Vet. Diagn. Invest. 3: 93–95. doi: 10.1177/104063879100300125 [DOI] [PubMed] [Google Scholar]
- 7.Liu M., Qu Y., Wang F., Liu S., Sun H.2015. Genotypic and pathotypic characterization of Newcastle disease virus isolated from racing pigeons in China. Poult. Sci. (in press). doi: 10.3382/ps/pev106 [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto K.2003. Proceeding of the slide-seminar held by the Livestock sanitation study group in 2002, part 2. Nichijyuukaishi 56: 728 (in Japanese).
- 9.Maier K., Olias P., Enderlein D., Klopfleisch R., Mayr S. L., Gruber A. D., Lierz M.2015. Parasite distribution and early-stage encephalitis in Sarcocystis calchasi infections in domestic pigeons (Columba livia f. domestica). Avian Pathol. 44: 5–12. doi: 10.1080/03079457.2014.978263 [DOI] [PubMed] [Google Scholar]
- 10.Olias P., Gruber A. D., Hafez H. M., Heydorn A. O., Mehlhorn H., Lierz M.2010. Sarcocystis calchasi sp. nov. of the domestic pigeon (Columba livia f. domestica) and the Northern goshawk (Accipiter gentilis): light and electron microscopical characteristics. Parasitol. Res. 106: 577–585. doi: 10.1007/s00436-009-1701-9 [DOI] [PubMed] [Google Scholar]
- 11.Olias P., Gruber A. D., Heydorn A. O., Kohls A., Hafez H. M., Lierz M.2010. Unusual biphasic disease in domestic pigeons (Columba livia f. domestica) following experimental infection with Sarcocystis calchasi. Avian Dis. 54: 1032–1037. doi: 10.1637/9303-031110-Reg.1 [DOI] [PubMed] [Google Scholar]
- 12.Olias P., Gruber A. D., Kohls A., Hafez H. M., Heydorn A. O., Mehlhorn H., Lierz M.2010. Sarcocystis species lethal for domestic pigeons. Emerg. Infect. Dis. 16: 497–499. doi: 10.3201/eid1603.090860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olias P., Gruber A. D., Heydorn A. O., Kohls A., Mehlhorn H., Hafez H. M., Lierz M.2009. A novel Sarcocystis-associated encephalitis and myositis in racing pigeons. Avian Pathol. 38: 121–128. doi: 10.1080/03079450902737847 [DOI] [PubMed] [Google Scholar]
- 14.Olias P., Maier K., Wuenschmann A., Reed L., Armien A. G., Shaw D. P., Gruder A. D., Lierz M.2014. Sarcocystis calchasi has an expanded host range and induces neurological disease in cockatiels (Nymphicus hollandicus) and North American rock pigeons (Columbia livia f. dom.). Vet. Parasitol. 200: 59–65. doi: 10.1016/j.vetpar.2013.11.012 [DOI] [PubMed] [Google Scholar]
- 15.Olias P., Meyer A., Klopfleisch R., Lierz M., Kaspers B., Gruber A. D.2013. Modulation of the host Th1 immune response in pigeon protozoal encephalitis caused by Sarcocystis calchasi. Vet. Res. 44: 10. doi: 10.1186/1297-9716-44-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olias P., Olias L., Krucken J., Lierz M., Gruber A. D.2011. High prevalence of Sarcocystis calchasi sporocysts in European Accipiter hawks. Vet. Parasitol. 175: 230–236. doi: 10.1016/j.vetpar.2010.10.025 [DOI] [PubMed] [Google Scholar]
- 17.Olias P., Olias L., Lierz M., Mehlhorn H., Gruber A. D.2010. Sarcocystis calchasi is distinct to Sarcocystis columbae sp. nov. from the wood pigeon (Columba palumbus) and Sarcocystis sp. from the sparrowhawk (Accipiter nisus). Vet. Parasitol. 171: 7–14. doi: 10.1016/j.vetpar.2010.03.021 [DOI] [PubMed] [Google Scholar]
- 18.Reiner A., Laverghetta A. V., Meade C. A., Cuthbertson S. L., Bottjer S. W.2004. An immunohistochemical and pathway tracing study of the striatopallidal organization of area X in the male zebra finch. J. Comp. Neurol. 469: 239–261. doi: 10.1002/cne.11012 [DOI] [PubMed] [Google Scholar]
- 19.Rimoldi G., Speer B., Wellehan J. F., Jr, Bradway D. S., Wright L., Reavill D., Barr B. C., Childress A., Shivaprasad H. L., Chin R. P.2013. An outbreak of Sarcocystis calchasi encephalitis in multiple psittacine species within an enclosed zoological aviary. J. Vet. Diagn. Invest. 25: 775–781. doi: 10.1177/1040638713502981 [DOI] [PubMed] [Google Scholar]
- 20.Shaheen S., Anjum A. D., Rizvi F.2005. Clinico-pathological observations of pigeons (Columba livia) suffering from Newcastle disease. Pakistan Vet. J. 25: 5–8. [Google Scholar]
- 21.Wünschmann A., Armien A. G., Reed L., Gruber A. D., Olias P.2011. Sarcocystis calchasi-associated neurologic disease in a domestic pigeon in North America. Transbound. Emerg. Dis. 58: 526–530. doi: 10.1111/j.1865-1682.2011.01254.x [DOI] [PubMed] [Google Scholar]


