Abstract
Rationale
Evidence suggests that differential rearing influences the function of a receptor subtype critical for maintaining glutamate homeostasis. Maintaining homeostatic glutamatergic function may be an important protector against drug abuse.
Objective
This study sought to determine if differential rearing influences the function of a receptor critical for glutamate homeostasis, which could in turn affect rates of amphetamine self-administration.
Methods
Rats were assigned to enriched (EC), isolated (IC), or standard (SC) conditions. After rearing for 30 days, rats were trained to lever press for sucrose reinforcement before the implantation of indwelling jugular catheters. After reaching stable responding for amphetamine (0.03 or 0.1 mg/kg/infusion), rats were injected with five doses (0, 0.3, 1.0, 3.0, and 5.0 mg/kg) of the mGluR5 antagonist, 3-((2-methyl-1,3-thiazol-4-yl)ethynyl) pyridine hydrochloride (MTEP), 30 min before self-administration sessions. Following fixed-ratio (FR-1) testing, rats were administered identical doses of MTEP on a progressive-ratio (PR) reinforcement schedule.
Results
MTEP (3.0 mg/kg) attenuated FR-1 self-administration (0.03 mg/kg/infusion) in IC rats. MTEP also dose-dependently attenuated amphetamine self-administration (0.1 mg/kg/infusion) during FR-1 and PR sessions, with 5.0 mg/kg MTEP attenuating amphetamine self-administration in IC and SC rats and 3.0 mg/kg MTEP attenuating amphetamine self-administration in EC and SC rats. PR results also revealed that IC rats not treated with MTEP were more motivated to self-administer the higher dose of amphetamine.
Conclusions
These results suggest that the mGlu5 receptor mediates differences in drug-taking behavior among differentially reared rats. Isolation also decreased sensitivity to MTEP, suggesting that environmental factors alter glutamate homeostasis which subsequently affects sensitivity and motivation to self-administer amphetamine.
Keywords: Environmental enrichment, Glutamate, Metabotropic glutamate receptor, Amphetamine, Self-administration, Schedule of reinforcement, Rat
Introduction
The early rearing environment impacts neuronal development and alters behavioral outcomes in adulthood (Bell and Carrillo 2007; Greenough et al. 1987). A differential rearing paradigm is used in rodents to examine the influence of the environment on the neurobiological and behavioral changes that occur (Fone and Porkess 2008; Renner and Rosenzweig 1987; van Praag et al. 2000). This paradigm typically uses enriched (EC) and isolated (IC) rearing conditions during the post-weaning period. The environments differ in the amount of social contact with conspecifics, handling by the experimenters, and physical activity. In addition, the rearing home cage environments differ in terms of the presence of novel objects, home cage flooring material, and size of the home cage (Renner and Rosenzweig 1987). Each of these factors are critical elements that create the enriched environment (Renner and Rosenzweig 1987).
These two environmental conditions are often compared to a standard condition (SC) in which rats are housed in pairs without novel stimuli. It is important to note that the standard condition does not control for all the differences present in the enriched and isolated conditions but is meant to serve as a known laboratory standard. Social stimulation alone is not sufficient to produce neurobiological changes similar to those observed with enrichment and generally results in a unique neurobiological phenotype when compared with those seen in enriched or isolated housing (Renner and Rosenzweig 1987; Rosenzweig et al. 1978); however, see (Cain et al. 2012; Gill et al. 2012; Wooters et al. 2011).
Given the substantial differences in the rearing environments, it is not surprising that enrichment results in a variety of behavioral and neurobiological changes, including altering the response to a variety of rewards and changes within the mesocorticolimbic pathway (Simpson and Kelly 2011; Stairs and Bardo 2009). Evidence suggests that the most consistent effects reside within the nucleus accumbens (NAcc) and medial prefrontal cortex (mPFC; Bardo and Hammer 1991; Del Arco et al. 2007; Melendez et al. 2004; Rahman and Bardo 2008; van Praag et al. 2000; Zhu et al. 2005; Zhu et al. 2004).
The mesocorticolimbic pathway receives substantial glutamatergic input that mediates the response to psychostimulants (Gass and Olive 2008; Kalivas 2009). Glutamatergic afferents from the mPFC synapse on NAcc neurons and augment glutamate release (Bonci et al. 2003; Jay 2003; Jones and Bonci 2005; Kalivas 2009; Nicola et al. 2000; Sesack et al. 2003). These afferents aid in regulation of glutamate release in the synapse and perisynaptic region to maintain glutamate homeostasis. Dysfunction in glutamate homeostasis within the NAcc and mPFC is hypothesized to contribute to drug addiction (Kalivas 2009).
Interestingly, differential rearing alters glutamatergic function in a variety of brain areas (Renner and Rosenzweig 1987; Simpson and Kelly 2011; van Praag et al. 2000), including within the NAcc (Rahman and Bardo 2008) and mPFC (Darna et al. 2015; Melendez et al. 2004). Enrichment enhances glutamate-dependent synaptic plasticity (Artola et al. 2006; Green and Greenough 1986; Renner and Rosenzweig 1987; Sharp et al. 1985; van Praag et al. 2000). Furthermore, EC and IC rats do not differ in the basal levels of glutamate within the NAcc, mPFC, or OFC (Darna et al. 2015; Melendez et al. 2004). However, when EC and IC rats are challenged with amphetamine, EC rats exhibit more glutamate release than IC rats in both the NAcc and mPFC (Darna et al. 2015; Rahman and Bardo 2008), suggesting a deficiency in glutamatergic tone in IC rats.
EC rats have increased expression of mGluR5 dimers in the PFC compared to IC rats, making them more capable of binding glutamate in this region (Melendez et al. 2004). This group I metabotropic receptor contributes to the maintenance of glutamate homeostasis (Kalivas 2009) via regulation of the cystine-glutamate exchanger (Kupchik et al. 2012). The mGLuR5 receptor is predominately located post-synaptically on GABAergic medium spiny neurons in the NAcc core (Mitrano and Smith 2007). Recent evidence suggests that antagonism of mGluR5 prevents activation of these GABAergic medium spiny neurons in response to glutamate overflow (Scofield and Kalivas 2014). Therefore, when an mGluR5 antagonist is administered, the response to psychostimulants is reduced. For example, removal or antagonism of mGluR5 reduces acute psychostimulant-induced hyperactivity, self-administration, and reinstatement (Chiamulera et al. 2001; Gass and Olive 2008; Gormley and Rompre 2010; Hao et al. 2010; Herzig and Schmidt 2004; Martin-Fardon et al. 2009; McGeehan and Olive 2003; Veeneman et al. 2011). We hypothesize that rearing-induced glutamatergic changes contribute to the effects of differential rearing on reward sensitivity. To begin to test this hypothesis, we previously examined the effects of 3-((2-methyl-1,3-thiazol-4-yl)ethynyl) pyridine hydrochloride (MTEP), a mGluR5 antagonist, on the expression of acute amphetamine-induced hyperactivity and amphetamine-induced sensitization (Gill et al. 2012). Antagonism of mGluR5 reduced acute amphetamine-induced hyperactivity in EC and SC rats, but not IC, only following a low dose of amphetamine. Interestingly, antagonism of mGluR5 reduced the expression of sensitization only in IC rats. These results suggest that differential rearing alters mGluR5 function.
To further test our hypothesis that rearing-induced glutamatergic changes contribute to differences in reward sensitivity, the current experiment tested the effects of mGluR5 antagonism during the self-administration of a low and moderate dose of amphetamine on both a fixed-ratio (FR-1) and progressive-ratio (PR) schedule of reinforcement. We predict that antagonism of mGluR5 will cause a greater decrease in the number of active lever presses on both the FR-1 and PR schedules during self-administration of a low dose (0.03 mg/kg/infusion) of amphetamine in IC rats when compared to EC and SC rats. In contrast, we predict that antagonism of mGluR5 receptors will not differentially affect EC and IC rats while responding for the high dose of amphetamine (0.1 mg/kg/infusion).
Method and materials
Subjects
Male Sprague-Dawley rats (Charles River, Portage, MI, USA) were housed in one of three environmental conditions: enriched (EC), isolated (IC), or standard (SC). Rats were given ad libitum access to food and water throughout the entire experiment, with the exception of lever press training. The colony room operated on a 12-h light-dark cycle and was maintained at approximately 22 °C, with humidity ranging from approximately 30 to 45 %. All behavioral tests were conducted during the light portion of the cycle. All procedures conducted and research reported were in accordance with the Institutional Animal Care and Use Committee at Kansas State University and complied with NIH guidelines (Council 2011).
Environmental conditions
Rats arrived in the lab at exactly 21 days of age and were randomly assigned to one of the three environmental conditions. EC rats lived with 9–13 other cohorts and were housed in a large metal cage (60×120×45 cm) that was lined with paper pulp bedding. To maintain novelty and to provide further enrichment, EC rats were also handled daily by experimenters. Fourteen objects (small children’s toys and PVC pipe) were regularly rotated and replaced inside the cage. Seven of the objects were changed daily, and all objects were changed two times weekly. IC rats were reared individually and were housed in hanging wire cages (17×24×20 cm). IC cages were composed of wire mesh on the front and bottom, with solid sides. The IC rats were not handled throughout the rearing process and were not exposed to novel objects or paper bedding. SC rats were housed in pairs in standard shoebox cages (20×43×20 cm). SC rats were exposed to the same bedding as EC rats but did not have any novel objects in their cage and were only handled during the weekly cage change. The inclusion of the SC group of rats was not implemented to control for differences between EC and IC rats but rather to provide us with a known laboratory standard for comparison. Rats remained in their respective environmental conditions for 30 days and remained in their housing conditions for the entire experimental period.
Apparatus: locomotor chambers
Locomotor experiments were conducted using six identical chambers (40.64×40.64×40.64 cm; Coulbourn Instruments, TruScan 2.01) that had clear Plexiglas walls with a plastic floor covered with bedding. Photobeams were arranged in a 16 (x-axis) photocell array, spaced 2.54 cm apart. Throughout every locomotor test session, a 70-db white noise was generated to mask any possible background noise.
Apparatus: operant chambers
Lever press training and amphetamine self-administration were conducted inside operant conditioning chambers (ENV-001, Med Associates, St. Albans, VT). Each chamber was enclosed in a sound-attenuating compartment and operated by a computer interface. During lever press training, sucrose was presented in a recessed food receptacle after the active lever was pressed. The two metal response levers were located on either side of the food tray 7.3 cm above the metal grid floor. A 28-V, 3-cm-diameter white cue light was centered above each response lever. Drug infusions were administered via a syringe pump (PHM-100, Med Associates) connected to a 10-ml syringe holding the correct amphetamine concentration based on each rat’s body weight.
Drugs: self-administration testing
3-((2-Methyl-4-thiazolyl)ethynyl)pyridine (MTEP; Abcam Biochemicals, MA, USA) was dissolved in 0.9 % saline (0.3, 1.0, 3.0, and 5.0 mg/kg; 1.0 mg/ml). MTEP was mixed and stored in frozen aliquots. On test days, MTEP was thawed and injected intraperitoneally (i.p.) 30 min prior to the locomotor test and amphetamine self-administration sessions.
D-amphetamine (Sigma Aldrich, MO, USA) was dissolved in 0.9 % saline (0.03 and 0.1 mg/kg/infusion) and was self-administered intravenously.
Behavioral procedures
Amphetamine self-administration
At approximately 52 days of age, rats were food-deprived to 85% of their free-feeding weights by restricting their intake of rat chow to 5–18 g per day until target weight was reached. Then, rats were trained to lever press on a single lever for 20% sucrose solution (dissolved in distilled water). To accomplish this, rats were familiarized with the chamber, the sound of the dipper, and the sucrose magazine. The next day, rats were trained on a fixed-ratio 1 (FR-1) schedule of sucrose reinforcement and were required to earn at least 100 presentations of sucrose. During the next four sessions, both the active and inactive levers were present in the chamber and rats lever-pressed for sucrose on FR-1 schedule. Responses made on the active lever led to sucrose reinforcement, whereas responses made on the inactive lever led to no programmed response. Data for both active and inactive lever presses were recorded. The same active lever (left or right) was maintained for each rat for both the sucrose training and amphetamine self-administration phases of the experiment.
Following sucrose training, rats were allowed to return to free-feeding weights for the remainder of the experiment. After returning to free-feeding weights, rats were deeply anesthetized with ketamine (80 mg/kg; 1 mg/ml, i.p.) and diazepam (5 mg/kg; 1 mg/ml, i.p.) prior to jugular catheter implantation. Polyurethane catheters measured approximately 12 cm in length and 0.2 mm in internal diameter (SAI Infusion Technologies) and were inserted through a dorsal incision on the rat’s back that led up under the skin and around into the rat’s left jugular vein. Catheter tubing from the jugular vein was connected subcutaneously to a 22-gauge back-mounted cannula (Plastics One; Roanoke, VA) secured and sutured to surgical mesh (Biomedical Structures; Warwick, RI). A stainless steel bolt covered the catheter cannula cap to prevent damage to the back mount. To maintain patency and to protect against infection, catheters were flushed daily with heparinized saline (10–30 IU/ml; 0.1 ml before self-administration and 0.1 ml after self-administration) and cefazolin (50 mg/ml; 0.1 ml intravenous (i.v.) infusion). To facilitate surgery recovery while maintaining the enriched environment, EC rats were held in shoebox cages with one other EC rat and a novel object the night following their surgery.
Following the post-surgical care period (9–12 days), rats were trained again in the operant chambers, this time self-administering amphetamine on an FR-1 schedule of reinforcement for 60-min sessions in which a response made on the active lever was recorded and followed by a 100-µl infusion over 5.9 s of either 0.03 mg/kg/infusion (experiment 1; n=37) or 0.1 mg/kg/infusion (experiment 2; n=25) d-amphetamine sulfate (Sigma Aldrich; dissolved in 0.9 % NaCl), with a 20-s timeout period signaled by the illumination of both cue lights immediately after each amphetamine infusion. Lever pressing during the timeout period was not included in the total active lever presses during the session. Inactive lever responding was recorded but had no programmed consequence. Rats were trained on an FR-1 schedule of amphetamine reinforcement until stable responding was achieved. The following criteria were used to determine stable responding: (1) 30 % or less variability in active lever presses across three sessions, (2) greater than a 2:1 ratio of active/inactive lever presses across the three sessions, and (3) at least ten infusions per session.
Some of the rats in experiment 1 failed to reach stability on the 0.03 mg/kg/infusion dose after 15 sessions and were therefore moved to a 0.1 mg/kg/infusion dose. In experiment 1, the following rats failed to reach stability on the 0.03 mg/kg/infusion dose: eight EC, five IC, and five SC rats. Once these rats stabilized for three consecutive sessions on the 0.1 mg/kg/infusion dose, they were adjusted back to the 0.03 mg/kg/infusion amphetamine dose for 2 days before they were tested on the MTEP doses.
After the first five sessions, as soon as the animal met the criteria for stable responding, MTEP test sessions commenced to determine the effect of mGluR5 antagonism and differential rearing on amphetamine self-administration.
FR-1 testing
On MTEP test sessions, rats were injected 30 min prior with MTEP (0.0, 0.3, 1.0, 3.0, or 5.0 mg/kg; i.p.) using a counterbalanced design. The 5.0 mg/kg dose was administered only in experiment 2. Intervening between each test session, rats experienced a minimum of two amphetamine self-administration sessions without MTEP to allow for the washout of any drug effect.
PR testing
After FR testing was complete, rats moved to a PR schedule of reinforcement. Due to the low response rate of the EC rats and previous literature demonstrating that MTEP was only effective during the first 90 min of the PR session (Gass et al. 2009), the PR session was only 60 min long and terminated regardless of lever press activity (Corrigall et al. 2001; Garcia et al. 2014; Neugebauer et al. 2014; Ross et al. 2007). Under this PR schedule, the number of responses required to earn each successive amphetamine infusion was determined by ROUND [5 × EXP (0.25 × infusion number) - 5] to produce the following sequence of required lever presses: 1, 3, 6, 9, 12, 17, 24, 32, 42, 56, 73, etc. (Richardson and Roberts 1996). Rats completed a minimum of two sessions on the PR schedule before MTEP testing. On MTEP test days, rats were injected 30 min prior with MTEP (0.0, 0.3, 1.0, 3.0, or 5.0 mg/kg; i.p.) using a counterbalanced design. The 5.0 mg/kg dose was administered only in experiment 2. Intervening between each test session, rats had a minimum of two amphetamine self-administration days without MTEP to allow for the washout of any drug effect. Following the last self-administration session during the FR-1 and the PR phase, catheter patency was verified by infusing Brevital (10 mg/ml; 0.1–0.15 ml, i.v.). The following rats failed their catheter patency checks at the end of either phase and, consequently, all of their corresponding data from that phase were excluded from the reported analyses: experiment I, one EC, three IC, and six SC rats; experiment II, three EC, four IC, and one SC rat.
Locomotor testing
To control for the possibility that MTEP suppressed general motor activity, we examined the effects of MTEP (3.0 mg/kg) on locomotor activity in EC, IC, and SC rats. Rats were administered MTEP (3.0 mg/kg) or saline 30 min prior to placement in the locomotor chambers for a 1-h session. Locomotor activity was measured by recording the amount of horizontal movements in centimeters.
Data analyses
Locomotor activity data were analyzed by a 3 (environmental condition) × 2 (MTEP or saline) mixed-factorial between-subjects analysis of variance (ANOVA), with total distance traveled (cm) as the dependent measure. For all analyses of active lever press responding, the number of active lever responses during the timeout period was not included. Active lever responding during the last three sessions of acquisition of the FR-1 schedule was investigated through a 3 (environmental condition) × 3 (session) repeated measures ANOVA measuring active lever pressing for the three sessions immediately prior to the initiation of MTEP treatment. Lever press behavior during MTEP test self-administration sessions was analyzed through a 4 (MTEP dose) × 3 (environmental condition) mixed factorial analysis of variance (ANOVA) in experiment 1 and a 5×3 mixed factorial ANOVA in experiment 2 in order to investigate the effects of MTEP treatment and environmental condition on active or inactive lever presses. Order effects of MTEP were also analyzed, and no significant effects were found. During both experiment 1 and experiment 2, each 1-h self-administration session was split into 12, 5-min bins. In order to ascertain when MTEP was actively suppressing amphetamine self-administration, 3 (environmental condition) × 12 (bin) repeated measures ANOVAs were conducted within each MTEP dose to analyze the effects of environmental condition and MTEP treatment across time. All alpha levels were set at p<0.05. Interactions were probed using planned comparisons to compare the effect of MTEP within each environmental condition, and to compare environmental conditions within each drug treatment. All planned comparisons were Bonferroni-corrected to control for family-wise error rate.
Results
Experiment 1: amphetamine 0.03 mg/kg/infusion and MTEP (0, 0.3, 1, and 3 mg/kg)
Acquisition
With the low dose of amphetamine (0.03 mg/kg/infusion), EC rats on average displayed fewer active lever presses than IC or SC rats throughout the three sessions preceding initial MTEP injections, but the differences were not significant.
FR-1 sessions following MTEP treatment
MTEP generally decreased amphetamine self-administration, as indicated by a main effect of MTEP treatment on active lever presses, F(3, 60)=12.19, p<.001. Specifically, when compared to saline treatment, MTEP at 3.0 mg/kg only reduced active lever presses in IC rats, F(1, 60)=28.94, p<.05, suggesting that MTEP at this dose was only effective in IC rats. In the absence of MTEP treatment, enrichment exerted a protective effect against low-dose amphetamine self-administration, with EC rats exhibiting significantly less active lever presses than IC rats, F(1, 60)=12.35, p<.05 (Fig. 1a).
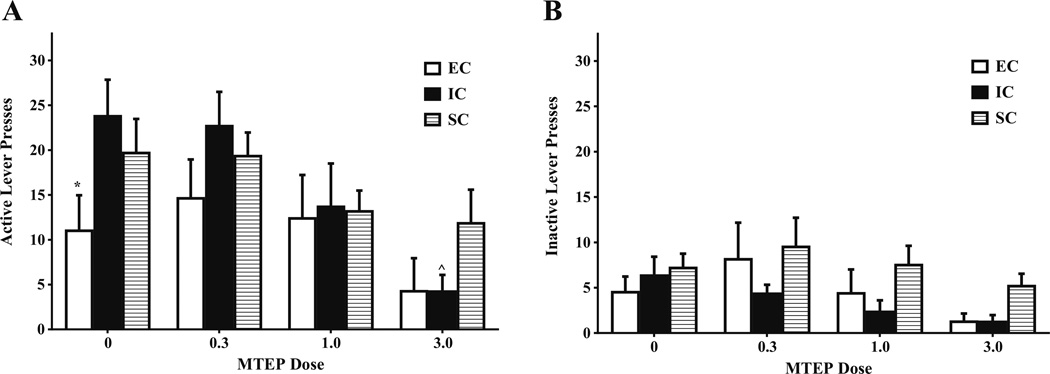
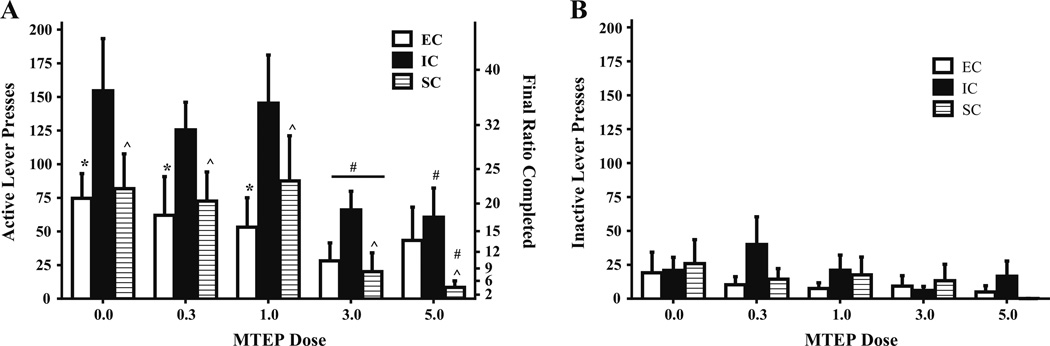
Fig. 1.
Mean ± SEM (a) active and (b) inactive lever presses for amphetamine (0.03 mg/kg/infusion) between EC, IC, and SC rats during FR-1 responding following MTEP (0.3, 1.0, 3.0 mg/kg) or saline. Caret sign (^) indicates that IC rats self-administered less amphetamine following 3.0 mg/kg MTEP than following saline. Asterisk (*) indicates that in the absence of treatment, EC rats self-administered less amphetamine than IC rats (p<.05). b There were no significant differences in inactive lever responding between EC, IC, or SC rats
There were no significant differences in inactive lever responding between EC, IC, or SC rats, as indicated by no significant main effects or interactions (Fig. 1b).
FR-1 time course
There were no significant baseline differences between environmental conditions on active lever pressing across the 1-h session, such that EC, IC, and SC rats treated with saline did not differ from each other on active lever responding between time bins. However, following 3.0 mg/kg MTEP, there was a significant bin × environmental condition interaction, F(22, 220)=1.91, p<.05, with MTEP at this dose suppressing active lever responding in IC rats compared to SC rats during bins 1, 7, and 8, all F(1, 220)=6.86–14.04, all p<.05 (Fig. 2a).
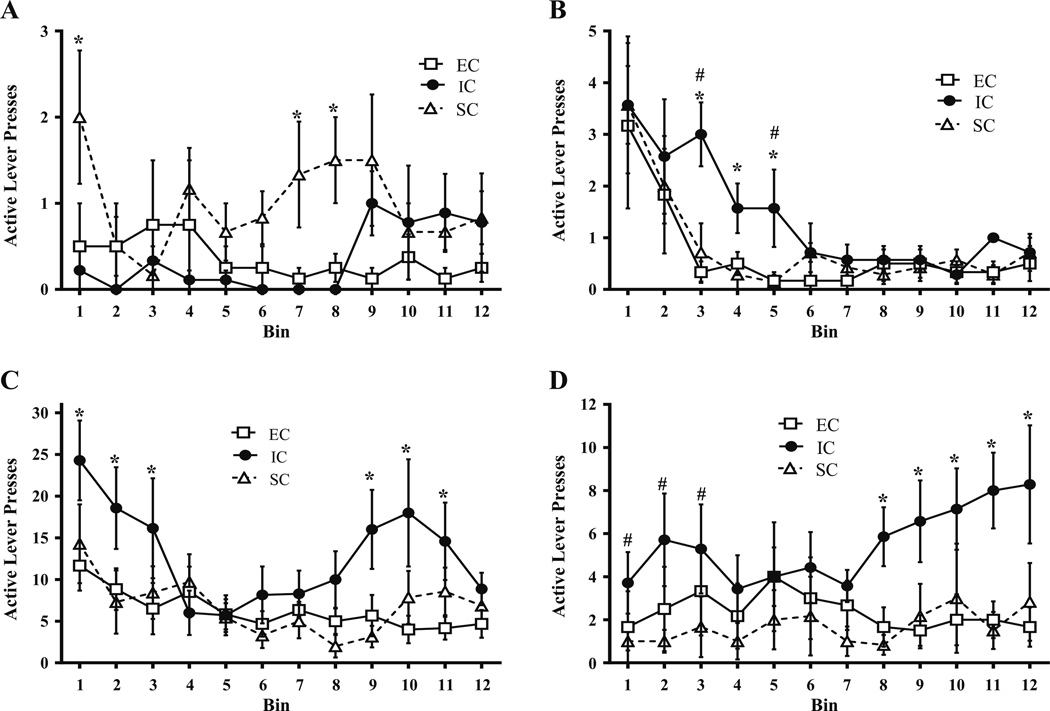
Fig. 2.
Mean ± SEM active lever presses for amphetamine (a 0.03 mg/kg/ infusion; b–d 0.1 mg/kg/infusion) across the 12 5-min bins within the 1-h test session between EC, IC, and SC rats. a FR session (0.03 mg/kg/ infusion) following 3.0 mg/kg MTEP treatment. Asterisks (*) indicate that SC rats exhibited significantly more active lever pressing than IC rats during bins 1, 7, and 8. b FR session (0.1 mg/kg/infusion) following 3.0 mg/kg MTEP treatment. Pound signs (#) indicate that IC rats exhibited significantly more active lever pressing than EC rats during bins 3 and 5. Asterisks (*) indicate that IC rats exhibited significantly more active lever pressing than SC rats during bins 3, 4, and 5. c PR session (0.1 mg/kg/infusion) following saline treatment. Asterisks (*) indicate that IC rats exhibited significantly more active lever responses than both SC and EC rats. d PR session (0.1 mg/kg/infusion) following 3.0 mg/kg MTEP. Asterisks (*) indicate IC rats exhibiting significantly more active lever responses than both SC and EC rats. Pound signs (#) indicate IC rats exhibiting more active lever responses than SC rats. (all p<.05).
PR sessions following MTEP treatment
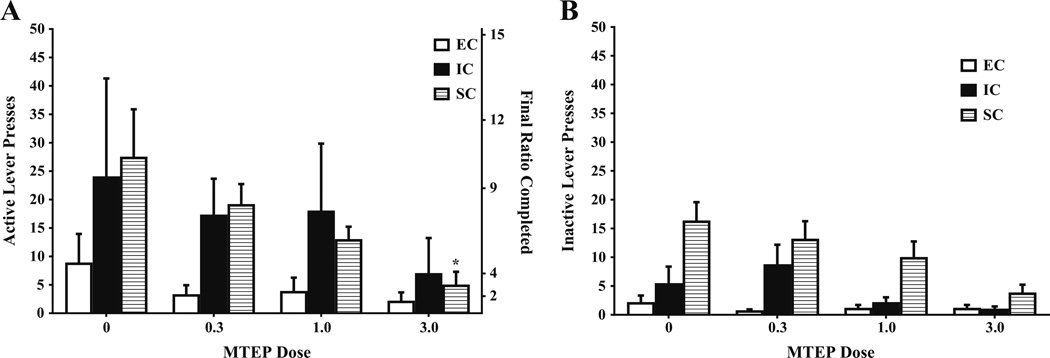
On a PR schedule of reinforcement, analyses revealed a main effect of MTEP dose (F(3,51)=5.04, p<.05) on active lever responding, such that MTEP generally decreased rates of amphetamine self-administration. There were no differences between any environmental conditions following saline treatment. However, compared to saline treatment, 3.0 mg/kg MTEP only resulted in a significant decrease in active lever responses in SC rats, F(1, 51)=9.63, p<.05 (Fig. 3a), suggesting that MTEP at this dose only had an effect in SC rats.
Fig. 3.
Mean ± SEM a active and b inactive lever presses for amphetamine (0.03 mg/kg/infusion) between EC, IC, and SC rats under a PR schedule of reinforcement following MTEP (0.3, 1.0, 3.0 mg/kg) or saline treatment. a Final ratio completed is included in the right y-axis. Asterisk (*) indicates that SC rats administered MTEP (3.0 mg/kg) exhibited significantly fewer active lever presses than SC rats administered saline (p<.05). b There were no significant differences in inactive lever responding between EC, IC, or SC rats under any MTEP dose
The analysis of inactive lever responding revealed a marginally significant MTEP dose × environmental condition interaction, F(6, 51)=2.20, p=0.06 (Fig. 3b).
Experiment 2: amphetamine 0.1 mg/kg/infusion and MTEP (0, 0.3, 1, 3, and 5 mg/kg)
Acquisition
As expected with the higher dose of amphetamine (0.1 mg/kg/ infusion), EC, IC, and SC rats did not differ in the acquisition of active lever pressing throughout the three sessions preceding initial MTEP injections.
FR-1 sessions following MTEP treatment
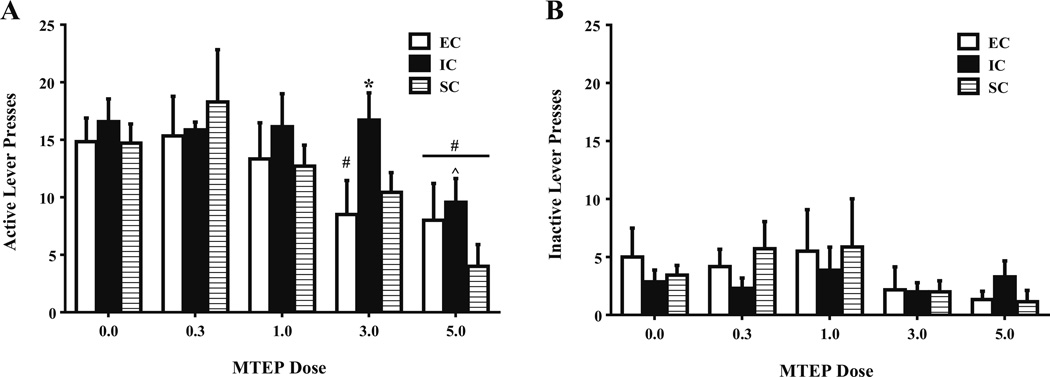
MTEP generally decreased amphetamine self-administration, as indicated by a main effect of MTEP treatment on active lever presses, F(4, 68)=12.81, p<.001. Specifically, when compared to saline treatment, both 3.0 mg/kg (F(1, 68)= 5.74, p<.05) and 5.0 mg/kg (F(1, 68)=6.69, p<.05) MTEP reduced active lever pressing in EC rats. Similarly, when compared to saline treatment, 5.0 mg/kg MTEP reduced the number of active lever presses in IC (F(1, 68)=8.18, p<.05) and SC (F(1, 68)=19.18, p<.05) rats. Although there were no differences in saline-pretreated rats between any environmental condition, IC rats displayed more active lever presses than EC rats (F(1, 68)=10.40, p<.05) and SC rats (F(1, 68)=6.60, p<.05) following the 3.0 mg/kg MTEP dose. Also, after the 5.0 mg/kg MTEP dose, IC rats displayed more active lever presses than SC rats, F(1, 68)=5.19, p<.05 (Fig. 4a). These results suggest a dose-dependent effect of MTEP and illustrate its ability to decrease self-administration between differentially reared rats, with significant attenuation following 5.0 mg/kg MTEP in all rats and significant attenuation following 3.0 mg/kg MTEP in EC and SC rats only.
Fig. 4.
Mean ± SEM a active and b inactive lever presses for amphetamine (0.1 mg/kg/infusion) between EC, IC, and SC rats during FR-1 responding following MTEP (0.3, 1.0, 3.0, 5.0 mg/kg) or saline. Pound signs (#) indicate that EC rats treated with 3.0 mg/kg MTEP, and all rats treated with 5.0 mg/kg MTEP exhibited less active lever responses than saline counterparts. Caret sign (^) indicates that following 5.0 mg/kg MTEP, IC rats displayed significantly more active lever presses than SC rats. Asterisk (*) indicates that IC rats administered 3.0 mg/kg MTEP exhibited significantly more active lever presses than EC and SC rats administered 3.0 mg/kg MTEP (p<.05). b There were no significant differences in inactive lever responding between EC, IC, or SC rats under any MTEP dose
There were no significant differences in inactive lever responding between EC, IC, or SC rats under any MTEP dose (Fig. 4b).
FR-1 time course
There were no significant baseline differences between environmental conditions on active lever pressing across the 1-h session, such that EC, IC, and SC rats treated with saline did not differ from each other. However, following 3.0 mg/kg MTEP, there was a significant main effect of bin, F(11, 187)=8.82, p<.001, and marginal effect of environmental condition, F(2, 17)=3.37, p=.058. Specifically, IC rats given 3.0 mg/kg MTEP pressed more on the active lever during bins 3 and 5 compared to EC rats given 3.0 mg/kg MTEP. IC rats also exhibited more active lever presses than SC rats during bins 3, 4, and 5, with no differences between EC and SC rats during any of the time bins (Fig. 2b).
PR sessions following MTEP treatment
On a PR schedule of reinforcement, analyses revealed a main effect of MTEP dose (F(4, 68)=14.66, p<.001) on active lever responding, such that MTEP generally decreased rates of amphetamine self-administration. Following saline, 0.3 mg/kg, and 1.0 mg/kg MTEP, IC rats displayed a greater level of responding than EC rats, all F(1, 68)=9.37–19.67, all p<.05. Furthermore, IC rats exhibited greater active lever responding than SC rats following all MTEP doses (all F(1, 68)=5.28–13.27, all p<.05). These results suggest that IC rats are more likely to work for amphetamine in the absence of MTEP treatment, indicated by their willingness to continue lever pressing with increasingly higher requirements to earn subsequent amphetamine infusions.
Compared to saline treatment, 3.0 mg/kg MTEP resulted in a significant decrease in active lever responses in all rats, with IC rats showing the greatest attenuation of responding (all F(1, 68)=4.65–19.70, all p<.05). Following 5.0 mg/kg, MTEP resulted in a significant decrease in active lever responses in both IC (F(1,68)=22.18, p<.05) and SC rats (F(1, 68)=9.56, p<.05). As can be seen in Fig. 5a, this suggests that MTEP was most effective at 3.0 and 5.0 mg/kg at decreasing amphetamine self-administration on a PR schedule of reinforcement, and environmental condition may play a role in altering this efficacy.
Fig. 5.
Mean ± SEM a active and b inactive lever presses for amphetamine (0.1 mg/kg/infusion) between EC, IC, and SC rats during PR responding following MTEP (0.3, 1.0, 3.0, 5.0 mg/kg) or saline. a Final ratio completed is included in the right y-axis. Asterisks (*) indicate that IC rats displayed significantly greater active lever presses than EC rats following saline, 0.3 mg/kg, and 1.0 mg/kg MTEP. Caret signs (^) indicate that IC rats exhibited more active lever responding than SC rats following all MTEP doses. Pound signs (#) indicate that compared to saline treatment, 3.0 mg/kg MTEP resulted in a significant decrease in active lever responses in all rats, and following 5.0 mg/kg MTEP resulted in a significant decrease in active lever responses in IC and SC rats (p<.05). b There were no significant differences in inactive lever responding between EC, IC, or SC rats under any MTEP dose
There were no significant differences in inactive lever responding between EC, IC, or SC rats under any MTEP dose (Fig. 5b).
PR time course
Contrary to the time course results of FR-1 active lever responding, there were baseline differences between EC, IC, and SC rats, as indicated by a significant main effect of bin, F(11, 187)=6.14, p<.001, and significant bin × environmental condition interaction, F(22, 187)=1.92, p<.05, suggesting that differential rearing alters amphetamine self-administration on a PR schedule of reinforcement. In the absence of MTEP treatment, IC rats displayed more active lever responding than EC and SC rats, toward both the beginning of the session and the latter portion of the session, all F(1, 187)= 6.10–18.50, all p<.05 (Fig. 2c).
Following 3.0 mg/kg MTEP, the effects of environmental condition on active lever presses across bins was marginally significant, F(2, 17)=3.26, p=.063. IC rats again displayed more active lever responding than both EC and SC rats, all F(1, 187)=3.04–17.40, all p<.05. Specifically, 3.0 mg/kg MTEP appeared to suppress active lever responding more toward the latter half of the 1-h session in EC and SC rats (Fig. 2d).
Locomotor test
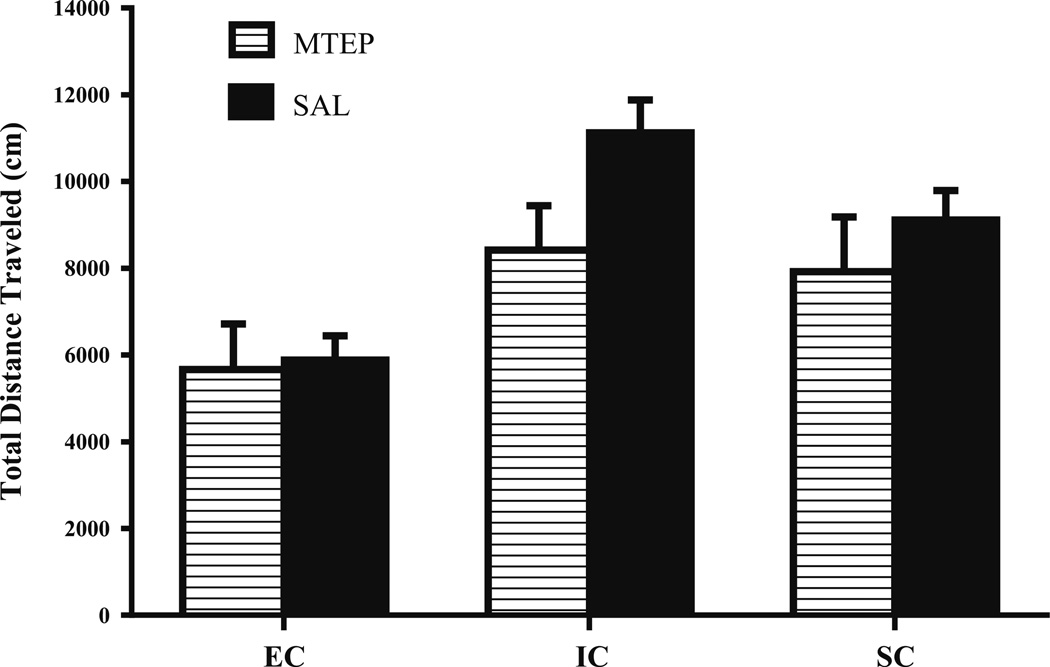
Results revealed a slight decrease in locomotor activity in MTEP-treated animals (3.0 mg/kg, i.p.) compared to saline controls. However, this difference was not significant, indicated by a non-significant main effect of MTEP (3.0 mg/kg) on total distance traveled (cm), F(1, 36)=3.33, p=.076. Results also revealed a nonsignificant Drug Treatment × Environmental Condition interaction, F(2, 36)=.931, p=.404 (Fig. 6), suggesting that the MTEP-induced attenuation of amphetamine self-administration observed in the current study was not confounded by a decrease in general motor activity.
Fig. 6.
Mean ± SEM total distance traveled (cm) in EC, IC, and SC rats following MTEP (3.0 mg/kg) or saline treatment. Drug treatment conditions: EC-saline (n=7), EC-MTEP (n=7), IC-saline (n=7), IC-MTEP (n= 7), SC-saline (n=7), and SC-MTEP (n=7). Results revealed no significant reductions in locomotor activity in MTEP-treated rats compared to saline-treated rats in any of the environmental conditions (p>.05)
Discussion
The current study suggests that differential rearing alters the function of the mGluR5 receptor, evident by dose-dependent changes in amphetamine self-administration following mGluR5 antagonism.
During self-administration on a FR-1 schedule, the 3.0 mg/kg dose of MTEP decreased responding of IC rats for the low dose of amphetamine (0.03 mg/kg/infusion) and decreased responding of EC rats for the high dose of amphetamine (0.1 mg/kg/infusion). These results suggest an interaction between amphetamine dose, differential rearing, and mGluR5 function. Our previous research observed that MTEP decreased repeated amphetamine-induced hyperactivity in IC rats, but not EC rats, following a low dose of amphetamine (Gill et al. 2012). The current results mimic this previous finding using a self-administration paradigm and suggest that repeated exposure to a higher dose of amphetamine may alter mGluR5 function differentially in EC and IC rats. In the current experiment, MTEP did not significantly alter inactive lever press behavior or locomotor activity. This finding is consistent with research suggesting that MTEP does not result in non-specific behavioral effects and suggests that the ability of MTEP to attenuate reward sensitivity is reinforcer specific (Gass et al. 2009; Gill et al. 2012; Hao et al. 2010; Martin-Fardon et al. 2009).
We also observed effects of differential rearing during amphetamine self-administration on a PR schedule. EC rats responded at extremely low rates on the PR schedule (less than 10 active lever presses per session) during experiment 1 (0.03 mg/kg/infusion amphetamine). Therefore, we were unable to evaluate the effects of MTEP on PR performance in EC rats. While responding for the low dose of amphetamine, only the SC rats had a decrease in responding following administration of the 3.0 mg/kg dose of MTEP. Therefore, these results suggest that IC rats are more resistant to the effects of MTEP than SC rats when responding for a low dose of amphetamine on a PR schedule.
Experiment 2 utilized a higher dose of amphetamine (0.1 mg/kg/infusion), and we observed much higher rates of lever pressing in EC rats under a PR schedule. IC rats displayed more active lever pressing than both SC and EC rats in the absence of MTEP treatment. Therefore, we compared active lever press behavior within rearing conditions and observed that while MTEP decreased active lever responding in all groups at the 3.0 mg/kg dose, it only decreased active lever responding in IC and SC rats at the 5.0 mg/kg dose. This suggests that when the motivation to respond is examined, EC rats are less sensitive than IC and SC rats to mGluR5 antagonism, and further suggests that the role of the mGluR5 receptor may differ between FR and PR schedules of reinforcement.
The effects of MTEP on responding for psychostimulants under a PR schedule of reinforcement have been less clear than its effects on responding under a FR schedule. Hao and colleagues (Hao et al. 2010) demonstrated that MTEP could decrease PR responding for rats with a history of short access to cocaine, but not in rats with a history of extended access to cocaine. Gass et al. (2009) observed that the 3.0 mg/kg dose of MTEP was only effective at reducing responding during the first 90 min of the PR session for methamphetamine (0.1 mg/kg/infusion). Therefore, we used a short 60-min PR session to ensure that MTEP was active throughout the entire session. Using the shorter session, we replicated these previous findings and also observed that MTEP can reduce responding for a lower dose of amphetamine on a PR schedule of reinforcement in SC rats. Taken together, these results suggest that MTEP is effective at reducing responding on a PR schedule for both low and high doses of psychostimulants.
Fixed- and progressive-ratio schedules of reinforcement are employed to measure the different neural substrates involved in addiction (Arnold and Roberts 1997). Typically, active lever press behavior of rats under a FR schedule is a measurement of reward sensitivity, while active lever pressing under a PR schedule reflects motivation to earn psychostimulant reinforcement (Richardson and Roberts 1996). Previous research has suggested that IC rats have enhanced reward sensitivity and motivation (Bardo and Dwoskin 2004; Beckmann and Bardo 2011; Kirkpatrick et al. 2013). The current study observed that IC rats responded more than EC rats on a FR schedule of reinforcement for a lower dose of amphetamine and add to the wealth of data suggesting that isolation enhances reward sensitivity (Bardo and Dwoskin 2004; Green et al. 2002; Grimm et al. 2008; Solinas et al. 2009; Stairs and Bardo 2009).
However, while previous literature demonstrates that isolation alters incentive motivation (Beckmann and Bardo 2011), the effects of isolation on motivation to respond on a PR schedule of reinforcement is less clear and suggests that isolation only enhances motivation for lower doses of amphetamine. Previous research has observed that IC rats respond more than EC rats for lower doses of amphetamine (0.03 to 0.06 mg/kg/infusion) on a PR schedule, and the current results support these findings (Alvers et al. 2012; Bardo et al. 2001; Green et al. 2002). Interestingly, this past literature has observed no differences in responding on a PR schedule between EC and IC rats for higher doses of psychostimulants (Alvers et al. 2012; Bardo et al. 2001; Green et al. 2002); however, the current experiment observed that IC rats still responded more than EC rats on the PR schedule for the higher dose of amphetamine, reflecting a higher degree of motivation in IC rats to self-administer amphetamine.
Environmental enrichment results in very low response rates for psychostimulants on a PR schedule (Alvers et al. 2012; Bardo et al. 2001; Green et al. 2002). Therefore, in order to ensure a higher response rate in EC rats, the current experiment used a short PR session utilized in the nicotine literature when response rates are low (Corrigall et al. 2001; Garcia et al. 2014; Neugebauer et al. 2014; Ross et al. 2007). Use of the shorter session length still resulted in very low response rates in EC rats for the lower dose of amphetamine (~10 lever presses) but resulted in a high response rate for the higher dose of amphetamine (~75 lever presses). This suggests that the use of a shorter PR session with higher doses of amphetamine results in high rates of lever press behavior in EC rats and offers an opportunity to examine treatments that will attenuate lever press behavior in EC rats. Importantly, the current results also suggest that isolation increases the motivation to respond for high doses of amphetamine and adds support to the hypothesis that enrichment attenuates both reward sensitivity and motivation (Bardo and Dwoskin 2004; Beckmann and Bardo 2011; Kirkpatrick et al. 2013).
While it is clear that differential rearing alters glutamate-dependent synaptic plasticity (Artola et al. 2006; Green and Greenough 1986; Renner and Rosenzweig 1987; Sharp et al. 1985; van Praag et al. 2000), relatively little is known about the effects of differential rearing on metabotropic glutamate receptors. The current study focused on the mGluR5 receptor because it contributes to maintaining glutamate homeostasis (Kalivas 2009) and alters the response to psychostimulants (Gass and Olive 2008; Hao et al. 2010; Herzig and Schmidt 2004; McGeehan and Olive 2003), and its expression within the PFC can be increased by enrichment (Melendez et al. 2004). We have previously demonstrated that MTEP attenuates acute amphetamine-induced locomotor activity in enriched and standard-housed rats compared to isolates (Gill et al. 2012) following low doses of amphetamine. Furthermore, we demonstrated that antagonism of mGluR5 reduced the expression of sensitization only in IC rats, suggesting that there is an interaction between amount of amphetamine exposure, amphetamine dose, and differential rearing on mGluR5 function. While our current experimental design did not allow us to examine the role of mGluR5 function during the acquisition of self-administration, our design did allow us to examine the role of amphetamine dose and strongly suggests that the ability of enrichment to alter mGluR5 function is dose dependent. Amphetamine results in an increase in extracellular glutamate in the striatum (Del Arco et al. 1999; Reid et al. 1997) and repeated amphetamine exposure increases extracellular glutamate levels within the NAcc (Vanderschuren and Kalivas 2000; Wolf 1998). Interestingly, mGluR5 rapidly desensitizes in response to glutamate (Gereau and Heinemann 1998), and therefore, our results suggest that differential rearing may be altering the downregulation of mGluR5 in response to repeated amphetamine.
The results of the current study support a role for mGluR5 in responding for psychostimulants. Limited research has been conducted investigating the effects of environmental enrichment on mGluR5, but here we show that both enrichment and isolation can alter the function of receptors critical for maintaining glutamate homeostasis, which can lead to altered drug-taking behaviors. We also show that this neural alteration, and ultimately the function of the mGluR5 receptor, may vary depending on reinforcement schedule, highlighting the impact of housing condition on altering receptors responsible for the sensitivity and motivation to self-administer psychostimulants.
Acknowledgments
Research reported in this article was supported by NIH through NIDA grant #: R15DA035435, and Kansas State University. We thank Michele Ulmer, Greg Erickson, Lauren Komer, Emily Reinhardt, Christy Peterson, and Alexander Howard for their assistance in daily experimental procedures, as well as Erik Garcia for providing surgical assistance.
References
- Alvers KM, Marusich JA, Gipson CD, Beckmann JS, Bardo MT. Environmental enrichment during development decreases intravenous self-administration of methylphenidate at low unit doses in rats. Behav Pharmacol. 2012;23:650–657. doi: 10.1097/FBP.0b013e3283584765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Artola A, von Frijtag JC, Fermont PC, Gispen WH, Schrama LH, Kamal A, Spruijt BM. Long-lasting modulation of the induction of LTD and LTP in rat hippocampal CA1 by behavioural stress and environmental enrichment. Eur J Neurosci. 2006;23:261–272. doi: 10.1111/j.1460-9568.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Dwoskin LP. Biological connection between novelty-and drug-seeking motivational systems. Nebr Symp Motiv. 2004;50:127–158. [PubMed] [Google Scholar]
- Bardo MT, Hammer RP., Jr Autoradiographic localization of dopamine D1 and D2 receptors in rat nucleus accumbens: resistance to differential rearing conditions. Neuroscience. 1991;45:281–290. doi: 10.1016/0306-4522(91)90226-e. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 2001;155:278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Beckmann JS, Bardo MT. Environmental enrichment reduces attribution of incentive salience to a food-associated stimulus. Behav Brain Res. 2011;226:331–334. doi: 10.1016/j.bbr.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SR, Carrillo N. Characteristics of effective summer learning programs in practice. New Dir Youth Dev. 2007:45–63. doi: 10.1002/yd.212. [DOI] [PubMed] [Google Scholar]
- Bonci A, Bernardi G, Grillner P, Mercuri NB. The dopamine-containing neuron: maestro or simple musician in the orchestra of addiction? Trends Pharmacol Sci. 2003;24:172–177. doi: 10.1016/S0165-6147(03)00068-3. [DOI] [PubMed] [Google Scholar]
- Cain ME, Mersmann MG, Gill MJ, Pittenger ST. Dose dependent effects of differential rearing on amphetamine-induced hyperactivity. Behav Pharmacol. 2012;23:744–752. doi: 10.1097/FBP.0b013e32835a38ec. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Zhang J, Adamson KL. GABA mechanisms in the pedunculopontine tegmental nucleus influence particular aspects of nicotine self-administration selectively in the rat. Psychopharmacology (Berl) 2001;158:190–197. doi: 10.1007/s002130100869. [DOI] [PubMed] [Google Scholar]
- Council NR. Guide for the care and use of laboratory animals. 8th edn. Washington, DC: National Academy Press. Institute of Laboratory Animal Resources; 2011. [Google Scholar]
- Darna M, Beckmann JS, Gipson CD, Bardo MT, Dwoskin LP. Effect of environmental enrichment on dopamine and serotonin transporters and glutamate neurotransmission in medial prefrontal and orbitofrontal cortex. Brain Res. 2015;1599:115–125. doi: 10.1016/j.brainres.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Arco A, Gonzalez-Mora JL, Armas VR, Mora F. Amphetamine increases the extracellular concentration of glutamate in striatum of the awake rat: involvement of high affinity transporter mechanisms. Neuropharmacology. 1999;38:943–954. doi: 10.1016/s0028-3908(99)00043-x. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Segovia G, Canales JJ, Garrido P, de Blas M, Garcia-Verdugo JM, Mora F. Environmental enrichment reduces the function of D1 dopamine receptors in the prefrontal cortex of the rat. J Neural Transm. 2007;114:43–48. doi: 10.1007/s00702-006-0565-8. [DOI] [PubMed] [Google Scholar]
- Fone KC, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32:1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Garcia KL, Le AD, Tyndale RF. Effect of food training and training dose on nicotine self-administration in rats. Behav Brain Res. 2014;274:10–18. doi: 10.1016/j.bbr.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Osborne MP, Watson NL, Brown JL, Olive MF. mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology. 2009;34:820–833. doi: 10.1038/npp.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gereau RW, Heinemann SF. Role of protein kinase C phosphorylation in rapid desensitization of metabotropic glutamate receptor 5. Neuron. 1998;20:143–151. doi: 10.1016/s0896-6273(00)80442-0. [DOI] [PubMed] [Google Scholar]
- Gill MJ, Arnold JC, Cain ME. Impact of mGluR5 during amphetamine-induced hyperactivity and conditioned hyperactivity in differentially reared rats. Psychopharmacology (Berl) 2012;221:227–237. doi: 10.1007/s00213-011-2565-0. [DOI] [PubMed] [Google Scholar]
- Gormley S, Rompre PP. Blockade of mGLUR5 receptors differentially alters amphetamine-induced enhancement of locomotor activity and of brain stimulation reward. J Psychopharmacol. 2010;25:393–401. doi: 10.1177/0269881110367460. [DOI] [PubMed] [Google Scholar]
- Green EJ, Greenough WT. Altered synaptic transmission in dentate gyrus of rats reared in complex environments: evidence from hippocampal slices maintained in vitro. J Neurophysiol. 1986;55:739–750. doi: 10.1152/jn.1986.55.4.739. [DOI] [PubMed] [Google Scholar]
- Green TA, Gehrke BJ, Bardo MT. Environmental enrichment decreases intravenous amphetamine self- administration in rats: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology (Berl) 2002;162:373–378. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Black JE, Wallace CS. Experience and brain development. Child Dev. 1987;58:539–559. [PubMed] [Google Scholar]
- Grimm JW, Osincup D, Wells B, Manaois M, Fyall A, Buse C, Harkness JH. Environmental enrichment attenuates cue-induced reinstatement of sucrose seeking in rats. Behav Pharmacol. 2008;19:777–785. doi: 10.1097/FBP.0b013e32831c3b18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Martin-Fardon R, Weiss F. Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine-escalated rats: factor in the transition to dependence. Biol Psychiatry. 2010;68:240–248. doi: 10.1016/j.biopsych.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig V, Schmidt WJ. Effects of MPEP on locomotion, sensitization and conditioned reward induced by cocaine or morphine. Neuropharmacology. 2004;47:973–984. doi: 10.1016/j.neuropharm.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol. 2003;69:375–390. doi: 10.1016/s0301-0082(03)00085-6. [DOI] [PubMed] [Google Scholar]
- Jones S, Bonci A. Synaptic plasticity and drug addiction. Curr Opin Pharmacol. 2005;5:20–25. doi: 10.1016/j.coph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick K, Marshall AT, Clarke J, Cain ME. Environmental rearing effects on impulsivity and reward sensitivity. Behav Neurosci. 2013;127:712–724. doi: 10.1037/a0034124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Moussawi K, Tang XC, Wang X, Kalivas BC, Kolokithas R, Ogburn KB, Kalivas PW. The effect of N-acetylcysteine in the nucleus accumbens on neurotransmission and relapse to cocaine. Biol Psychiatry. 2012;71:978–986. doi: 10.1016/j.biopsych.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Baptista MA, Dayas CV, Weiss F. Dissociation of the effects of MTEP [3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine] on conditioned reinstatement and reinforcement: comparison between cocaine and a conventional reinforcer. J Pharmacol Exp Ther. 2009;329:1084–1090. doi: 10.1124/jpet.109.151357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeehan AJ, Olive MF. The mGluR5 antagonist MPEP reduces the conditioned rewarding effects of cocaine but not other drugs of abuse. Synapse. 2003;47:240–242. doi: 10.1002/syn.10166. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Gregory ML, Bardo MT, Kalivas PW. Impoverished rearing environment alters metabotropic glutamate receptor expression and function in the prefrontal cortex. Neuropsychopharmacology. 2004;29:1980–1987. doi: 10.1038/sj.npp.1300507. [DOI] [PubMed] [Google Scholar]
- Mitrano DA, Smith Y. Comparative analysis of the subcellular and subsynaptic localization of mGluR1a and mGluR5 metabotropic glutamate receptors in the shell and core of the nucleus accumbens in rat and monkey. J Comp Neurol. 2007;500:788–806. doi: 10.1002/cne.21214. [DOI] [PubMed] [Google Scholar]
- Neugebauer NM, Cortright JJ, Sampedro GR, Vezina P. Exposure to nicotine enhances its subsequent self-administration: contribution of nicotine-associated contextual stimuli. Behav Brain Res. 2014;260:155–161. doi: 10.1016/j.bbr.2013.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- Rahman S, Bardo MT. Environmental enrichment increases amphetamine-induced glutamate neurotransmission in the nucleus accumbens: a neurochemical study. Brain Res. 2008;1197:40–46. doi: 10.1016/j.brainres.2007.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Hsu K, Jr, Berger SP. Cocaine and amphetamine preferentially stimulate glutamate release in the limbic system: studies on the involvement of dopamine. Synapse. 1997;27:95–105. doi: 10.1002/(SICI)1098-2396(199710)27:2<95::AID-SYN1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Renner MJ, Rosenzweig MR. Enriched and impoverished environments: effects on brain and behavior. New York: Springer-Verlag; 1987. [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL, Hebert M, Morimoto H. Social grouping cannot account for cerebral effects of enriched environments. Brain Res. 1978;153:563–576. doi: 10.1016/0006-8993(78)90340-2. [DOI] [PubMed] [Google Scholar]
- Ross JT, Corrigall WA, Heidbreder CA, LeSage MG. Effects of the selective dopamine D3 receptor antagonist SB-277011A on the reinforcing effects of nicotine as measured by a progressive-ratio schedule in rats. Eur J Pharmacol. 2007;559:173–179. doi: 10.1016/j.ejphar.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Scofield MD, Kalivas PW. Astrocytic dysfunction and addiction: consequences of impaired glutamate homeostasis. Neuroscientist. 2014;20:610–622. doi: 10.1177/1073858413520347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann N Y Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- Sharp PE, McNaughton BL, Barnes CA. Enhancement of hippocampal field potentials in rats exposed to a novel, complex environment. Brain Res. 1985;339:361–365. doi: 10.1016/0006-8993(85)90105-2. [DOI] [PubMed] [Google Scholar]
- Simpson J, Kelly JP. The impact of environmental enrichment in laboratory rats-Behavioural and neurochemical aspects. Behav Brain Res. 2011;222:246–264. doi: 10.1016/j.bbr.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Solinas M, Thiriet N, El Rawas R, Lardeux V, Jaber M. Environmental enrichment during early stages of life reduces the behavioral, neurochemical, and molecular effects of cocaine. Neuropsychopharmacology. 2009;34:1102–1111. doi: 10.1038/npp.2008.51. [DOI] [PubMed] [Google Scholar]
- Stairs DJ, Bardo MT. Neurobehavioral effects of environmental enrichment and drug abuse vulnerability. Pharmacol Biochem Behav. 2009;92:377–382. doi: 10.1016/j.pbb.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Veeneman MM, Boleij H, Broekhoven MH, Snoeren EM, Guitart Masip M, Cousijn J, Spooren W, Vanderschuren LJ. Dissociable roles of mGlu5 and dopamine receptors in the rewarding and sensitizing properties of morphine and cocaine. Psychopharmacology (Berl) 2011;214:863–876. doi: 10.1007/s00213-010-2095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Wooters TE, Bardo MT, Dwoskin LP, Midde NM, Gomez AM, Mactutus CF, Booze RM, Zhu J. Effect of environmental enrichment on methylphenidate-induced locomotion and dopamine transporter dynamics. Behav Brain Res. 2011;219:98–107. doi: 10.1016/j.bbr.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Green TA, Bardo MT, Dwoskin LP. Environmental enrichment enhances sensitization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex. Behav Brain Res. 2004;148:107–117. doi: 10.1016/s0166-4328(03)00190-6. [DOI] [PubMed] [Google Scholar]
- Zhu J, Apparsundaram S, Bardo MT, Dwoskin LP. Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. J Neurochem. 2005;93:1434–1443. doi: 10.1111/j.1471-4159.2005.03130.x. [DOI] [PubMed] [Google Scholar]