Abstract
Direct targeting of RAS, which is frequently mutated, has proven to be challenging, and inhibition of individual downstream RAS mediators has resulted in limited clinical efficacy. We designed a chemical screen to identify compounds capable of potentiating mTOR inhibition in mutant RAS-positive leukemia, and identified a Wee1 inhibitor. Synergy was observed in both mutant NRAS- and mutant KRAS-positive acute myelogenous leukemia (AML) cell lines and primary patient samples. The observed synergy enhanced dephosphorylation of AKT, 4E-BP1 and S6K, and correlated with increased apoptosis. The specificity of Wee1 as the target of MK-1775 was validated by Wee1 knockdown (KD), as well as partial reversal of drug combination-induced apoptosis by a CDK1 inhibitor. Importantly, we also extended our findings to other mutant RAS-expressing malignancies, including mutant NRAS-positive melanoma, and mutant KRAS-positive colorectal cancer, pancreatic cancer, and lung cancer. We observed favorable responses with combined Wee1/mTOR inhibition in human cancer cell lines from multiple malignancies, and inhibition of tumor growth in in vivo models of mutant KRAS lung cancer and leukemia. The present study introduces for the first time Wee1 inhibition combined with mTOR inhibition as a novel therapeutic strategy to the selective treatment of mutant RAS-positive leukemia and other mutant RAS-expressing malignancies.
Keywords: acute myeloid leukemia, RAS mutations, Wee1, mTOR, drug resistance, synergy
Introduction
Point mutations in the RAS family, which is comprised of NRAS, KRAS, and HRAS, are the most frequently occurring mutation in human proto-oncogenes, with around 20-30% of all human malignancies characterized by constitutively activated RAS1. The incidence of mutations in NRAS and KRAS in AML has been reported to be approximately 10-11% and 5%, respectively, and around 12% for myelodysplastic syndromes2,3. The incidence of NRAS mutations has been reported to be approximately 10% in childhood ALL4.
Despite its prevalence and significance with respect to transformation, direct molecular inhibition of mutant RAS has thus far been difficult due to its biochemistry and structure, although KRAS (G12C) mutant-specific inhibitors, which depend on mutant cysteine for their selective inactivation of this mutant, have recently been reported and are in early stages of development5,6. The potential to interrupt mutant RAS signaling by inhibiting the major signaling pathways of RAS, PI3K/PTEN/AKT/mTOR and Raf/MEK/ERK, has prompted the development of selective inhibitors of these pathways. However, the redundant nature and cross-talk of the highly complex signaling cascades associated with RAS can lead to drug resistance and therefore make developing individual targeted therapies a challenge. There is thus potential clinical benefit to be gained from the use of a multi-targeted therapy approach as opposed to a more narrowly focused strategy to enhance efficacy and circumvent the emergence of drug resistance.
One promising approach to improving the clinical efficacy of inhibitors of RAS-mediated transformation is the identification of combinations of drugs that target either multiple RAS-driven pathways, or block bypass or resistance pathways. As the mammalian target of rapamycin, or mTOR, is an established and important downstream effector of RAS in leukemia and mTOR inhibitors are under clinical investigation for the treatment of mutant RAS-associated malignancies (reviewed in7), we developed a chemical screen to identify agents capable of enhancing the activity of an mTOR inhibitor, Torin 18. As anticipated, this screen identified inhibitors of phosphatidylinositol 3-kinase (PI3K) and MAPK/ERK kinase (MEK), but unexpectedly also identified MK-1775, an inhibitor of Wee1, which has not previously been linked to RAS signaling.
Wee1 is a protein kinase and inhibitory regulator of the G2/M checkpoint that prevents cells from going through mitosis by inhibiting the activity of cell division cycle 2 (Cdc2; also known as cyclin-dependent kinase 1 (Cdk1))9-11. In response to DNA damage, such as that induced in tumor cells by cytotoxic agents, Wee1 inactivates Cdc2 through phosphorylation of its Tyr15 residue, thus leading to G2 arrest; this allows transformed cells the time needed for repair of damaged DNA and thus confers a survival advantage9-11. Inhibition of Wee1 by MK-1775 results in the removal of Cdc2 Tyr15 phosphorylation, activation of Cdc2, and the entrance of cells into the mitotic phase of the cell cycle12.
Wee1 inhibition has been thought to potentiate the effects of chemotherapy by causing transformed cells with damaged DNA to go through an untimely mitosis and undergo apoptosis13. In fact, MK-1775 has been shown to enhance the activity of chemotherapy agents against a variety of malignancies14,15. However, little has been reported on the ability of MK-1775 to potentiate the effects of targeted and selective small molecule inhibitors that offer the potential benefit of less adverse effects and better tolerability as compared to standard cytotoxic agents. The present study is the first report of the ability of Wee1 inhibition to increase the effectiveness of targeted inhibition of RAS signaling against mutant RAS-positive AML. We also demonstrate the ability of MK-1775 to potentiate mTOR inhibition in the broader context of additional mutant RAS-expressing malignancies, including acute lymphoblastic leukemia (ALL), melanoma, colorectal cancer, pancreatic cancer, and lung cancer.
Materials and Methods
LINCS library chemical screen
The Kinase Inhibitor Focused Library, LINCS, was utilized for a chemical screen designed to identify selective kinase inhibitors capable of synergizing with the mTOR inhibitor, Torin116, against mutant NRAS-driven cells (see schematic, Figure 1A). The LINCS library is available from Harvard Medical School/NIH LINCS program (https://lincs.hms.harvard.edu/) and contains 202 known selective and potent kinase inhibitors.
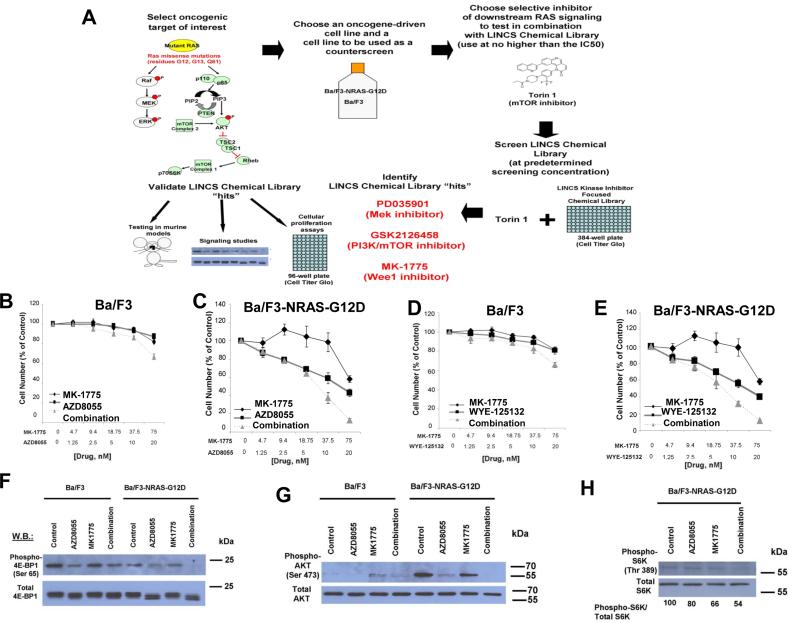
Figure 1. Identification of Wee1 inhibitor, MK-1775, as able to potentiate the effects of mTOR inhibition against mutant NRAS-expressing cells via inhibition of AKT, 4E-BP1, and S6K phosphorylation.
(A) Schematic of LINCS library chemical screen. 384-well plates were seeded with either parental Ba/F3 cells cultured in the presence of 15% WEHI (as a source of IL-3), which were used as a control to eliminate inhibitors that exhibit off-target toxicity and interfere with IL-3-mediated signaling, or Ba/F3-NRAS- G12D cells (growth factor-independent). Included in the screen were cell-containing plates that only received LINCS library drugs (300 nM, determined to be the optimal screening concentration) for the purpose of assessing single agent activity of the LINCS library compounds, or plates that contained LINCS library compounds (300 nM) plus the mTOR inhibitor, Torin 1 (20 nM, which was determined to be close to the IC50 against mutant NRAS-expressing cells). DMSO (vehicle) wells and Torin 1-only wells were included as controls in the plates. Following pintool administration of the library compounds, the 384-well plates were incubated for two days prior to administration of Cell Titer Glo (Promega, Madison, WI) and analysis of bioluminescence using a plate reader. Ideal candidates for further investigation were LINCS library compounds showing minimal activity as single agents against parental Ba/F3 cells, yet potentiation of the efficacy of Torin 1 against NRAS-driven cells. “Hits” were validated for synergizing potential using several approaches, including cellular proliferation assays, signaling studies, and in vivo analysis. (B-E) Approximately two-day proliferation studies performed with MK-1775, combined with AZD8055 or WYE-125132 against parental Ba/F3 and Ba/F3-NRAS-G12D cells. (F-H) Effect of approximately two-hr treatment of Ba/F3 or Ba/F3-NRAS-G12D cells with MK-1775 (75 nM), alone and combined with mTOR inhibitor, AZD8055 (20 nM), on phosphorylation of 4E-BP1, AKT, and S6K, as measured by immunoblotting.
Cell lines and cell culture
IL-3–dependent murine Ba/F3 cells were transduced with NRAS G12D– or KRAS G12D containing murine stem cell virus (MSCV) retroviruses harboring an IRES-GFP and rendered growth factor-independent via IL-3 withdrawal from the culture media (to develop Ba/F3-NRAS-G12D and Ba/F3-KRAS-G12D cells, respectively). Ba/F3-NRAS G12D myrAKT cells (expressing constitutively-active, myristoylated AKT) were created by transducing pMIG myrAKT to Ba/F3-NRAS-G12D-neo and sorting GFP-positive cells.
The human mutant NRAS-expressing AML line, P31-FUJ, and mutant KRAS-expressing AML lines, SKM-1 (KRAS K117N), Nomo-1 (KRAS G13D), and NB4 (KRAS A18D), were obtained from Dr. Gary Gilliland. Mutant NRAS-expressing ALL lines, PF-382 and NALM6, were obtained by Dr. Thomas Look and Dr. David Weinstock, respectively. The human AML-derived, wild-type (wt) RAS-expressing line, MOLM1417, was provided by Dr. Scott Armstrong. MOLM14 cells were transduced with the FUWLuc-mCherry-puro lentivirus as previously described18. The wt RAS-expressing line, HEL, was purchased from ATCC (Manassas, VA, USA).
Information about culturing conditions and solid tumor cell lines are provided in the supplementary section.
Chemical compounds and biologic reagents
A listing of chemical compounds and biologic reagents is shown in the supplementary section.
Proliferation studies, cell cycle analysis, and apoptosis assay
Details are provided in the supplementary section.
Antibodies and immunoblotting
A listing of antibodies is shown in the supplementary section.
Protein lysate preparation and immunoblotting were carried out as previously described19.
Drug combination studies
The Chou-Talalay method20 was employed for drug combination studies. Additional details are provided in the supplementary section.
AML patient cells
Mononuclear cells were isolated from samples from AML patients identified as harboring mutant NRAS. Cells were tested in liquid culture (DMEM, supplemented with 20% FBS) in the presence of different concentrations of single and combined agents. All blood and bone marrow samples from AML patients were obtained under approval of the Dana Farber Cancer Institute Institutional Review Board.
Mutant NRAS-positive AML5 cells were derived from a 46 year old patient with AML, with maturation, harboring NRAS-G13D, with 49% bone marrow blasts. Mutant NRAS-positive AML6 cells were derived from a 69 year old patient with AML (acute myelomonocytic leukemia), harboring NRAS-G13D, with 43% bone marrow blasts.
KD of Wee1 by shRNA
pLKO.1puro lentiviral shRNA vector particles against Wee1 were purchased from Sigma-Aldrich (St. Louis, MO). Cells were incubated with the viral particles in the presence of 8 μg/ml Polybrene for 24 hours, and the cells were selected with 1-2 μg/ml puromycin for 72 hours. Following selection, cells were used for the studies described.
The sequence of shRNA are follows:
GFP : ACAACAGCCACAACGTCTATA
Wee1: CTAGAAAGAGTGCAGAACAAT
Mouse studies
In vivo model of inducible mutant KRAS-positive lung cancer
A well-established inducible mutant Kras lung cancer mouse model has been described previously21. Additional details are provided in the supplementary section.
In vivo model of mutant KRAS-positive leukemia
NB4 cells were transduced with a retrovirus encoding firefly luciferase (MSCV-Luc), and selected with neomycin (NB4-luc+). Six week-old female NSG mice (Jackson Laboratories, Bar Harbor, ME) were administered 1 × 106 NB4- luc+ cells via tail vein injection, followed four days later by imaging and quantification of total body bioluminescence as previously described19. Treatment cohorts with matched tumor burden were established and mice were orally administered 1X daily 10mg/kg MK-1775, 10mg/kg Torin 2, or a combination. Mice were treated on day 1 of dosing with 15mg/kg Torin 2 alone or in combination with 10mg/kg MK-1775, and thereafter, due to drug tolerance concerns, the dose of Torin 2 was lowered to 10mg/kg for six consecutive days before the final imaging. Only viable mice were censored for assessment of leukemia burden via serial bioluminescence imaging on days 3 and 7 of drug treatment. Studies were performed with Animal Care and Use Committee protocols at Dana-Farber Cancer Institute.
For both in vivo studies, MK-1775 was dissolved in a solution containing 0.5% methycellulose and 0.5% tween 80 (Sigma, St. Louis, MO). Torin 2 was dissolved in 30% capsitol (Sigma, St. Louis, MO).
Results
Identification of Wee1 inhibitor, MK-1775, as able to potentiate the effects of mTOR inhibition against mutant NRAS-expressing cells
Single agent monotherapies targeting either the PI3K/mTOR or MEK/ERK pathway, the two most critical signaling cascades downstream of activated RAS, have shown only modest effects in pre-clinical in vivo studies and in human clinical trials. However, a number of drug combinations centered on MEK inhibition, including addition of PI3K/mTOR/AKT inhibitors or conventional chemotherapy agents, have led to promising results in multiple RAS-expressing malignancies. In contrast, effective treatment combinations focused on mTOR inhibition are limited up to date. Taking advantage of the LINCS chemical library, we performed a synergy screen to search for drugs with a favorable pharmacological profile that could synergize with the mTOR inhibitor, Torin 1 (See schematic, Figure 1A). We carried out our initial screen in a mutant NRAS-transformed Ba/F3 cell line with parental Ba/F3 as baseline control. As expected, both MEK (PD0325901) and PI3K (GSK2126458) inhibitors were observed to synergize with Torin 1 against mutant NRAS-expressing cells (See schematic, Figure 1A and Figure 2), partially validating this strategy.
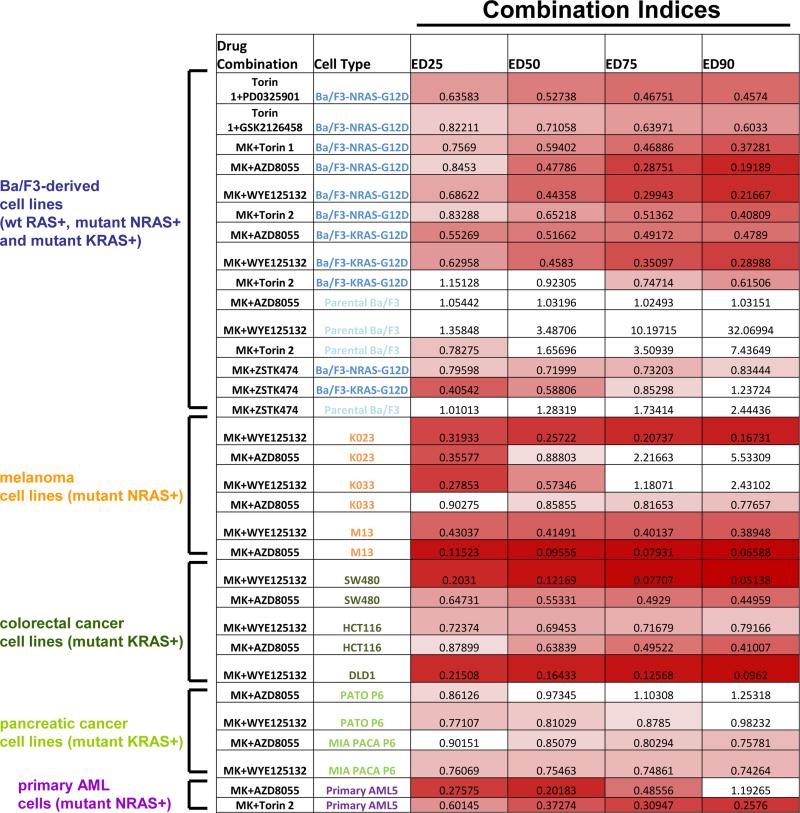
Figure 2. Combination indices for proliferation studies testing MK-1775 alone and combined with targeted inhibitors of mutant RAS signaling.
MK-1775 is abbreviated as “MK.” Values less than 0.9 indicate synergy and are in shades of red (darker shades mean higher synergy). Values greater than 0.9 do not indicate synergy and are colored white. Mutant RAS-expressing Ba/F3 cells are shown in dark blue font. Parental Ba/F3 cells are shown in light blue font. Mutant NRAS-positive melanoma cell lines are shown in orange font. Mutant KRAS-positive colorectal cancer cell lines are shown in dark green font. Mutant KRAS-expressing pancreatic cancer cell lines are shown in light green font. Primary AML patient cells are shown in dark purple font.
Surprisingly, however, the screen also identified the Wee1 inhibitor, MK-1775, which has not been previously shown to enhance anti-tumor effects of mTOR inhibition, despite prior evidence supporting its role as a synergistic partner for cytotoxic chemotherapy (see schematic, Figure 1A and Figure 2). We further validated the observed synergy using additional selective mTOR inhibitors, AZD8055, WYE125132, and Torin 2, and confirmed its activity against Ba/F3-NRAS-G12D cells, but not parental Ba/F3 cells (Figure 1B-E and Figure 2).
The combination of MK-1775 and mTOR inhibition against Ba/F3-NRAS-G12D cells correlates with inhibition of AKT, 4E-BP1, and S6K phosphorylation
mTOR inhibitors as single agents led to substantial G1 arrest in Ba/F3-NRAS-G12D cells approximately 48 hours after treatment, but not parental Ba/F3 cells (Supplementary Figure 1). However, addition of MK-1775 did not result in significant differences in cell cycle progression as compared to mTOR inhibitor only-treated cells (mTOR inhibitor=20 nM, MK-1775 =75 nM) (Supplementary Figure 1), suggesting that the role of Wee1 as a cell cycle entry checkpoint regulator may not have a significant contribution to the observed synergy. We detected enhanced suppression of phosphorylation of signaling molecules downstream of mutant RAS within the PI3K/mTOR/AKT pathway, including 4E-BP1 and AKT, in mutant NRAS-expressing Ba/F3 cells with the combination of Wee1 and mTOR inhibition (Figure 1F-G). In contrast, no drug combination effects were observed in wild-type RAS-expressing parental Ba/F3 cells (Figure 1F-G). The drug combination also led to greater suppression of S6 kinase phosphorylation (Figure 1H), although no further decrease in MAPK phosphorylation in mutant RAS-expressing cells, as compared to single agent effects, was observed (Supplementary Figure 2). These results suggest the MAPK pathway may play a less important role for the synergy of the two drugs in this particular genetic setting.
Combined Wee1 and mTOR inhibition is effective against Ba/F3-KRAS-G12D expressing cells
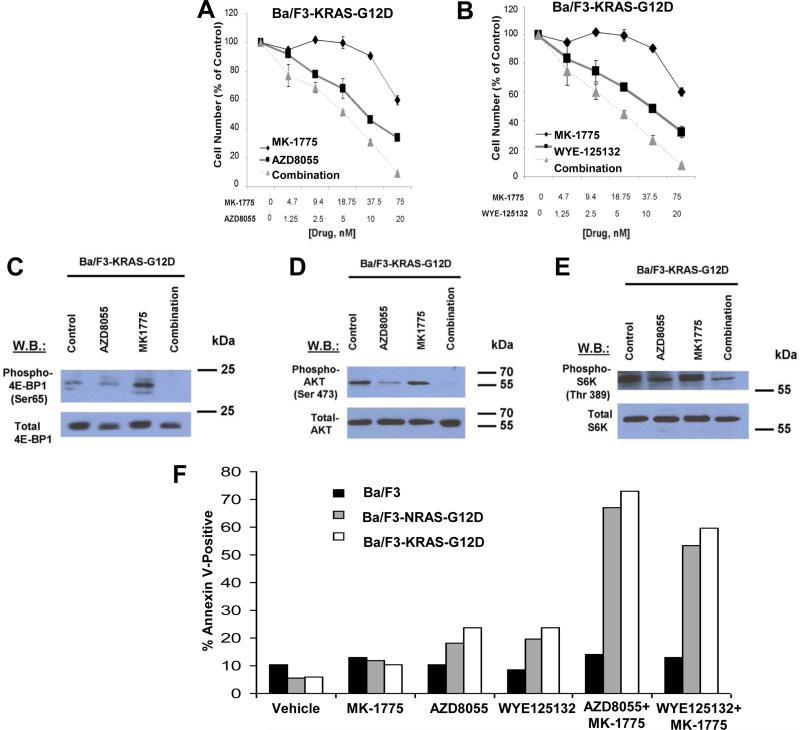
Because of the critical function of PI3K/mTOR/AKT pathway in the downstream signaling of multiple RAS isotypes, we asked the question whether combined Wee1 and mTOR inhibition also leads to enhanced inhibitory effects in mutant KRAS-expressing cells. Using a Ba/F3 cell line dependent on the expression of a KRAS G12D mutant, we confirmed the synergistic effects between MK-1775 and mTOR inhibition (Figure 3A-B and Figure 2). Consistent with the results in mutant NRAS-expressing Ba/F3 cells, we also observed decreased activity of phospho-4E-BP1, phospho-AKT, and phospho-S6K in Ba/F3-KRAS-G12D cells (Figure 3C-E).
Figure 3. Combined Wee1 and TORC inhibition is effective against Ba/F3-KRAS-G12D expressing cells and induces apoptosis in mutant NRAS- and mutant KRAS-expressing cells.
(A-B) Approximately two-day proliferation studies performed with MK-1775, combined with AZD8055 or WYE-125132 against Ba/F3-KRAS-G12D cells. (C-E) Effect of approximately two-hr treatment of Ba/F3-KRAS-G12D cells with MK-1775 (75 nM), alone and combined with mTOR inhibitor, AZD8055 (20 nM), on phosphorylation of 4E-BP1, AKT, and S6K, as measured by immunoblotting. (F) Approximately three-day treatment of Ba/F3, Ba/F3-NRASG12D, and Ba/F3-KRAS-G12D cells, respectively, with MK-1775 (75 nM), AZD8055 (20 nM), WYE125132 (20 nM), or a combination of MK-1775+AZD8055 or MK-1775+WYE125132 prior to analysis of apoptotic death.
Combined Wee1 and mTOR inhibition induces apoptosis in mutant RAS-expressing cells
Importantly, consistent with the increased mTOR/AKT inhibition, the combination of MK-1775 and mTOR inhibition led to a higher increase in apoptotic cell death than either agent alone when tested against mutant NRAS- and KRAS-expressing cells (Figure 3F), confirming the observed synergy for anti-cancer effects. This finding is further supported by increased phosphorylated histone H2A.X, a marker for DNA damage, which is required for DNA fragmentation during apoptosis and phosphorylated in response to apoptotic signals (Supplementary Figure 3).
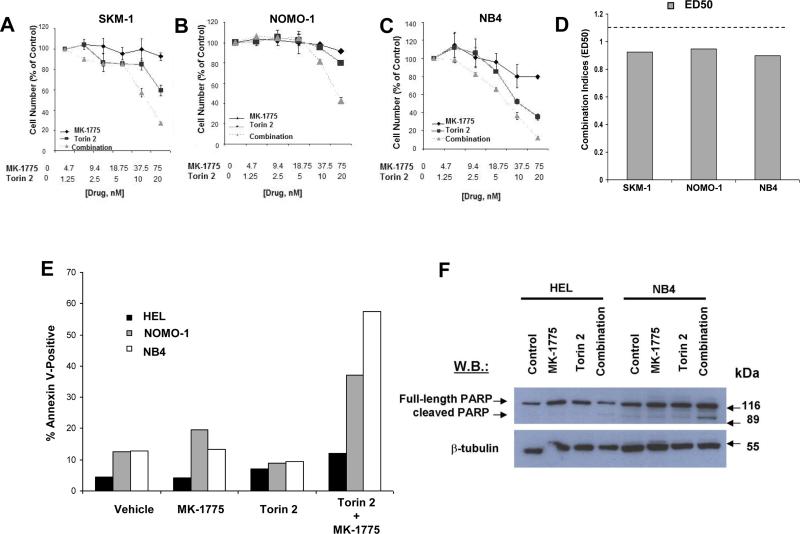
MK-1775 selectively potentiates the anti-leukemic effects of mTOR inhibition on proliferation of human mutant KRAS-expressing acute leukemia cells
We validated our findings from the Ba/F3 system in mutant KRAS-expressing human acute leukemia cell lines. As shown in Figure 4A-D, the combination of Wee1 and mTOR inhibition was synergistic against human mutant KRAS-expressing AML cell lines, SKM-1, Nomo-1, and NB4. In addition, the drug combination corresponded to a higher fraction of apoptotic cells and PARP cleavage than either drug alone in mutant KRAS-expressing cells, as compared to wild-type RAS-expressing cells (Figure 4E-F).
Figure 4. MK-1775 selectively potentiates the anti-leukemic effects of mTOR inhibition on proliferation of human mutant KRAS-expressing AML cells via induction of apoptosis.
(A-C) Dose-response curves for mutant KRAS-expressing human AML cells (SKM-1, Nomo-1, and NB4) showing single agent versus drug combination effects following approximately three days of treatment. (D) Calcusyn combination indices derived from six-point concentration proliferation experiments. The cut-off for nearly additive effects (C.I.: 1.1) is marked by a dashed line. (E) Approximately three-day treatment of HEL, Nomo-1, and NB4 cells, respectively, with MK-1775 (75 nM), Torin 2 (20 nM), or a combination of MK-1775+Torin 2 prior to analysis of apoptotic death. (F) Immunoblot showing effect of MK-1775+/-Torin 2 on PARP cleavage HEL and NB4 cells.
Wee1 kinase is the main target of MK-1775 that mediates synergy with mTOR inhibitors
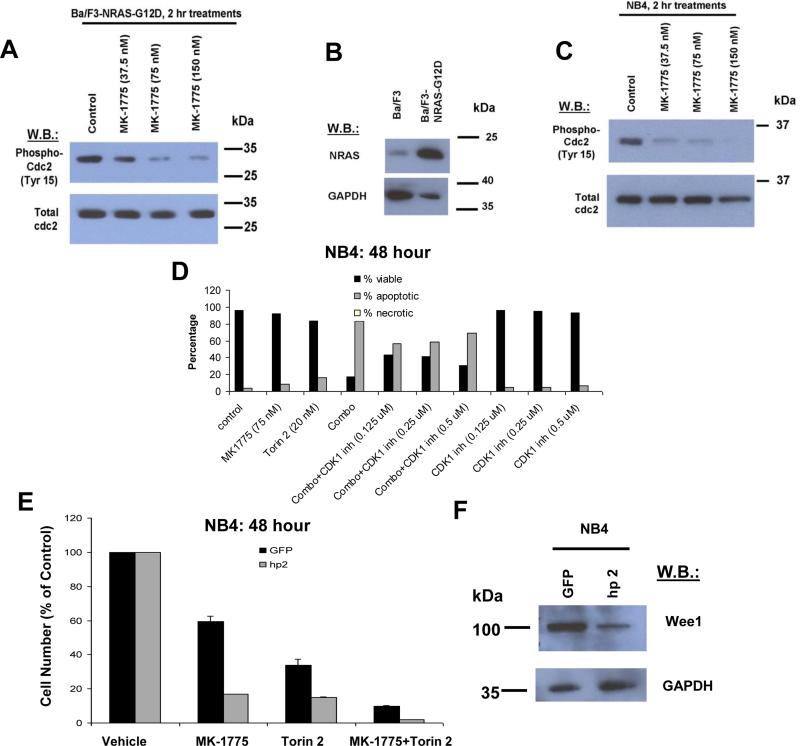
To exclude potential off target effects of MK-1775 that may contribute to the observed synergy with mTOR inhibition, we first confirmed that MK-1775 potently decreased the levels of phosphorylated cdc2 (CDK1), a direct substrate of Wee1, in a concentration-dependent manner (Figure 5A) in Ba/F3-NRAS-G12D cells that expressed higher protein levels of NRAS than parental Ba/F3 cells (Figure 5B). Similarly, MK-1775 inhibited phosphorylation of CDK1 in human active KRAS-expressing NB4 cells (Figure 5C).
Figure 5. Wee1 kinase is the main target of MK-1775 that mediates synergy with TORC inhibitors.
(A, C) Effect of approximately two hr treatment of Ba/F3-NRAS-G12D cells or NB4 cells with MK-1775 on phosphorylation of cdc2 (CDK1), as measured by immunoblotting. (B) NRAS expression in Ba/F3 versus Ba/F3-NRAS-G12D cells. (D) Measurement of apoptosis following approximately 48 hour treatment of NB4 cells with MK-1775, Torin 2, MK-1775+Torin 2, or MK-1775+Torin 2 in the presence of a CDK1 inhibitor at the indicated concentrations. (E) Proliferation of approximately 48 hour MK-1775 (75 nM)- and Torin 2 (20 nM)-treated NB4 cells following KD of Wee1 by shRNA (hp2) as compared to GFP control. (F) Validation of Wee1 KD efficiency in NB4 cells.
In order to confirm that Wee1 inhibition plays a role in the observed drug combination-induced apoptosis and synergy through CDK1 activation, we tested the ability of the CDK1 inhibitor, R0-3306, to block Wee1 and mTOR inhibitor-induced apoptosis in mutant RAS-expressing cells. Co-treatment of NB4 cells with MK-1775+Torin 2 and R0-3306 led to a partial rescue of cells from the proapoptotic effects of the drug combination (Figure 5D). Modest rescue of drug combination effects on cellular proliferation was also observed with R0-3306 treatment (Supplementary Figure 4). Importantly, shRNA KD of Wee1 in mutant KRAS-expressing NB4 cells led to an increased sensitivity of cells to the anti-proliferative effects of MK-1775 and Torin 2, both as single agents and combined (Figure 5E-F). These results suggest that the synergy observed between MK-1775 and mTOR inhibition is at least in part due to targeting of Wee1 by MK-1775.
MK-1775 selectively potentiates the anti-leukemic effects of mTOR inhibition on proliferation of human mutant NRAS-expressing acute leukemia cells
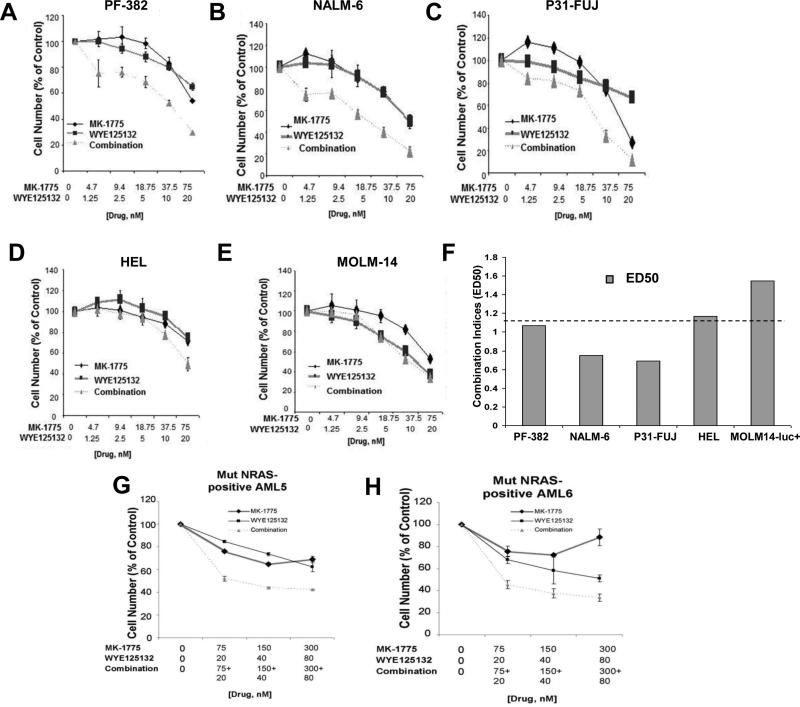
We next validated our findings from the Ba/F3 system in mutant NRAS-expressing human acute leukemia cell lines. Similar to the Ba/F3 results, MK-1775 was observed to effectively combine respectively with the selective mTOR inhibitor, WYE125132, against the mutant NRAS-expressing lines, PF-382 (ALL), NALM6 (ALL), and P31-FUJ (AML) (Figure 6A-C, F). In contrast, our results suggest potential antagonism between MK-1775 and WYE125132 against wt RAS-expressing lines, such as HEL or MOLM14 (Figure 6D-F). Similar results were observed when MK-1775 was combined respectively with mTOR inhibitors Torin 1, Torin 2, or AZD8055 (data not shown).We also tested the combined Wee1 and mTOR inhibition in mutant NRAS-expressing primary AML patient cells, and observed more cell death with the combination than either agent alone (Figure 6G-H, Supplementary Figure 5), with combination indices suggestive of synergy (Figure 2). These data support the potential clinical utility of this drug combination for mutant NRAS-positive AML.
Figure 6. MK-1775 selectively potentiates the anti-leukemic effects of mTOR inhibition on proliferation of human mutant NRAS-expressing acute leukemia cells.
(A-E) Dose-response curves for mutant NRAS-expressing acute leukemia cells (PF-382, NALM6, P31-FUJ) or wt RAS-expressing acute leukemia cells (HEL, MOLM14) showing single agent (MK-1775 or WYE125132) versus drug combination effects following approximately two days of treatment. (F) Calcusyn combination indices derived from six-point concentration proliferation experiments. The cut-off for nearly additive effects (C.I.: 1.1) is marked by a dashed line. (G-H) Mutant NRAS-positive AML patient samples treated with MK-1775, WYE125132, or a combination of both.
AKT partially mediates potentiation of mTOR inhibition by MK-1775
To assess the significance of AKT in the observed synergy between MK-1775 and mTOR inhibitors, we compared the effects of drug treatment on Ba/F3-NRAS-G12D cells and Ba/F3-NRAS-G12D cells expressing constitutively active, myristoylated AKT (Ba/F3-NRASG12D-AKT (myr)). Whereas the activity of MK-1775 was not substantially different between the two cell lines, Ba/F3-NRAS-G12D-AKT (myr) cells were less sensitive to the effects of mTOR inhibition (Supplementary Figure 6). There was a modest rightward shift in the drug combination dose-response curve in Ba/F3-NRAS-G12D-AKT (myr) cells, as compared to Ba/F3-NRAS-G12D cells, as well as corresponding higher combination indices (Supplementary Figure 6), suggesting that AKT may at least in part contribute to the observed synergy between MK-1775 and mTOR inhibition.
MK-1775 potentiates the effects of PI3K inhibition against mutant RAS-positive cells
As the PI3K/AKT/mTOR pathway may be particularly important for the observed synergy, we investigated whether MK-1775 was able to synergize with inhibitors targeting components within the PI3K/AKT/mTOR pathway other than targeted mTOR inhibitors. The results suggest that MK-1775 selectively synergized with ZSTK474, an inhibitor of PI3K isoforms, against mutant RAS-expressing Ba/F3 cells, however this drug combination was antagonistic against parental Ba/F3 cells (Supplementary Figure 7).
MK-1775 potentiates the effects of mTOR inhibition against mutant RAS-expressing solid tumors
Having established the effectiveness of MK-1775 combined with mTOR inhibition against mutant RAS-positive acute leukemia, we investigated whether this particular targeting strategy is also efficacious against other mutant RAS-positive malignancies. The incidence of NRAS mutations in human melanoma has been reported to be as high as 28%22, while the incidence of KRAS mutations ranges between 20-40% in colorectal cancer23, 75-95% in pancreatic cancer24, and 15-30% in lung adenocarcinoma25. Both AZD8055 and WYE125132 respectively synergized with MK-1775 against mutant NRAS-positive melanoma cell lines, K023, K033, and M13 (Supplementary Figure 8 and Figure 2). In addition, comparable potentiating effects of MK-1775 for mTOR inhibitors were also observed in three mutant KRAS-expressing colorectal cancer cell lines, HCT116, DLD1, and SW480 (Supplementary Figure 9 and Figure 2), and in two mutant KRAS-expressing pancreatic cancer cell lines, PATO P6 and MIA PaCa (Supplementary Figure 10 and Figure 2). MK-1775 also enhanced the effects of mTOR inhibition against active KRAS-expressing human lung cancer lines, A549, H1355, H441, H1944, and H1792 (Figure 7A and Supplementary Figure 11). Finally, MK-1775 synergized with Torin 1 or Torin 2 against p53-kd/KRAS and Lkb-1/p53-kd/KRAS expressing murine lung cancer cell lines derived from in vivo models (Supplementary Figure 12, Supplementary Table 1).
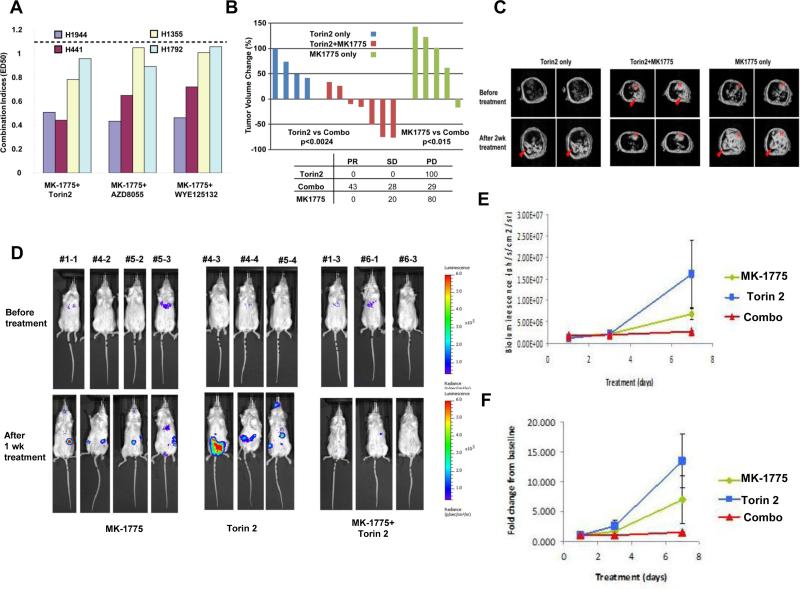
Figure 7. In vivo investigation of the combined effects of Wee1 and mTOR inhibition in murine models of active KRAS.
(A) Calcusyn combination indices derived from six-point concentration proliferation experiments, investigating the combination of MK-1775 and mTOR inhibitors against human active KRAS-expressing lung cancer cell lines. The cut-off for nearly additive effects (C.I.: 1.1) is marked by a dashed line. (B) In vivo effects of Torin 2 (20mg/kg 1X daily), MK-1775 (10mg/kg 1X daily), or a combination of the two agents using an inducible active KRAS model of lung cancer. Each column represents tumor volume changes after 2 weeks of indicated treatment for an individual mouse by comparing total lung tumor volume before and after treatment. Negative and positive percentage indicates tumor regression and progression respectively. A two-tailed t test was used to calculate the p value between treatment groups. (C) Representative MRI scans detecting tumor burden changes before and after indicated treatments. Arrows, tumors; H, Heart. (D-F) In vivo effects of Torin 2, MK-1775, or a combination of the two agents using a murine model of mutant KRAS-positive leukemia. (D) Mouse bioluminescence images taken before treatment (baseline) and after one week of drug treatment of NSG mice harboring NB4-luc+ cells. (E) Bioluminescence values plotted over time for single agent- and combination-treated mice. (F) Fold leukemia induction plotted over time for single agent- and combination-treated mice. Combination-treated mice were tested in a pilot titration study testing for Torin 2 tolerance/toxicity in which they received 0, 5, or 10mg/kg Torin 2 1X daily for 1 week prior to tail vein-injection of NB4-luc+ cells. Drug administration was terminated several days prior to cell inoculation and observed to have no influence on starting leukemia burden.
Combination of MK-1775 and Torin 2 is effective against mutant KRAS lung cancer and mutant KRAS-positive leukemia in mouse models
Having demonstrated the ability of MK-1775 to potentiate the efficacy of mTOR inhibition against mutant NRAS-positive leukemia and melanoma in vitro, and given the observed synergy between Wee1 inhibition and mTOR inhibition against mutant KRAS-positive colorectal, pancreatic, and lung cancer in vitro, we tested this combination against RAS positive lung cancer in a murine model. We used a well-established genetically engineered mouse lung cancer model that harbors an activating KRAS mutation (G12D)21. Specifically, mice harboring conditional activating mutation (G12D) at the endogenous Kras locus were activated by nasal instillation of 5×106 pfu of adenovirus encoding CRE recombinase . Expression of cre recombinase in infected lung epithelial cells removes loxp flanked stop cassette located before ATG start codon of the mutant Kras gene (Supplementary Figure 13). Induced KRAS mice express activating KRAS G12D mutant in lung epithelial cells, and have a disease latency of 13-15 weeks after induction.
With this model, we were able to detect significant tumor regression when tumor-bearing mice were treated by the MK-1775 and Torin 2 combination (Figure 7B, 7C). Progressive disease (PD) is defined as 20% progression in tumor volume over the baseline scan performed before treatment starts. Partial response (PR) is defined as 30% or more regression in tumor volume compared to baseline. Stable disease (SD) is between 20% progression and 30% regression. The combination therapy achieved a nearly 81% disease control rate (43% PR and 28% SD). In contrast, single agent treatment with either MK-1775 or Torin 2 showed 100% and 80% PD respectively (Figure 7B, 7C).
Increased disease suppression by the Wee1 and mTOR inhibitor combination, as compared to single agents, was similarly observed in a murine model of mutant KRAS-positive AML. The combination of MK-1775 and Torin 2 was more effective at suppressing the growth of NB4-luc+ cells than either drug alone following 1 week of drug administration (Figure 7D-F). The comparable trend observed for the drug combination versus single agent effects in the mutant RAS leukemia model supports results observed in the mutant RAS lung cancer model. Taken together, these results suggest that the combination of a Wee1 inhibitor and an mTOR inhibitor may be of potential clinical value in inhibiting the progression of mutant RAS-driven malignancies.
Discussion
Mediation of the effects of RAS by prominent signaling pathways such as PI3K//PTEN/AKT/mTOR and Raf/MEK/ERK has prompted the testing of targeted inhibitors of these pathways as a strategy to treat mutant RAS-driven malignancies. Unfortunately, inhibition of PI3K or mTOR alone has limited efficiency (reviewed in26) and has further contributed to the clinical sense that targeting RAS in general has been disappointing27,28. In part, the limited efficacy of inhibitors of Raf/MEK/Erk signaling or PI3K/Akt in mutant RAS-positive cancer is believed to be due to negative feedback loops and compensatory activation of bypass signaling pathways, leading the many current efforts to simultaneously target multiple effectors in mutant RAS-positive cancers.
In the present study, we introduce a novel chemical screen approach, utilizing the LINCS chemical library, to identify agents with activity in the presence of mTOR inhibition in mutant RAS-expressing cells. LINCS library compounds were anticipated to be useful because of their limited spectrum of kinase targets, therefore giving the potential of more successfully identifying real target kinases. Consistent with what has been reported in the literature, mTOR inhibition was potentiated by inhibitors of PI3K/AKT and MEK derived from the screened chemical library. We also surprisingly observed enhancement of the effects of mTOR inhibition by the targeted Wee1 inhibitor, MK-1775. The notion that the relevant target of MK-1775 was indeed Wee1 was supported by initial studies with an inhibitor relevant to Wee1 signaling, as well as by KD of Wee1 expression.
Our studies utilized several well-characterized mTOR inhibitors, including AZD8055, a pan-mTOR inhibitor developed by AstraZeneca, which is being investigated in a clinical trial (NCT01316809) aimed at drug-resistant glioma and other cancers29,30. Also included was WYE125132 (Wyeth (Pfizer)), which shows 5000-fold higher selectivity for mTOR over PI3K and has demonstrated anti-cancer activity against solid tumors, including gliomas and cancers of the kidney, breast, and lung31. Finally, we used Torin 232, a second-generation ATP-competitive inhibitor that was developed by optimizing the earlier generation inhibitor, Torin18. Torin 2 potently inhibits the signaling complex, mTORC1, with around 800-fold selectivity for cellular mTOR versus PI3K, and potently inhibits additional targets including the PI3K-like kinase (PIKK) family kinases (including ATM, DNA-PK, and ATR)33.
mTORC1 promotes protein synthesis by phosphorylation of 4E-BP1 and p70S6K, whereas mTORC2 promotes survival and migration through phosphorylation of AKT and activation of RhoGTPase (reviewed in34). In the present study, the strongest suppression of phosphorylation of AKT and 4E-BP1 was observed by the combination of MK-1775 and mTOR inhibition in mutant RAS-expressing cells, suggesting MK-1775 potentiation of mTOR inhibitor effects in this system.
Both 4E-BP1 and AKT play significant roles in apoptosis, and loss of their activity correlates with the increased apoptosis observed in mutant RAS-expressing cells by the MK-1775+mTOR inhibitor combination. Specifically, as activated AKT phosphorylates and inhibits pro-apoptotic Bcl-2 family members35, the increased dephosphorylation of AKT observed with MK-1775+mTOR inhibition supports a possible role for AKT in enhanced induction of apoptosis in mutant RAS-positive cells. Inhibition of mTOR signaling and dephosphorylation of 4E-BP1 precede apoptosis, and in apoptotic cells, caspase-dependent cleavage of 4E-BP1 leads to inhibition of its phosphorylation and an increase in its association with eukaryotic initiation factor 4E (eIF4)36. The increased dephosphorylation of 4E-BP1 observed with MK-1775+mTOR inhibition may similarly correlate with a possible role for 4E-BP1 in increased apoptosis in mutant RAS-expressing cells.
It is unknown which malignancies will respond to- or show resistance to- the pan mTOR inhibitors that are currently in clinical trials. For instance, KRAS was identified in one study as being a significant genetic marker for resistance to PP-242, an inhibitor of both mTORC1 and mTORC2 that is presently in early stage clinical trials37. The degree of effectiveness of mTOR inhibition in that study correlated with the degree of inhibition of phosphorylation of 4E-BP1. RAS activation also leads to hyper-activation of the serine/threonine kinase, AKT, and increased AKT activation may account for resistance to therapy38. The complete inhibition of phosphorylation of 4E-BP1 and AKT by the combination of MK-1775 and mTOR inhibition, versus only partial inhibition of these molecules by mTOR inhibition alone, points toward a potentially effective strategy to override insensitivity of mutant RAS-expressing cells to mTOR inhibition.
Importantly, cdc2 (CDK1), a direct substrate of Wee1 that is inhibited by Wee1, has been described as a proapoptotic mediator39. Specifically, increased CDK1 activity has been observed to correlate with apoptosis and studies involving CDK1 inhibition have suggested that this increased activity may be significant for cell death39. In the present study we have shown that MK-1775, which inhibits Wee1, leads to dephosphorylation- and presumed activation- of CDK1. Co-treatment of mutant RAS-positive cells with a CDK1 inhibitor partially reversed the inductive effect of MK-1775+mTOR inhibition on apoptosis in these cells, suggesting a possible contributory role of CDK1 activity in the increased apoptosis observed with the drug combination.
CDK1 directly phosphorylates various mTORC1-related signaling factors, and inter-regulation between CDK1 and mTORC1 kinase complexes, which may be dependent on exact timing and localization of these proteins during mitosis, has been proposed40. It is possible that such direct communication contributes mechanistically to the synergy we have observed between Wee1 and mTOR inhibition in mutant RAS-positive cells.
MK-1775 has recently been shown pre-clinically to exhibit single agent anti-tumor activity against solid tumors including sarcomas and neuroblastomas41-43. Of note, compared to the IC50s (300-500 nM) reported for MK-1775 against these solid tumors, we tested a considerably lower range of MK-1775 concentrations for synergy in our present studies that could be considered desirable for clinical usage. Specifically, concentrations of MK-1775 less than 75 nM, which showed little single agent activity, potentiated the effects of low nM concentrations of mTOR inhibitors, as well as PI3K inhibition, against mutant RAS-expressing leukemia cells.
Generally, the potentiating effects of MK-1775 on radiotherapy or cytotoxic DNA-damaging therapies have been more extensively investigated than single agent activity14,15,44-47 and such combination approaches constitute the majority of MK-1775-related clinical trials. The use of nonselective cytotoxic agents, though, is generally not ideal due to adverse effects and limited tolerance48. There is thus a need for more efficacious and safer therapeutic strategies, and combination therapy based on small molecule, targeted pharmacological inhibitors offers a promising alternative.
Here, we have focused primarily on the effects of Wee1 and mTOR inhibition, combined, against mutant RAS-driven acute leukemia. Our findings additionally extend to a variety of solid tumors characterized as harboring RAS mutations. High expression of Wee1 was reported in malignant melanoma and was found to correlate with poor disease-free survival, and abnormal Wee1 expression was found in lung carcinoma49,50. These findings suggest that there is potential clinical benefit to be gained from using a Wee1 inhibitor as part of a treatment strategy against these diseases. Importantly, however, malignancies driven by or characterized by mutated RAS expression represent especially challenging disease targets due to the difficulties associated with directly targeting the RAS protein, as well as feedback loops and the redundancies and complexities of RAS signaling that counteract effective targeting of signaling factors downstream of RAS.
Our findings support the notion that an inhibitor of Wee1 may be effectively combined with a targeted inhibitor of mutant RAS signaling against malignancies characterized by RAS mutations. This novel combination strategy thus warrants further investigation as a therapeutic approach that could potentially override drug resistance associated with mTOR inhibition and that therefore may be of potential clinical benefit in mutant RAS-driven malignancies.
Supplementary Material
Acknowledgments
N.S. Gray is supported by NIH LINCS grant HG006097. J.D.G. receives research support and has a financial interest with Novartis Pharma AG.
Footnotes
Conflict of Interest: None of the authors included in this manuscript have a financial conflict of interest.
Supplementary information is available at Leukemia's website.
References
- 1.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 2.Bowen DT, Frew ME, Hills R, Gale RE, Wheatley K, Groves MJ, et al. RAS mutation in acute myeloid leukemia is associated with distinct cytogenetic subgroups but does not influence outcome in patients younger than 60 years. Blood. 2005;106:2113–2119. doi: 10.1182/blood-2005-03-0867. [DOI] [PubMed] [Google Scholar]
- 3.Bacher U, Haferlach T, Schoch C, Kern W, Schnittger S. Implications of NRAS mutations in AML: a study of 2502 patients. Blood. 2006;107:3847–3853. doi: 10.1182/blood-2005-08-3522. [DOI] [PubMed] [Google Scholar]
- 4.Nakao M, Janssen JW, Seriu T, Bartram CR. Rapid and reliable detection of N-ras mutations in acute lymphoblastic leukemia by melting curve analysis using LightCycler technology. Leukemia. 2000;14:312–315. doi: 10.1038/sj.leu.2401645. [DOI] [PubMed] [Google Scholar]
- 5.Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-RAS (G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503:548–51. doi: 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim SM, Westover KD, Ficarro SB, Harrison RA, Choi HG, Pacold ME, et al. Therapeutic targeting of oncogenic K-RAS by a covalent catalytic site inhibitor. Angew Chem Int Ed Engl. 2014;53:199–204. doi: 10.1002/anie.201307387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Franklin RA, Montalto G, et al. RAS/Raf/MEK/ERK and PI3K/PTEN/AKT/mTOR cascade inhibitors: how mutations can result in therapy resistance and how to overcome resistance. Oncotarget. 2012;3:1068–1111. doi: 10.18632/oncotarget.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, et al. An ATP-competitice mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Igarashi M, Nagata A, Jinno S, Suto K, Okayama H. Wee1+-like gene in human. Nature. 1991;353:80–83. doi: 10.1038/353080a0. [DOI] [PubMed] [Google Scholar]
- 10.Parker LL, Piwnica-Worms H. Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science. 1992;257:1955–1957. doi: 10.1126/science.1384126. [DOI] [PubMed] [Google Scholar]
- 11.McGowan CH, Russell P. Human Wee1 kinase inhibits cell division by phosphorylating p34cdc2 exclusively on Tyr15. EMBO J. 1993;12:75–85. doi: 10.1002/j.1460-2075.1993.tb05633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin P, Gu Y, Morgan DO. Role of inhibitory CDC2 phosphorylation in radiation-induced G2 arrest in human cells. J Cell Biol. 1996;134:963–970. doi: 10.1083/jcb.134.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Witt Hamer PC, Mir SE, Noske D, Van Noorden CJF, Wurdinger T. Wee1 kinase targeting combined with DNA-damaging cancer therapy catalyzes mitotic catastrophe. Clin Cancer Res. 2011;17:4200–4207. doi: 10.1158/1078-0432.CCR-10-2537. [DOI] [PubMed] [Google Scholar]
- 14.Hirai H, Arai T, Okada M, Nishibata T, Kobayashi M, Sakai N, et al. MK-1775, a small molecule Wee1 inhibitor, enhances anti-tumor efficacy of various DNA-damaging agents, including 5-fluorouracil. Cancer Biol Ther. 2010;9:514–522. doi: 10.4161/cbt.9.7.11115. [DOI] [PubMed] [Google Scholar]
- 15.Rajeshkumar NV, De Oliveira E, Ottenhof N, Watters J, Brooks D, Demuth T, et al. MK-1775, a potent Wee1 inhibitor, synergizes with gemcitabine to achieve tumor regressions, selectively in p53-deficient pancreatic cancer xenografts. Clin Cancer Res. 2011;17:2799–2806. doi: 10.1158/1078-0432.CCR-10-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Q, Chang JW, Wang J, Kang SA, Thoreen CC, Markhard A, et al. Discovery of 1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benzo[h][1,6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J Med Chem. 2010;53:7146–7155. doi: 10.1021/jm101144f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuo Y, MacLeod RA, Uphoff CC, Drexler HG, Nishizaki C, Katayama Y, et al. Two acute monocytic leukemia (AML-M5a) cell lines (MOLM13 and MOLM14) with interclonal phenotypic heterogeneity showing MLL-AF9 fusion resulting from an occult chromosome insertion, ins(11;9)(q23;p22p23). Leukemia. 1997;11:1469–1477. doi: 10.1038/sj.leu.2400768. [DOI] [PubMed] [Google Scholar]
- 18.Kimbrel EA, Davis TN, Bradner JE, Kung AL. In vivo pharmacodynamic imaging of proteosome inhibition. Mol Imaging. 2009;8:140–147. [PubMed] [Google Scholar]
- 19.Weisberg E, Manley PW, Breitenstein W, Bruggen J, Cowan-Jacob SW, Ray A, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7:129–41. doi: 10.1016/j.ccr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 21.Chen Z, Cheng K, Walton Z, Wang Y, Ebi H, Shimamura T, et al. A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature. 2012;483:613–617. doi: 10.1038/nature10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omholt K, Karsberg S, Platz A, Kanter L, Ringborg U, Hansson J. Screening of N-rascodon 61 mutations in paired primary and metastatic cutaneous melanomas: mutations occur early and persist throughout tumor progression. Clin Cancer Res. 2002;8:3468–3474. [PubMed] [Google Scholar]
- 23.Arrington AK, Heinrich EL, Lee W, Duldulao M, Patel S, Sanchez J, et al. Prognostic and predictive roles of KRAS mutation in colorectal cancer. Int J Mol Sci. 2012;13:12153–68. doi: 10.3390/ijms131012153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laghi L, Orbetegli O, Bianchi P, Zerbi A, Di Carlo V, Boland CR, et al. Common occurrence of multiple K-RAS mutations in pancreatic cancers with associated precursor lesions and in biliary cancers. Oncogene. 2002;21:4301–6. doi: 10.1038/sj.onc.1205533. [DOI] [PubMed] [Google Scholar]
- 25.Pao W, Wang TY, Riely GJ, Miller VA, Pan Q, Ladanyi M, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ward AF, Braun BS, Shannon KM. Targeting oncogenic RAS signaling in hematologic malignancies. Blood. 2012;120:3397–406. doi: 10.1182/blood-2012-05-378596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karp JE, Lancet JE, Kaufmann SH, et al. Clinical and biologic activity of the farnesyltransferase inhibitor R115777 in adults with refractory and relapsed acute leukemias: a phase I clinical-laboratory correlative trial. Blood. 2001;97:3361–3369. doi: 10.1182/blood.v97.11.3361. [DOI] [PubMed] [Google Scholar]
- 28.Downward J. Targeting RAS signaling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 29.Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, Chritchlow SE, et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010;70:288–298. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- 30.Banerji U, Aghajanian C, Raymond E, Kurzrock R, Blanco-Codesido M, Oelmann E, et al. First results from a phase I trial of AZD8055, a dual mTORC1 and mTORC2 inhibitor. J Clin Oncol. 2011;29(suppl) abstr 3096. [Google Scholar]
- 31.Yu K, Shi C, Toral-Barza L, Lucas J, Shor B, Kim JE, et al. Beyond rapalog therapy: preclinical pharmacology and antitumor activity of WYE 125132, an ATP-competitive and specific inhibitor of mTORC1 and mTORC2. Cancer Res. 2010;70:621–631. doi: 10.1158/0008-5472.CAN-09-2340. [DOI] [PubMed] [Google Scholar]
- 32.Liu Q, Wang J, Kang SA, Thoreen CC, Hur W, Ahmed T, et al. Discovery of 9 (6 aminopyridin-3 yl)-1 (3-(trifluoromethyl)phenyl)benzo[h][1,6] naphthyridin 2(1H)-one (Torin 2) as a potent, selective, and orally available mammalian target of rapamycin (mTOR) inhibitor for treatment of cancer. J Med Chem. 2011;54:1473–1480. doi: 10.1021/jm101520v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Q, Xu C, Kirubakaran S, Zhang X, Hur W, Liu Y, et al. Characterization of Torin 2, an ATP-competitive inhibitor of mTOR, ATM, and ATR. Cancer Res. 2013;73:2574–2586. doi: 10.1158/0008-5472.CAN-12-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bracho-Valdes I, Moreno-Alvarez P, Valencia-Martinez I, Robles-Molina E, Chavez-Vargas L, Vazquez-Prado J. mTORC1- and mTORC2-interacting proteins keep their multifunctional partners focused. IUBMB Life. 2011;63:896–914. doi: 10.1002/iub.558. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Tang N, Hadden TJ, Rishi AK. AKT, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011;1813:1978–86. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Proud CG. The eukaryotic initiation factor 4E-binding proteins and apoptosis. Cell Death Differ. 2005;12:541–6. doi: 10.1038/sj.cdd.4401588. [DOI] [PubMed] [Google Scholar]
- 37.Ducker GS, Atreya CE, Simko JP, Hom YK, Matli MR, Benes CH, et al. Incomplete inhibition of phosphorylation of 4E-BP1 as a mechanism of primary resistance to ATP-competitive mTOR inhibitors. Oncogene. 2013 doi: 10.1038/onc.2013.92. e-pub ahead of print April 2013; doi: 10.1038/onc.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cassinelli G, Zuco V, Gatti L, Lanzi C, Zaffaroni N, Colombo D, et al. Targeting the AKT kinase to modulate survival, invasiveness and drug resistance of cancer cells. Curr Med Chem. 2013;20:1923–1945. doi: 10.2174/09298673113209990106. [DOI] [PubMed] [Google Scholar]
- 39.Castedo M, Perfettini JL, Roumier T, Kroemer G. Cyclin-dependent kinase-1: linking apoptosis to cell cycle and mitotic catastrophe. Cell Death Differ. 2002;9:1287–93. doi: 10.1038/sj.cdd.4401130. [DOI] [PubMed] [Google Scholar]
- 40.Gwinn DM, Asara JM, Shaw RJ. Raptor is phosphorylated by cdc2 during mitosis. PLoS One. 2010;5:e9197. doi: 10.1371/journal.pone.0009197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kreahling JM, Gemmer JY, Reed D, Letson D, Bui M, Altiok S. MK-1775, a selective Wee1 inhibitor, shows single-agent antitumor activity against sarcoma cells. Mol Cancer Ther. 2012;11:174–182. doi: 10.1158/1535-7163.MCT-11-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guertin AD, Li J, Liu Y, Hurd MS, Schuller AG, Long B, et al. Preclinical evaluation of the WEE1 inhibitor MK-1775 as Single-Agent Anticancer Therapy. Mol Cancer Ther. 2013;12:1442–1452. doi: 10.1158/1535-7163.MCT-13-0025. [DOI] [PubMed] [Google Scholar]
- 43.Russell MR, Levin K, Rader J, Belcastro L, Li Y, Martinez D, et al. Combination therapy targeting the Chk1 and Wee1 kinases shows therapeutic efficacy in neuroblastoma. Cancer Res. 2013;73:776–84. doi: 10.1158/0008-5472.CAN-12-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarcar B, Kahali S, Prabhu AH, Shumway SD, Xu Y, Demuth T, et al. Targeting radiation-induced G(2) checkpoint activation with the Wee-1 inhibitor MK-1775 in glioblastoma cell lines. Mol Cancer Ther. 2011;10:2405–2414. doi: 10.1158/1535-7163.MCT-11-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bridges KA, Hirai H, Buser CA, Brooks C, Liu H, Buchholz TA, et al. MK-1775, a novel Wee1 kinase inhibitor, radiosensitizes p53-defective human tumor cells. Clin Cancer Res. 2011;17:5638–5648. doi: 10.1158/1078-0432.CCR-11-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aarts M, Sharpe R, Garcia-Murillas I, Gevensleben H, Hurd MS, Shumway SD, et al. Forced mitotic entry of S-phase cells as a therapeutic strategy induced by inhibition of Wee1. Cancer Discov. 2012;2:524–539. doi: 10.1158/2159-8290.CD-11-0320. [DOI] [PubMed] [Google Scholar]
- 47.Tibes R, Bogenberger JM, Chaudhuri L, Hagelstrom RT, Chow D, Buechel ME, et al. RNAi screening of the kinome with cytarabine in leukemias. Blood. 2012;119:2863–2872. doi: 10.1182/blood-2011-07-367557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramamoorthy K, Ramesh P, Al Bahar S. Primary treatment of acute myeloid leukemia (non M3) in elderly: a review. Gulf J Oncolog. 2008;4:19–26. [PubMed] [Google Scholar]
- 49.Yoshida T, Tanaka S, Mogi A, Shitara Y, Kuwano H. The clinical significance of cyclin B1 and Wee1 expression in non-small-cell lung cancer. Ann Oncol. 2004;15:252–256. doi: 10.1093/annonc/mdh073. [DOI] [PubMed] [Google Scholar]
- 50.Magnussen GI, Holm R, Emilsen E, Rosnes AK, Slipicevic A, Florenes VA. High expression of Wee1 is associated with poor disease-free survival in malignant melanoma: potential for targeted therapy. PLoS One. 2012;7:e38254. doi: 10.1371/journal.pone.0038254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.