Abstract
Reducing the number of host-vector interactions is an effective way to reduce the spread of vector-borne diseases. Repellents are widely used to protect humans from a variety of protozoans, viruses, and nematodes. DEET (N,N-Diethyl-meta-toluamide), a safe and effective repellent, was developed during World War II. Fear of possible side effects of DEET has created a large market for “natural” DEET-free repellents with a variety of active ingredients. We present a comparative study on the efficacy of eight commercially available products, two fragrances, and a vitamin B patch. The products were tested using a human hand as attractant in a Y-tube olfactometer setup with Aedes aegypti (Linnaeus) and Aedes albopictus (Skuse), both major human disease vectors. We found that Ae. albopictus were generally less attracted to the test subject’s hand compared with Ae, aegypti. Repellents with DEET as active ingredient had a prominent repellency effect over longer times and on both species. Repellents containing p-menthane-3,8-diol produced comparable results but for shorter time periods. Some of the DEET-free products containing citronella or geraniol did not have any significant repellency effect. Interestingly, the perfume we tested had a modest repellency effect early after application, and the vitamin B patch had no effect on either species. This study shows that the different active ingredients in commercially available mosquito repellent products are not equivalent in terms of duration and strength of repellency. Our results suggest that products containing DEET or p-menthane-3,8-diol have long-lasting repellent effects and therefore provide good protection from mosquito-borne diseases.
Keywords: repellents, Aedes, yellow fever mosquito, Asian tiger mosquito, DEET
Mosquitoes are hosts for an array of different protozoan parasites, nematodes, and viruses (Marquardt 2004, Enserink 2007, Murray et al. 2010). Controlling mosquito populations is an effective tool for the fight against such pathogens. Several different methods for mosquito control have been developed, e.g., source reduction, physical exclusion (nets, screens, etc.), pesticide application, biological control methods, sterile insect technique, and release of genetically modified mosquitoes (Rose 2001, Phuc et al. 2007, Alphey et al. 2010). Unfortunately and for a variety of reasons, these approaches can be difficult to implement in many locations (Peter et al. 2005). Widespread insecticide resistance in disease-carrying mosquito populations also poses a significant problem.
On an individual level, mosquito repellents are widely used to avoid disease exposure (Barnard 2000, Barnard and Xue 2004). Repellents, even though they can never guarantee complete protection, can significantly lessen the chance of contracting vector-borne diseases (Kahn et al. 1975, Barnard et al. 1998, Barnard and Xue 2004, Rowland et al. 2004, Hill et al. 2007). They are especially useful when used where human activity coincides with the diurnal activity patterns of mosquitoes, e.g., outdoor activities that take place at dusk and dawn, e.g., hunting and fishing.
The sense of smell is one of the most important senses that mosquitoes use for long range host seeking (Potter 2014). Insect olfaction has been extensively studied leading to the identification of the key proteins involved: odorant receptors, odorant receptor co-receptors, gustatory receptors, and odorant binding proteins (Kaupp 2010, Suh et al. 2014). The processing of olfactory information in different regions of the insect brain has also attracted a lot of research interest (Galizia 2014). Various mosquito attractants and repellents have been identified, many of which are produced by human metabolism or the bacterial degradation of sweat components. Lactic acid and 1-octen-3-ol are two components that act as strong mosquito attractants (Zwiebel and Takken 2004). Carbon dioxide from breath is another strong attractant that sensitizes mosquitoes to other odorants (Dekker et al. 2005). Studies have shown that different insect repellents use a similar mode-of-action. Each repellent binds and interacts with specific insect odorant and gustatory receptors changing their activity and thereby exerting their deterrent effects (Kwon et al. 2010, Dickens and Bohbot 2013, Xu et al. 2014). The most widely used insect repellent, DEET (N,N-diethyl-m-toluamide), has been in use for about 70 yr. DEET is considered a very safe repellent (Osimitz and Grothaus 1995, Koren et al. 2003, Sudakin and Trevathan 2003). Nevertheless, fear of possible side effects of DEET and general chemophobia has resulted in the development of a multitude of “DEET-free” mosquito repellents with a variety of active ingredients. Plant-based repellents usually contain essential plant oils as active ingredients.
There are several approaches for evaluating the efficacy of insect repellents. Some of the bioassays that have been used include spatial repellency assay, host attraction-inhibition assay, landing inhibition assay, effective dose and duration assays, taxis cage assays, etc. (Lorenz et al. 2013, Afify et al. 2014, Menger et al. 2014). Olfactometers are useful tools used in attraction-inhibition assays to test repellent efficacy. They allow the experimenter to perform the tests under very controlled conditions, thereby eliminating many variables that may alter experimental results in more open systems (McIndoo 1926).
Here, we report experiments performed with a Y-tube olfactometer that was constructed according to a blueprint published by the World Health Organization in its publication “Guidelines for efficacy testing of spatial repellents” (World Health Organization [WHO] 2013). We performed host attraction-inhibition assays and tested the efficacy of eight commercially available mosquito repellent products, a perfume, a bath oil, and a vitamin B patch.
Materials and Methods
Mosquito Culture
Ae. aegypti UGAL strain and Ae. albopictus F10 strain were acquired from the Malaria Research and Reference Reagent Resource Center (ATCC 2015). Mosquito culture was executed as described in Marquardt(2004), using chicken for a blood meal source. Larvae were reared in 13” by 20” pans filled with 2 liters of deionized water held at 27°C. Larvae were given five dry cat food pellets (Special Kitty-Wal-Mart Stores, Inc. Bentonville, AR) every 3 d; water was changed after 5 d. The mosquitoes were allowed to mature for 5 d in a BugDorm-1 Insect Rearing Cage (30 by 30 by 30 cm, BugDorm Store, Taichung, Taiwan) before the experiment commenced. The adult mosquitoes were maintained on 20% sucrose solution, ad libitum, up to 24 h before the experiment began. The cages were placed in an incubation room that was maintained at 80% humidity and 27°C with a photoperiod of 14:10 (L:D) h. Mosquitoes were starved 24 h before each Y-tube assay.
Institutional Review Board Approval
This study has been approved by the New Mexico State University Institutional Review Board. Title: “Efficacy of different insect repellents,” study no. 11505A.
Selection of Commercially Available Repellents
Repellents were purchased locally in Las Cruces, NM, or ordered online via Amazon. Table 1 lists the products tested, active ingredients, manufacturer and location, and the manufacturer’s estimated protection times. The Mosquito Skin Patch was applied 2 h before the start of the experiment.
Table 1.
Active ingredients, manufacturers, and estimated protection time of the repellents, fragrances, and patch
| Product name | Product type | Active ingredient(s) | Manufacturer/distributor | Estimated protection timea |
|---|---|---|---|---|
| Repel 100 insect repellent | Repellent spray | DEET (98.11%) | WPC Brands, Inc. | 10 h |
| OFF deep woods insect repellent VIII | Repellent spray | DEET (25%) | S.C. Johnson & Son, Inc. | Not provided |
| Cutter skinsations insect repellent | Repellent spray | DEET (7.0%) | Spectrum Division of United Industries Corporation | Not provided |
| Cutter natural insect repellent | Repellent spray | Geraniol (5%) | Spectrum Division of United Industries Corporation | 2 h |
| Soybean oil (2%) | ||||
| EcoSmart organic insect repellent | Repellent spray | Geraniol (1.0%) | EcoSMART Technologies Inc. | 2 h |
| Rosemary oil (0.5%) | ||||
| Cinnamon oil (0.5%) | ||||
| Lemongrass oil (0.5%) | ||||
| Cutter lemon eucalyptus insect repellent | Repellent spray | Oil of lemon eucalyptus (30%) | Spectrum Division of United Industries Corporation | 6 h |
| This oil contains 65% p-menthane-3-8-diol | ||||
| Avon Skin So Soft Bug Guard | Repellent spray | Oil of citronella (10%) | Avon Products, Inc. | 2 h |
| Avon Skin So Soft Bath Oil | Fragrance | Unknown | Avon Products, Inc. | Not Recommended |
| Victoria Secret Bombshell | Fragrance | Unknown | Victoria Secret | Not Recommended |
| Mosquito skin patch | Patch | Thiamin B1 (300 mg) | AgraCo Technologies International, LLC | 36 h |
a Manufacturer provided estimated protection time.
Attraction-Inhibition Assay
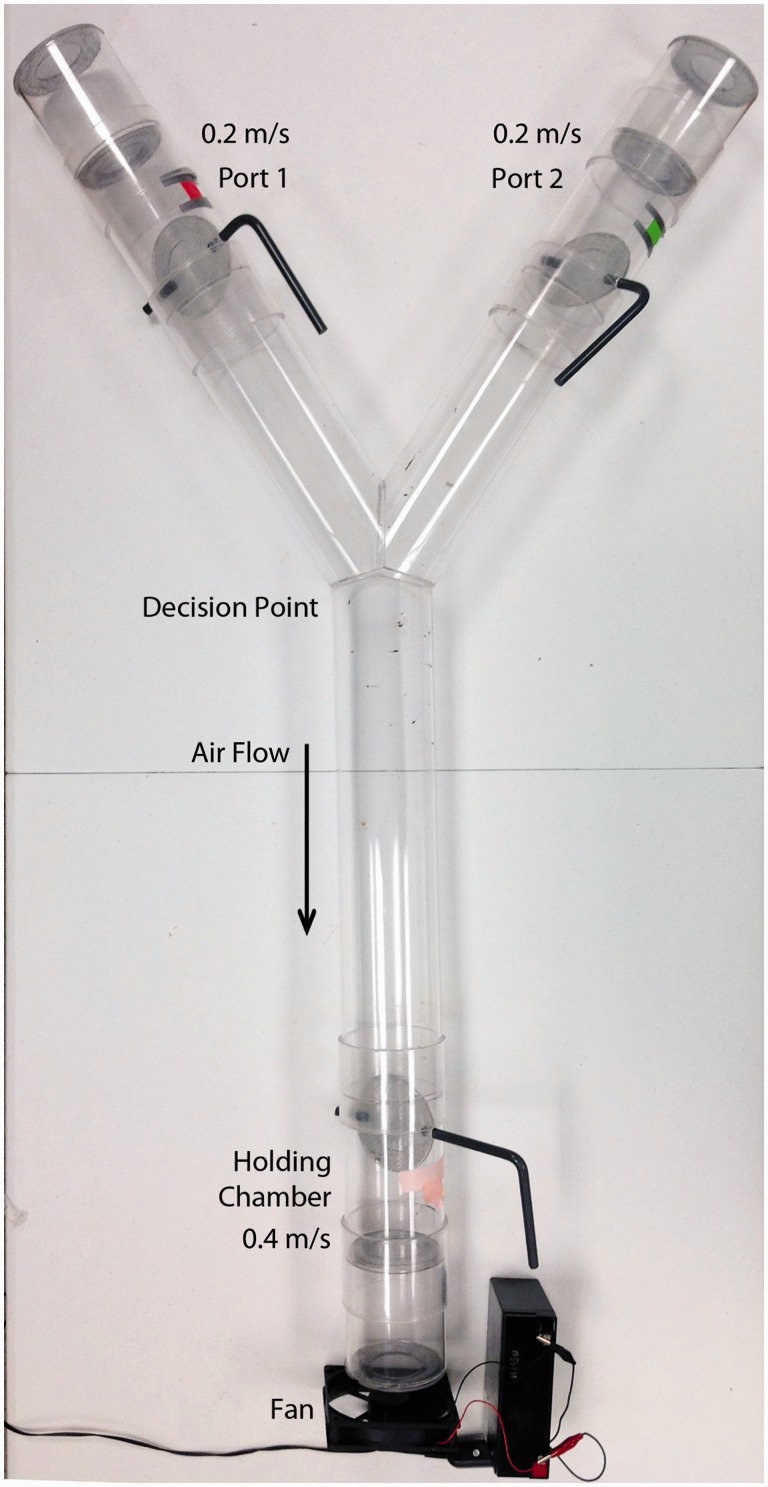
The Y-tube (see Fig. 1 and Supp Material 1 [online only]) was constructed according to the WHO schematic with modifications (WHO 2013). A constant air current was produced by a computer fan that was placed at the bottom of the Y-tube. The air flow was adjusted to 0.4 m/s in the base leg (0.2 m/s in each port) by placing the probe of an anemometer within the different shafts of the Y-tube and moving the fan in relation to the tube opening until the correct air flow speed was achieved. Experiments were all performed between 0800 and 1200 to avoid diurnal changes in mosquito activity. One of the co-authors was selected as the attractant for Y-tube assays based on preliminary attraction studies that found her to be a superior attractant. The volunteer was not allowed to wash her hands, wear perfume, or take a shower in the morning prior to the experiments. One of the attractant’s hands was sprayed with approximately 0.5 ml of liquid repellent; the other hand was covered with a nitrile glove. The hand was sprayed on both sides and allowed to air dry. Trap doors 1 and 2 were opened and the mosquitoes were placed in the holding chamber of the Y-tube. The treated hand was then placed in one of the decision ports (from here on referred to as the “hand port”); the other, untreated gloved hand was inserted into the other port (control port). Alternating decision ports were used for the biological replicates to ensure there was no bias. The mosquitoes were given 30 s to acclimate to their environment while exposed to the odor on the hand before they were released from the holding chamber by opening trap door 3. The mosquitoes were given 2 min to relocate within the tube. After a 2-min period, all trap doors were closed. Three groups of mosquitoes were counted and recorded: the ones that stayed in the holding port, the ones that arrived in either decision port, and the ones that stayed in the shaft of the Y-tube. The mosquitoes that were not captured at either decision port or in the holding port were considered wandering. For each replicate, there were a total of 20 mosquitoes placed in the holding chamber. We evaluated efficacy of the repellent over a 4-h time period with evaluation points at: 0 min, 30 min, 120 min, and 240 min post application. The experiments commenced in the early morning and ran for 4 h, the time of commencement represented the laboratory mosquitoes’ dawn. Five replicates were performed for each time point, and the experiments were performed over a 3-mo period. Attraction rate (%) was calculated as the number of mosquitoes in the treated hand port divided by the total number of mosquitoes in the replicate.
Fig. 1.
The Y-tube used in the attraction-inhibition assays.
Statistical Methods
To evaluate the efficacy of each repellent, a one-way repeated measures analysis of variance was used. The dependent variable was the rank-transformed ratio of the number of mosquitoes that ended up in the port containing the hand or the holding port vs. the total number of mosquitoes in the test. One test was performed for the mosquitoes in the hand port and a second for those in the holding chamber. Dunnett’s multiple comparison procedure was used, with the untreated time zero control group as the control.
Results
Ae. aegypti Attraction-Inhibition Assays
Table 2 lists the overall attraction rates of Ae. aegypti females to an untreated control hand and hands treated with various repellents. The overall attraction rate was determined as an average of five replicates, calculating the number of mosquitoes in the treated hand port out of the total mosquitoes in the replicate.
Table 2.
Average percentage of Ae. aegypti mosquitoes trapped in the port with the hand
| Treatments (N = 5) | Initial (±SE) | 30 min (±SE) | 120 min (±SE) | 240 min (±SE) |
|---|---|---|---|---|
| Control | 61 (±4.30) | 61 (±4.00) | 58 (±2.00) | 68 (±3.39) |
| Repel 100 insect repellent | 10** (±1.58) | 18** (±3.39) | 15** (±5.24) | 14** (±4.85) |
| OFF deep woods insect repellent VIII | 6** (±1.87) | 17** (±1.22) | 14** (±1.00) | 29** (±3.32) |
| Cutter skinsations insect repellent | 11** (±3.67) | 22** (±5.15) | 17** (±2.55) | 30** (±5.24) |
| Cutter natural insect repellent | 57 (±3.39) | 47 (±4.06) | 64 (±1.87) | 65 (±6.12) |
| EcoSmart organic insect repellent | 9** (±1.87) | 55 (±3.16) | 68 (±2.00) | 67 (±3.39) |
| Cutter lemon eucalyptus insect repellent | 9** (±2.45) | 8** (±3.00 | 13** (±4.64) | 18** (±3.74) |
| Avon Skin So Soft Bug Guard | 48 (±4.06) | 42* (±2.55) | 52 (±2.55) | 67 (±5.15) |
| Avon Skin So Soft Bath Oil | 31** (±1.00) | 35** (±5.70) | 43* (±6.04) | 53 (±8.00) |
| Victoria Secret Bombshell | 17** (±5.39) | 15** (±5.24) | 18** (±3.00) | 47 (±5.39) |
| Mosquito skin patch | 68 (±5.10) | 67 (±5.61) | 48 (±4.90) | 68 (±5.15) |
N, number of replicates.
*Significantly different than the control, P < 0.05.
**Significantly different than the control, P < 0.01.
The control, bare hand, elicited an attraction rate of 61% (±1.6) from Ae. aegypti female mosquitoes during the initial evaluation. As expected, controls at time 30 min, 120 min, and 240 min were not significantly different from the control at time 0 min.
Application of sprays containing DEET (Repel 100 Insect Repellent, OFF Deep Woods Insect Repellent VIII, and Cutter Skinsations Insect Repellent) resulted in a strong reduction of attraction. At the 120 min, all three spray treatments still confer maximal protection, while at the 240 min, this effect starts to wear off with the lower DEET concentrations.
Application of DEET-free repellent sprays (Cutter Lemon Eucalyptus, Ecosmart Organic Insect Repellent, Cutter Natural insect repellent, and Avon Skin So Soft Bug Guard) produced different results. Two of the sprays (Cutter Natural insect repellent and Avon Skin So Soft Bug Guard) did not result in any reduction of attraction. Ecosmart Organic Insect Repellent resulted in a strong reduction of attraction at 0 min, but this effect did not persist after 30 min or any of the later time points.
Avon Skin So Soft bath oil resulted in significant reduction of attraction nearly half at the initial time point when compared with the control that had a 61% attraction rate. The effect was still significant at 120 min, but this effect was not seen at 240 min. Interestingly, Victoria Secret Bombshell repelled mosquitoes quite effectively 120 min post application. The Mosquito Skin Patch did not reduce attraction rates.
Ae. albopictus Attraction-Inhibition Assays
Table 3 lists the overall attraction rates of Ae. albopictus females to an untreated control hand and hands treated with various repellents. The overall attraction rates were determined as described above.
Table 3.
Average percentile of Ae. albopictus mosquitoes trapped in the port with the hand
| Treatments (N = 5) | Initial (±SE) | 30 (±SE) | 120 (±SE) | 240 (±SE) |
|---|---|---|---|---|
| Control | 41 (±6.96) | 50 (±4.18) | 41 (±3.32) | 47 (±7.68) |
| Repel 100 insect repellent | 10** (±2.24) | 7** (±3.39) | 14** (±4.30) | 14** (±2.45) |
| OFF deep woods insect repellent VIII | 7** (±3.00) | 13** (±3.74) | 15** (±3.54) | 27 (±2.55) |
| Cutter skinsations insect repellent | 20** (±4.74) | 18** (±5.39) | 18** (±4.64) | 30 (±4.74) |
| Cutter natural insect repellent | 33 (±3.74) | 26 (±2.92) | 37 (±3.00) | 50 (±4.47) |
| EcoSmart organic insect repellent | 5** (±2.74) | 15** (±2.74) | 23* (±5.61) | 15** (±3.16) |
| Cutter lemon eucalyptus insect repellent | 9** (±2.92) | 18** (±7.00) | 23* (±4.06) | 22* (±2.00) |
| Avon Skin So Soft Bug Guard | 21** (±1.87) | 27 (±3.39) | 20** (±3.54) | 38 (±6.44) |
| Avon Skin So Soft Bath Oil | 31 (±3.67) | 36 (±3.67) | 36 (±2.92) | 56 (±5.79) |
| Victoria Secret Bombshell | 14** (±4.58) | 24* (±3.32) | 17**(±3.74) | 35(±4.74) |
| Mosquito skin patch | 40 (±4.18) | 42 (±5.15) | 34 (±1.87) | 39 (±2.92) |
N, number of replicates.
*Significantly different than the control, P < 0.05.
**Significantly different than the control, P < 0.01.
The control, bare hand, elicited an initial attraction rate of 41% (±6.96). As expected, controls at 30 min, 120 min, and 240 min were not significantly different from the control at 0 min.
The DEET-containing sprays reduced attraction rates significantly for 120 min. The repellency effect was lost at 240 min resulting in no significant difference between the control and the treated hand with the two lower concentrated DEET sprays (OFF Deep Woods Insect Repellent VIII and Cutter Skinsations Insect Repellent).
The DEET-free repellent sprays produced varied results. Cutter Natural Insect Repellent produced no significant reduction in attraction. Avon Skin So Soft Bug Guard achieved a significant reduction in attraction of Ae. albopictus at 0 min and at 120 min after application.
Avon Skin So Soft bath oil had no effect on the attraction of Ae. albopictus. Victoria Secret Bombshell repelled mosquitoes quite effectively during the first 120 min after application. The Mosquito Skin Patch did not reduce attraction rates for both species.
Discussion
In the last centuries, mosquitoes of the genus Aedes have extended their range from tropical and subtropical habitats to more temperate climates (Lounibos 2002). Both Ae. aegypti and Ae. albopictus mosquitoes are vectors for human diseases (Brunkard et al. 2007, Shepard et al. 2011, Joy et al. 2012).
Mosquito repellents are an effective way to reduce vector/host contacts and incidence of vector-borne diseases on an individual level. The more effective and long-lasting the efficacy of a repellent is, the higher the level of protection it provides to the consumer. Therefore, information on the efficacy of repellents is extremely valuable for the individual consumer to make informed choices. A multitude of studies has addressed this problem using different techniques (Barnard et al. 1998, Kline et al. 2003, Barnard and Xue 2004, Trongtokit et al. 2005, Tsetsarkin et al. 2007).
For this study, we chose to test the efficacy of several commercially available repellents, two fragrances, and a mosquito repellent patch on two different Aedes species. We found that overall Ae. albopictus was not as strongly attracted to the control hand as Ae. aegypti. This result confirms the deduction by Lupi et al. which reviewed 102 publications on this topic (Lupi et al. 2013, Faherty 2015).
Ae. aegypti was strongly repelled by DEET-containing sprays (Table 1). Two of the DEET-free sprays produced little or no repellency effect (Cutter Natural Insect Repellent and Avon Skin So Soft Bug Guard). EcoSmart Organic Insect Repellent produced a strong but very short-duration repellency toward Ae. aegypti. One of the DEET-free sprays we tested (Cutter Lemon Eucalyptus Insect repellent) conferred long-lasting, strong repellency against this species. Avon Skin So Soft bath oil has been used as a home remedy for mosquito repellency and, in this study, has shown some level of protection for 120 min.
Interestingly, other studies have shown that floral scents attract mosquitoes, and it is suggested to avoid floral scented perfumes to reduce mosquito attraction (Beever 2006). Surprisingly, the perfume we tested, Victoria Secret Bombshell (Fragrance type: Fruity floral notes: Purple passion fruit, Shangri-la peony, Vanilla orchid [2015]) has shown to be a strong repellent with effects lasting longer than 120 min. It must be noted that the concentration of perfume we used in this test was rather high and that lower concentrations of the same fragrance might have different effects.
Mosquito repellent patches for the transdermal delivery of thiamine (vitamin B1) have been available to consumers for decades. The proposed mode-of-action is that the patch alters blood chemistry and human smell making the user less attractive to mosquitoes. Our results show that the Mosquito Skin Patch did not provide any repellency against Ae. aegypti or Ae. albopictus, which confirms a study from 1969 with similar results (Khan et al. 1969, Kahn et al. 1975).
Ae. albopictus females were also strongly repelled by DEET-containing sprays similar to Ae. aegypti females (see Tables 2 and 3). However; the results obtained from the DEET-free sprays differed from the results Ae. aegypti female repellency tests in two instances. While Cutter Natural Insect Repellent did not work in either species, Avon Skin So Soft Bug Guard reduced the number of Ae. albopictus mosquitoes attracted to the hand by almost 50%. Interestingly, EcoSmart Organic Insect Repellent lost its effect on Ae. aegypti after 30 min of exposure; however, the effect on Ae. albopictus was still prominent after 240 min of exposure.
Another notable result is that Avon Skin So Soft Bath Oil in contrast to our results with Ae. aegypti females did not confer any significant protection from Ae. albopictus females. The perfume (Victoria Secret Bombshell) had similar results in both species. Lastly, as has been shown in prior studies, the Mosquito Skin Patch had no effect on either species (Revay et al. 2013).
In summary, the results of this study show that not all commercially available mosquito repellents are effective in repelling mosquitoes and that efficacy is also dependent on the species of mosquito that is repelled. Overall, the results from this study confirm that DEET repellents are the most effective mosquito repellents in the market. Although, based on the results from this study, a lemon-eucalyptus oil containing p-menthane-3,8-diol has similar efficacy compared with DEET repellents. This DEET-free repellent had similar rates and duration of repellency as the commercially available DEET repellents. Our results challenge the notion that floral perfume-scented sprays, in general, attract mosquitoes. Floral fragrances may provide a masking odor resulting in low mosquito attraction rates but over a shorter duration of time. Our study has confirmed earlier results that vitamin B1 is not a systemic insect repellent.
Supplementary Data
Supplementary data are available at Journal of Insect Science online.
Acknowledgments
We would like to thank Dean Rodriguez for building the Y-tube olfactometer.
References Cited
- Afify A., Horlacher B., Roller J., Galizia C. G. 2014. Different repellents for Aedes aegypti against blood-feeding and oviposition. PLoS One 9: e103765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alphey L., Benedict M., Bellini R., Clark G. G., Dame D. A., Service M. W., Dobson S. L. 2010. Sterile-insect methods for control of mosquito-borne diseases: an analysis. Vector Borne Zoonotic Dis. 10: 295–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (ATTC) American Type Culture Collection. 2015. Malaria Research and Reference Reagent Resource Center. https:// www. mr4. org/. [Google Scholar]
- Barnard D. R. 2000. Repellents and toxicants for personal protection: a WHO position paper. World Health Organization, Geneva. [Google Scholar]
- Barnard D. R., Xue R. D. 2004. Laboratory evaluation of mosquito repellents against Aedes albopictus, Culex nigripalpus, and Ochierotatus triseriatus (Diptera: Culicidae). J. Med. Entomol. 41: 726–730. [DOI] [PubMed] [Google Scholar]
- Barnard D. R., Posey K. H., Smith D., Schreck C. E. 1998. Mosquito density, biting rate and cage size effects on repellent tests. Med. Vet. Entomol. 12: 39–45. [DOI] [PubMed] [Google Scholar]
- Beever R. 2006. Mosquito repellent effectiveness: a placebo controlled trial comparing 95% DEET, Avon Skin So Soft, and a “special mixture.” BC Med. J. 48: 226–231. [Google Scholar]
- Brunkard J. M., Robles Lopez J. L., Ramirez J., Cifuentes E., Rothenberg S. J., Hunsperger E. A., Moore C. G., Brussolo R. M., Villarreal N. A., Haddad B. M. 2007. Dengue fever seroprevalence and risk factors, Texas-Mexico border, 2004. Emerg. Infect. Dis. 13: 1477–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker T., Geier M., Cardé R. T. 2005. Carbon dioxide instantly sensitizes female yellow fever mosquitoes to human skin odours. J. Exp. Biol. 208: 2963–2972. [DOI] [PubMed] [Google Scholar]
- Dickens J. C., Bohbot J. D. 2013. Mini review: mode of action of mosquito repellents. Pestic. Biochem. Physiol. 106: 149–155. [Google Scholar]
- Enserink M. 2007. Infectious diseases—Chikungunya: no longer a third world disease. Science 318: 1860–1861. [DOI] [PubMed] [Google Scholar]
- Faherty G. W. 2015. Apropos—the efficacy of repellents against Aedes, Anopheles, Culex and Ixodes spp. A literature review. Travel Med. Infect. Dis. 13: 207. [DOI] [PubMed] [Google Scholar]
- Galizia C. G. 2014. Olfactory coding in the insect brain: data and conjectures. Eur. J. Neurosci. 39: 1784–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill N., Lenglet A., Arnez A. M., Carneiro I. 2007. Plant based insect repellent and insecticide treated bed nets to protect against malaria in areas of early evening biting vectors: double blind randomised placebo controlled clinical trial in the Bolivian Amazon. BMJ 335: 1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy T. K., Gutierrez E.H.J., Ernst K., Walker K. R., Carriere Y., Torabi M., Riehle M. A. 2012. Aging field collected Aedes aegypti to determine their capacity for dengue transmission in the southwestern United States. PLoS One 7: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn A. A., Maibach H. I., Skidmore D. L. 1975. Addition of vanillin to mosquito repellents to increase protection time. Mosq. News. 35: 223–225. [Google Scholar]
- Kaupp U. B. 2010. Olfactory signalling in vertebrates and insects: differences and commonalities. Nat. Rev. Neurosci. 11: 188–200. [DOI] [PubMed] [Google Scholar]
- Khan A. A., Maibach H. I., Strauss W. G., Fenley W. R. 1969. Vitamin B1 is not a systemic mosquito repellent in man. Trans. St Johns Hosp. Dermatol. Soc. 55: 99–102. [PubMed] [Google Scholar]
- Kline D. L., Bernier U. R., Posey K. H., Barnard D. R. 2003. Olfactometric evaluation of spatial repellents for Aedes aegypti. J. Med. Entomol. 40: 463–467. [DOI] [PubMed] [Google Scholar]
- Koren G., Matsui D., Bailey B. 2003. DEET-based insect repellents: safety implications for children and pregnant and lactating women. Can. Med. Assoc. J. 169: 209–212. [PMC free article] [PubMed] [Google Scholar]
- Kwon Y., Kim S. H., Ronderos D. S., Lee Y., Akitake B., Woodward O. M., Guggino W. B., Smith D. P., Montell C. 2010. Drosophila TRPA1 channel is required to avoid the naturally occurring insect repellent citronellal. Curr. Biol. 20: 1672–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz L. M., Keane A., Moore J. D., Munk C. J., Seeholzer L., Mseka A., Simfukwe E., Ligamba J., Turner E. L., Biswaro L. R., et al. 2013. Taxis assays measure directional movement of mosquitoes to olfactory cues. Parasit. Vectors 6: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos L. P. 2002. Invasions by insect vectors of human disease. Annu. Rev. Entomol. 47: 233–266. [DOI] [PubMed] [Google Scholar]
- Lupi E., Hatz C., Schlagenhauf P. 2013. The efficacy of repellents against Aedes, Anopheles, Culex and Ixodes spp.—a literature review. Travel Med. Infect. Dis. 11: 374–411. [DOI] [PubMed] [Google Scholar]
- Marquardt W. H. 2004. Biology of disease vectors. Academic Press, Amsterdam. [Google Scholar]
- McIndoo N. 1926. An insect olfactometer. J. Econ. Entomol. 19: 545–571. [Google Scholar]
- Menger D., Van Loon J., Takken W. 2014. Assessing the efficacy of candidate mosquito repellents against the background of an attractive source that mimics a human host. Med. Vet. Entomol. 28: 407–413. [DOI] [PubMed] [Google Scholar]
- Murray K. O., Mertens E., Despres P. 2010. West Nile virus and its emergence in the United States of America. Vet. Res. 41: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osimitz T., Grothaus R. H. 1995. The present safety assessment of deet. J. Am. Mosq. Control Assoc. 11: 274–278. [PubMed] [Google Scholar]
- Peter R. J., Van Den Bossche P., Penzhorn B. L., Sharp B. 2005. Tick, fly, and mosquito control–lessons from the past, solutions for the future. Vet. Parasitol. 132: 205–215. [DOI] [PubMed] [Google Scholar]
- Phuc H. K., Andreasen M. H., Burton R. S., Vass C., Epton M. J., Pape G., Fu G., Condon K. C., Scaife S., Donnelly C. A., et al. 2007. Late-acting dominant lethal genetic systems and mosquito control. BMC Biol. 5: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter C. J. 2014. Stop the biting: targeting a mosquito's sense of smell. Cell 156: 878–881. [DOI] [PubMed] [Google Scholar]
- Revay E. E., Junnila A., Xue R. D., Kline D. L., Bernier U. R., Kravchenko V. D., Qualls W. A., Ghattas N., Muller G. C. 2013. Evaluation of commercial products for personal protection against mosquitoes. Acta Trop. 125: 226–230. [DOI] [PubMed] [Google Scholar]
- Rose R. I. 2001. Pesticides nd public health: Integrated methods of mosquito management. Emerg. Infect. Dis. 7: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland M., Downey G., Rab A., Freeman T., Mohammad N., Rehman H., Durrani N., Reyburn H., Curtis C., Lines J., et al. 2004. DEET mosquito repellent provides personal protection against malaria: a household randomized trial in an Afghan refugee camp in Pakistan. Trop. Med. Int. Health 9: 335–342. [DOI] [PubMed] [Google Scholar]
- Shepard D. S., Coudeville L., Halasa Y. A., Zambrano B., Dayan G. H. 2011. Economic impact of dengue illness in the Americas. Am. J. Trop. Med. Hyg. 84: 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakin D. L., Trevathan W. R. 2003. DEET: a review and update of safety and risk in the general population. J. Toxicol. Clin. Toxicol. 41: 831–839. [DOI] [PubMed] [Google Scholar]
- Suh E., Bohbot J., Zwiebel L. J. 2014. Peripheral olfactory signaling in insects. Curr. Opin. Insect Sci. 6: 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trongtokit Y., Rongsriyam Y., Komalamisra N., Apiwathnasorn C. 2005. Comparative repellency of 38 essential oils against mosquito bites. Phytother. Res. 19: 303–309. [DOI] [PubMed] [Google Scholar]
- Tsetsarkin K. A., Vanlandingham D. L., Mcgee C. E., Higgs S. 2007. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 3: e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoria’s Secret. 2015. Bombshell Eau de Parfum. https:// www. victoriassecret. com/ beauty/ victorias- secret- bombshell/ bombshell- eau- de- parfum- victorias- secret?cm_ sp=& ProductID= 262444& CatalogueType= OLS. [Google Scholar]
- (WHO) World Health Organization. 2013. Guidelines for efficacy testing of spatial repellents, p. 58 WHO Press, Geneva. [Google Scholar]
- Xu P., Choo Y. M., De La Rosa A., Leal W. S. 2014. Mosquito odorant receptor for DEET and methyl jasmonate. Proc. Natl. Acad. Sci. USA. 111: 16592–16597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwiebel L. J., Takken W. 2004. Olfactory regulation of mosquito-host interactions. Insect Biochem. Mol. Biol. 34: 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]