Abstract
Background
The effectiveness of low-fat diets for long-term weight loss has been debated for decades, with dozens of randomized trials (RCTs) and recent reviews giving mixed results.
Methods
We conducted a random effects meta-analysis of RCTs to estimate the long-term effect of low-fat vs. higher fat dietary interventions on weight loss. Our search included RCTs conducted in adult populations reporting weight change outcomes at ≥1 year, comparing low-fat with higher fat interventions, published through July 2014. The primary outcome measure was mean difference in weight change between interventions.
Findings
Fifty-three studies met inclusion criteria representing 68,128 participants. In the setting of weight loss trials, low-carbohydrate interventions led to significantly greater weight loss than low-fat interventions (n comparisons=18; weighted mean difference [WMD]=1.15 kg, 95% CI=0.52 to 1.79; I2=10%). Low-fat did not lead to differences in weight change compared with other moderate fat weight loss interventions (n=19; WMD=0.36, 95% CI=-0.66 to 1.37; I2=82%), and were superior only when compared with “usual diet” (n=8; WMD=-5.41, 95% CI=-7.29 to −3.54; I2=68%). Similarly, non-weight loss trials and weight maintenance trials, for which there were no low-carbohydrate comparisons, had similar effects for low-fat vs moderate fat interventions, and were superior compared with “usual diet”. Weight loss trials achieving a greater difference in fat intake at follow-up significantly favored the higher fat dietary interventions, as indicated by difference of ≥5% of calories from fat (n=18; WMD=1.04, 95% CI=0.06 to 2.03; I2=78%) or by difference in change serum triglycerides of ≥5 mg/dL (n=17; WMD=1.38, 95% CI=0.50 to 2.25; I2=62%).
Interpretation
These findings suggest that the long-term effect of low-fat diets on body weight depends on the intensity of intervention in the comparison group. When compared to dietary interventions of similar intensity, evidence from RCTs does not support low-fat diets over other dietary interventions.
Introduction
Identifying effective strategies for long-term weight control will be critical to reduce the alarming prevalence of overweight and obesity worldwide. The macronutrient composition of the diet, or the proportions of calories contributed by fat, carbohydrate, and protein, has received significant attention in past decades for its potential relevance in weight loss and weight maintenance. Numerous short- and long-term randomized trials across a variety of general and clinical populations have attempted to identify the optimal ratio of macronutrients for weight loss. Lowering the proportion of daily calories consumed from total fat has been targeted for many reasons, one of which is that a single gram of fat contains more than twice the calories of a gram of carbohydrates or protein (9 kcal/gram vs. 4 kcal/gram). Thus, reducing total fat intake may theoretically lead to an appreciable impact on total calories consumed. However, randomized trials have failed to consistently demonstrate that reducing the percent of energy from total fat leads to long-term weight loss compared to other dietary interventions.
This systematic review and meta-analysis aimed to summarize the large body of evidence from randomized control trials (RCTs) lasting ≥1 year in which weight changes on low-fat diets vs. other dietary intervention groups were compared. Trials were included regardless of whether weight loss was intended or not, for example in studies evaluating lipids or cancer endpoints. We considered stratification by characteristics of the interventions that may affect differences in weight loss, including whether the intervention arms received similar attention and intervention intensity, or the composition of the comparison diet. We hypothesized that low-fat diets would not be associated with greater weight loss when differences in these intervention characteristics were taken into account, and that differences in weight loss favoring higher fat interventions would be larger when adherence was greater.
Methods
Search strategy and inclusion criteria
Predefined search strategy, study eligibility criteria, and statistical methodological approaches, were detailed in our unpublished research protocol. Full details of our literature search (Page 2) and PRISMA checklist (Pages 7–10) are outlined in the Appendix. Briefly, we used the MEDLINE, EMBASE, CENTRAL, and Cochrane Database of Systematic Reviews to identify eligible trials. We included trials lasting ≥1 year comparing weight change on a low-fat diet (as defined by authors) with any higher fat dietary intervention, including “usual diet” among non-pregnant adults. Trials of shorter duration were excluded because weight-loss trials frequently observe an initial maximal weight loss around 6 months with subsequent weight regain.
The outcome of interest was long-term (≥1 year) change in body weight (reported as mean change from baseline, mean change difference, or mean body weight at end of follow-up). Efforts were made to contact authors to obtain variance measures, if not reported, but were ultimately excluded if unavailable. We excluded trials if one intervention group included a non-dietary weight loss component (e.g., exercise regimen, pharmaceutical intervention) while the other did not. We did not make exclusions based on concomitant dietary components (e.g., increase fruits and vegetables). Nonrandomized trials were excluded as well as dietary supplements or meal replacement drink interventions as these were beyond the scope of our investigation. If trial results were published more than once, the paper with the most complete follow-up was included in the main analysis. Screening of abstracts for relevance was conducted by two reviewers (DT, MC) and eligible full texts were reviewed with an inclusion/exclusion criteria sheet independently and in duplicate by two reviewers (DT, MC).
Data extraction
Variables captured from the final accepted studies included study level information (authors, country, center), study population characteristics, intervention details, including weight loss intention (yes, no, maintain) and the relative intensity of each intervention, as described by study authors (i.e., systematically greater attention, time spent with study clinicians, dieticians, program materials, etc for one intervention group over the other), and outcomes by treatment arm. We also recorded dietary adherence, including change in serum triglyceride levels and the percent calories from fat during follow-up. We analyzed the intention-to-treat estimates, when reported.
We evaluated the trials’ potential for bias using the Cochrane risk of bias assessment tool.(1) Data were extracted independently by two investigators (DT, MC), and discrepancies resolved with a third reviewer (FH), if necessary.
Data analysis
We calculated the mean difference in body weight change from baseline by subtracting the mean change of the comparison diet group from the mean change in the low-fat diet group. If the mean change was not reported we compared the groups’ final mean body weights, under the assumption that randomization resulted in similar average baseline body weights between treatment arms. We estimated the pooled weighted mean difference and 95% confidence interval (CI) with a DerSimonian and Laird random effects model. P<0.05 was considered statistically significant.
We assessed heterogeneity from the Mantel-Haenszel model and I2 values (the percent of variance in the pooled estimate due to between-study differences), with I2>50% indicating moderate heterogeneity.(2) Analyses established a priori were conducted to evaluate potential heterogeneity by the whether the trial was designed with the intention of weight loss, the composition of the comparison diet (low-carbohydrate, other moderate fat/”healthful” diet, or usual diet), the interventions’ relative intensity, , whether either, neither, or both of the interventions included caloric restriction, and the baseline health status of the participants. Additionally, we stratified by change in triglyceride levels and in attained self-reported percent calories from fat, with an increase in triglycerides reflecting a relative decrease in fat intake.(3)
Finally, we conducted sensitivity analyses to assess the robustness of findings. We evaluated the impact of removing the largest study or studies, based on their percent weight in the pooled estimates and restricted to trials conducting intention-to-treat analyses and with ≥100 participants. Primary analyses were repeated using an inverse variance weighted fixed effect model. The Begg (4) and Egger (5, 6) tests were conducted to test for the potential of publication bias by plotting the inverse of the variance against the treatment effect. Analyses were performed using STATA® version 13.1.
Role of the funding source
The funding sources did not participate in the design or conduct of the study; collection, management, analysis or interpretation of the data; preparation, review, or approval of the manuscript. DT had full access to all of the data and the final responsibility to submit for publication.
RESULTS
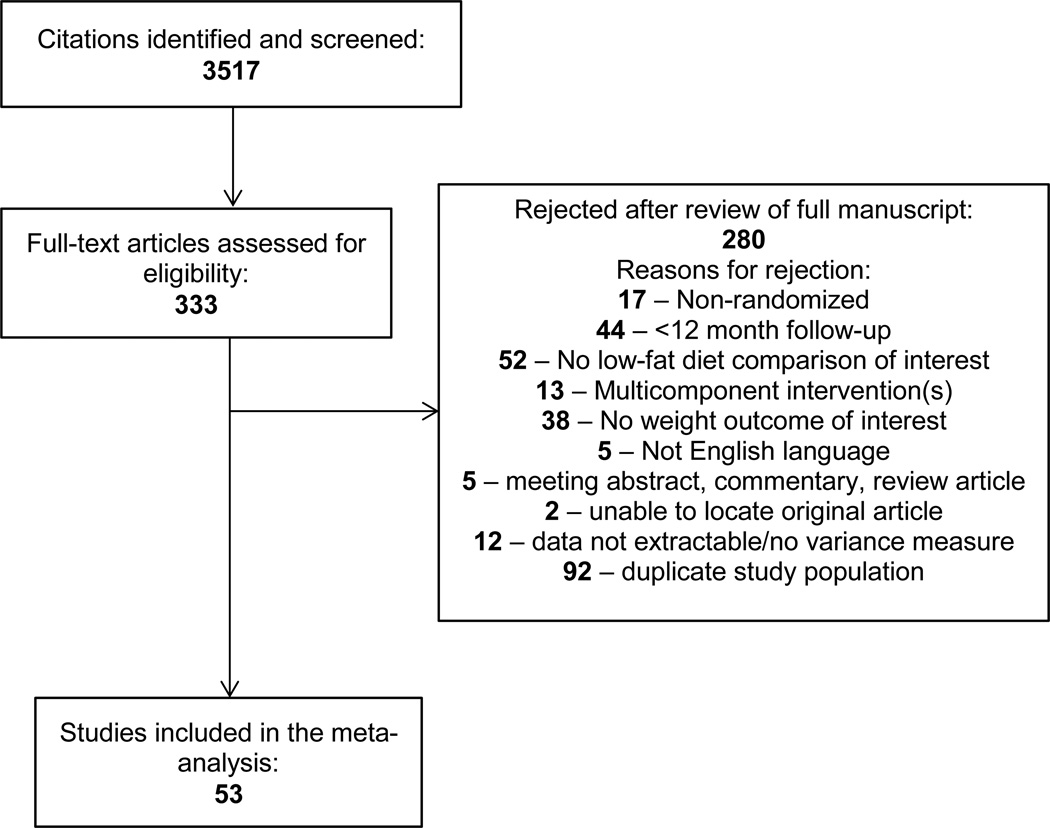
Our search yielded 3,517 citations (Figure 1), of which 53 RCTs were eligible for inclusion in our analysis (Table 1). The majority of trials were conducted in North America (n=37) and were 1 year in duration (n=27). Twenty trials specifically enrolled participants with prevalent chronic diseases, including breast cancer,(7–10) hypercholesterolemia,(11–13) and type 2 diabetes.(14–22) In addition to 35 weight loss trials, there were 13 trials with no intended intervention on weight, (7–10, 12, 13, 22–28) and 5 weight maintenance trials designed to maintain baseline body weight. (11, 29–32)
Figure 1.
PRISMA Flow Diagram
Table 1.
Randomized trials of low-fat versus other dietary interventions of at least 1 year duration among adults, included in the meta-analysis.
| Trial Name | N randomized; Population |
Country | Weight loss goal |
Low-fat diet(s) intervention |
Comparator diet(s) intervention |
Follow- up duration (years) |
|---|---|---|---|---|---|---|
| A to Z (36) | 311; Overweight, premenopausal women |
US | Yes | [1] LEARN (reduced calorie); [2] Ornish (<10% fat; reduced calorie) |
[1] Atkins low-carbohydrate; [2] Zone (30% fat; reduced calorie) |
1 |
| Anderson 1992 (11) |
117; Moderate hypercholesterolemia |
US | Maintain | American Heart Association Phase II (25% fat) |
Usual diet | 1 |
| Barnard 2009 (14) |
99; Type 2 diabetes | US | Yes | Vegan (10% fat) | American Diabetes Association Diet 2003 (30% fat; reduced calorie) |
1.4 |
| Bazzano 2014 (37) |
148; Obese | US | Yes | National Cholesterol Education Program (<30% fat) |
Low carbohydrate | 1 |
| Bertz 2012 (38) | 68; Breastfeeding mothers |
Sweden | Yes | Nordic Nutrition Guidelines (<30% fat; reduced calorie) |
Usual diet | 1 |
| Boyd 1990 (29) | 295; Women at high breast cancer risk |
Canada | Maintain | 15% fat | Canadian Food Guide (No fat intake advice) |
1 |
| Breast Cancer Prevention Program (23) |
194; Women at high breast cancer risk |
US | No | 15% fat | Usual diet | 1 |
| Brehm 2009 (15) |
124; Overweight/obese with type 2 diabetes |
US | Yes | High carbohydrate (25% fat; reduced calorie) |
High mono-unsaturated fat (40% fat; reduced calorie) |
1 |
| BRIDGES (7) | 172; Women with recent breast cancer |
US | No | Nutrition Education Program (20% fat) |
Usual diet | 1 |
| Brinkworth 2009 (39, 40) |
118; At risk for metabolic syndrome |
Australia | Yes | 30% fat (reduced calorie) | Atkins low-carbohydrate (61% fat; reduced calorie) |
1 |
| CALERIE Phase I (41) |
34; Overweight | US | Yes | High glycemic index, food provided (20% fat; reduced calorie) |
Low glycemic index, food provided (30% fat; reduced calorie) |
1 |
| Canadian Diet and Breast Cancer Prevention Study (30) |
4690; Women at high breast cancer risk |
Canada | Maintain | 15% fat | Canadian Food Guide (No fat intake advice) |
10 |
| Dansinger 2005 (42) |
160; At risk for cardiovascular disease |
US | Yes | Ornish (<10% fat) | [1] Atkins low-carbohydrate; [2] Zone (30% fat); [3] Weight Watchers (reduced calorie) |
1 |
| Davis 2009 (16) | 105; Type 2 diabetes | US | Yes | Diabetes Prevention Program diet (25% fat) |
Atkins low-carbohydrate | 1 |
| DEER (12) | 377; Hypercholesterolemia |
US | No | National Cholesterol Education Program (<30% fat) |
Usual diet | 1 |
| The Dietary Alternatives Study (13) |
508; Men with hypercholesterolemia |
US | No | [1] 26% fat; [2] 22% fat; [3] 18% fat |
30% fat | 1 |
| DIRECT (17) | 322; Type 2 diabetes, cardiovascular disease, or obese |
Israel | Yes | American Heart Association (30% fat; reduced calorie) |
[1] Mediterranean diet (35% fat; reduced calorie); [2] Atkins low-carbohydrate |
2 |
| Ebbeling 2007 (43) |
73; Obese young adults |
US | Yes | 20% fat | Low glycemic-index carbohydrates (35% fat) |
1.5 |
| Elhayany 2010 (18) |
259; Type 2 diabetes | Israel | Yes | [1] American Diabetes Association 2003 (30% fat; reduced calorie); [2] Low-fat Mediterranean (30% fat; reduced calorie) |
Low carbohydrate Mediterranean diet (45% fat; reduced calorie) |
1 |
| Esposito 2009 (19) |
215; Type 2 diabetes | Italy | Yes | American Heart Association 2000 (<30% fat; reduced calorie) |
Mediterranean diet (>30% fat; reduced calorie) |
4 |
| Foster 2003 (44) |
63; Obese | US | Yes | 25% fat (reduced calorie) | Atkins low-carbohydrate | 1 |
| Foster 2010 (45) |
307; Obese | US | Yes | 30% fat (reduced calorie) | Atkins low-carbohydrate | 2 |
| Guldbrand 2012 (20) |
61; Type 2 diabetes | Sweden | Yes | <30% fat (reduced calorie) | Low-carbohydrate (50% fat; reduced calorie) |
2 |
| Harvey-Berino 1999 (46) |
80; Overweight/obese |
US | Yes | 20% fat | Low-calorie | 1.5 |
| Iqbal 2010 (21) | 144; Type 2 diabetes, obese |
US | Yes | <30% fat (reduced calorie) | Low-carbohydrate | 2 |
| Keogh 2007 (47) |
44; Overweight/obese |
Australia | Yes | 20% fat (reduced calorie) | Low-carbohydrate (27% fat; reduced calorie) |
1 |
| Klemsdal 2010 (48) |
202; Metabolic syndrome |
Norway | Yes | 30% fat (reduced calorie) | Low glycemic load (35–40% fat; reduced calorie) |
1 |
| Kristal 2005 (49) |
93; Overweight/obese with esophageal metaplasia |
US | Yes | 20% fat (reduced calorie) | Usual diet | 3 |
| Lapointe 2010 (50) |
68; Overweight/obese postmenopausal women |
Canada | Yes | Reduce fat intake | Increase fruits and vegetables |
1.5 |
| Lim 2010 (51) | 113; High cardiovascular disease risk |
Australia | Yes | Food provided (10% fat; reduced calorie) |
[1] Low-carbohydrate, food provided (60% fat; reduced calorie); [2] High unsaturated fat, food provided (30% fat; reduced calorie); [3] Usual diet |
1.25 |
| McAuley 2006 (52) |
96; Women overweight/obese with insulin resistance |
New Zealand |
Yes | Diabetes and Nutrition Study Group of the European Association for the Study of Diabetes (<30% fat) |
[1] low carbohydrate Atkins diet; [2] Zone diet (30% fat) |
1 |
| McManus 2001 (53) |
101; Overweight | US | Yes | 20% fat (reduced calorie) | 35% fat (reduced calorie) | 1.5 |
| Nutrition and Exercise in Women Study (54) |
439; Postmenopausal overweight/obese women |
US | Yes | <30% fat (reduced calorie) | Usual diet | 1 |
| Nutrition and Breast Health Study (31) |
122; Premenopausal women at risk of breast cancer |
US | Maintain | (1) 15% fat; (2) High fruits and vegetables (15% fat) |
(1) Usual diet; (2) High fruits and vegetables |
1 |
| Pilkington 1960 (24) |
58; Men with ischemic heart disease |
UK | No | 20 g fat/day | Increase unsaturated fats | 1.5 |
| Polyp Prevention Trial (25) |
2079; Recent colorectal adenoma |
US | No | 20% fat | Usual diet | 3.1 |
| Pounds Lost Trial (55) |
811; Overweight/obese |
US | Yes | [1] 20% fat (reduced calorie); [2] high protein (20% fat; reduced calorie) |
[1] 40% fat (reduced calorie); [2] high protein (40% fat; reduced calorie) |
2 |
| PREDIMED (26) |
7447; High cardiovascular disease risk |
Spain | No | Reduce fat intake | Mediterranean Diet + [1] increase extra-virgin olive oil intake, [2] mixed nuts intake |
4.8 |
| PREMIER (56) | 810; Prehypertension | US | Yes | DASH (<25% fat; reduced calorie) |
30% fat (reduced calorie) | 1.5 |
| Shah 1996 (57) | 122; Obese women | US | Yes | 20 g fat/day | 30% fat (reduced calorie) | 1 |
| SMART Study (58) |
200; Overweight/obese |
Germany | Yes | German Nutrition Society (30% fat; reduced calorie) |
Low-carbohydrate (35% fat; reduced calorie) |
1 |
| Stern 2004 (59, 60) |
132; Morbidly obese | US | Yes | NHLBI (30% fat; reduced calorie) |
Low-carbohydrate | 1 |
| Swinburn 2001 (28) |
176; Glucose intolerance |
New Zealand |
No | Reduce fat | Usual diet | 5 |
| Tapsell 2004 (22) |
63; Type 2 diabetes | Australia | No | 27% fat | 37% fat | 1 |
| Tehran Lipid and Glucose Study (61) |
100; Overweight/obese |
Iran | Yes | 20% fat (reduced calorie) | 30% fat (reduced calorie) | 1.2 |
| Turner- McGrievy 2007 (62) |
64; Overweight/obese postmenopausal women |
US | Yes | Vegan (10% fat) | National Cholesterol Education Program (<30% fat) |
2 |
| Viegener 1990 (63) |
85; Overweight/obese women |
US | Yes | 15–25% fat (reduced calorie) | 30% fat (reduced calorie) | 1 |
| Women’s Health Initiative Dietary Modification Trial (32) |
48835; Postmenopausal women |
US | Maintain | 20% fat | Usual diet | 7.5 |
| Women’s Health Trial Vanguard Study (27) |
303; Women at high breast cancer risk |
US | No | 20% fat | Usual diet | 2 |
| Women’s Healthy Eating and Living (WHEL) (9) |
3088; Women with previous breast cancer |
US | No | 15–20% fat | USDA guidelines (<30% fat) | 7.3 |
| Women’s Intervention Nutrition Study (WINS) (8) |
2437; Women with breast cancer |
US | No | 15% fat | General counseling on nutritional adequacy |
5 |
| Women’s Intervention Nutrition Study (WINS) Feasibility (10) |
290; Women with postmenopausal breast cancer |
US | No | 20% fat | General counseling on nutritional adequacy |
1.5 |
| Wood 1991 (64) | 294; Overweight/obese |
US | Yes | National Cholesterol Education Program (<30% fat; reduced calorie) |
Usual diet | 1 |
The low-fat dietary interventions ranged from very low-fat ≤10% of calories from fat, to more moderate goals of ≤30% of calories from fat. Comparator diets of higher fat intake were diverse, ranging from a single baseline interaction with instructions to maintain “usual diet”, to a variety of other dietary interventions, including low-carbohydrate and other moderate-to-high-fat diets. The intensity of the interventions varied from pamphlets or instructions given at baseline only, to multicomponent programs integrating counseling sessions, regular meetings with dieticians, food diaries, cooking lessons, etc., to feeding studies, in which participants were given a significant portion of their food. Caloric restriction was a component of many weight loss interventions, but not all. For example, despite being a weight loss intervention, a low-carbohydrate Atkins-style diet is often ad libitum (i.e., eat until satiated).
Our primary meta-analysis included 68,128 adults from eligible randomized clinical trials, reporting a mean weight loss of 2.71 kg (SD=2.8) after a median of 1 year of follow-up, and 3.75 kg (SD=2.7) among weight loss trials. Figure 2 presents the overall results according to weight loss trial design (yes, no, or maintain) and composition of comparator intervention (low-carbohydrate, other higher fat intervention, or usual diet). No difference between low-fat and higher fat dietary interventions was observed when all weight loss trials were combined, although there was significant between-study heterogeneity. Low-carbohydrate weight loss interventions led to an average 1.15 kg greater long-term weight loss than low-fat weight loss interventions, with minimal between-study heterogeneity. No difference, however, was observed between low-fat and other higher fat dietary interventions. Compared with groups only following their usual diet, low-fat weight loss interventions led to 5.41 kg greater weight loss. Non-weight loss trials and weight maintenance trials also found a significant but smaller magnitude of weight loss in low-fat interventions when compared with usual diet, and no difference between low-fat and other higher fat dietary interventions. No long-term non-weight loss or weight maintenance trials compared low-fat with low-carbohydrate dietary interventions.
Figure 2.
Random effects pooled weighted mean difference (kg) for low-fat vs. comparator dietary interventions from 53 randomized trials reporting at least 1 year of follow-up, by weight loss intention and comparator intervention.
Table 2 presents analyses stratified by additional trial characteristics, limited to trials of similar intensity to minimize bias from one group receiving more attention and higher intervention intensity. Only 4 of the 17 comparisons among trials without a weight loss goal (13, 22, 24) and 1 of the 6 comparisons among weight maintenance trials (31) remained, limiting our ability to stratify further; thus, Table 2 includes weight loss trials only, which trended towards greater weight loss for higher fat interventions. Stratifying by caloric restriction indicated no significant difference in weight loss between low-fat and higher fat dietary weight loss interventions when interventions were concordant for caloric restriction. Calorie-restricted low-fat diets, however, fared significantly worse compared with non-calorie restricted higher fat interventions. Results were similar for weight loss trials among participants with or without a specific chronic disease at baseline (e.g., breast cancer).
Table 2.
Random effects pooled weighted mean difference (kg) for low-fat vs. comparator dietary interventions from 36 randomized weight loss trials reporting at least 1 year of follow-up, stratified by trial characteristics.
| N Comparisons | WMD (95% CI) | p- value |
I2 (p-value for heterogeneity) | |
|---|---|---|---|---|
| Weight Loss Goal | ||||
| Similar Intervention Intensity | 33 | 0.62 (−0.08, 1.32) | 0.084 | 71.6% (p<0.0001) |
| Comparator Diet | ||||
| Low-Carbohydrate | 18 | 1.15 (0.52, 1.79) | <0.001 | 10.4% ( p=0.33) |
| Other Higher Fat Intervention | 19 | 0.36 (−0.66, 1.37) | 0.49 | 82.0% (p<0.0001) |
| Usual Diet | 0 | -- | -- | |
| Caloric Restriction | ||||
| Both Interventions | 18 | 0.74 (−0.19, 1.68) | 0.12 | 78.4% (p <0.0001) |
| Neither Intervention | 8 | 0.33 (−1.18, 1.83) | 0.67 | 65.1% (p=0.005) |
| Low-Fat Only | 6 | 1.49 (0.53, 2.45) | 0.002 | 7.7% (p=0.37) |
| Comparator Only | 5 | −0.62 (−1.95, 0.72) | 0.37 | 15.5% (p=0.32) |
| Chronic Disease Population | ||||
| No | 25 | 0.77 (−0.15, 1.69) | 0.10 | 76.1% (p <0.0001) |
| Yes | 8 | 0.37 (−0.33, 1.07) | 0.30 | 10.3% (p=0.35) |
| Difference in Fat Intake at Follow-up (% Calories) | ||||
| <5% Difference in Fat Intake | 8 | 0.14 (−0.80, 1.09) | 0.77 | 30.1% ( p=0.19) |
| ≥5% Difference in Fat Intake | 18 | 1.04 (0.06, 2.03) | 0.038 | 77.7% (p<0.0001) |
| Difference in Triglycerides at Follow-up (mg/dL Change) | ||||
| <5 mg/dL Change Difference | 8 | −0.21 (−0.86, 0.43) | 0.52 | 0.0% (p =0.92) |
| ≥5 mg/dL Greater Change in Low-Fat Group | 17 | 1.38 (0.50, 2.25) | 0.002 | 62.3% (p<0.0001) |
| No Weight Loss Goal | ||||
| Similar Intervention Intensity | 4 | −1.71 (−4.52, 1.10) | 0.23 | 59.3% (p=0.061) |
| Comparator Diet | ||||
| Low-Carbohydrate | 0 | -- | -- | -- |
| Other Higher Fat Intervention | 4 | −1.71 (−4.52, 1.10) | 0.23 | 59.3% (p=0.061) |
| Usual Diet | 0 | -- | -- | |
| Caloric Restriction | ||||
| Both Interventions | 0 | -- | -- | |
| Neither Intervention | 2 | −1.47 (−5.85, 2.91) | 0.51 | 76.3% (p=0.04) |
| Low-Fat Only | 0 | -- | -- | |
| Comparator Only | 0 | -- | -- | |
| Chronic Disease Population | ||||
| No | 0 | -- | -- | |
| Yes | 4 | −1.71 (−4.52, 1.10) | 0.23 | 59.3% (p=0.061) |
| Difference in Fat Intake at Follow-up (% Calories) | ||||
| <5% Difference in Fat Intake | 1 | NA | NA | NA |
| ≥5% Difference in Fat Intake | 2 | −2.18 (−6.19, 1.83) | 0.29 | 45.0% (p=0.18) |
| Difference in Triglycerides at Follow-up (mg/dL Change) | ||||
| <5 mg/dL Change Difference | 1 | NA | NA | NA |
| ≥5 mg/dL Greater Change in Low-Fat Group | 1 | NA | NA | NA |
WMD=DerSimonian and Laird random effects weighted mean difference, in kg; Negative value favors low-fat dietary intervention; Positive value favors higher fat comparator intervention
When groups differed by >5% calories from fat at follow-up, higher fat led to significantly greater weight loss than low-fat weight loss interventions. Similarly, weight loss trials with a ≥5 mg/dL greater change in triglycerides for low-fat vs. higher fat interventions, led to significantly greater weight loss for the higher fat groups.
Excluding the Women’s Health Initiative trial (96.90% of weight) from weight maintenance trials, did not impact findings (n=5; WMD=-0.77 kg, 95% CI=-1.50 to −0.04, p=0.039; I2=0.0%, p-heterogeneity=0.95). Results were similar when restricted studies conducting to intention-to-treat analyses (Appendix pages 3–4) and when excluding smaller trials of <100 total participants, although few non-weight loss or weight maintenance trials remained eligible according to these criteria. The fixed effect meta-analysis (Appendix pages 5–6), which gives less weight to smaller trials with greater variance, estimated 0.44 kg greater weight loss for the comparator vs. low-fat interventions among the weight loss trials. Fixed effect analyses stratified by comparator group also indicated greater weight loss for “other higher fat interventions” vs. low-fat in trials with and without a weight loss goal, which showed no difference in the random effects analysis.
Results from the Cochrane risk of bias assessment tool (Appendix pages 10–12) were variable and evaluation was limited for many studies by a lack of reporting. Incomplete outcome data was a high potential source of bias for 39 trials due to dropout and lost-to-follow-up rates exceeding 5%. Differential intervention intensity was deemed a source of bias for 20 trials. Both the Begg and Egger’s tests for small-study effects did not indicate publication bias (p=0.83 and p=0.85, respectively). Visual inspection of the funnel plot demonstrated an approximately symmetrical distribution of the inverse variances, which is consistent with these findings (Appendix page 13).
Discussion
Results from this comprehensive meta-analysis of RCTs with at least 1 year of follow-up indicate low-fat dietary interventions do not lead to greater weight loss when compared with higher fat dietary interventions of similar intensity, regardless of the weight loss intention of the trial. In fact, in the setting of weight loss trials, higher fat, low-carbohydrate dietary interventions led to a modest but significant greater long-term weight loss than low-fat interventions. Other higher fat dietary interventions led to similar weight loss as the low-fat groups, whether the trial had a weight loss goal or not. Low-fat interventions were favored only in comparison with interventions of lesser intensity, particularly those in which controls were only asked to maintain their usual diet. Furthermore, trials achieving greater differences in dietary fat intake and serum triglyceride concentrations resulted in greater weight loss under the higher fat interventions. Although these are not perfect measures of dietary fat intake, given the potential for measurement error in self-reported diet and confounding by weight loss for triglycerides as a marker of fat intake, results were consistent between these two methods.
This systematic literature review and meta-analysis highlights several important points. First, of the 53 eligible RCTs, 19 included higher fat comparator groups which maintained their usual intake, while the low-fat groups underwent interventions with more frequent and/or more intense interaction with research staff. Such comparisons do not provide evidence to support the effect of the low-fat diets themselves, since the effect of lowering total fat intake cannot be distinguished from the other components of the intervention. Stratifying by this type of comparator group (Figure 2), it is clear that lowering fat intake was not an independent contributor to weight loss. Second, despite concerted efforts among motivated clinical trial participants and staff, the average weight loss in all groups after a median 1 year of follow-up was a modest 2.7 kg, and 3.8 kg when calculated among weight loss trials only.
Our findings contrast with the findings of a previous systematic review and meta-analysis, which concluded that reduction in total fat intake leads to clinically meaningful weight loss, reporting 1.57 kg (95% CI=1.97 to 1.16) greater weight loss for low-fat vs. other diet interventions.(33) The main differences in their study selection criteria from ours were their inclusion of trials with <1 year of follow-up and their deliberate exclusion of trials with any weight loss intention. Trials of short duration (e.g. 6 months) are unlikely to demonstrate effects representative of long-term effects of diet on weight. Additionally, evaluating low-fat diets for weight loss exclusively among trials without a weight loss goal excluded a substantial proportion of the available literature, giving a pooled estimate that was over-weighted by trials comparing low-fat with “usual diet”, as well as trials conducted among populations at high risk for specific non-body weight related endpoints of interest (e.g., cholesterol-lowering, breast cancer prevention, etc). In our current meta-analysis among trials without a weight loss goal and at least 1 year duration, we found that after removing comparisons between low-fat and “usual diet”, low-fat interventions did not lead to greater weight loss that higher fat interventions (n=7; WMD=0.26 kg, 95% CI=-0.39 to 0.91). In fact, of the 33 trials included in their overall analysis, only 8 comparisons were conducted among trials giving similar attention to the low-fat and comparator treatment arms, and only 1 of these lasted at least 1 year. Furthermore, only 3 were among healthy participants. Therefore, generalizability of their findings to overall populations intending to lose weight is highly questionable, and their estimated effects of reducing fat intake are likely to be seriously confounded by differences in comparator group intensity, which was demonstrated to be a major source of heterogeneity in our analysis.
Johnston, et al, conducted a network meta-analysis among trials comparing named popular diet programs.(34) Pooling both direct (i.e., head-to-head comparison of two interventions within a single RCT) and indirect comparison (i.e., non-randomized comparisons of two intervention effects derived from separate trials) produced estimates similar to ours, indicating significant weight loss at 12 months for low-fat interventions compared with “usual diet”, and no significant benefit when compared with other dietary interventions of similar intensity. Limitations of indirect comparisons, however, include the inability to control for between-study and between-participant differences that may confound the pooled estimates. Another recent meta-analysis evaluated 13 trials of low-fat vs. very low-carbohydrate diet interventions with at least 12 months of follow-up.(35) Their pooled estimate indicated a 0.91 kg (95% CI=1.65 to 0.17) greater weight loss for very low-carbohydrate compared with low-fat diet interventions, consistent with our pooled estimate of 1.15 kg for low-carbohydrate vs. low-fat weight loss interventions.
A limitation of this meta-analysis is the substantial heterogeneity within several strata, indicating inconsistent effects across studies. Heterogeneity to some degree would be expected given the various intervention designs, baseline characteristics of the participants, and comparator diets. Stratified analyses reduced heterogeneity in many cases. Additionally, our manuscript did not have a pre-published protocol, and our search was limited to English language publications, did not include other potential databases, or a search of grey literature, which may have missed trials. Finally, the majority of RCTs of ≥1 year duration were not feeding trials, since large-scale long-term trials of this nature can be costly; therefore, our analysis addresses the effectiveness of dietary interventions, and not necessarily the diets themselves.
The strength of evidence of the literature included in this systematic review is variable with a high concern for attrition bias from significant drop-out and loss-to-follow-up rates in the majority of trials. Retaining participants for long-term lifestyle interventions can be difficult and bias is a concern when attrition is related to intervention assignment. Other bias measures were difficult to assess as a whole, without details of methods for randomization and allocation concealment, and whether staff members measuring outcomes were blinded.
Findings from our systematic literature review and meta-analysis of RCTs fail to support the efficacy of low-fat diet interventions over higher fat diet interventions of similar intensity for significant long-term clinically meaningful weight control. Previous trials comparing low-fat diet interventions with “usual diet” or minimal intensity control groups have mislead perceptions of the efficacy of reductions in fat intake as a strategy for long-term weight loss. In fact, comparisons of similar intervention intensity conclude that dietary interventions lower in total fat intake lead to significantly less weight loss compared with higher fat, low-carbohydrate diets. Health and nutrition guidelines should cease recommending low-fat diets for weight loss given the clear lack of long-term efficacy over other similar intensity dietary interventions. Additional research is needed to identify optimal intervention strategies for long-term weight loss and weight maintenance, including the need to look beyond variations in macronutrient composition.
Supplementary Material
Acknowledgements
This study was supported by grants from the National Institutes of Health (DK082730, HL34594, HL60712, CA176726, DK58845, DK46200, DK103720, and CA155626). Dr. Tobias was supported by a fellowship from the American Diabetes Association (7-12-MN-34). The funding sources did not participate in the design or conduct of the study; collection, management, analysis or interpretation of the data; preparation, review, or approval of the manuscript.
Dr. Ludwig received royalties for books on nutrition and obesity. Dr. Hu has received research support from California Walnut Commission and Metagenics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest statement
Drs. Tobias, Chen, Manson, and Willett have no disclosures.
Author contributions
Dr. Tobias developed the study protocol, conducted the literature search, data extraction, analysis, and interpretation, and draft manuscript. Dr. Chen conducted the literature search, and data extraction. Drs. Manson, Ludwig, Willett, and Hu contributed to study protocol and key data interpretation and manuscript review.
References
- 1.Higgins JPTAD, JAC Sterne, editors. Assessing risk of bias in included studies. 2011. Cochrane Handbook for Systematic Reviews of Interventions Version 510 [Internet] 2011 Available from www.cochrane-handbook.org.: The Cochrane Collaboration.
- 2.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willett W, Stampfer M, Chu NF, Spiegelman D, Holmes M, Rimm E. Assessment of questionnaire validity for measuring total fat intake using plasma lipid levels as criteria. Am J Epidemiol. 2001;154(12):1107–1112. doi: 10.1093/aje/154.12.1107. [DOI] [PubMed] [Google Scholar]
- 4.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 5.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295(6):676–680. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 7.Hebert JR, Ebbeling CB, Olendzki BC, et al. Change in women’s diet and body mass following intensive intervention for early-stage breast cancer. J Am Diet Assoc. 2001;101(4):421–431. doi: 10.1016/S0002-8223(01)00109-2. [DOI] [PubMed] [Google Scholar]
- 8.Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: Interim efficacy results from the women’s intervention nutrition study. Journal of the National Cancer Institute. 2006;98(24):1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 9.Pierce JP, Natarajan L, Caan BJ, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298(3):289–298. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chlebowski RT, Blackburn GL, Buzzard IM, et al. Adherence to a dietary fat intake reduction program in postmenopausal women receiving therapy for early breast cancer. The Women’s Intervention Nutrition Study. J Clin Oncol. 1993;11(11):2072–2080. doi: 10.1200/JCO.1993.11.11.2072. [DOI] [PubMed] [Google Scholar]
- 11.Anderson JW, Garrity TF, Wood CL, Whitis SE, Smith BM, Oeltgen PR. Prospective, randomized, controlled comparison of the effects of low-fat and low-fat plus high-fiber diets on serum lipid concentrations. Am J Clin Nutr. 1992;56(5):887–894. doi: 10.1093/ajcn/56.5.887. [DOI] [PubMed] [Google Scholar]
- 12.Stefanick ML, Mackey S, Sheehan M, Ellsworth N, Haskell WL, Wood PD. Effects of diet and exercise in men and postmenopausal women with low levels of HDL cholesterol and high levels of LDL cholesterol. N Engl J Med. 1998;339(1):12–20. doi: 10.1056/NEJM199807023390103. [DOI] [PubMed] [Google Scholar]
- 13.Knopp RH, Walden CE, Retzlaff BM, et al. Long-term cholesterol-lowering effects of 4 fat-restricted diets in hypercholesterolemic, combined hyperlipidemic men. The Dietary Alternatives Study. JAMA. 1997;278(18):1509–1515. [PubMed] [Google Scholar]
- 14.Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr. 2009;89(5):1588S–1596S. doi: 10.3945/ajcn.2009.26736H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brehm BJ, Lattin BL, Summer SS, et al. One-year comparison of a high-monounsaturated fat diet with a high-carbohydrate diet in type 2 diabetes. Diabetes Care. 2009;32(2):215–220. doi: 10.2337/dc08-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis NJ, Tomuta N, Schechter C, et al. Comparative study of the effects of a 1-year dietary intervention of a low-carbohydrate diet versus a low-fat diet on weight and glycemic control in type 2 diabetes. Diabetes Care. 2009;32(7):1147–1152. doi: 10.2337/dc08-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359(3):229–241. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 18.Elhayany A, Lustman A, Abel R, Attal-Singer J, Vinker S. A low carbohydrate Mediterranean diet improves cardiovascular risk factors and diabetes control among overweight patients with type 2 diabetes mellitus: a 1-year prospective randomized intervention study. Diabetes Obes Metab. 2010;12(3):204–209. doi: 10.1111/j.1463-1326.2009.01151.x. [DOI] [PubMed] [Google Scholar]
- 19.Esposito K, Maiorino MI, Ciotola M, et al. Effects of a Mediterranean-style diet on the need for antihyperglycemic drug therapy in patients with newly diagnosed type 2 diabetes: a randomized trial. Ann Intern Med. 2009;151(5):306–314. doi: 10.7326/0003-4819-151-5-200909010-00004. [DOI] [PubMed] [Google Scholar]
- 20.Guldbrand H, Dizdar B, Bunjaku B, et al. In type 2 diabetes, randomisation to advice to follow a low-carbohydrate diet transiently improves glycaemic control compared with advice to follow a low-fat diet producing a similar weight loss. Diabetologia. 2012;55(8):2118–2127. doi: 10.1007/s00125-012-2567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iqbal N, Vetter ML, Moore RH, et al. Effects of a low-intensity intervention that prescribed a low-carbohydrate vs. a low-fat diet in obese, diabetic participants. Obesity (Silver Spring) 2010;18(9):1733–1738. doi: 10.1038/oby.2009.460. [DOI] [PubMed] [Google Scholar]
- 22.Tapsell LC, Hokman A, Sebastiao A, et al. The impact of usual dietary patterns, selection of significant foods and cuisine choices on changing dietary fat under ‘free living’ conditions. Asia Pac J Clin Nutr. 2004;13(1):86–91. [PubMed] [Google Scholar]
- 23.Simon MS, Heilbrun LK, Boomer A, et al. A randomized trial of a low-fat dietary intervention in women at high risk for breast cancer. Nutr Cancer. 1997;27(2):136–142. doi: 10.1080/01635589709514515. [DOI] [PubMed] [Google Scholar]
- 24.Pilkington TR, Stafford JL, Hankin VS, Simmonds FM, Koerselman HB. Practical Diets for Lowering Serum Lipids. British medical journal. 1960;1(5165):23–25. doi: 10.1136/bmj.1.5165.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schatzkin A, Lanza E, Corle D, et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. Polyp Prevention Trial Study Group. N Engl J Med. 2000;342(16):1149–1155. doi: 10.1056/NEJM200004203421601. [DOI] [PubMed] [Google Scholar]
- 26.Babio N, Toledo E, Estruch R, et al. Mediterranean diets and metabolic syndrome status in the PREDIMED randomized trial. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2014;186(17):E649–E657. doi: 10.1503/cmaj.140764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henderson MM, Kushi LH, Thompson DJ, et al. Feasibility of a randomized trial of a low-fat diet for the prevention of breast cancer: dietary compliance in the Women’s Health Trial Vanguard Study. Prev Med. 1990;19(2):115–133. doi: 10.1016/0091-7435(90)90014-b. [DOI] [PubMed] [Google Scholar]
- 28.Swinburn BA, Metcalf PA, Ley SJ. Long-term (5-year) effects of a reduced-fat diet intervention in individuals with glucose intolerance. Diabetes Care. 2001;24(4):619–624. doi: 10.2337/diacare.24.4.619. [DOI] [PubMed] [Google Scholar]
- 29.Boyd NF, Cousins M, Beaton M, Kriukov V, Lockwood G, Tritchler D. Quantitative changes in dietary fat intake and serum cholesterol in women: results from a randomized, controlled trial. Am J Clin Nutr. 1990;52(3):470–476. doi: 10.1093/ajcn/52.3.470. [DOI] [PubMed] [Google Scholar]
- 30.Martin LJ, Li Q, Melnichouk O, et al. A randomized trial of dietary intervention for breast cancer prevention. Cancer Res. 2011;71(1):123–133. doi: 10.1158/0008-5472.CAN-10-1436. [DOI] [PubMed] [Google Scholar]
- 31.Djuric Z, Poore KM, Depper JB, et al. Methods to increase fruit and vegetable intake with and without a decrease in fat intake: compliance and effects on body weight in the nutrition and breast health study. Nutr Cancer. 2002;43(2):141–151. doi: 10.1207/S15327914NC432_4. [DOI] [PubMed] [Google Scholar]
- 32.Howard BV, Manson JE, Stefanick ML, et al. Low-fat dietary pattern and weight change over 7 years: the Women’s Health Initiative Dietary Modification Trial. JAMA. 2006;295(1):39–49. doi: 10.1001/jama.295.1.39. [DOI] [PubMed] [Google Scholar]
- 33.Hooper L, Abdelhamid A, Moore HJ, Douthwaite W, Skeaff CM, Summerbell CD. Effect of reducing total fat intake on body weight: systematic review and meta-analysis of randomised controlled trials and cohort studies. BMJ. 2012;345:e7666. doi: 10.1136/bmj.e7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnston BC, Kanters S, Bandayrel K, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA. 2014;312(9):923–933. doi: 10.1001/jama.2014.10397. [DOI] [PubMed] [Google Scholar]
- 35.Bueno NB, de Melo IS, de Oliveira SL, da Rocha Ataide T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110(7):1178–1187. doi: 10.1017/S0007114513000548. [DOI] [PubMed] [Google Scholar]
- 36.Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297(9):969–977. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 37.Bazzano LA, Hu T, Reynolds K, et al. Effects of low-carbohydrate and low-fat diets: a randomized trial. Ann Intern Med. 2014;161(5):309–318. doi: 10.7326/M14-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertz F, Brekke HK, Ellegard L, Rasmussen KM, Wennergren M, Winkvist A. Diet and exercise weight-loss trial in lactating overweight and obese women. Am J Clin Nutr. 2012;96(4):698–705. doi: 10.3945/ajcn.112.040196. [DOI] [PubMed] [Google Scholar]
- 39.Brinkworth GD, Noakes M, Buckley JD, Keogh JB, Clifton PM. Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. Am J Clin Nutr. 2009;90(1):23–32. doi: 10.3945/ajcn.2008.27326. [DOI] [PubMed] [Google Scholar]
- 40.Tay J, Brinkworth GD, Noakes M, Keogh J, Clifton PM. Metabolic effects of weight loss on a very-low-carbohydrate diet compared with an isocaloric high-carbohydrate diet in abdominally obese subjects. J Am Coll Cardiol. 2008;51(1):59–67. doi: 10.1016/j.jacc.2007.08.050. [DOI] [PubMed] [Google Scholar]
- 41.Das SK, Gilhooly CH, Golden JK, et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr. 2007;85(4):1023–1030. doi: 10.1093/ajcn/85.4.1023. [DOI] [PubMed] [Google Scholar]
- 42.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293(1):43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
- 43.Ebbeling CB, Leidig MM, Feldman HA, Lovesky MM, Ludwig DS. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA. 2007;297(19):2092–2102. doi: 10.1001/jama.297.19.2092. [DOI] [PubMed] [Google Scholar]
- 44.Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348(21):2082–2090. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 45.Foster GD, Wyatt HR, Hill JO, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med. 2010;153(3):147–157. doi: 10.1059/0003-4819-153-3-201008030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harvey-Berino J. Calorie restriction is more effective for obesity treatment than dietary fat restriction. Ann Behav Med. 1999;21(1):35–39. doi: 10.1007/BF02895031. [DOI] [PubMed] [Google Scholar]
- 47.Keogh JB, Brinkworth GD, Clifton PM. Effects of weight loss on a low-carbohydrate diet on flow-mediated dilatation, adhesion molecules and adiponectin. British Journal of Nutrition. 2007;98(4):852–859. doi: 10.1017/S0007114507747815. [DOI] [PubMed] [Google Scholar]
- 48.Klemsdal TO, Holme I, Nerland H, Pedersen TR, Tonstad S. Effects of a low glycemic load diet versus a low-fat diet in subjects with and without the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2010;20(3):195–201. doi: 10.1016/j.numecd.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 49.Kristal AR, Blount PL, Schenk JM, et al. Low-fat, high fruit and vegetable diets and weight loss do not affect biomarkers of cellular proliferation in Barrett esophagus. Cancer Epidemiol Biomarkers Prev. 2005;14(10):2377–2383. doi: 10.1158/1055-9965.EPI-05-0158. [DOI] [PubMed] [Google Scholar]
- 50.Lapointe A, Weisnagel SJ, Provencher V, et al. Comparison of a dietary intervention promoting high intakes of fruits and vegetables with a low-fat approach: long-term effects on dietary intakes, eating behaviours and body weight in postmenopausal women. Br J Nutr. 2010;104(7):1080–1090. doi: 10.1017/S0007114510001716. [DOI] [PubMed] [Google Scholar]
- 51.Lim SS, Noakes M, Keogh JB, Clifton PM. Long-term effects of a low carbohydrate, low fat or high unsaturated fat diet compared to a no-intervention control. Nutr Metab Cardiovasc Dis. 2010;20(8):599–607. doi: 10.1016/j.numecd.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 52.McAuley KA, Smith KJ, Taylor RW, McLay RT, Williams SM, Mann JI. Long-term effects of popular dietary approaches on weight loss and features of insulin resistance. Int J Obes (Lond) 2006;30(2):342–349. doi: 10.1038/sj.ijo.0803075. [DOI] [PubMed] [Google Scholar]
- 53.McManus K, Antinoro L, Sacks F. A randomized controlled trial of a moderate-fat, low-energy diet compared with a low fat, low-energy diet for weight loss in overweight adults. Int J Obes Relat Metab Disord. 2001;25(10):1503–1511. doi: 10.1038/sj.ijo.0801796. [DOI] [PubMed] [Google Scholar]
- 54.Foster-Schubert KE, Alfano CM, Duggan CR, et al. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring) 2012;20(8):1628–1638. doi: 10.1038/oby.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sacks FM, Bray GA, Carey VJ, et al. The new England journal of medicine: Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. New England Journal of Medicine. 2009;360(9):859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elmer PJ, Obarzanek E, Vollmer WM, et al. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18-month results of a randomized trial. Ann Intern Med. 2006;144(7):485–495. doi: 10.7326/0003-4819-144-7-200604040-00007. [DOI] [PubMed] [Google Scholar]
- 57.Shah M, Baxter JE, McGovern PG, Garg A. Nutrient and food intake in obese women on a low-fat or low-calorie diet. Am J Health Promot. 1996;10(3):179–182. doi: 10.4278/0890-1171-10.3.179. [DOI] [PubMed] [Google Scholar]
- 58.Frisch S, Zittermann A, Berthold HK, et al. A randomized controlled trial on the efficacy of carbohydrate-reduced or fat-reduced diets in patients attending a telemedically guided weight loss program. Cardiovasc Diabetol. 2009;8:36. doi: 10.1186/1475-2840-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stern L, Iqbal N, Seshadri P, et al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Ann Intern Med. 2004;140(10):778–785. doi: 10.7326/0003-4819-140-10-200405180-00007. [DOI] [PubMed] [Google Scholar]
- 60.Samaha FF, Iqbal N, Seshadri P, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348(21):2074–2081. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]
- 61.Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi F. Better dietary adherence and weight maintenance achieved by a long-term moderate-fat diet. Br J Nutr. 2007;97(2):399–404. doi: 10.1017/S0007114507328602. [DOI] [PubMed] [Google Scholar]
- 62.Turner-McGrievy GM, Barnard ND, Scialli AR. A two-year randomized weight loss trial comparing a vegan diet to a more moderate low-fat diet. Obesity (Silver Spring) 2007;15(9):2276–2281. doi: 10.1038/oby.2007.270. [DOI] [PubMed] [Google Scholar]
- 63.Viegener BJ, Perri MG, Nezu AM, Renjilian DA, McKelvey WF, Schein RL. Effects of an intermittent, low-fat, low-calorie diet in the behavioral treatment of obesity. Behavior Therapy. 1990;21(4):499–509. [Google Scholar]
- 64.Wood PD, Stefanick ML, Williams PT, Haskell WL. The effects on plasma lipoproteins of a prudent weight-reducing diet, with or without exercise, in overweight men and women. N Engl J Med. 1991;325(7):461–466. doi: 10.1056/NEJM199108153250703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.