Abstract
Background
Food cravings are more prevalent and potentially problematic for many individuals with obesity. Initial evidence suggests that bariatric surgery has some short-term beneficial effects on cravings in adults, but little is known about the effect on adolescents or the trajectory beyond 6 months.
Methods
The purpose of the present study was to determine the longitudinal effect of Roux-en-Y gastric bypass (RYGB) on food cravings in a sample of adolescents with severe obesity (body mass index (BMI) ≥40 kg/m2). Sixteen adolescents were recruited and underwent RYGB. Participants completed the Food Craving Inventory before RYGB, and 3, 6, 12, 18, and 24 months postoperatively. The present study took place in a single pediatric tertiary care hospital.
Results
RYGB produced a negative (cravings decreased as time increased) nonlinear trend for total food cravings as well as for each individual subscale (sweets, high fat foods, carbohydrates, fast food) over the 24-month study period. This means that while cravings decrease postsurgically, there is a decline in the slope with the line reaching asymptote at approximately 18 months. BMI change was not a significant predictor of food cravings, but low statistical power may account for this lack of significance.
Conclusion
These findings provide preliminary evidence that RYGB decreases food cravings in adolescents.
Keywords: Food cravings, Roux-en-Y gastric bypass, Adolescents, Longitudinal data
Food craving is commonly defined as the subjective experience of an intense desire to consume food categorized in 3 naturally occurring food classes (e.g., carbohydrates, sweets, and fats), and one environmentally derived category (fast-food fats) [1]. People who experience food cravings report multiple episodes each week (both using retrospective report and 5 weeks of prospective assessment), which are typically followed by eating the craved food or a similar type of food [2–4]. Self-reported food cravings are usually higher among obese individuals compared to normal weight controls [1]. Moreover, it appears that the relationship between body mass index (BMI) and cravings for high-fat foods [1] as well as fast-food fats and carbohydrates is positive and linear [5]. Behavioral weight loss programs have demonstrated that restricting food intake produces changes in craved foods with low (i.e., 1000–1200 kcal/d) and very low calorie diets (i.e., 400 kcal/d), yielding reductions that generalize to all types of craved foods [6–8].
Initial evidence suggests that bariatric surgery for weight loss also has some short-term effects on sweet cravings in adult samples [9–11]. However, methodological problems including limited assessment of the type of foods craved and use of novel instruments make it difficult to extrapolate the findings [11]. In the most rigorous prospective study to date, a bariatric sample of adults demonstrated significant elevations in food cravings presurgically relative to normal weight controls; postsurgically the bariatric surgery group demonstrated significant and sustained reductions in total food cravings at 3 and 6 months postoperatively. However, no changes were noted in the levels of high-fat cravings. Moreover, a return to presurgical levels was noted in the carbohydrates scale at 6 months, and an upward trend (toward initial baseline values) was apparent in the sweet cravings scale. This raises questions regarding the stability of food cravings following bariatric surgery suggesting that changes are nonlinear with a pattern of initial decrease followed by rebound in cravings. Moreover, the best available data is limited to 6 months postsurgically [11]. Finally, the current literature is limited to adult populations.
The aims of the current pilot study were to ascertain for the first time the effect of Roux-en-Y gastric bypass (RYGB) on food cravings in adolescent (ages 13–17) patients. The assessment of food cravings at multiple postoperative time points across the first 24 months extends knowledge beyond that of the limited adult literature (e.g., 6 months) [11] and allows exploration of how food cravings may change as a function of BMI in adolescents [12,13]. Based on the limited adult literature, as well as nonlinear trends of both anthropometric and psychosocial variables in bariatric populations [12,13], it was hypothesized that food cravings would demonstrate a negative nonlinear trend.
Methods
Participants and procedures
Data presented in this manuscript were collected as part of a prospective observational examination of psychosocial outcomes for adolescents undergoing RYGB conducted within a bariatric surgery program for adolescents at a single pediatric tertiary care hospital [12,14]. Data were collected preoperatively (baseline) and at 3-month, 6-month, 12-month, 18-month, and 24-month postoperative intervals. The local institutional review board approved the protocol described below.
Participants
Eligibility for the present study required participants to have previously met criteria (e.g., BMI ≥40 kg/m2) for program enrollment based on published adolescent guidelines [15], as well as obtain insurance approval for bariatric surgery. Additional inclusion criteria were: 14–17 years of age at the time of surgery and having no developmental disabilities due to the high reading demands of the research protocol. During the study enrollment period, 16 consecutive eligible patients were approached, and all gave written informed parental consent and adolescent assent to participate.
Study retention was high with data obtained at all 5 follow-up time points for 75% of the sample and at 4 time points for 94%. Data collection was completed for 88% of the sample (n = 14) at the 24-month interval. Data collection occurred using several different methods to reduce participant burden and maximize retention. Height and weight were assessed, and self-report measures were administered at scheduled clinical visits when possible. If participants were unable to attend in-person, self-reported height and weight were obtained, and questionnaires were administered by trained study staff in-home, by telephone, or by regular mail at 18-month (47% mail; 7% in-home) and 24-month (7% mail; 36% in-home) time points. Participants were compensated for their involvement.
Measures
Demographic characteristics and BMI at baseline
Participants were primarily girls (62.5%), nonHispanic Caucasian (87.5%), and from a wide geographic area (7 states). The mean age for participants was 16.2 ± 1.4 years. The mean BMI for the sample was M = 59.91 ± 8.71 kg/m2 at baseline. Half of the participants’ mothers were married (n = 8), while 43.8% were divorced or separated (n = 7) and one mother was single (6.3%). All families had at least one other child living in the home (M = 2.13 ± 1.03 children). The majority of families were living on $30,000 or less per year (n = 9).
Food cravings
Food cravings were assessed using the Food Craving Inventory (FCI) [1]. The FCI is a 28-item instrument that asks the respondent to rate how often over the past month he or she has experienced a craving for a particular food. Respondents answer using a 0–4 scale with the following anchors: 0 = Never, 1 = Rarely (once or twice), 2 = Sometimes, 3 = Often, 4 = Always/Almost Every Day. Therefore, higher scores indicate greater food cravings. The FCI provides a total score (mean of the 28 items) and 4 subscale scores including cravings for sweets (e.g., ice cream), high-fat foods (e.g., fried chicken), fast food (e.g., hamburger), and carbohydrates (e.g., rolls). The scale has demonstrated good psychometric properties in college student populations (mean age 20.30 ± 2.98) with good internal consistency estimates (α = .76–0.93) and test-retest reliability ranging from .79 to 0.91 [1].
Statistical analysis
Hierarchical linear modeling using Mplus version 6.12 (Los Angeles, CA) [16] was used to estimate the average growth trajectory of food cravings over time. Complete data were available for 75% of the sample, however, Mplus allows for the use of full information maximum likelihood estimation, which handles missing data by estimating a complete covariance matrix and retaining all participants with any data points in the analysis. In growth curve analysis, significant power gains are available to models using 4 or more points of data. Even with these power gains, however, only large effect sizes will be detectable with a sample of n = 16 patients [17].
As stated above, a nonlinear trend was hypothesized over time based on the established nonlinear trend of both BMI, hedonic hunger, and some components of food cravings in bariatric populations [11–13]. We used a transformation of the time variable itself (log[month + .5]) as a single nonlinear function of time [(L)time]. A log transformation produces a curve similar to the quadratic polynomial for monotonic curves [18]. Each response variable was analyzed using a 3-step procedure: (1) a fixed-effect model was estimated first, where response variable changes over (L) time are not allowed to vary across participants followed by (2) a random-effect model that tested for the presence of significant variation across participants in response variable changes over (L)time, and (3) BMI was added as a covariate to assess the effect of changes in anthropometric values on food cravings.
Results
Frequency of food craving
To characterize the severity of food cravings in the current sample, we examined frequency data at baseline. In terms of total food cravings, adolescents used approximately the midpoint of the 0–4 scale (mode = 2) which corresponds to a response of “sometimes” craving the foods assessed by the FCI. Of the subscales contributing to the total score, the largest elevation was seen in the fast-food scale with these types of cravings occurring “often.” The remainder of the subscales were qualitatively in the “sometimes” range (Table 1).
Table 1.
Means and standard deviations of BMI and cravings over time
| Variable | Baseline (n = 16) | 3-mo FU (n = 15) | 6-mo FU (n = 14) | 12-mo FU (n = 15) | 18-mo FU (n = 15) | 24-mo FU (n = 14) |
|---|---|---|---|---|---|---|
| BMI | 59.9 ± 8.7 | 49.0 ± 9.1 | 42.2 ± 7.8 | 36.9 ± 4.9 | 35.2 ± 5.1 | 38.4 ± 7.5 |
| Crave total | 2.2 ± .7 | 2.3 ± .9 | 1.9 ± .8 | 1.9 ± .7 | 1.7 ± .5 | 1.7 ± .5 |
| Crave fat | 2.0 ± .7 | 2.1 ± .9 | 1.8 ± .8 | 1.7 ± .6 | 1.5 ± .4 | 1.5 ± .3 |
| Crave sweet | 2.3 ± .9 | 2.2 ± .9 | 1.9 ± .9 | 2.0 ± .8 | 1.8 ± .6 | 1.8 ± .6 |
| Crave carb | 2.1 ± .7 | 2.2 ± 1.0 | 2.0 ± .9 | 2.0 ± .9 | 1.7 ± .7 | 1.7 ± .7 |
| Crave fast | 2.8 ± .9 | 2.9 ± 1.2 | 2.3 ± 1.0 | 2.2 ± .8 | 2.3 ± 1.0 | 2.3 ± 1.0 |
BMI = Body mass index; Carb = Carbohydrates; Crave = Food cravings; Fast = Fast food fats; FU = Follow-up; Mo = Months.
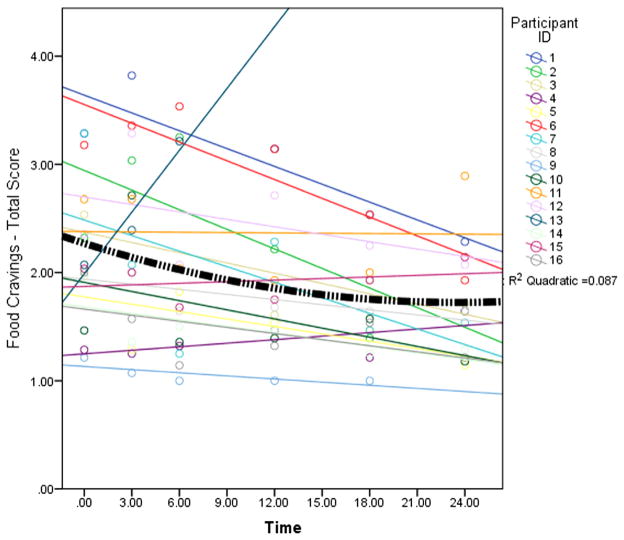
Results of the food cravings models revealed significant (P < .05) negative nonlinear effects over (L)time, and medium to large r2 effect sizes, for the FCI total score (β = −.20, r2 = .33; Fig. 1), fat (β = −.16, r2 = .18), carbohydrate (β = −.19, r2 = .27), sweet (β = −.23, r2 = . 29), and fast food (β = −.17, r2 = .12) scales. Specifically, the effects were negative indicating the largest effect on food cravings occurred in the first 6 months followed by minimal decreases at 24 months. Food cravings did not vary as a function of changes in BMI (P = .09).
Fig. 1.
Individual food cravings scores (circles), the individual regression lines (straight lines), and the overall non-linear effect of time on total food cravings for the sample (thick black dashed line). Note: Frequency of food cravings measured at baseline, 3 months, 6 months, 12 months, 18 months, and 24 months postoperatively.
Discussion
The present study examined the effect of RYGB on food cravings in adolescents. Consistent with the study hypothesis, the data from this sample indicate that food cravings follow a nonlinear trend. Specifically, there were steep decreases in the first 6 months and a slowed rate of decline out to 24 months. Our findings diverge from initial short-term (i.e., 6-month) outcome data in adults [11], as there was no recurrence of food cravings for the adolescent patient group as a function of time. However, as can be seen from the individual plots (Fig. 1), there are several patterns of variability within the data that may indicate heterogeneity in the food-craving response to RYGB. These preliminary data provide cause for cautious optimism that RYGB can positively affect food cravings in adolescents with severe obesity. Nonetheless, replication of these findings with a considerably larger sample is imperative.
Several possible explanations for these changes in food cravings following RYGB are offered. Most obvious, due to the restrictive nature of the surgery (small gastric pouch) and subsequent complications (e.g., the dumping syndrome), RYGB recipients would be unable to eat the desired levels of craved foods as they had before surgery due to the newly restricted stomach size. This is analogous to a low or very low calorie diet, which has been demonstrated to decrease food cravings in adults [6,7]. Indeed, one study by Martin et al. [7] demonstrated a similar curve in food cravings using behavioral dietary restriction. Participants on a very low calorie diet demonstrated a similar nonlinear curve in carbohydrate cravings with decreases through the 6-week assessment window followed by a slowed rate of change out to 24 months. In the present study, a similar pattern emerged with steep changes through 6 months and a decelerated rate of change through 24 months. Moreover, Martin et al. [7] noted that cravings were not associated with BMI, a finding that is mirrored in the present study. This suggests that future studies may be needed to determine whether it is calorie restriction or the RYGB procedure that yields the change in cravings.
Moreover, preference for high-sugar, high-carbohydrate foods is often reduced immediately after surgery because the body typically can no longer tolerate these foods. For example, as noted by Leahey et al., reduced cravings postsurgically may be the result of the dumping syndrome, which is characterized by nausea, etc. after consuming foods high in sugar, which could make these foods more aversive and therefore avoided. However, in the present study, the frequency of the dumping syndrome and other gastrointestinal-related events were not assessed but could be a logical contributor to reduced food cravings in the early postoperative period and represent an important avenue for future investigation. Indeed, this seems a reasonable hypothesis given that even mild nausea is sufficient to reduce intake of highly palatable foods in animal models [19].
While there is reason to believe that the mechanical effects of surgery may lead to changes in food cravings, it is also possible that physiology (specifically anorexic peptides) plays a role. Changes in glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) do appear to be tied to hedonic hunger following RYGB [20–22], with one study providing some evidence that RYGB, indeed modifies hedonic hunger and dietary intake [23]. Moreover, multiple studies point to the fact that surgery type (banding or RYGB) produce differntial changes in dietary patterns [24], and peptide levels, and hedonic hunger [21–23]. It may be that changes in GLP-1, PYY or other peptides also precede and predict the changes in food cravings. Similar to our speculation regarding the mechanical precursors, we did not collect data on changes in physiology, but such an investigation will be important for moving this area forward.
There are a number of limitations in the current data. First, while the sample was large enough to detect important statistical trends, 16 participants may be too small to draw definitive inferences about the total population of adolescents who receive bariatric surgery. Recent work in adult patients has revealed that guilt related to food cravings may serve as an important mediator of weight-related outcome [25]. Therefore, it may be the presence of food cravings in combination with the individual’s interpretation of the cravings that lead to problems with weight regain. The present study lacks a comparison condition. Therefore, observation, maturation, and repeated assessment all pose a risk as potential confounders. Finally, the present study did not assess dietary intake, which is an important downstream correlate of food cravings. Future studies should assess both craving and eating related to cravings in adolescent samples. Another direction for future work will be to specifically target those individuals for whom cravings do not decline as a function of time. These individuals may be in more need of therapeutic interventions targeting eating behavior.
In conclusion, it appears that RYGB decreases food cravings relative to their baseline levels in adolescent patients. It is important to note, however, that the effect is nonlinear, and descriptive data in Table 1 reveal steep reductions in the first 6 months postoperatively and minimal decreases in the following 18 months. One of the significant strengths of the current data is that it is possible to observe the rate of change over a 2-year study window, suggesting stability in the trend. The possible differential effects of RYGB on cravings across types of subjective drives (i.e., hedonic hunger versus cravings) and populations (adult versus adolescent samples) suggest that this should be a fertile area for additional research inquiry.
Acknowledgments
This research was funded by a CReFF award (Zeller, PI) Cincinnati Children’s Hospital Medical Center – General Clinical Research Center (USPHS Grant #M01 RR 08084 from the General Clinical Research Centers Program, National Center for Research Resources/NIH) and R03 DK0788901 (Zeller, PI), Cassie Brode was funded by T32 DK 063929 (Zeller, PI). We thank Christina Ramey and Lindsay Wilson for assistance with data collection and participant retention efforts.
Footnotes
Disclosures
The authors have no commercial associations that might be a conflict of interest in relation to this article.
References
- 1.White MA, Whisenhunt BL, Williamson DA, Greenway FL, Netemeyer RG. Development and validation of the food-craving inventory. Obesity. 2002;10:107–14. doi: 10.1038/oby.2002.17. [DOI] [PubMed] [Google Scholar]
- 2.Weingarten HP, Elston D. Food cravings in a college population. Appetite. 1991;17:167–75. doi: 10.1016/0195-6663(91)90019-o. [DOI] [PubMed] [Google Scholar]
- 3.Hill AJ, Heaton-Brown L. The experience of food craving: a prospective investigation in healthy women. J Psychosom Res. 1994;38:801–14. doi: 10.1016/0022-3999(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 4.Hill AJ, Weaver CFL, Blundell JE. Food craving, dietary restraint and mood. Appetite. 1991;17:187–97. doi: 10.1016/0195-6663(91)90021-j. [DOI] [PubMed] [Google Scholar]
- 5.Burton P, Smit JH, Lightowler JH. The influence of restrained and external eating patterns on overeating. Appetite. 2007;49:191–7. doi: 10.1016/j.appet.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Harvey J, Wing RR, Mullen M. Effects on food cravings of a very low calorie diet or a balanced, low calorie diet. Appetite. 1993;21:105–15. doi: 10.1016/0195-6663(93)90003-3. [DOI] [PubMed] [Google Scholar]
- 7.Martin CK, O’Neil PM, Pawlow L. Changes in food cravings during low-calorie and very-low-calorie diets. Obesity. 2006;14:115–21. doi: 10.1038/oby.2006.14. [DOI] [PubMed] [Google Scholar]
- 8.Lappalainen R, Sjoden P, Hursti T, Vesa V. Hunger/craving responses and reactivity to food stimuli during fasting and dieting. Int J Obes. 1990;14:679–88. [PubMed] [Google Scholar]
- 9.Busetto L, Segato G, De Marchi F, et al. Outcome predictors in morbidly obese recipients of an adjustable gastric band. Obes Surg. 2002;12:83–92. doi: 10.1381/096089202321144649. [DOI] [PubMed] [Google Scholar]
- 10.Burgmer R, Grigutsch K, Zipfel S, et al. The influence of eating behavior and eating pathology on weight loss after gastric restriction operations. Obes Surg. 2005;15:684–91. doi: 10.1381/0960892053923798. [DOI] [PubMed] [Google Scholar]
- 11.Leahey TM, Bond DS, Raynor H, et al. Effects of bariatric surgery on food cravings: do food cravings and the consumption of craved foods “normalize” after surgery? Surg Obes Relat Dis. 2012;8:84–91. doi: 10.1016/j.soard.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeller MH, Reiter-Purtill J, Ratcliff MB, Inge TH, Noll JG. Two-year trends in psychosocial functioning after adolescent Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2011;7:727–32. doi: 10.1016/j.soard.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cushing CC, Benoit SC, Peugh JL, Reiter-Purtill J, Inge TH, Zeller MH. Longitudinal trends in hedonic hunger following Roux-en-Y gastric bypass in adolescents. Surg Obes Relat Dis. 2013;10(1):125–30. doi: 10.1016/j.soard.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeller MH, Modi AC, Noll JG, Long JD, Inge TH. Psychosocial functioning improves following adolescent bariatric surgery. Obesity. 2009;17:985–90. doi: 10.1038/oby.2008.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inge TH, Krebs NF, Garcia VF, et al. Bariatric surgery for severely overweight adolescents: concerns and recommendations. Pediatrics. 2004;114:217–23. doi: 10.1542/peds.114.1.217. [DOI] [PubMed] [Google Scholar]
- 16.Muthén LK, Muthén BO. Mplus user’s guide. 6. Los Angeles, CA: Muthén & Muthén; 1988–2011. [Google Scholar]
- 17.Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum; 1988. [Google Scholar]
- 18.Royston P, Altman DG. Regression using fractional polynomials of continuous covariates: parsimonious parametric modelling. J Appl Stat. 1994;43(3):429–67. [Google Scholar]
- 19.Benoit SC, Air EL, Wilmer K, et al. Two novel paradigms for the simultaneous assessment of conditioned taste aversion and food intake effects of anorexic agents. Physiol Behav. 2003;79:761–6. doi: 10.1016/s0031-9384(03)00189-6. [DOI] [PubMed] [Google Scholar]
- 20.Xanthakos SA. Bariatric surgery for extreme adolescent obesity: indications, outcomes, and physiologic effects on the gut-brain axis. Pathophysiology. 2008;15:135–46. doi: 10.1016/j.pathophys.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schultes B, Ernst B, Wilms B, Thurnheer M, Hallschmid M. Hedonic hunger is increased in severely obese patients and is reduced after gastric bypass surgery. Am J Clin Nutr. 2010;92:277–83. doi: 10.3945/ajcn.2009.29007. [DOI] [PubMed] [Google Scholar]
- 22.le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–14. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ullrich J, Ernst B, Wilms B, Thurnheer M, Schultes B. Roux-en Y gastric bypass surgery reduces hedonic hunger and improves dietary habits in severly obese subjects. Obes Surg. 2013;23:50–5. doi: 10.1007/s11695-012-0754-5. [DOI] [PubMed] [Google Scholar]
- 24.Ernst B, Thurnheer M, Wilms B, Schultes B. Differential changes in dietary habits after gastric bypass versus gastric banding operations. Obes Surg. 2009;19:274–80. doi: 10.1007/s11695-008-9769-3. [DOI] [PubMed] [Google Scholar]
- 25.Crowley NM, LePage ML, Goldman RL, O’Neil PM, Borckardt JJ, Byrne TK. The food craving questionnaire-trait in a bariatric surgery seeking population and ability to predict post-surgery weight loss at six months. Eat Behav. 2012;13:366–70. doi: 10.1016/j.eatbeh.2012.07.003. [DOI] [PubMed] [Google Scholar]