Abstract
Background
Pneumonia remains a leading cause of under-five morbidity and mortality globally. Comprehensive incidence, epidemiologic and etiologic data are needed to update prevention and control strategies.
Methods
We conducted active, population-based surveillance for hospitalized cases of acute lower respiratory tract infections (ALRI) among children <5 years of age in rural thailand. ALRI cases were systematically sampled for an etiology study that tested nasopharyngeal specimens by polymerase chain reaction; children without ALRI were enrolled as controls from outpatient clinics.
Results
We identified 28,543 hospitalized ALRI cases from 2005 to 2010. Among the 49% with chest radiographs, 63% had findings consistent with pneumonia as identified by 2 study radiologists. The hospitalized ALRI incidence rate was 5772 per 100,000 child-years (95% confidence interval: 5707, 5837) and was higher in boys versus girls (incidence rate ratio 1.38, 95% confidence interval: 1.35–1.41) and in children 6–23 months of age versus other age groups (incidence rate ratio 1.76, 95% confidence interval: 1.69–1.84). Viruses most commonly detected in ALRI cases were respiratory syncytial virus (19.5%), rhinoviruses (18.7%), bocavirus (12.8%) and influenza viruses (8%). Compared with controls, ALRI cases were more likely to test positive for respiratory syncytial virus, influenza, adenovirus, human metapneumovirus and parainfluenza viruses 1 and 3 (P ≤ 0.01 for all). Bloodstream infections, most commonly Streptococcus pneumoniae and nontyphoidal Salmonella, accounted for 1.8% of cases.
Conclusions
Our findings underscore the high burden of hospitalization for ALRI and the importance of viral pathogens among children in Thailand. Interventions targeting viral pathogens coupled with improved diagnostic approaches, especially for bacteria, are critical for better understanding of ALRI etiology, prevention and control.
Keywords: pneumonia, respiratory illness, Thailand, pediatric
An estimated 156 million childhood clinical pneumonia cases occur annually (150 million in developing nations), causing nearly 2 million deaths in children <5 years, making pneumonia the most common cause of mortality among young children worldwide.1 Previous reports have noted a high pneumonia incidence in Southeast asia,1 although few country-specific estimates exist. A 2008 Bulletin of the World Health OrGAnization (WHO) concluded that “a comprehensive and accurate description of the epidemiology and etiology” of childhood pneumonia is an essential first step for the prevention and management of disease.2
The International Emerging Infections Program at the Global Disease Detection Center, part of a collaboration between the Thailand Ministry of Public Health (MoPH) and United States Centers for Disease Control and Prevention (CDC), has conducted surveillance for acute lower respiratory tract infection (ALRI) in 2 rural provinces in Thailand since 2003. Data collected for over 140,000 ALRI hospitalizations include demographics, clinical characteristics, chest radiograph (CXR) results and, for a large subset of patients, etiologic data. In this article, we present a comprehensive summary of our data on the incidence, clinical characteristics, seasonal trends and etiology of hospitalized ALRI in children <5 years in rural Thailand.
METHODS
Population
Thailand is a middle-income nation with a population of 4.36 million children <5 years old and a low infant mortality rate (8 deaths/1000 live births).3 Basic health care services at public facilities are free or available for a nominal fee. From 2006 to 2010, UNICEF estimated that 84% of children <5 years old with suspected pneumonia were taken to a health care provider. Neither pneumococcal conjuGAte vaccine nor Haemophilus influenzae type b (Hib) conjuGAte vaccine is part of Thailand’s expanded Program on immunization. Although both vaccines are available on the private market, coverage among children <5 years old in the provinces under surveillance is <2% (unpublished data).
ALRI Surveillance
Active, population-based surveillance for hospitalized ALRI cases beGAn in Sa Kaeo Province (eastern Thailand, bordering Cambodia; population 549,844 persons; 32,960 <5 years of age) in 2002 and was expanded to Nakhon Phanom Province (northeastern Thailand, bordering laos; population 751,251 persons; 48,064 <5 years of age) in 2003.4 These 2 provinces account for approximately 2% of Thailand’s population.
Surveillance was conducted at all district (16) and military (2) hospitals (10–140 inpatient beds) and at both provincial hospitals (225 and 327 inpatient beds), which represent all acute care hospitals in these provinces; there are no private hospitals. ALRI surveillance methods have been described in detail elsewhere.5–7 in brief, surveillance officers reviewed admission logbooks daily to identify patients with diagnoses related to possible respiratory illness for further data collection. Newborns hospitalized since birth were excluded.
ALRI was defined as evidence of acute infection (reported fever, documented temperature >38.0%C, elevated age-specific white blood cell count or abnormal differential) and symptoms/ signs of respiratory illness (abnormal breath sounds, tachypnea based on documented respiratory rate, cough, sputum production, hemoptysis, chest pain or dyspnea) at the time of admission. Previous reports from these surveillance sites have used this case definition.7–10 CXRs were performed at clinician discretion. If done, CXrs were digitized and interpreted by 3 radiologists using standardized criteria.11 A patient was considered to have radiographically confirmed pneumonia if 2 of 3 independent radiologists interpreted the CXR as consistent with probable or definite pneumonia.
Etiology Evaluation
A systematic sample of ALRI patients was selected for possible enrollment in an etiology study. Between 2005 and 2007, eligibility was limited to ALRI patients for whom a CXR was performed within 48 hours of hospital admission (reGArdless of results). Beginning in January 2008, every other patient with ALRI was considered eligible (ie, 50% sample), whether a CXR was performed. Participants provided nasopharyngeal (NP) swab specimens and, during 2005, paired serum specimens (at enrollment and 3–5 weeks later). NP swab specimens were collected from all participants, using polyester swabs (Puritan, guilford, ME) from 2005 to June 2010 and using flocked swabs (FLOQSwabs, Copan, Murrieta, CA) since July 2010. NP specimens were inoculated in viral transport media, stored at 4–8°C for up to 24 hours before being frozen at −70°C and transported weekly on dry ice to Bangkok. This study was approved by a CDC Institutional Review Board (Protocol 3754) and the ethical review Committee of the Thailand MoPH. Guardians provided written consent for their child’s participation.
From January 2005 to December 2007, we tested NP swab specimens from a convenience sample of controls: outpatients without fever, cough, sore throat or diarrhea within the previous 3 days. Controls were enrolled in the outpatient departments of the 2 provincial hospitals.
Specimens were tested at the uS CDC, Atlanta, GA, through mid-2005 with all subsequent testing at the Thailand National Institute of Health. Laboratory methods have been described previously.10 in brief, testing for viruses was performed using a combination of conventional (first 6 months of 2005) and real-time (last 6 months of 2005 through 2010) reverse-transcription polymerase chain reaction (PCR), serology and viral culture. rhinoviruses and coronaviruses were tested only in 2005.8,12 Serology and viral culture were performed during 2005 only. Infections were determined by a positive PCR or culture or a 4-fold rise in antibody titer. Specimens from controls were tested only for viruses by PCR.
Blood cultures were collected at clinician discretion, processed using the Bact/alert 3d System (bioMeriéux) and subcultured using standard methods.9,13 We sought to collect 4 ml of blood for culture, but a previous analysis found that only 45% of children with blood culture performed had 4 ml collected and 27.7% had <2 ml.14
Statistical Analysis
Analyses were conducted in Stata, version 9.1 (StataCorp lP, College Station, tX). to minimize double counting the same ALRI episode, readmissions within 14 days of a previous hospital discharge and referrals/transfers from other hospitals in the same province (n = 1109) were excluded from all incidence calculations; however, data from these 1109 admissions and referrals were used for etiologic findings and patient outcomes.
Confidence intervals (CI) for incidence rates (IRs) were calculated using the standard formula for counts, while Cis for incidence rate ratios (IRRs) were calculated using exact methods. An adjusted number of CXR-confirmed cases was estimated by applying the proportion of CXRs with evidence of pneumonia among those with interpretations to the total number of cases with CXRs performed.
RESULTS
During 2005–2010, 28,543 ALRI hospitalizations occurred among children <5 years of age. Forty-nine percent (n = 14,047) had a CXR performed. Of those, 10,484 (75%) had a final radiologist reading, of which 6645 (63%) had radiographic evidence of pneumonia. More than one-third (37%) of abnormal CXRs had a nonconsolidated alveolar infiltrate, 10.4% showed a consolidation and two-thirds showed an interstitial infiltrate (Table 1). Ninety-three percent met the definition for severe acute respiratory infection, as defined by the WHO.15 Males accounted for 59.0% of ALRI cases (n = 16,846), which was similar when stratifying by age and province. Overall, 1.1% (n = 317) required intubation, 22% (n = 6407) received supplemental oxygen and 9% (n = 520) had recorded oxygen saturation <90% on admission. Ninety-eight hospitalized ALRI case-patients died (0.3%, population mortality rate 19.0 per 100,000 person-years); these deaths were most common among the youngest cases, those with bacteremia and those who were intubated (Table 1). Death occurred in 0.8% of those with radiographically confirmed pneumonia compared with 0.2% of those without radiographically confirmed pneumonia (P < 0.01). Among cases with CXRs obtained and with evidence of alveolar consolidation, death occurred in 2.9%. Fifty-eight patients were known to be HiV positive.
TABLE 1.
Clinical Characteristics, Hospital Course, and Patient Outcomes of Children <5 Years of Age Hospitalized With ALRI, Sa Kaeo and Nakhon Phanom provinces, Thailand, 2005–2010
| ALRI |
Radiographically Confirmed Pneumonia (n = 6645) % |
Pathogen Identified |
||||||
|---|---|---|---|---|---|---|---|---|
| Overall (n = 28,543) % |
Age <28 Days (n = 195) % |
Age 28 Days–6 months (n = 2375) % |
6–23 Months (n = 14,384 % |
24–59 Months (n = 11,589) % |
Bacterial*
(n = 145) % |
Viral (n = 2695) % |
||
| Met SARI† definition | 93.0 | 48.2 | 87.0 | 93.8 | 94.0 | 93.9 | 89.7 | 97.1 |
| Tachypnea‡ | 23.0 | 23.6 | 26.2 | 25.1 | 19.7 | 32.6 | 31.0 | 29.8 |
| Cough | 96.6 | 53.3 | 93.0 | 97.0 | 97.6 | 98.0 | 91.7 | 99.0 |
| Chest indrawing | 8.3 | 24.6 | 12.1 | 7.9 | 6.3 | 12.3 | 9.0 | 8.0 |
| Dyspnea | 58.8 | 61.5 | 66.7 | 60.6 | 55.0 | 77.7 | 59.3 | 71.3 |
| Acute infection | ||||||||
| Fever§ | 46.4 | 21.0 | 32.0 | 46.1 | 50.1 | 44.9 | 60.0 | 53.4 |
| Fever history (self-reported) | 94.8 | 72.8 | 91.4 | 95.6 | 94.9 | 94.6 | 95.9 | 97.4 |
| Abnormal white blood cell count¶ | 28.8 | 17.2 | 23.5 | 28.3 | 30.9 | 28.3 | 40.2 | 22.3 |
| CXR data | ||||||||
| CXR completed | 49.2 | 53.3 | 61.9 | 50.5 | 45.0 | — | 61.4 | 83.9 |
| CXR interpreted∥ | 74.6 | 55.8 | 75.4 | 75.4 | 73.7 | — | 58.7 | 80.4 |
| Evidence of pneumonia** | 63.4 | 46.6 | 61.1 | 65.4 | 61.5 | — | 77.8 | 67.0 |
| Nonconsolidated alveolar infiltrate†† | 36.5 | 66.7 | 51.3 | 38.1 | 29.3 | — | 38.1 | 32.9 |
| Consolidated alveolar infiltrate | 10.4 | 14.8 | 14.6 | 9.9 | 10.0 | — | 21.4 | 9.6 |
| Interstitial infiltrate | 66.2 | 25.9 | 50.6 | 65.8 | 71.9 | — | 47.6 | 72.5 |
| Hospital course | ||||||||
| Intubation | 1.1 | 16.9 | 4.6 | 0.7 | 0.6 | 2.5 | 6.9 | 0.6 |
| Supplemental oxygen | 22.5 | 45.6 | 40.7 | 24.4 | 15.9 | 36.7 | 42.1 | 29.9 |
| Surgical intervention/thoracentesis | 0.1 | 0.5 | 0.3 | 0.1 | 0.1 | 0.2 | 2.8 | 0.1 |
| Outcome | ||||||||
| Discharged | 96.8 | 85.1 | 92.4 | 97.1 | 97.6 | 96.6 | 91.0 | 97.7 |
| Transferred | 1.9 | 10.8 | 5.4 | 1.7 | 1.4 | 1.9 | 3.5 | 1.3 |
| Death | 0.3 | 2.1 | 1.4 | 0.3 | 0.2 | 0.8 | 3.5 | 0.1 |
| Other | 0.9 | 2.1 | 0.8 | 1.0 | 0.8 | 0.8 | 2.1 | 0.9 |
Including all isolates from blood cultures not considered contaminants.
SARI, severe acute respiratory infection, as defined by WHO (an acute respiratory infection with a history of fever or measured fever of ≥38°C and cough, with onset within the past 7 days, requiring hospitalization).
Tachypnea was present if respiratory rate was >60 for infants <2 months; >50 for children 2 months to 1 year; >40 for children 1–5 years.
Fever was present if a measured in-hospital fever was ≥38.0°C.
Proportions displayed are among those with white blood cell counts measured. Normal age-specific white blood cell count ranges were 9,000-30,000/mm3 for age < 7 days; 5,000-20,000/mm3 for 7 days to 1 month; 6,000-17,500/mm3 for 1 month to 23 months; 6,000-17,000/mm3 for 2-3 years; 5,500-15,500/mm3 for 4 years.32
The proportion of CXRs interpreted was calculated as the number of CXRs with official readings divided by the number of CXRs completed.
The proportion of CXRs with evidence of pneumonia was calculated as the number CXRs with pneumonia divided by the number of CXRs with interpretations.
The proportion of CXRs with infiltrates was calculated as the number with each specific type of infiltrate divided by the number of CXRs with evidence of pneumonia.
CXRs were obtained more often for patients who were intubated or who died in hospital (86% and 80% of those who were intubated or died had CXRs vs. 49% and 50% of those who were not intubated or did not die, respectively). Nasopharyngeal swab collection was less common among intubated or fatal ALRI cases (19%) compared with nonintubated, nonfatal cases (26%).
Neonates (<28 days old, median age 15 days) comprised 0.7% (n = 195) of hospitalized ALRI cases. Compared with children in other age groups, neonates were less likely to have cough (53% vs. 97%) and more likely to have chest indrawing (25% vs. 6–12%) (Table 1). A lower proportion of neonates had radiographically confirmed pneumonia compared with the older age categories although a larger proportion required mechanical ventilation.
Incidence
The IR of ALRI receiving hospitalization among children <5 years old was 5772 cases per 100,000 person-years (Table 2). Compared with patients <6 months of age, those 6–23 months of age had a higher IR (IRR 1.76, 95% Ci: 1.69–1.84), whereas the irr for those 24–59 months of age was 0.69 (95% Ci: 0.66–0.72). incidence was nearly 40% higher among boys than girls (IRR 1.38, 95% CI: 1.35–1.41); this relationship remained after stratification by province and age. the IR of radiographically confirmed pneumonia was 1800 cases per 100,000 personyears (95% Ci: 1774–1827); comparisons by sex, age and province followed similar patterns as those observed for hospitalized ALRI cases overall (Table 2). the ir of ALRI among neonates <28 days old was 2897 per 100,000 person-years (95% Ci: 2520–3274). Table, Supplemental Digital Content 1, http://links. lww.com/INF/B816, shows incidence of both ALRI and radiographically confirmed ALRI hospitalizations by month of age.
TABLE 2.
Incidence Rate (Per 100,000 Person-Years) of ALRI and Radiographically Confirmed Pneumonia, Stratified by Province, Sex and Age, in Hospitalized Children <5 years, Sa Kaeo and Nakhon Phanom Provinces, Thailand, 2005–2010
| ALRI (n = 28,543) |
Radiographically Confirmed* (n = 6645) |
|||||||
|---|---|---|---|---|---|---|---|---|
| IR | 95% CI | IRR | 95% CI | IR | 95% CI | IRR | 95% CI | |
| Overall | 5772 | 5707–5837 | 1800 | 1774–1827 | ||||
| Girls | 4833 | 4748–4919 | 1.0 | 1492 | 1458–1525 | 1.0 | ||
| Boys | 6671 | 6574–6769 | 1.38 | 1.35–1.41 | 2096 | 2056–2136 | 1.41 | 1.34–1.48 |
| <6 months | 5561 | 5352–5770 | 1.0 | 2059 | 1963–2155 | 1.0 | ||
| 6–23 months | 9795 | 9643–9947 | 1.76 | 1.69–1.84 | 3230 | 3168–3293 | 1.57 | 1.45–1.7 |
| 24–59 months | 3848 | 3779–3916 | 0.69 | 0.66–0.72 | 1064 | 1038–1091 | 0.52 | 0.48–0.56 |
| Sa Kaeo | 5303 | 5204–5401 | 1.0 | 1664 | 1626–1701 | 1.0 | ||
| Nakhon Phanom | 6086 | 6000–6172 | 1.15 | 1.12–1.18 | 1888 | 1852–1924 | 1.13 | 1.08–1.19 |
| 2005 | 5716 | 5563–5869 | 1.0 | 1720 | 1660–1779 | 1.0 | ||
| 2006 | 5609 | 5453–5765 | 0.98 | 0.94–1.02 | 1705 | 1643–1766 | 0.99 | 0.91–1.08 |
| 2007 | 5590 | 5432–5748 | 0.98 | 0.94–1.02 | 1683 | 1615–1751 | 0.98 | 0.9–1.07 |
| 2008 | 5943 | 5779–6106 | 1.04 | 1–1.08 | 1875 | 1815–1936 | 1.09 | 1.01–1.18 |
| 2009 | 5091 | 4939–5243 | 0.89 | 0.85–0.93 | 1506 | 1446–1565 | 0.88 | 0.8–0.95 |
| 2010 | 6691 | 6519–6863 | 1.17 | 1.13–1.22 | 2327 | 2251–2403 | 1.35 | 1.25–1.46 |
Applying proportion of CXRs with evidence of pneumonia to the total number of CXRs performed.
Seasonal Trends
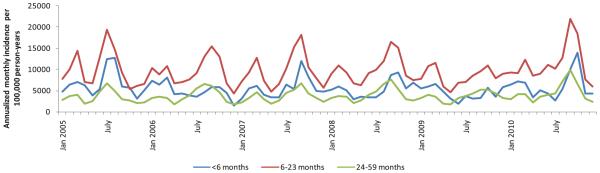
For all age categories, we observed 2 annual seasonal ALRI elevations in hospitalized ALRI: an increase from February to March (IRR 1.48, 95% CI: 1.39–1.58) and a larger increase from June through October (IRR 1.76, 95% CI: 1.65–1.86), both measures compared with incidence during nonpeak months (april, May, november, december and January) (Fig. 1). The 2 peaks were similarly sized in children <6 months; however, the June to October peak was significantly larger compared with the February to March peak among children >6 months (data not shown).
FIGURE 1.
Seasonal patterns of hospitalized ALRI incidence among children <5 years of age, Sa Kaeo and Nakhon Phanom provinces, Thailand, 2005–2010.
Etiology
Virus testing was performed for 7388 (25.6%) ALRI patients (Table 3). Other than rhinoviruses and bocavirus, the most commonly identified pathogens were respiratory syncytial virus (RSV) (n = 1430, 19.5%) and influenza viruses (n = 602, 8.4%). The proportion with rSV was highest in children <6 months of age and decreased with increasing age, while the proportion with influenza viruses increased with age. In patients with mixed viral Infections (n = 237), the most common combinations were RSV with influenza a (n = 46) and RSV with rhinovirus (n = 42). We did not find substantial differences in CXR pattern by type of viral infection. Of 20 neonates tested, 8 had viruses detected, including RSV (n = 3), RSV/human parainfluenza virus (HPiV) 2 coinfection (n = 1), rhinovirus (n = 2), influenza B (n = 1) and adenovirus (n = 1).
TABLE 3.
Viral Pathogens* Detected Among Children <5 Years of Age Hospitalized With ALRI, Sa Kaeo and Nakhon Phanom Provinces, Thailand, 2005–2010
| Pathogen* | Total (n = 7388) | Age <6 Months (n = 638) |
Age 6–23 Months (n = 3877) |
Age 24–59 Months (n = 2873) |
CXR-Confirmed Pneumonia (n = 2866) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| n/Tested | % | n/Tested | % | n/Tested | % | n/Tested | % | n/Tested | % | |
| RSV | 1430/7324 | 19.5 | 151/637 | 23.7 | 776/3840 | 20.2 | 503/2847 | 17.7 | 704/2836 | 24.8 |
| Influenza A | 454/7324 | 6.2 | 27/637 | 4.2 | 221/3840 | 5.8 | 206/2847 | 7.2 | 177/2836 | 6.2 |
| Influenza B | 148/7324 | 2 | 10/637 | 1.6 | 65/3840 | 1.7 | 73/2847 | 2.6 | 43/2836 | 1.5 |
| Adenovirus | 260/7324 | 3.5 | 15/637 | 2.4 | 166/3840 | 4.3 | 79/2847 | 2.8 | 95/2836 | 3.3 |
| HPIV 1 | 119/3810 | 3.1 | 7/397 | 1.8 | 67/2063 | 3.2 | 45/1350 | 3.3 | 52/1761 | 3 |
| HPIV 2 | 30/3810 | 0.8 | 2/397 | 0.5 | 16/2063 | 0.8 | 12/1350 | 0.9 | 12/1761 | 0.7 |
| HPIV 3 | 198/3810 | 5.2 | 16/397 | 4 | 123/2063 | 6 | 59/1350 | 4.4 | 100/1761 | 5.7 |
| HMPV | 111/3810 | 2.9 | 8/397 | 2 | 57/2063 | 2.8 | 46/1350 | 3.4 | 60/1761 | 3.4 |
| Rhinoviruses | 169/903 | 18.7 | 17/97 | 17.5 | 101/500 | 20.2 | 51/306 | 16.7 | 91/478 | 19 |
| Bocavirus | 39/304 | 12.8 | 2/35 | 5.7 | 24/165 | 14.5 | 13/104 | 12.5 | 23/191 | 12 |
| Coronaviruses | 8/304 | 2.6 | 2/35 | 5.7 | 6/165 | 3.6 | 0/104 | 0 | 8/191 | 4.2 |
RSV, influenza A and B, and adenovirus were tested during all years under study. HPIV and HMPV were tested from January 2005–December 2007. Rhino-, boca- and corona-viruses were tested during the first 9 months of 2005 only.
RSV Infections occurred most frequently during June to October (87% of all RSV cases), coinciding with the large peak in ALRI. Influenza a and B viruses displayed 2 peaks: one during February to March and the other during June to October.
During 2005–2007, 667 controls <5 years of age were enrolled with a mean age of 1.7 years; 22% were <6 months, 31% were 6–23 months and 47% 24–59 months. ALRI cases were significantly more likely to test positive for RSV, influenza A and B viruses, adenovirus, HPIV 1 and 3 and human metapneumovirus (HMPV), but not for rhinoviruses or coronaviruses39 (Table 4).
TABLE 4.
Viral Testing Results Among Hospitalized ALRI Cases and Outpatient Controls* With Age-Adjusted Odds Ratios (AOR) and 95% CI, 2005–2007
| ALRI Cases (n = 3845) |
Controls (n = 667) |
|||||
|---|---|---|---|---|---|---|
| n/Tested | % | n/Tested | % | AOR | 95% CI | |
| RSV | 776/3809 | 20.4 | 7/589 | 1.2 | 21.3 | 10.1–45.1 |
| Influenza A | 227/3809 | 6.0 | 3/589 | 0.5 | 12.4 | 3.9–38.9 |
| Influenza B | 71/3809 | 1.9 | 2/589 | 0.3 | 5.3 | 1.3–21.9 |
| Adenovirus | 128/3809 | 3.4 | 5/589 | 0.8 | 3.4 | 1.4–8.5 |
| HPIV 1 | 119/3809 | 3.1 | 2/589 | 0.3 | 8.9 | 2.2–36.3 |
| HPIV 2 | 30/3809 | 0.8 | 1/589 | 0.2 | 4.7 | 0.6–34.7 |
| HPIV 3 | 198/3809 | 5.2 | 1/589 | 0.2 | 29.8 | 4.2–213 |
| HMPV | 111/3809 | 2.9 | 1/658 | 0.2 | 19.5 | 2.7–140 |
| Rhinoviruses | 169/903 | 18.7 | 14/116 | 12.1 | 1.6 | 0.9–2.9 |
Blood cultures were collected from 28% (n = 7975) of all ALRI patients hospitalized from 2005 to 2010, with the younger age groups more likely to have blood cultures collected (35% of children <6 months) (Table 5). Overall, 88.8% (n = 7082) of cultures were neGAtive and 1.8% (n = 145) were positive for a bacterial pathogen. Nontyphoidal Salmonella, Streptococcus pneumoniae and Haemophilus influenzae were the most commonly identified pathogens. Likely contaminants were isolated in 8.1% (n = 649). Fifty-two percent (n = 101) of neonates <28 days of age had blood cultures and 2 were positive: one for Staphylococcus aureus and the other for Mycobacterium tuberculosis complex (later confirmed as Mycobacterium bovis Bacille Calmette-guérin).16
TABLE 5.
Blood Culture Results for Children <5 Years Hospitalized With ALRIs, Sa Kaeo and Nakhon Phanom provinces, Thailand, 2005–2010
| Total (n = 28,543) |
Age <6 months (n = 2570) |
Age 6–23 Months (n = 14,384) |
Age 24–59 Months (n = 11,589) |
CXR-Confirmed Pneumonia (n = 6645) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | %* | n | %* | n | %* | n | %* | n | %* | |
| Blood culture collected | 7975 | 27.9 | 893 | 34.7 | 4049 | 28.1 | 3033 | 26.2 | 2272 | 34.2 |
| Pathogen isolated† | 145 | 1.8 | 25 | 2.8 | 78 | 1.9 | 42 | 1.4 | 40 | 1.8 |
| Streptococcus pneumoniae | 19 | 13.1 | 1 | 4 | 13 | 16.7 | 5 | 11.9 | 5 | 12.5 |
| Escherichia coli | 13 | 9 | 5 | 20 | 6 | 7.7 | 2 | 4.8 | 0 | 0 |
| Haemophilus influenzae | 14 | 9.7 | 3 | 12 | 8 | 10.3 | 3 | 7.1 | 7 | 17.5 |
| Acinetobacter baumannii | 11 | 7.6 | 3 | 12 | 3 | 3.8 | 5 | 11.9 | 2 | 5 |
| Salmonella, nontyphoid | 21 | 14.5 | 4 | 16 | 14 | 17.9 | 3 | 7.1 | 7 | 17.5 |
| Staphylococcus aureus | 10 | 6.9 | 4 | 16 | 3 | 3.8 | 3 | 7.1 | 5 | 12.5 |
| Klebsiella spp. | 6 | 4.1 | 2 | 8 | 2 | 2.6 | 2 | 4.8 | 1 | 2.5 |
| Moraxella catarrhalis | 8 | 5.5 | 0 | 0 | 6 | 7.7 | 2 | 4.8 | 2 | 5 |
| Burkholderia pseudomallei | 6 | 4.1 | 0 | 0 | 1 | 1.3 | 5 | 11.9 | 3 | 7.5 |
Denominators differ for each level: for blood cultures collected, the denominator is all patients; for pathogen isolated, the denominator is number of patients with blood cultures and for individual pathogens, the denominator is the total number of pathogens.
Only fully speciated pathogens of clear clinical significance for which >4 cases were observed are displayed. Pathogens not included in the table include: Acinetobacter, non-baumannii (14), Bacillus cereus (7), Brevundimonas (3), Enterobacter cloacae (n = 3), Enterobacter, other (2), Enterococcus faecium (n = 1), Enterococcus, other (2), Mycobacterium tuberculosis complex (later confirmed as Mycobacterium bovis BCG16, n = 1), Streptococcus agalactiae (n = 1), Citrobacter freundii (n = 1), Ralstonia (n = 1) and Proteus species (1). Several Streptococcus species that were likely contaminants were identified: Streptococcus anginosus (n = 4), Streptococcus mitis (n = 11) and Streptococcus salivarius (n = 4) (not included in count of pathogens isolated).
Among children with CXR-confirmed pneumonia and blood cultures collected, pathogen detection was more common among those with alveolar consolidation [9/312 (2.9%)] compared with those with other infiltrates [25/1827 (1.4%)] (P < 0.05).
DISCUSSION
We found a high incidence of hospitalized ALRI in thai children <5 years of age (5772 cases per 100,000 person-years) with higher rates among boys, among children 6–23 months of age and during June through October. Complicated illness requiring intubation affected ~1% of cases overall but was more common among neonates (17%) and infants 28 days to <6 months old (5%). RSV was the most common viral pathogen associated with ALRI, followed by influenza, while the most common bacterial pathogens were nontyphoidal Salmonella, S. pneumoniae and H. influenzae. Only 1.8% of cases with blood culture had confirmed bacteremia.
Our estimates were similar to minimum estimates of hospitalized pneumonia incidence in a systematic review in China (6000 cases per 100,000 person-years)17 and somewhat higher than estimates from taiwan (3965–4984 per 100,000 person-years)18 and Bangladesh (5020 per 100,000 person-years).19 The Bangladeshi study was most similar to ours, in that it incorporated active surveillance methods in 7 hospitals. Our incidence estimates were twice as high as estimates from the Child Health Epidemiology Reference Group, which estimated the incidence of severe and very severe ALRI hospitalizations among children <5 years of age in developing countries to be 19.7 and 5.1 cases per 1000 child-years, respectively.20 Analyzing data from published studies, Child Health Epidemiology Reference Group estimated that the incidence of severe ALRI in Southeast asia to be 17.8 cases per 1000 child-years.
Our IRs may have been influenced by active case finding and a lower threshold for hospitalization in the setting of very good health care access,21 thereby elevating IRs and lowering the proportion of those with severe disease and poor outcome. Furthermore, our sensitive ALRI case definition captured a broader group of cases than only those with pneumonia. Only 23% of ALRI cases had elevated respiratory rates compared with the age-specific norms used for the WHO definition of nonsevere pneumonia,22 although our data collection procedures were not standardized for this sign.
However, it is worth noting that 93% of our ALRI cases met the WHO case definition for severe acute respiratory infection.15 We prioritized a sensitive case definition to improve capture of cases with respiratory pathogens; for example, 67% of 1430 RSV-associated and 77% of 602 influenza virus-associated hospitalizations would have been missed if we had used WHO case definitions of pneumonia (severe or nonsevere). By contrast, our definition of CXR-confirmed pneumonia based on standardized readings from 3 radiologists was more specific and thereby provided a minimum (yet still substantial) IR of hospitalized ALRI in rural Thailand.
Our estimates are also 3.5 times higher than those reported by the Thailand MoPH (1580 cases per 100,000 person-years in children <5 years in 2010),23 which relies on passive reporting based on clinician diagnosis. CXR-confirmed pneumonia IRs in our analysis are similar to the MoPH’s overall rate, suggesting that Thai clinicians may base reporting of pneumonia cases on CXR data.
Our finding of higher ALRI IRs in boys is consistent with previous studies.18–20,24,25 Cultural traditions in Thailand do not display a male preference, so this difference is unlikely related to increased health care–seeking behavior for sons.21 There is some evidence that asthma is more prevalent among boys than girls in Thailand26 and the united States.27,28 Although many factors may have a role, it is possible that despite equal exposure to respiratory pathogens, boys are more likely to develop Infections that warrant hospitalization due to their higher prevalence of underlying asthma. Additional investiGAtion into higher ALRI rates among boys is needed.
Despite a lack of empiric data, pneumonia burden among neonates is believed to be substantial.1 Neonatal ALRI is difficult to study due to variable illness presentation, clinical overlap with sepsis and other neonatal conditions and the challenges of obtaining adequate samples for etiology studies.29 We estimated a neonatal ALRI IR of 2897 cases per 100,000 person-years, 70% lower than the rate among those 6–23 months of age. Although this estimate is subject to the aforementioned limitations, our standardized data collection over 6 years offers insight into neonatal disease burden, symptomatology, outcomes and pathogen distribution.
Similar to our previously published work, we found a consistent seasonal peak of hospitalized ALRI in February to March and a larger peak during June to October.6,7,10 Influenza and parainfluenza viruses contributed prominently to both peaks of ALRI incidence, whereas RSV displayed only 1 large June to October elevation. understanding ALRI seasonal patterns is essential for effective resource allocation and planning of preventive and therapeutic interventions, including the distribution of vaccines, medications and personnel. Consistent timing of annual RSV peaks supports the feasibility of interventions that are best timed with seasonal epidemics, such as palivizumab for high-risk infants30 or possibly, in the future, vaccines.
We found that a high proportion of ALRI hospital admissions had the detection of bocavirus or rhinoviruses, but the etiologic contribution of these viruses remains uncertain. Bocavirus infection was previously shown to be more common among hospitalized ALRI cases in Sa Kaeo Province than among controls although 91% of bocavirus-positive ALRI patients <5 years of age were coinfected with another virus.31 Rhinovirus infection in the current analysis was not significantly more common among cases than among controls, but differences approached statistical significance and were consistent with previously published work from our sites that did show a significant association between hospitalized ALRI and rhinovirus detection.8 These data highlight the challenges of etiologic attribution with increasingly sensitive diagnostic assays for an increasing number of potential pathogens and underscore the value of testing controls without respiratory illness to assess causality. However, lack of association between ALRI and pathogen prevalence cannot definitively exclude the possibility that a pathogen is part of the causal pathway for disease. Our control design may not have been optimal to evaluate causality for all pathogens as we could not evaluate prolonged shedding from prior infection or shedding prior to the onset of symptoms. In addition to RSV, influenza a and B viruses, HMPV and HPIV1 and 3, we found that hospitalized ALRI was significantly associated with the detection of adenovirus, which differed from previous studies in Alaska (United States)33 and Kenya.34
Our data underestimate the bacterial contribution to childhood ALRI. We did not perform testing for Mycoplasma pneumoniae and Chlamydia pneumoniae during the analysis period, but a previous report from these sites found that only ~1% of ALRI cases among children <5 years of age were positive for each.10 testing for tuberculosis was not done, and testing for other bacterial pathogens associated with severe pneumonia, such as S. pneumoniae and Haemophilus influenzae, was limited to blood culture. The sensitivity of blood cultures is low, and low blood volumes and prior antibiotic administration (~25%) likely limited the yield further.14 The relative frequency of nontyphoidal Salmonella (n = 21) and Escherichia coli (n = 13) was unexpected; enteric Gram-neGAtive bacteria are generally easier to isolate than Grampositives like S. pneumoniae and may have been less affected by antibiotic Pretreatment. Previous studies have identified invasive Salmonella infection among children with pneumonia, including a study from Thailand that identified cases with radiographic evidence of pulmonary infiltrates.35,36 Even under ideal testing conditions, only a fraction of bacterial pneumonia cases are bacteremic. Based on a pneumococcal conjuGAte vaccine probe study in South africa, only 3.4% of pneumococcal clinical pneumonia cases had positive blood cultures.37
Our conclusions were limited by surveillance that only captured ALRI cases requiring hospitalization, which missed the substantial outpatient burden and biased descriptions of seasonality and pathogen distribution toward cases severe enough to need hospitalization. Through community surveys, we believe that hospital-based surveillance captures 58–80% of all ALRI in these 2 provinces.21,38 Our findings provide informative data for rural Thailand, but it is not clear that results can be generalized to more urban settings or other nations.
This analysis demonstrates a high incidence of hospitalized ALRI among young children in Thailand and suggests that important differences in IR exist by age, sex and season. Virus Infections account for the largest proportion of known pathogens, but a substantial proportion remain of unknown etiology. Our results underscore the critical need for effective prevention and control measures for high-burden pathogens such as RSV, highlight the potential impact of proven prevention measures (eg, influenza and pneumococcal conjuGAte vaccines) and emphasize the need for continued efforts to better define ALRI etiology, including the need for improved molecular-based diagnostic approaches in young children.
ACKNOWLEDGMENTS
We thank Peera Areerat and Asadang Ruayajin of the Provincial Health Offices in Nakhon Phanom and Sa Kaeo, respectively, for their collaboration and advocacy for this work; Pornpak Khunatorn, Thantapat Akarachotpong and Nuanpan Chaoprakoon of the United States CDC, International Emerging Infections Program, Nonthaburi, Thailand, for data management and graphics support; Matt Moore and Zahid Samad of the US CDC, Atlanta, GA, for their technical input and critical review; and Barameht Piralam, Darunee Ditsungnoen, Ying Lu, and Jakkarin Seema for their overall project support.
R. H. was supported by the CDC Foundation for the completion of this work. Support for this project was also provided by Pneumococcal Vaccines accelerated development and introduction Plan, which was funded by the GAvialliance and is based at the Johns Hopkins Bloomberg School of Public Health. Support for this project was also provided by the CDC Global Disease Detection Program and the Thailand Ministry of Public Health.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HtMl and PdF versions of this article on the journal’s website (www.pidj.com).
The authors have no other funding or conflicts of interest to disclose.
REFERENCES
- 1.Rudan I, Boschi-Pinto C, Biloglav Z, et al. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86:408–416. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott JA. The global epidemiology of childhood pneumonia 20 years on. Bull World Health Organ. 2008;86:494–496. doi: 10.2471/BLT.08.052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNICEF Thailand Statistics 2010 Available at: http://www.UNICEF.org/infobycountry/Thailand_statistics.html.
- 4.National Economic and Social Development Board of Thailand Population Projections of Thailand 2000–2030. Available at: http://www.nesdb.go.th/temp_social/pop.zip.
- 5.Olsen SJ, Laosiritaworn Y, Siasiriwattana S, et al. The incidence of pneumonia in rural Thailand. Int J Infect Dis. 2006;10:439–445. doi: 10.1016/j.ijid.2006.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simmerman JM, Chittaganpitch M, Levy J, et al. Incidence, seasonality and mortality associated with influenza pneumonia in Thailand: 2005-2008. PLoS One. 2009;4:e7776. doi: 10.1371/journal.pone.0007776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fry AM, Chittaganpitch M, Baggett HC, et al. The burden of hospitalized lower respiratory tract infection due to respiratory syncytial virus in rural Thailand. PLoS One. 2010;5:e15098. doi: 10.1371/journal.pone.0015098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fry AM, Lu X, Olsen SJ, et al. Human rhinovirus infections in rural Thailand: epidemiological evidence for rhinovirus as both pathogen and bystander. PLoS One. 2011;6:e17780. doi: 10.1371/journal.pone.0017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baggett HC, Peruski LF, Olsen SJ, et al. Incidence of pneumococcal bacteremia requiring hospitalization in rural Thailand. Clin Infect Dis. 2009;48(suppl 2):S65–S74. doi: 10.1086/596484. [DOI] [PubMed] [Google Scholar]
- 10.Olsen SJ, Thamthitiwat S, Chantra S, et al. Incidence of respiratory pathogens in persons hospitalized with pneumonia in two provinces in Thailand. Epidemiol Infect. 2010;138:1811–1822. doi: 10.1017/S0950268810000646. [DOI] [PubMed] [Google Scholar]
- 11.Javadi M, Subhannachart P, Levine S, et al. Diagnosing pneumonia in rural Thailand: digital cameras versus film digitizers for chest radiograph teleradiology. Int J Infect Dis. 2006;10:129–135. doi: 10.1016/j.ijid.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dare RK, Fry AM, Chittaganpitch M, et al. Human coronavirus infections in rural Thailand: a comprehensive study using real-time reverse-transcription polymerase chain reaction assays. J Infect Dis. 2007;196:1321–1328. doi: 10.1086/521308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perilla MJ, Ajello G, Bopp C, et al. Manual for the Laboratory Identification and Antimicrobial Susceptibility Testing of Bacterial Pathogens of Public Health Importance in the Developing World. World Health Organization; Geneva: 2003. [Google Scholar]
- 14.Rhodes J, Hyder JA, Peruski LF, et al. Antibiotic use in Thailand: quantifying impact on blood culture yield and estimates of pneumococcal bacteremia incidence. Am J Trop Med Hyg. 2010;83:301–306. doi: 10.4269/ajtmh.2010.09-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO Global Influenza Programme Surveillance and Epidemiology team WHO Interim Global Epidemiological Standards for Influenza. 2012 Available at: http://www.who.int/influenza/resources/documents/INFSURVMANUAL.pdf.
- 16.Thamthitiwat S, Marin N, Baggett HC, et al. Mycobacterium bovis (Bacille Calmette-Guérin) bacteremia in immunocompetent neonates following vaccination. Vaccine. 2011;29:1727–1730. doi: 10.1016/j.vaccine.2010.12.089. [DOI] [PubMed] [Google Scholar]
- 17.Guan X, Silk BJ, Li W, et al. Pneumonia incidence and mortality in Mainland China: systematic review of Chinese and English literature, 1985-2008. PLoS One. 2010;5:e11721. doi: 10.1371/journal.pone.0011721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu PS, Chang IS, Tsai FY, et al. Epidemiology and impacts of children hospitalized with pneumonia from 1997 to 2004 in Taiwan. Pediatr Pulmonol. 2009;44:162–166. doi: 10.1002/ppul.20969. [DOI] [PubMed] [Google Scholar]
- 19.Baqui AH, Rahman M, Zaman K, et al. A population-based study of hospital admission incidence rate and bacterial aetiology of acute lower respiratory infections in children aged less than five years in Bangladesh. J Health Popul Nutr. 2007;25:179–188. [PMC free article] [PubMed] [Google Scholar]
- 20.Nair H, Simões EA, Rudan I, et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 2013;6736:4–6. doi: 10.1016/S0140-6736(12)61901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan HT, Prapasiri P, Areerat P, et al. A comparison of population-based pneumonia surveillance and health-seeking behavior in two provinces in rural Thailand. Int J Infect Dis. 2009;13:355–361. doi: 10.1016/j.ijid.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization Cough or Difficult Breathing Pocketbook of Hospital Care for children: Guidelines for the Management of Common Illnesses With Limited Resources. 2005 [Google Scholar]
- 23.Bureau of Epidemiology, Center for Epidemiological Information & Thailand Ministry of Public Health. National Disease Surveillance (Report 506) 2010 [Google Scholar]
- 24.Selwyn BJ. The epidemiology of acute respiratory tract infection in young children: comparison of findings from several developing countries. Coordinated Data Group of BOSTID Researchers. Rev Infect Dis. 1990;12(suppl 8):S870–S888. doi: 10.1093/clinids/12.supplement_s870. [DOI] [PubMed] [Google Scholar]
- 25.Mathisen M, Strand TA, Sharma BN, et al. RNA viruses in communityacquired childhood pneumonia in semi-urban Nepal; a cross-sectional study. BMC Med. 2009;7:35. doi: 10.1186/1741-7015-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuchinda M, Habananada S, Vareenil J, et al. Asthma in Thai children: a study of 2000 cases. Ann Allergy. 1987;59:207–211. [PubMed] [Google Scholar]
- 27.Zahran HS, Bailey C, Garbe P. Vital signs: asthma prevalence, disease characteristics, and self-management education—United States, 2001–2009. MMWR Morbid MortalWkly Rep. 2011;60:547–552. [PubMed] [Google Scholar]
- 28.Wright AL, Stern DA, Kauffmann F, et al. Factors influencing gender differences in the diagnosis and treatment of asthma in childhood: the Tucson Children’s Respiratory Study. Pediatr Pulmonol. 2006;41:318–325. doi: 10.1002/ppul.20373. [DOI] [PubMed] [Google Scholar]
- 29.Graham SM, English M, Hazir T, et al. Challenges to improving case management of childhood pneumonia at health facilities in resource-limited settings. Bull World Health Organ. 2008;86:349–355. doi: 10.2471/BLT.07.048512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Committee on Infectious Diseases Modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. Pediatrics. 2009;124:1694–1701. doi: 10.1542/peds.2009-2345. [DOI] [PubMed] [Google Scholar]
- 31.Fry AM, Lu X, Chittaganpitch M, et al. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J Infect Dis. 2007;195:1038–1045. doi: 10.1086/512163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brooks WA, Goswami D, Rahman M, et al. Influenza is a major contributor to childhood pneumonia in a tropical developing country. Pediatr Infect Dis J. 2010;29:216–221. doi: 10.1097/INF.0b013e3181bc23fd. [DOI] [PubMed] [Google Scholar]
- 33.Singleton RJ, Bulkow LR, Miernyk K, et al. Viral respiratory infections in hospitalized and community control children in Alaska. J Med Virol. 2010;82:1282–1290. doi: 10.1002/jmv.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammitt LL, Kazungu S, Morpeth SC, et al. A preliminary study of pneumonia etiology among hospitalized children in Kenya. Clin Infect Dis. 2012;54(suppl 2):S190–S199. doi: 10.1093/cid/cir1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Punpanich W, Netsawang S, Thippated C. Invasive salmonellosis in urban Thai children: a ten-year review. Pediatr Infect Dis J. 2012;31:e105–e110. doi: 10.1097/INF.0b013e31825894b0. [DOI] [PubMed] [Google Scholar]
- 36.Brent AJ, Oundo JO, Mwangi I, et al. Salmonella bacteremia in Kenyan children. Pediatr Infect Dis J. 2006;25:230–236. doi: 10.1097/01.inf.0000202066.02212.ff. [DOI] [PubMed] [Google Scholar]
- 37.Madhi SA, Kuwanda L, Cutland C, et al. The impact of a 9-valent pneumococcal conjugate vaccine on the public health burden of pneumonia in HIV-infected and -uninfected children. Clin Infect Dis. 2005;40:1511–1518. doi: 10.1086/429828. [DOI] [PubMed] [Google Scholar]
- 38.Chamany S, Burapat C, Wannachaiwong Y, et al. Assessing the sensitivity of surveillance for pneumonia in rural Thailand. Southeast Asian J Trop Med Public Health. 2008;39:549–556. [PubMed] [Google Scholar]
- 39.Dare RK, Fry AM, Chittaganpitch M, et al. Human coronavirus infections in rural Thailand: a comprehensive study using real-time reverse-transcription polymerase chain reaction assays. J Infect Dis. 2007;196:1321–1328. doi: 10.1086/521308. [DOI] [PMC free article] [PubMed] [Google Scholar]