Abstract
Isocyanates have been a leading chemical cause of occupational asthma since their utility for generating polyurethane was first recognized over 60 years ago, yet the mechanisms of isocyanate asthma pathogenesis remain unclear. The present study provides in vivo evidence that a GSH mediated pathway underlies asthma-like eosinophilic inflammatory responses to respiratory tract isocyanate exposure. In naïve mice, a mixture of GSH reaction products with the chemical allergen, methylene-diphenyl diisocyanate (MDI), induced innate immune responses, characterized by significantly increased airway levels of Chitinase YM-1 and IL-12/IL-23β (but not α) subunit. However, in mice immunologically sensitized to MDI via prior skin exposure, identical GSH–MDI doses induced substantially greater inflammatory responses, including significantly increased airway eosinophil numbers and mucus production, along with IL-12/IL-23β, chitinases, and other indicators of alternative macrophage activation. The “self”-protein albumin in mouse airway fluid was uniquely modified by GSH–MDI at position 414K, a preferred site of MDI reactivity on human albumin. The 414K–MDI conjugation appears to covalently cross-link GSH to albumin via GSH's NH2-terminus, a unique conformation possibly resulting from cyclized mono(GSH)–MDI or asymmetric (S,N′-linked) bis(GSH)–MDI conjugates. Together, the data support a possible thiol mediated transcarbamoylating mechanism linking MDI exposure to pathogenic eosinophilic inflammatory responses.
Introduction
Isocyanates (N=C=O) are reactive chemicals with many commercial/industrial uses, especially the di- and poly isocyanates essential to polyurethane production.1 Adverse respiratory health effects from isocyanate exposure were first reported in 1951, yet global production and usage continues to increase with economic demand for polyurethane products.2 Methylene-diphenyl diisocyanate (MDI) is the most abundantly produced isocyanate, with specialized applications for making rigid foams, including spray polyurethane foam insulation.3,4 Despite widespread recognition of MDI and other isocyanates' toxicity and regulation of permissible workplace airborne levels, these chemicals remain among the leading chemical causes of occupational asthma throughout the world.5,6
The mechanisms by which isocyanates cause asthma remain unclear, hampering disease prevention, diagnosis, and treatment.7 It is assumed the reactive nature of N=C=O groups underlies isocyanate asthma, with a hapten-based mechanism as the most obvious pathway to pathogenesis.8 However, the critical “self” reaction targets for isocyanate in vivo remain uncertain. Free primary amine groups on specific lysine side chains of albumin are preferred reactants under physiologic conditions, and isocyanate–albumin adducts can be found circulating in peripheral blood of exposed workers.7,9–11 Antibodies triggered by isocyanate exposure specifically recognize isocyanate conjugated albumin but not other carrier proteins, suggesting that albumin is the major reaction target for isocyanate in vivo.8,10,12 However, isocyanate–albumin specific IgE antibodies are commonly undetectable among hypersensitive individuals, questioning the mechanistic role of albumin in isocyanate asthma pathogenesis.7,13
Accumulating evidence suggests free thiol groups on the tripeptide GSH, a major antioxidant of airway fluid, may be the primary self reactant for isocyanate in vivo.14 Glutathione conjugation of small reactive chemicals generally comprises part of a metabolic/detoxification pathway and may be especially relevant to isocyanates since they can react directly with GSH without the transferase enzymes typically required for conjugation.15–19 However, S-linked bonds that orm with N=C=O are quasi-stable and strongly depend upon temperature and pH.20,21 Further reactivity of S-linked isocyanate with water reverses the thiocarbamate linkage and hydrolyzes the original N=C=O to an amine.16 In contrast, further reactivity of GSH-S-isocyanate with free amine groups on proteins (e.g., albumin), results in stable transcarbamoylation and distinct antigenic changes specifically recognized by serum IgG from exposed workers.18,19,22,23 Together, these data suggest a critical role for GSH in the response to isocyanate exposure.
We presently describe the inflammatory activity of GSH– MDI reaction products in vivo in the airways. The data support the proposed role of GSH as a reaction intermediate in the pathogenesis of isocyanate asthma and may help to explain some of the unusual features of the disease.
Experimental Methods
Caution
This chemical is dangerous. Methylene diphenyl diisocyanate is hazardous and is a well-recognized immune-sensitizing chemical. Nitrile gloves, protective clothing, and goggles should be used for personal protection.
Reaction of GSH with MDI
Reduced glutathione, GSH (CAS no. 70-18-8) and 4,4′-methylenebis(phenyl isocyanate) or MDI (CAS no. 101-68-8) were from Sigma-Aldrich (St. Louis, MO) and were of ≥98.0% purity. GSH was reacted with MDI under conditions that yield reaction products with the greatest capacity to carbamoylate human albumin, among the conditions tested in previously published studies.18 Briefly, 50 μL of 10% (w/v) MDI in acetone from JT Baker (Phillipsburg, NJ) was added dropwise with stirring to 25 mL of 10 mM GSH in 200 mM sodium phosphate, pH 7.4. The reaction mixture was rotated end-over-end for 2 h at 37 °C and then centrifuged at 10 000g, 0.2 μm filtered, aliquoted, and used immediately or snap frozen in LN2 and stored at −80 °C until analysis could be performed.
Reverse-Phase HPLC Analysis and Purification of GSH–MDI Reaction Products
GSH–MDI reaction products were fractionated on a Hewlett-Packard 1090 HPLC system equipped with an Isco model 2150 peak separator and a 1 mm × 25 cm Vydac C-18 (5 μm particle size, 300 Å pore size) reverse-phase column.7 The column was equilibrated, and samples were loaded in 98% buffer A (0.06% TFA) and 2% buffer B (0.052% TFA, 80% acetonitrile) and eluted by increasing buffer B to 37% over the course of 1 h, followed by a stepwise increase to 60% acetonitrile over the next 60 min and 98% washout after 2 h. Autopeak detection was based on A210, with simultaneous measurement at A245.
Skin Sensitization and Airway Exposure in Mice
Female Balb/C mice 8 weeks of age were housed under specific pathogen-free conditions, with automated water supply and 12 h day/night light cycles. Mice were immunologically sensitized to MDI via skin exposure, as previously described.24 Briefly, a region on the back was shaved 24 h prior to application of 50 μL of 1% (w/v) MDI in acetone or (in preliminary experiments, not shown) acetone alone as control. For respiratory tract exposure, 50 μL of GSH–MDI reaction products or control solutions (MDI reacted in solvent without GSH = MDI-m, GSH mock reacted without MDI = GSH-m) were delivered intranasally. All exposures were performed under isoflurane sedation. Mice received skin exposures on days 0 and 7 and respiratory tract exposure on days 15, 16, 19, and 20, or in one experiment, mice received skin exposure on days 0, 7, and 30, followed by respiratory tract exposure on days 61, 62, 65, and 66. All animal studies followed guidelines established in the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources, National Research Council, and published by the National Academy Press [revised 1996].
Bronchoalveolar Lavage (BAL), Cell Count/Differential, and Lung Histology
BAL (3 × 0.8 mL), lung tissue samples, and peripheral blood were obtained 48 h following the last respiratory tract exposure, as previously described.24 BAL was centrifuged at 800g, and the supernatant was collected, aliquoted, and stored frozen at −20 °C. The cell pellets were treated with RBC lysis buffer, washed, and resuspended in PBS for cytospin and cell counting. Total BAL cell numbers were calculated using a hemacytometer, and differential counts were performed on 200 cytospun cells that had been stained with diff quick (Polysciences Inc.; Warrington, PA). For histology, lung tissue was perfused in situ and fixed in 10% buffered formalin. Tissue sections were visualized after hematoxylin/eosin or Periodic acid–Schiff stains. Histology slides and BAL cytospins were viewed and photographed on a Zeiss (Pittsburgh, PA) microscope.
Western Blot of Airway Fluid Protein
Electrophoresis and western blot of BAL fluid were performed as previously described using precast sodium dodecyl sulfate (SDS) acrylamide gels (4–15% gradient) from BioRad (Hercules, CA), under reducing or non-reducing conditions as noted, and protein transfer to nitrocellulose membrane.24 Nitrocellulose membranes were incubated for 2 h with mAbs or polyclonal antisera specific for YM-1, also known as mouse Chitinase 3-like 3, from R&D Systems (Minneapolis, MN), CLCA3, also known as mouse CLCA1, from Santa Cruz Biotechnology, Inc. (Dallas, TX), biotin anti-mouse IL-12/IL-23 p40 from BioLegend (San Diego, CA), polyclonal HRP-anti-IgG Fc (Santa Cruz Biotechnology, Inc.), or biotinylated anti-MDI DA5 developed in our laboratory.25 After extensive washing with PBS containing 0.05% Tween 20, strips were incubated with appropriate secondary antibody and developed with enhanced chemiluminescence substrate from Thermo Fisher Scientific Inc. (Rockford, IL).
iTRAQ Analysis
Pooled airway lavage samples were prepared for iTRAQ analysis using a CHCl3/MeOH precipitation after diluting each to 100 μL with water. Four hundred microliters of MeOH was then added and vortexed extensively prior to the addition of 100 μL of CHCl3. An additional 300 μL of water was added prior to vortexing and centrifuging at 14 000g for 1 min. The top aqueous layer was removed and discarded, and an additional 400 μL of MeOH was added. After a 2 min centrifugation at 14 000g, the MeOH was removed without disturbing the pellet. The pellet was dried in a SpeedVac and dissolved in 50 μL of 0.5 M tetraethylammonium bicarbonate with 0.2% SDS. One hundred micrograms of each sample was used for labeling, which was determined based on a nanodrop measurement at A280 versus a buffer blank. Disulfide reduction was performed by incubating with 5 mM tris(2-carboxyethyl)phosphine at 60 °C for 1 h. Alkylation was then performed by incubating with 20 mM methylmethanethiolsulfonate at room temperature for 10 min. Samples were digested using a 1:10 w/w ratio protein/trypsin and incubated at 37 °C for 16 h. Each dried iTRAQ label was dissolved in 50 μL of 100% isopropanol. Reporter ion tags 114 and 115 were used to respectively label the proteins of the BAL samples from experiment 1, which compared control mice (MDI-m) and GSH–MDI exposed mice that had been skin exposed three times (see above). The 116 and 117 tags were used, respectively, in experiment 2, which compared MDI-m and GSH–MDI exposed mice that had received only two skin exposures. After vortexing, each reconstituted iTRAQ reagent was transferred to the appropriate vial and incubated at room temperature for 2 h. At this point, the tagged sample digests were mixed together and acidified with 1 M phosphoric acid to a pH less than 3.0 and then separated on a Hewlett-Packard 1090 HPLC system fitted with a polySulfethylA column (The Nest Group, Southborough, MA). At 0.5 mL/min of buffer A (10 mM KH2PO4, pH 3.0, 25% acetonitrile), a gradient of 0–100% buffer B (10 mM KH2PO4, 1 M NaCl, pH 3.0, 25% acetonitrile) was established over 120 min. Fractions were collected at 1 min intervals. The broad, unresolved A214 peak was pooled into 10 fractions according to absorbance.
The samples were dried, dissolved in 20 μL 70% formic acid (FA) and diluted with 300 μL 0.1% trifluoroacetic acid (TFA), prior to desalting using a Macrospin C18 (The Nest Group Inc., South-borough, MA). The bound peptides were eluted with 360 μL of 80% acetonitrile (ACN) containing 0.1% TFA, and the elution was repeated by washing with an additional 180 μL of the same solution. Samples were dried in a SpeedVac and dissolved in 3 μL of FA mixed with 8 μL of 0.1% TFA. Three micrograms of each strong cation exchange fraction (N = 10) was separated and analyzed on a Waters (Milford, MA) nanoAcquity UPLC system equipped with a 5600 TripleTOF (AB SCIEX; Framingham, MA) fitted with a Nanospray III source (AB SCIEX) and a pulled quartz tip as the emitter (New Objectives, Woburn, MA). The Waters nanoAcquity UPLC system uses a Waters Symmetry C18, 180 μm × 20 mm trap column, and a 1.7 μm, 75 μm × 150 mm nanoAcquity UPLC column (45 °C) for peptide separation of the multiplexed samples. Trapping was done at 15 μL/min, with 99% buffer A (99.9% water and 0.1% FA) for 1 min. Peptide separation was performed at 500 nL/min with buffer A and buffer B (99.925% acetonitrile and 0.075% FA). The gradient was 99% A under initial conditions with a linear gradient to 35% B at 160 min and 95% B at 160.3 min.
The combined raw MS/MS files (*.wiff) from Analyst TF 1.6 were analyzed by Mascot and with the Paragon search algorithm26 of ProteinPilot (version 4.5). Data was searched against the SwissProt protein database (April 2014, 544 996 sequences) with a mouse taxonomy filter (16 676 sequences). For each ratio, the iTRAQ peak areas (for each peptide) are corrected for both observed bias correction and background correction. The false discovery analysis conducted by the ProteinPilot software utilized a reversed-sequence decoy database to determine the false discovery rate. Peptides identified by ProteinPilot were filtered using the auto setting to include only unique peptides, no missed cleavages, and at least two iTRAQ ions per peptide. To minimize iTRAQ ratios from low-intensity ions, ProteinPilot also required peptides to maintain a signal-to-noise ratio greater than 9 from the combined intensities of the contributing iTRAQ ions. Additionally, each protein quantified by ProteinPilot required two or more peptides.
Protein fold ratios were calculated and expressed from a pairwise comparison of two iTRAQ channels (114 vs 116 or 115 vs 117). The final ratio shown in the Yale protein expression database (YPED) is based on a weighted averaged of the corrected peak areas. All iTRAQ results were uploaded into the YPED (http://yped.med.yale.edu/repository/).27
Cytokine Protein Array and ELISAs
BAL fluid was analyzed using a cytokine antibody microarray from RayBiotech Inc. (Norcross GA). Pooled samples from N = 6 MDI skin sensitized mice, exposed to either GSH–MDI or MDI control (MDI reacted without GSH = MDI-m as described above), were incubated on different array membranes overnight at 4 °C. Microarrays were developed according to the manufactures specifications using enhanced chemiluminescence with substrate from Thermo Fisher Scientific Inc. and Carestream Kodak BioMax light film from Sigma-Aldrich. Additional cytokine analyses were performed by ELISA using kits from Biolegend (IL-4, IL-5, IL-10, IL-12p40, IL-23), R&D Diagnostics (IL-13, IL-12p70), or eBiosciences (San Diego, CA) (IL-10) and RayBiotech Inc. (IL-10) according to the manufacturers' recommendations.
Statistical Analyses
Statistical significance of differences in BAL cell numbers or cytokine levels was determined with an unpaired Student's t test or with analysis of covariance when data from more than one experiment were pooled.
Results
Glutathione–MDI (GSH–MDI) Reaction Products Induce Airway Inflammation
In vivo studies were performed in mice to determine the biological activity of GSH–MDI in the respiratory tract. GSH–MDI reaction products containing a mixture of bis(GSH)–MDI and mono(GSH)–MDI conjugates (see Supporting Information Figure S1) were delivered intranasally to naïve mice or mice immunologically sensitized to MDI via prior skin exposure as previously described.24 Mice that received GSH–MDI exhibited significantly (p < 0.01) greater numbers of cells in the airway and mucosal lung tissue vs mice that received control reaction products of GSH reacted without MDI (GSH-m) or MDI reacted without GSH (MDI-m, essentially hydrolyzed MDI), as shown in Figures 1 and 2. Mice that were immunologically sensitized to MDI exhibited exaggerated and qualitatively distinct inflammatory responses to GSH–MDI compared with that in naïve animals, with significantly (p < 0.02) greater airway eosinophilia, total cell numbers, and mucus production (Figures 1 and 2).
Figure 1.
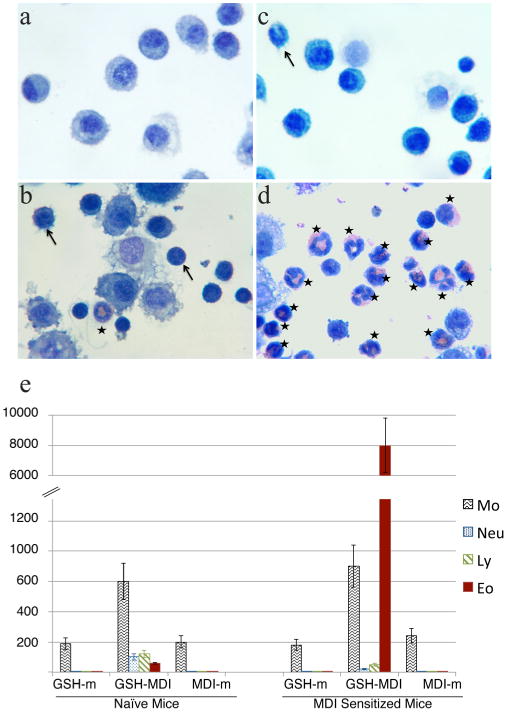
Airway inflammation evoked by GSH–MDI reaction products. Cytospun, stained airway lavage cells from representative naïve (a, b) or MDI sensitized mice (c, d) exposed via the respiratory tract to control stimuli [GSH-m = GSH reacted without MDI (a); MDI-m = MDI reacted without GSH (c)] or GSH–MDI reaction products (b, d). Asterisks highlight eosinophils, and arrows highlight neutrophils or lymphocytes. (e) Mean number of cells (×10−3) ± standard error (Y-axis) derived from airway lavage samples of naïve or MDI sensitized mice exposed to control stimuli, GSH-m, MDI-m, or GSH–MDI. Data are derived from three separate experiments with a total of N = 18 mice/group.
Figure 2.
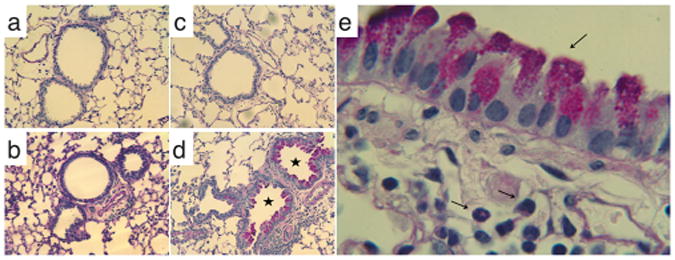
Lung tissue inflammation and mucus production evoked by GSH–MDI reaction products. Lung tissue sections from naïve mice exposed to control stimuli, GSH-m (a), or GSH–MDI (b) or from MDI sensitized mice exposed to control stimuli, MDI-m (c), or GSH– MDI (d) were subject to periodic acid–Schiff (PAS) staining to highlight mucus production (airways with asterisk). (e) PAS stained lung tissue section from a representative MDI sensitized, GSH–MDI exposed host under higher magnification to highlight the mucus containing goblet cells lining the airways and submucosal eosinophils.
Cytokines in the Airways of GSH–MDI Exposed Mice
Inflammatory mediators induced by GSH–MDI were initially characterized by analysis of airway fluid samples using broad-spectrum cytokine/chemokine monoclonal antibody-based arrays. Data comparing immunologically sensitized mice exposed to GSH–MDI vs controls (Figure 3A) suggested that GSH–MDI induced substantial increases in the beta (p40) subunit of IL-12 but not the complete, heterodimeric (α/β) IL-12 cytokine (e.g., p70). Subsequent ELISA studies (Figure 3B) confirmed that both naïve and immunologically sensitized mice exposed to GSH–MDI had significantly (p < 0.05) elevated IL-12 beta airway fluid levels, without measurable increases in IL-4, IL-5, IL-12p70, IL-13, or IL-23, which uses the same beta (p40) subunit as IL-12.28 Western blot analysis of airway fluid from GSH–MDI exposed mice vs controls revealed the presence of IL-12 p40 monomers as well as homodimers (which are known to modulate IgE, IL-12 receptor signaling, and other immune responses in vivo)29,30 rather than conventional heterodimeric IL-12/IL-23 α/β pairings (Figure 3C).
Figure 3.
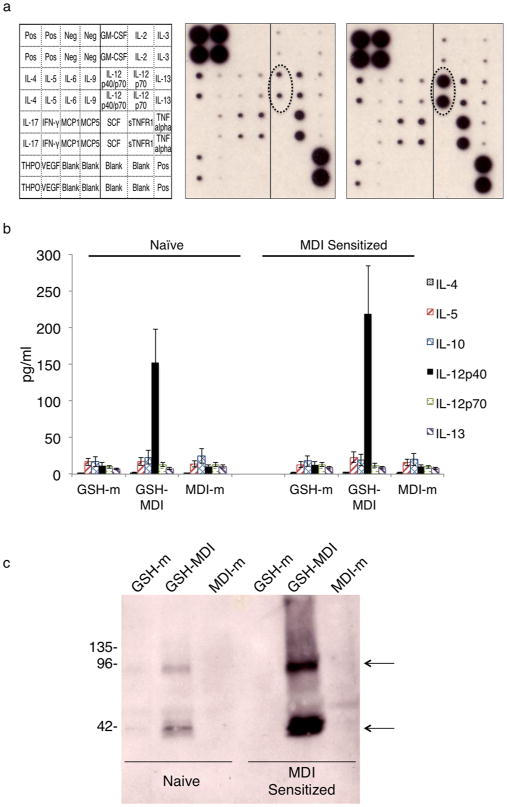
GSH–MDI increases airway levels of IL-12/IL-23β. (a) Monoclonal antibody-based array (key to the left) was used to screen for changes in cytokine levels of pooled airway fluid samples (N = 6 each) from MDI sensitized hosts exposed to control stimuli MDI-m (MDI reacted without GSH) vs GSH–MDI (middle and far right respectively). (b) Graph representing the mean concentration in pg/ mL ± standard error of different cytokines in airway fluid (Y-axis) from N = 18 each naïve or MDI sensitized mice from three separate experiments, exposed to GSH–MDI or control stimuli, as labeled. (c) Anti-IL-12/IL-23β western blot on airway fluid from naïve or MDI sensitized mice exposed to GSH–MDI or control stimuli (GSH-m = GSH reacted without MDI; MDI-m = MDI reacted without GSH) was performed under nonreducing conditions. Arrows highlight banding due to monomeric p40 (lower) and homodimeric p80 (upper) IL-12/ IL-23β.
Airway Protein Analysis Using Isobaric Tags for Relative and Absolute Quantitation (iTRAQ)
To better understand the GSH–MDI exposure induced changes in the airway microenvironment, additional proteomic analyses of airway fluid from GSH exposed mice were performed using iTRAQ.31 Two separate iTRAQ experiments of pooled airway fluid, from sensitized mice exposed to GSH–MDI vs control animals, identified the greatest average differences in the 25 proteins listed in Table 1. The protein most increased in the airways of MDI sensitized, GSH–MDI exposed animals was CLCA1, also known as gob-5, which induces mucus production.32 Western blot analyses (Figure 4) confirmed the increase in glycosylated full-length CLCA1 (∼130 kDa)33 levels in airway fluid of GSH–MDI exposed mice previously sensitized to MDI, but not naïve animals, consistent with histology data shown in Figure 2. The second, third, and fourth most increased proteins in airways of MDI sensitized, GSH–MDI exposed mice are well-described biomarkers of alternatively activated macrophages (chitinases YM-1 and YM-2 and RELMα/Fizz1).34 Western blot analyses (Figure 4) verified that airway fluid YM-1 levels were increased in both naïve and MDI immune sensitized mice upon GSH–MDI exposure. A number of additional proteins upregulated in MDI sensitized, GSH–MDI exposed mice are known to have specialized roles in the immune system and/or have been linked to human asthma.35
Table 1. Airway Proteins Whose Relative Levels Are Most Affected by Exposure to GSH–MDI Based on iTRAQ Analysis.
| avg Δ | protein ID | protein name | MW | % coverage | scoreb | p valueb | 114/116 ratio | 114/116 Nb | 115/117 ratio | 115/117 Nb |
|---|---|---|---|---|---|---|---|---|---|---|
| 13.23 | CLCA1 | Ca2+ activated chloride channel regulator 1 (aka gob-5) | 107 580 | 55.4 | 6544 | 0 | 11.6940 | 102 | 14.771 | 152 |
| 12.84 | CHIL3 | Chitinase-like protein 3 (aka YM-1) | 48 663 | 60.1 | 10 517 | 0 | 19.5440 | 262 | 6.131 | 251 |
| 11.08 | CHIL4 | Chitinase-like protein 4 (aka YM-2) | 49 035 | 59.5 | 3131 | 0 | 16.7220 | 58 | 5.437 | 69 |
| 9.98 | RETNA | Resistin-like alpha (aka RELMα or Fizz-1) | 13 477 | 20.7 | 204 | 7.30 × 10−17 | 6.6150 | 2 | 13.335 | 3 |
| 9.06 | MD1L1a | Mitotic spindle assembly checkpoint protein MAD1 | 91 094 | 32.8 | 28 | 0.038 | 13.6520 | 2 | 4.467 | 2 |
| 4.72 | RGS9a | Regulator of G-protein signaling 9 | 85 137 | 17.6 | 58 | 0.024 | 7.7440 | 9 | 1.704 | 9 |
| 3.75 | RES3G | Regenerating islet-derived protein 3-gamma | 21 134 | 55.2 | 427 | 3.50 × 10−39 | 4.2240 | 12 | 3.271 | 11 |
| 3.33 | C1QBa | Complement C1q subcomponent subunit B | 29 234 | 19 | 35 | 0.024 | 3.6650 | 2 | 3.004 | 2 |
| 3.16 | CHAD | Chondroadherin | 44 295 | 28.5 | 160 | 1.80 × 10−12 | 4.3380 | 3 | 1.986 | 3 |
| 2.69 | PIGR | Polymeric immunoglobulin receptor | 92 309 | 29.7 | 1737 | 0 | 3.2070 | 37 | 2.176 | 40 |
| 2.57 | CATS | Cathepsin S | 42 736 | 25.3 | 335 | 5.40 × 10−30 | 2.7010 | 11 | 2.441 | 10 |
| 2.49 | HA10 | H-2 class I histocompatibility antigen, Q10 alpha chain | 38 896 | 11.7 | 99 | 0.0000019 | 2.5160 | 3 | 2.458 | 3 |
| 2.24 | PSME2 | Proteasome activator complex subunit 2 | 30 583 | 45.2 | 146 | 4.10 × 10−11 | 1.9150 | 2 | 2.563 | 3 |
| 2.21 | VCAM1 | vascular cell adhesion protein 1 | 89 611 | 20.8 | 81 | 0.00012 | 2.5420 | 3 | 1.878 | 3 |
| 2.14 | PAFA | Platelet-activating factor acetylhydrolase | 53 748 | 14.3 | 79 | 0.00021 | 2.3640 | 3 | 1.925 | 4 |
| 2.01 | IGHA | IgA constant region | 39 611 | 23.3 | 756 | 4.30 × 10−72 | 2.1810 | 18 | 1.838 | 11 |
| 0.52 | ADIPO | Adiponectin | 28 924 | 27.5 | 367 | 3.10 × 10−33 | 0.5840 | 12 | 0.448 | 13 |
| 0.50 | TMPSDa | Transmembrane protease serine 13 | 64 166 | 16.8 | 37 | 0.004 | 0.5340 | 3 | 0.466 | 3 |
| 0.48 | FLNB | Filamin-B | 305 321 | 13.5 | 31 | 0.018 | 0.5310 | 2 | 0.431 | 2 |
| 0.47 | BPIA1 | BPI fold-containing family A member 1 (aka PLUNC) | 30 363 | 61.5 | 1045 | 5.40 × 10−101 | 0.5150 | 31 | 0.428 | 32 |
| 0.44 | CBPM | Carboxypeptidase M | 54 669 | 17.6 | 75 | 0.00058 | 0.5010 | 2 | 0.383 | 2 |
| 0.44 | NAPSAa | Napsin-A | 47 788 | 2.1 | 75 | 0.00051 | 0.4720 | 4 | 0.4 | 4 |
| 0.43 | MAL2a | Protein MAL2 | 19 598 | 6.3 | 26 | 0.06 | 0.5740 | 2 | 0.29 | 2 |
| 0.42 | XRCC1 | DNA repair protein XRCC1 | 75 323 | 8.4 | 53 | 0.091 | 0.3110 | 3 | 0.521 | 2 |
| 0.29 | INMT | Indolethylamine N-methyltransferase | 32 662 | 23.9 | 132 | 1.20 × 10−09 | 0.3450 | 3 | 0.238 | 3 |
Only 1 unique peptide with significant identity.
Score and p value are based on Mascot Probability; N = number of peptides from which experimental ratios are calculated.
Figure 4.
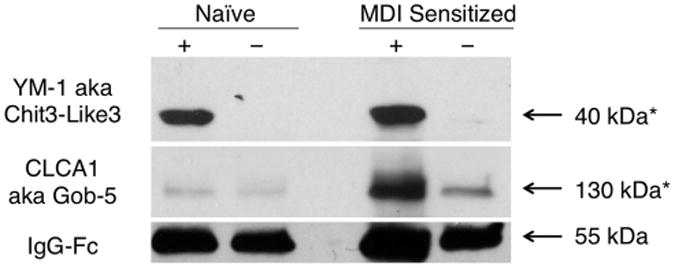
GSH–MDI increases airway chitinase YM-1 and calcium-activated chloride channel CLCA1. Pooled airway fluid from N = 6 naïve or MDI sensitized mice exposed to GSH–MDI (+) or control stimuli GSH-m (−) for naïve or MDI-m for sensitized mice were western blotted under reducing (YM-1, IgG Fc) or nonreducing conditions (CLCA1). Arrows highlight the 39 kDa band corresponding to YM-1, the ∼130 kDa mature glycosylated CLCA1, and the ∼50 kDa heavy chain of IgG, as a loading control.
A limited number of proteins were consistently decreased (>2-fold) in the BAL fluid of MDI sensitized, GSH–MDI exposed vs control mice (Table 1). The most underrepresented proteins (>3.4-fold decreased) were indolethylamine N-methyltransferase (INMT), a major lung N-methyltransferase that inactivates histamine in vivo,36 and DNA repair enzyme XRCC1 (>2.4-fold decreased), which is underexpressed in monocytes.37 Other airway fluid proteins reduced 2-fold below control levels included PLUNC-1, Napsin A, Filamin, carboxypeptidase M, and a particular transmembrane protease (TMPSD). The significance of these proteins' underexpression in GSH–MDI induced airway inflammation remains unclear at the present time.
MDI Conjugated Albumin in GSH–MDI Exposed Mice: Evidence of Transcarbamoylation in Vivo
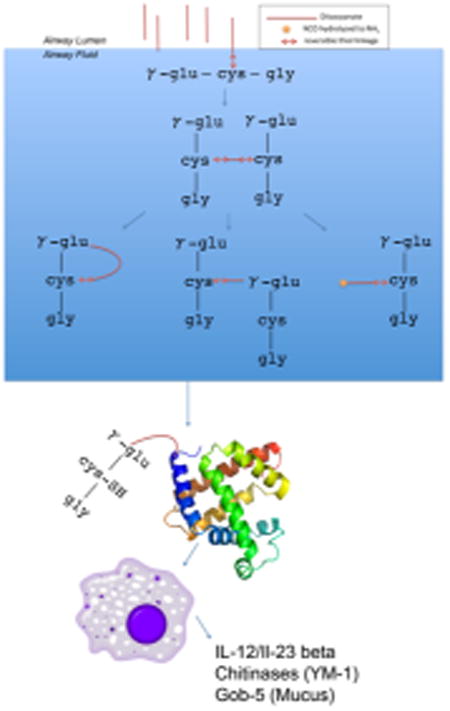
One mechanism through which GSH–MDI might mediate airway inflammation is transcarbamoylation, or transfer of MDI, from the unstable S-linkage with GSH to a stable N-linkage with a protein. Transcarbamoylation may alter a protein's conformation, thereby creating new antigens (neoepitopes) recognized as foreign by the immune system,18 or it could alter protein function in a way that evokes inflammation. To begin assessing this possibility, we probed for MDI modification of host airway fluid proteins by western blot using an anti-MDI mAb (Figure 5A). The data identified a single major MDI-conjugated airway fluid protein in GSH–MDI exposed mice, with an apparent molecular weight (∼68 kDa) consistent with that of mouse albumin.38 Retrospective reanalysis of mass spectrometry data from the iTRAQ experiments (above) further supported MDI conjugation of airway fluid mouse albumin (see Supporting Information Tables S1 and S2). When the data were queried for the 614 Da modification due to transcarbamoylation by GSH–MDI and subsequent carbamidomethylation during the mass spec sample workup, as previously described in vitro,18 a single peptide was uniquely identified in GSH–MDI exposed vs control animals' BAL. The data identify in vivo modification of mouse albumin on the lysine residue at position 414 of the mature protein, a preferential MDI conjugation site of human albumin in vitro.9,18 As depicted in Figure 5B and supported by MS/MS data (Figure S2), the albumin-Lys414 appears to be cross-linked via one MDI molecule to the NH2-terminus of GSH (e.g., via γ-glutamate), a configuration also described for HDI in vitro.19 The observed modification could result from reactivity with cyclized mono(GSH)–MDI, asymmetric (S,N′-linked) bis-(GSH)–MDI, or via intramolecular rearrangement, before or after, reactivity with the symmetric (S,S′-linked) bis(GSH)– MDI. Together, the data support the hypothetical role for GSH in mediating the modification of self proteins, leading to subsequent airway inflammation and asthma.
Figure 5.
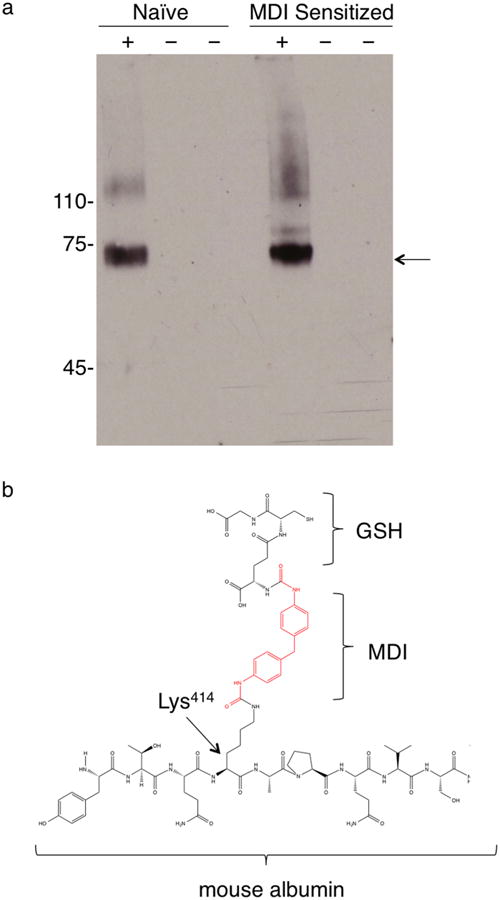
Modification of airway fluid albumin by GSH–MDI in vivo. (a) Pooled airway fluid samples from N = 6 each naïve or MDI sensitized mice exposed to GSH–MDI (+) or control stimuli (−), GSH-m and MDI-m, respectively, were subject to reducing SDS-PAGE and western blotted with biotin labeled MDI-specific mAb DA5. Arrow highlights dominant band ∼68 kDa. (b) Chemical structure depicting the unique GSH–MDI modification of airway fluid albumin detected through LC-MS/MS (see Supporting Information Tables S1 and S2).
Discussion
The present data define a possible thiol mediated biochemical link between occupational exposure to the chemical allergen, MDI, and asthma-like airway inflammation in vivo. The tripeptide, glutathione, which is normally present at high levels (>100 μM) in the airway fluid,14 reacts rapidly with MDI under physiologic conditions, resulting in a mixture of mono(GSH)– MDI and bis(GSH)–MDI conjugates. These GSH–MDI reaction products transcarbamoylate host proteins and induce localized innate immune responses in the airway. In mice immunologically sensitized to MDI via prior skin exposure, GSH–MDI induces significantly greater airway eosinophilia and mucus production, two pathological hallmarks of asthma. Local airway inflammatory response to GSH–MDI are characterized by markers of alternative macrophage activation and selective increases in the shared beta subunit of IL-12/IL-23 but not in the respective alpha subunits or other asthma-associated Th2-type cytokines (IL-4, IL-5, IL-13). Together, the findings describe a glutathione mediated pathway that may distinguish the pathogenesis of isocyanate asthma from that triggered by other allergens. The findings may explain some of the puzzling characteristics of isocyanate asthma that challenge disease recognition and prevention and may extend to airway diseases caused by other reactive chemicals.
A glutathione mediated transcarbamoylation mechanism, as described above, could drive potentially distinct pathways of immune activation vs direct isocyanate reactivity (nucleophilic addition) with host molecules. Glutathione conjugation with isocyanate would protect the parent chemical from hydrolysis or conjugation with protective barrier molecules, allowing deeper penetration into lung tissue and reactivity with host molecules otherwise sheltered from isocyanate by their microenvironment. In addition to causing MDI modifications identical to those produced by direct nucleophilic addition with MDI, transcarbamoylation of proteins via GSH–MDI can produce additional unique epitopes, whose clinical significance remains to be tested. Most importantly, however, GSH mediated carbamoylation could cause functional (vs structural/antigenic) changes in host proteins that trigger airway inflammation without evoking chemical-specific adaptive immune responses, such as isocyanate-specific IgE.
In the present study, a single mouse airway fluid protein, albumin, was detectably carbamoylated by GSH–MDI, at a single loci (Lys414), the same site previously identified as a preferential isocyanate target on human albumin in vitro.9,18 The Lys414 modification consisted of covalent linkage to a MDI molecule that was capped by the γ-glutamate of GSH, which, to the best of our knowledge, represents a previously undescribed in vivo protein modification. The data are consistent with clinical investigations suggesting albumin as the major carrier protein for isocyanate in occupationally exposed workers,8,12 but they do not rule out the possibility that additional targets of GSH mediated carbamoylation exist in the airway epithelial tissue.39
Consistent with potentially distinct mechanisms of isocyanate induced pathogenesis, the inflamed airways of MDI sensitized, GSH–MDI exposed mice contained strikingly different cytokine profiles vs that observed in prototypical mouse asthma models (e.g., ovalbumin).40 Rather than T-cell derived cytokines (IL-4, IL-5, and IL-13), GSH–MDI increased the shared IL-12/IL-23β (but not α) subunit, which is produced largely by macrophages/dendritic cells and, to a lesser extent, B-cells.41 IL-12/IL-23β monomers and dimers have biological activity distinct from IL-12 or IL-23 α/β hetrodimers, including macrophage chemoattraction and the ability to counter-regulate IL-12 activity.29,30,41,42 Moreover, IL-12 signaling plays a critical role in regulating IgE production, which is further modified by genetic polymorphisms and unusual RNA editing of IL-12 receptor transcript. 43,44 Thus, the IL-12 axis may play a more prominent role in isocyanate asthma vs that induced by other allergens and could help to explain the paradoxical lack of isocyanate-specific and/or low total IgE levels in isocyanate-hypersensitive individuals.
In addition to distinct cytokines, another striking feature of the GSH–MDI induced airway inflammatory response was the prominent protein profile of alternatively activated macrophages (AAMs), including three of the four proteins most increased in the airway fluid (YM-1, YM-2, and RELMα/Fizz1). GSH–MDI induction of murine AAMs in vivo is consistent with previous in vitro studies demonstrating isocyanate induced activation of human macrophages, including chitinase upregulation and associations of monocyte-derived cytokines with clinical response to respiratory tract exposure.45–48 The in vitro human response to isocyanate, like the present in vivo murine response to GSH–MDI, occurs without measurable increases in critical cytokines (IL-4/IL-13) known to polarize monocyte responses34 and may define novel pathways by which macrophages are alternatively activated.
Besides YM-1, YM-2, and RELMα/Fizz1, a number of other airway proteins were markedly affected by GSH–MDI exposure. The most upregulated proteins included molecules previously associated with asthma (CLCA1 or gob-5, VCAM1, CTSS) and specific components of the mucosal/IgA immune system (IgA constant region, and nonpolymorphic H-2 class I-Q10a). The two most downregulated proteins (XRCC1 and INMT) are thought to function in repairing or limiting tissue damage, specifically that involving single-strand DNA breaks, xenobiotics, or endogenous amino, sulfo, or selenium compounds.36,37 Overall, the protein changes in the airways of sensitized, GSH–MDI exposed mice are distinct compared with those induced by other respiratory tract exposures49–51 and might serve as biomarkers of MDI exposure and/or asthma.
The relationship of the exposure doses used in this study to human exposure levels in occupational settings is crucial to interpreting the potential clinical relevance of the present findings. The 1% MDI (w/v) skin exposure dose, used for inducing systemic immune sensitization, is roughly equivalent to the final MDI concentration in polyol/diisocyanate mixtures used to make polyurethane foam.1 The GSH–MDI dose delivered via the intranasal route is more challenging to compare with human occupational respiratory tract MDI (vapor/aerosol) exposure, due to uncertainty regarding the relative rates of GSH–MDI formation and hydrolysis, and requires some extrapolation. On the basis of the amount of MDI used to make the GSH–MDI starting material (assuming 100% GSH–MDI product formation and subsequent release of active MDI), each intranasal exposure delivered the potential equivalent of 10 μg of MDI, approximately 100-fold below the predicted inhaled MDI dose (1 mg) for a human during an 8 h work shift, if exposed to MDI vapor/aerosol at the permissible exposure limit established by the U.S. Occupational Health and Safety Administration (0.2 mg/m3), assuming a mean minute ventilation rate ranging between 10 and 20 L/min.52–56
In summary, this article provides evidence that isocyanate– glutathione reaction products could play a unique role in the pathogenesis of isocyanate asthma and may help to explain some of the unusual features of the disease. The findings confirm the rapid, spontaneous reactivity of GSH with MDI under physiological conditions and further demonstrate the ability of GSH–MDI to carbamoylate self proteins in vivo and trigger airway eosinophilia and mucus production. The cytokines and alternative pattern of macrophage activation observed in response to GSH–MDI suggest these reaction products may trigger distinct signaling cascades vs conventional asthma-causing allergens. Together, the data provide proof-of-principle that GSH can mediate pathogenic responses to respiratory tract MDI exposure in vivo and suggest that GSH, or functionally related thiols, might represent targets for disease prevention and/or intervention.
Supplementary Material
Acknowledgments
We wish to acknowledge Ted Voss, Mary Lopresti, Jean Kanyo, and Tom Abbott from the Yale Keck Center for sample preparation and MS/MS analyses.
Funding: Funding was provided by U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health grant nos. OH010438 and OH010494.
Abbreviations
- BAL
bronchoalveolar lavage
- CLCA1 aka gob-5
calcium activated chloride channel regulator 1
- CHIL3 aka YM-1
chitinase-like protein 3
- CHIL4 aka YM-2
chitinase-like protein 4
- GSH
reduced glutathione
- INMT
indolethylamine N-methyltransferase
- iTRAQ
isobaric tags for relative and absolute quantitation
- MDI
methylene diphenyl diisocyanate
- PAS
periodic acid–Schiff
- RELMα aka Fizz-1
resistin-like alpha
Footnotes
Supporting Information: LC-MS characterization of GSH–MDI reaction products, Tables of peptides matched to albumin or modified albumin, and MS/MS data on the GSH–MDI modified albumin peptide containing residues 411–428. This material is available free of charge via the Internet at http://pubs.acs.org.
Notes: The authors declare no competing financial interest.
References
- 1.Allport DC, Gilbert DS, Outterside SM. MDI and TDI: Safety, Health and the Environment: A Source Book and Practical Guide. Wiley; Chichester: 2003. pp. 1–438. [Google Scholar]
- 2.Fuchs S, Valade P. Clinical and experimental study of some cases of poisoning by Desmodur T (1-2-4 and 1–2-6 di-isocyanates of toluene) Arch Mal Prof. 1951;12:191–196. [PubMed] [Google Scholar]
- 3.Rodziewicz P, Goclon J. Structural flexibility of 4,4′-methylene diphenyl diisocyanate (4,4′-MDI): evidence from first principles calculations. J Mol Model. 2014;20:2097. doi: 10.1007/s00894-014-2097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lesage J, Stanley J, Karoly WJ, Lichtenberg FW. Airborne methylene diphenyl diisocyanate (MDI) concentrations associated with the application of polyurethane spray foam in residential construction. J Occup Environ Hyg. 2007;4:145–155. doi: 10.1080/15459620601133779. [DOI] [PubMed] [Google Scholar]
- 5.Kenyon NJ, Morrissey BM, Schivo M, Albertson TE. Occupational asthma. Clin Rev Allergy Immunol. 2012;43:3–13. doi: 10.1007/s12016-011-8272-0. [DOI] [PubMed] [Google Scholar]
- 6.Booth K, Cummings B, Karoly WJ, Mullins S, Robert WP, Spence M, Lichtenberg FW, Banta J. Measurements of airborne methylene diphenyl diisocyanate (MDI) concentration in the U.S. workplace. J Occup Environ Hyg. 2009;6:228–238. doi: 10.1080/15459620902724060. [DOI] [PubMed] [Google Scholar]
- 7.Wisnewski AV, Liu J, Redlich CA. Antigenic changes in human albumin caused by reactivity with the occupational allergen diphenylmethane diisocyanate. Anal Biochem. 2010;400:251–258. doi: 10.1016/j.ab.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Q, Wisnewski AV. Recent developments in diisocyanate asthma. Ann Allergy, Asthma, Immunol. 2003;90:35–41. doi: 10.1016/s1081-1206(10)61647-x. [DOI] [PubMed] [Google Scholar]
- 9.Hettick JM, Siegel PD. Comparative analysis of aromatic diisocyanate conjugation to human albumin utilizing multiplexed tandem mass spectrometry. Int J Mass Spectrom. 2012;169:168–175. [Google Scholar]
- 10.Wisnewski AV, Stowe MH, Cartier A, Liu Q, Liu J, Chen L, Redlich CA. Isocyanate vapor-induced antigenicity of human albumin. J Allergy Clin Immunol. 2004;113:1178–1184. doi: 10.1016/j.jaci.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Johannesson G, Sennbro CJ, Willix P, Lindh CH, Jonsson BA. Identification and characterisation of adducts between serum albumin and 4,4′-methylenediphenyl diisocyanate (MDI) in human plasma. Arch Toxicol. 2004;78:378–383. doi: 10.1007/s00204-004-0555-2. [DOI] [PubMed] [Google Scholar]
- 12.Wass U, Belin L. Immunologic specificity of isocyanate-induced IgE antibodies in serum from 10 sensitized workers. J Allergy Clin Immunol. 1989;83:126–135. doi: 10.1016/0091-6749(89)90487-9. [DOI] [PubMed] [Google Scholar]
- 13.Redlich CA, Karol MH. Diisocyanate asthma: clinical aspects and immunopathogenesis. Int Immunopharmacol. 2002;2:213–224. doi: 10.1016/s1567-5769(01)00174-6. [DOI] [PubMed] [Google Scholar]
- 14.Cantin AM, North SL, Hubbard RC, Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol. 1987;63:152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- 15.Day BW, Jin R, Basalyga DM, Kramarik JA, Karol MH. Formation, solvolysis, and transcarbamoylation reactions of bis(S-glutathionyl) adducts of 2,4- and 2,6-diisocyanatotoluene. Chem Res Toxicol. 1997;10:424–431. doi: 10.1021/tx960201+. [DOI] [PubMed] [Google Scholar]
- 16.Reisser M, Schmidt BF, Brown WE. Synthesis, characterization, and solvolysis of mono- and bis-S-(glutathionyl) adducts of methylene-bis-(phenylisocyanate) (MDI) Chem Res Toxicol. 2002;15:1235–1241. doi: 10.1021/tx0255020. [DOI] [PubMed] [Google Scholar]
- 17.Wisnewski AV, Hettick JM, Siegel PD. Toluene diisocyanate reactivity with glutathione across a vapor/liquidinterface and subsequent transcarbamoylation of human albumin. Chem Res Toxicol. 2011;24:1686–1693. doi: 10.1021/tx2002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wisnewski AV, Liu J, Redlich CA. Connecting glutathione with immune responses to occupational methylene diphenyl diisocyanate exposure. Chem –Biol Interact. 2013;205:38–45. doi: 10.1016/j.cbi.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wisnewski AV, Mhike M, Hettick JM, Liu J, Siegel PD. Hexamethylene diisocyanate (HDI) vapor reactivity with glutathione and subsequent transfer to human albumin. Toxicol In Vitro. 2013;27:662–671. doi: 10.1016/j.tiv.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slatter JG, Rashed MS, Pearson PG, Han DH, Baillie TA. Biotransformation of methyl isocyanate in the rat. Evidence for glutathione conjugation as a major pathway of metabolism and implications for isocyanate-mediated toxicities. Chem Res Toxicol. 1991;4:157–161. doi: 10.1021/tx00020a006. [DOI] [PubMed] [Google Scholar]
- 21.Chipinda I, Stetson SJ, Depree GJ, Simoyi RH, Siegel PD. Kinetics and mechanistic studies of the hydrolysis of diisocyanate-derived bis-thiocarbamates of cysteine methyl ester. Chem Res Toxicol. 2006;19:341–350. doi: 10.1021/tx050311t. [DOI] [PubMed] [Google Scholar]
- 22.Fleischel O, Gimenez-Arnau E, Lepoittevin JP. Nuclear magnetic resonance studies on covalent modification of amino acids thiol and amino residues by monofunctional aryl 13C-isocyanates, models of skin and respiratory sensitizers: transformation of thiocarbamates into urea adducts. Chem Res Toxicol. 2009;22:1106–1115. doi: 10.1021/tx9000539. [DOI] [PubMed] [Google Scholar]
- 23.Johansson MT, Lindberg S, Astot C. Novel glutathione conjugates of phenyl isocyanate identified by ultra- performance liquid chromatography/electrospray ionization mass spectrometry and nuclear magnetic resonance. J Mass Spectrom. 2014;49:68–79. doi: 10.1002/jms.3306. [DOI] [PubMed] [Google Scholar]
- 24.Wisnewski AV, Xu L, Robinson E, Liu J, Redlich CA, Herrick CA. Immune sensitization to methylene diphenyl diisocyanate (MDI) resulting from skin exposure: albumin as a carrier protein connecting skin exposure to subsequent respiratory responses. J Occup Med Toxicol. 2011;6:6. doi: 10.1186/1745-6673-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wisnewski AV, Liu J. Molecular determinants of humoral immune specificity for the occupational allergen, methylene diphenyl diisocyanate. Mol Immunol. 2013;54:233–237. doi: 10.1016/j.molimm.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shilov IV, Seymour SL, Patel AA, Loboda A, Tang WH, Keating SP, Hunter CL, Nuwaysir LM, Schaeffer DA. The Paragon Algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol Cell Proteomics. 2007;6:1638–1655. doi: 10.1074/mcp.T600050-MCP200. [DOI] [PubMed] [Google Scholar]
- 27.Shifman MA, Li Y, Colangelo CM, Stone KL, Wu TL, Cheung KH, Miller PL, Williams KR. YPED: a web-accessible database system for protein expression analysis. J Proteome Res. 2007;6:4019–4024. doi: 10.1021/pr070325f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 29.Walter MJ, Kajiwara N, Karanja P, Castro M, Holtzman MJ. Interleukin 12 p40 production by barrierepithelial cells during airway inflammation. J Exp Med. 2001;193:339–351. doi: 10.1084/jem.193.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russell TD, Yan Q, Fan G, Khalifah AP, Bishop DK, Brody SL, Walter MJ. IL-12 p40 homodimer-dependent macrophage chemotaxis and respiratory viral inflammation are mediated through IL-12 receptor beta 1. J Immunol. 2003;171:6866–6874. doi: 10.4049/jimmunol.171.12.6866. [DOI] [PubMed] [Google Scholar]
- 31.Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJ. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics. 2004;3:1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 32.Nakanishi A, Morita S, Iwashita H, Sagiya Y, Ashida Y, Shirafuji H, Fujisawa Y, Nishimura O, Fujino M. Role of gob-5 in mucus overproduction and airway hyperresponsiveness in asthma. Proc Natl Acad Sci U S A. 2001;98:5175–5180. doi: 10.1073/pnas.081510898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mundhenk L, Alfalah M, Elble RC, Pauli BU, Naim HY, Gruber AD. Both cleavage products of the mCLCA3 protein are secreted soluble proteins. J Biol Chem. 2006;281:30072–30080. doi: 10.1074/jbc.M606489200. [DOI] [PubMed] [Google Scholar]
- 34.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Holt PG, Macaubas C, Stumbles PA, Sly PD. The role of allergy in the development of asthma. Nature. 1999;402:B12–B17. doi: 10.1038/35037009. [DOI] [PubMed] [Google Scholar]
- 36.Herman KS, Bowsher RR, Henry DP. Synthesis of N pi-methylhistamine and N alpha-methylhistamine by purified rabbit lung indolethylamine N-methyltransferase. J Biol Chem. 1985;260:12336–12340. [PubMed] [Google Scholar]
- 37.Bauer M, Goldstein M, Heylmann D, Kaina B. Human monocytes undergo excessive apoptosis following Temozolomide activating the ATM/ATR pathway while dendritic cells and macrophages are resistant. PLoS One. 2012;7:e39956. doi: 10.1371/journal.pone.0039956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feldhoff RC, Steffen MC, Geoghegan TE, Ledford BE. Purification of transferrin and albumin from mouse ascites fluid. Prep Biochem. 1985;15:221–236. doi: 10.1080/00327488508062442. [DOI] [PubMed] [Google Scholar]
- 39.Monks TJ, Anders MW, Dekant W, Stevens JL, Lau SS, van Bladeren PJ. Glutathione conjugate mediated toxicities. Toxicol Appl Pharmacol. 1990;106:1–19. doi: 10.1016/0041-008x(90)90100-9. [DOI] [PubMed] [Google Scholar]
- 40.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conte E, Nigro L, Fagone E, Drago F, Cacopardo B. Quantitative evaluation of interleukin-12 p40 gene expression in peripheral blood mononuclear cells. Immunol Invest. 2008;37:143–151. doi: 10.1080/08820130701690824. [DOI] [PubMed] [Google Scholar]
- 42.Gillessen S, Carvajal D, Ling P, Podlaski FJ, Stremlo DL, Familletti PC, Gubler U, Presky DH, Stern AS, Gately MK. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur J Immunol. 1995;25:200–206. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- 43.Kondo N, Matsui E, Kaneko H, Aoki M, Kato Z, Fukao T, Kasahara K, Morimoto N. RNA editing of interleukin-12 receptor beta2, 2451 C-to-U (Ala 604 Val) conversion, associated with atopy. Clin Exp Allergy. 2004;34:363–368. doi: 10.1111/j.1365-2222.2004.01901.x. [DOI] [PubMed] [Google Scholar]
- 44.Peng JC, Abu-Bakar S, Richardson MM, Jonsson JJ, Frazer IH, Nielsen LK, Morahan G, Thomas R. IL10 and IL12B polymorphisms each influence IL-12p70 secretion by dendritic cells in response to LPS. Immunol Cell Biol. 2006;84:227–232. doi: 10.1111/j.1440-1711.2006.01419.x. [DOI] [PubMed] [Google Scholar]
- 45.Wisnewski AV, Liu Q, Liu J, Redlich CA. Human innate immune responses to hexamethylene diisocyanate (HDI) and HDI-albumin conjugates. Clin Exp Allergy. 2008;38:957–967. doi: 10.1111/j.1365-2222.2008.02982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lummus ZL, Alam R, Bernstein JA, Bernstein DI. Diisocyanate antigen-enhanced production of monocyte chemoattractant protein-1, IL-8, and tumor necrosis factor-alpha by peripheral mononuclear cells of workers with occupational asthma. J Allergy Clin Immunol. 1998;102:265–274. doi: 10.1016/s0091-6749(98)70095-8. [DOI] [PubMed] [Google Scholar]
- 47.Silva A, Nunes C, Martins J, Dinis TC, Lopes C, Neves B, Cruz T. Respiratory sensitizer hexamethylene diisocyanate inhibits SOD 1 and induces ERK-dependent detoxifying and maturation pathways in dendritic-like cells. Free Radical Biol Med. 2014;72:238–246. doi: 10.1016/j.freeradbiomed.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Verstraelen S, Wens B, Hooyberghs J, Nelissen I, Witters H, Schoeters G, Cauwenberge PV, Heuvel RV. Gene expression profiling of in vitro cultured macrophages after exposure to the respiratory sensitizer hexamethylene diisocyanate. Toxicol In Vitro. 2008;22:1107–1114. doi: 10.1016/j.tiv.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 49.Ali M, Umstead TM, Haque R, Mikerov AN, Freeman WM, Floros J, Phelps DS. Differences in the BAL proteome after Klebsiella pneumoniae infection in wild type and SPA-/- mice. Proteome Sci. 2010;8:34. doi: 10.1186/1477-5956-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Juang YM, Lai BH, Chien HJ, Ho M, Cheng TJ, Lai CC. Changes in protein expression in rat bronchoalveolar lavage fluid after exposure to zinc oxide nanoparticles: an iTRAQ proteomic approach. Rapid Commun Mass Spectrom. 2014;28:974–980. doi: 10.1002/rcm.6866. [DOI] [PubMed] [Google Scholar]
- 51.Kumar Y, Liang C, Limmon GV, Liang L, Engelward BP, Ooi EE, Chen J, Tannenbaum SR. Molecular analysis of serum and bronchoalveolar lavage in a mouse model of influenza reveals markers of disease severity that can be clinically useful in humans. PLoS One. 2014;9:e86912. doi: 10.1371/journal.pone.0086912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Preventing Asthma and Death from Diisocyanate Exposure. National Institute for Occupational Safety and Health (NIOSH), CDC; Atlanta, GA: 1996. DHHS (NIOSH) Publication Number 96-11. http://www.cdc.gov/niosh/docs/96-111/ [Google Scholar]
- 53.Exposure Factors Handbook. U.S. Environmental Protection Agency, Office of Research and Development, National Center for Environmental Assessment; Research Triangle Park, NC: 1997. http://www.epa.gov/ncea/pdfs/efh/front.pdf. [Google Scholar]
- 54.Layton DW. Metabolically consistent breathing rates for use in dose assessments. Health Phys. 1993;64:23–36. doi: 10.1097/00004032-199301000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Mackey M, Ellis E, Nicholls M. Breathing patterns and heart rate during simulated occupational upper limb tasks in normal subjects. Physiother Res Int. 1998;3:83–99. doi: 10.1002/pri.128. [DOI] [PubMed] [Google Scholar]
- 56.Zuurbier M, Hoek G, van den Hazel P, Brunekreef B. Minute ventilation of cyclists, car and bus passengers: an experimental study. Environ Health. 2009;8:48. doi: 10.1186/1476-069X-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


