Abstract
Tissue turnover is a regular feature of higher eukaryotes, either as part of normal wear and tear (homeostasis) or in response to injury (regeneration). Cell replacement is achieved either through replication of existing cells or differentiation from a self-renewing pool of stem cells. The major distinction regards cellular potential, because stem cells by definition have a capacity to differentiate, while replication implies that cells adopt a single fate under physiologic conditions. A hybrid model, the facultative stem cell (FSC) model, posits that tissues contain cells that normally exhibit unipotency but have the capacity to function as stem cells upon injury. The FSC paradigm is well established in urodele amphibians, but the nature and role of FSCs in mammals is less defined. Here, we review the evidence for FSCs in two mammalian organs, the liver and the pancreas, and discuss alternative models that could account for regeneration in these organs.
Keywords: stem cells, facultative stem cells, liver, pancreas, regeneration
INTRODUCTION
fac·ul·ta·tive \□fa-kəl-□tā-tiv, British -tə-tiv\; a : taking place under some conditions but not under others <facultative diapause> b : exhibiting an indicated lifestyle under some environmental conditions but not under others <facultative anaerobes>.
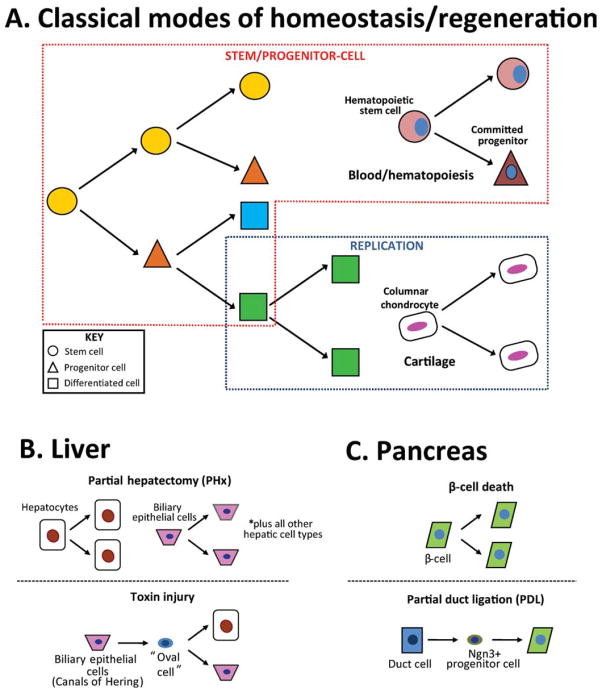
Stem cells are distinct from other mature cellular populations due to their unique ability to both self-renew (give rise to more stem cells) and differentiate into other cell types (Potten and Loeffler, 1990). The latter ability becomes more restricted as development progresses, resulting in a stem cell hierarchy based on the extent of potency (Slack, 2008). For instance, early on in development, cells from the inner cell mass of the blastocyst are considered to be pluripotent stem cells, because they are able to give rise to all cell lineages except for extraembryonic tissues. With the onset of organogenesis later in development, stem cell potential becomes restricted as commitment to distinctive tissue-specific lineages occurs (Eckfeldt et al., 2005; Slack, 2008). An example of this is the male germline, in which the potential of self-renewing spermatogonial stem cells is limited to spermatogonia for the lifetime of a male organism (de Rooij, 2001). Adult tissues have two mechanisms for replacing cells lost during routine cellular turnover. In some tissues, adult stem cells are the source of new cells throughout life, while other tissues are devoid of adult stem cells and maintain homeostasis through replication of existing cells. The skin, intestine, and blood are examples of tissues that continuously generate new cells from stem cells, while bone, kidney, and cartilage are examples of tissues in which stem cells play a limited, if any, role in normal organ homeostasis (Fig. 1A).
Fig. 1.
Schematic depiction of mechanisms used for maintaining homeostasis and regeneration in various adult mammalian tissues. A: Two classical mechanisms for tissue homeostasis/regeneration involve differentiation of a stem/progenitor population (red box) or proliferation of differentiated cells (blue box). Hematopoietic stem cells have an apparently unlimited self renewal capacity that enables them to continuously supply new blood cells, while cellular maintenance of the cartilage anlagen occurs by means of chondrocyte proliferation within the columnar region. B: Following partial hepatectomy (PHx; top panel), differentiated liver cells undergo replication to make-up for the surgical loss of mass. In a toxin injury (bottom panel), a new cell type known as an “oval cell” is proposed to arise from BECs and function as a bipotent facultative stem cell (FSC). C: The β-cell loss by surgery or targeted genetic ablation is replaced by replication of remaining β-cells (top panel). Following partial duct ligation (PDL), duct cells are proposed to adopt an Ngn3+ progenitor identity and give rise to new β-cells (bottom panel).
In contrast to normal tissue turnover, regeneration describes the process whereby new cells arise to replace those lost by injury. As with normal homeostasis, both stem cell-dependent and stem cell-independent mechanisms for regeneration are used by different tissues. However, under conditions of both homeostasis and injury, the relative balance between stem cell-dependent and -independent mechanisms of recovery has not been quantified. Thus, for most tissues, the relative degree to which stem cells contribute to tissue maintenance and regeneration remains undefined.
The nature of the injury may also play a role in determining the recovery mechanism used by a given tissue. It has been postulated that following particular types of injury, a subset of differentiated cells can, in certain tissues, adopt a “stem cell-like” state (Zipori, 2004). These cells have been termed facultative stem cells (FSCs) due to their ability to acquire multipotent qualities during conditions other than homeostasis, despite being initially unipotent. Such a potential blurs the stem cell-progeny paradigm that has been used by developmental biologists for decades. Thus, the biology of FSCs has relevance not only for tissue regeneration but could also serve to greatly inform our understanding of the multipotent or pluripotent state. Despite the potential importance of FSCs, the evidence supporting their existence remains largely circumstantial.
Historically, three major assays have been used to document stem cell activity: clonogenic (in vitro) growth, cellular transplantation, and lineage tracing (Slack, 2006). Each technique has both advantages and limitations. For example, clonogenic growth can provide evidence of self-renewal and multi-lineage differentiation. Moreover, as an in vitro culture system, clonogenic growth can be technically straightforward. However, such assays do not necessarily indicate “stemness” in vivo. Moreover, clonogenic growth assays assume that the progeny of the putative stem cell are “stable” in vitro. This latter point is critical, because the appearance of multiple cell types in a colony arising from a single cell is commonly taken as evidence of multi-potency, yet this interpretation would be incorrect if differentiated cells placed in culture have the capacity to interconvert or “transdifferentiate.” Likewise, cell transplantation assays have been tremendously important in the identification and study of stem cells, particularly hematopoietic stem cells. However, transplantation assays can also be subject to confounding phenomena. One of the most important of these confounders is cell fusion, which can occur with many different types of cells and which can give a false impression regarding potency (Wagers and Weissman, 2004).
The use of in vivo lineage tracing is a key technique for determining the origin of new cells. The most commonly used technique for lineage tracing in the mouse is Cre-Lox technology, which permits labeling to occur in a cell-type–specific manner. Additionally, a variant of the Cre recombinase fused to a mutated estrogen receptor (Cre-ERT2) allows temporal control, labeling cells at a desired time point during development or adulthood. Through such genetic labeling, a cell’s subsequent fate and that of its progeny can be followed, as genetic lineage labeling constitutes a heritable marking. Such labeling of putative stem cell populations allows for stringent testing of stem cell properties of self-renewal and pluripotency, and can provide insight into the cellular mechanisms of regeneration. However, Cre-Lox–based cell labeling uses the use of “tissue-specific” promoters to label cells, and thus the technique relies upon the specificity of such promoters. Hence, studies which use lineage labeling to determine whether stem cells contribute to homeostasis and/or regeneration are ultimately constrained by the specificity of the particular promoters and mouse strains used.
LIVER REGENERATION AND THE ORIGINS OF THE FSC MODEL
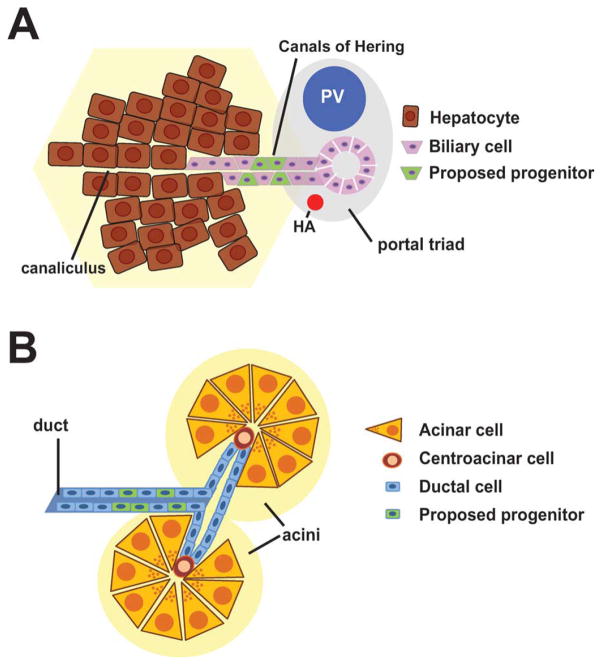
For several decades, the liver has stood the model organ for mammalian regenerative studies. The liver has multiple functions in normal physiology—including production of plasma proteins, synthesis of bile for fat emulcification, and detoxification—and liver failure is incompatible with life. The liver’s regenerative ability is apparent when, after removing two-thirds of its mass (partial hepatectomy, PHx), the remnant third is able to grow back to its original size and restore normal function (Higgins, 1931). The liver consists of several cellular components—two epithelial cell types (hepatocytes and biliary epithelial cells, or BECs) and several “non-parenchymal cells” (endothelial cells, macrophage-like “Kuppfer cells,” and fibroblasts)—organized into structures referred to as hepatic lobules. Hepatocytes comprise the vast majority of cells in the center of the lobule, while BEC-lined bile ducts are found in regions known as portal tracts at the periphery of the lobule. Bile made by hepatocytes drains into the bile ducts through tubular structures known as the “Canals of Hering,” which constitutes a transitional zone between hepatocytes and BECs (Fig. 2A).
Fig. 2.
Schematic view of the location of putative locations of FSCs in the liver and pancreas. A: Schematic depiction of a portal tract in the liver. Bile made by hepatocytes drains into the canalicular space and subsequently through the Canals of Hering into the bile duct. The precursors of oval cells in the liver have been proposed to reside within the Canals of Hering, the transitional zone between hepatocytes and biliary cells. HA, hepatic artery; PV, portal vein. B: Schematic depiction of two acinar units in the pancreas. Pancreatic cells of the ductal lineage (which include pancreatic centroacinar cells) have been proposed to adopt progenitor-like properties and give rise to β-cells.
The dominant cellular mechanism by which the liver regrows is proliferation (Fig. 1B, top panel; Michalopoulos, 2007). Studies using thymidine-H3 incorporation during various phases of PHx recovery in rodents have demonstrated that cell replication following partial hepatectomy follows reproducible kinetics. Hepatocytes constitute the first cell population to enter the cell cycle, followed by the biliary epithelial cells and then nonparenchymal cells. Serial transplantation studies have demonstrated an almost inexhaustible self-renewal capacity of hepatocytes, with calculations suggesting that a single hepatocyte is competent to give rise to many entire livers (Overturf et al., 1997).
Despite the robust regenerative capacity of hepatocytes, it is widely believed that an alternative mechanism for adult regeneration, involving FSCs, is used following certain types of liver injury (Fig. 1B, bottom panel). This hypothesis initially emerged from studies in which the rat liver was forced to regenerate following exposure to one of several hepatotoxic carcinogens. In rats, it is believed these cells emerge as a result of impaired hepatocyte replication, although in the mouse, counterparts of these cells still appear in injured livers despite the ability of hepatocytes to proliferate (Ghoshal et al., 1983; Wang et al., 2003a). Under such conditions, a distinctive histological picture was noted, characterized by the emergence of a heterogenous population of small oval-shaped cells with biliary properties (Farber, 1956). Subsequent work revealed that cells with a similar appearance are observed in many or most models of hepatocarcinogenesis or toxin-induced injury (Roskams et al., 2003; Lee et al., 2006). These cells have been referred to by many different names including “ductular hepatocytes,” “intermediate hepatobiliary cells,” “atypical ductular proliferation,” or more commonly as “oval cells,” a term adopted from rodent studies (Gerber et al., 1983; Factor et al., 1994; Preisegger et al., 1999; Zhou et al., 2007). They are characterized by small size, an ovoid nucleus, scant cytoplasm, poorly defined lumen, and lack of basement membranes (Gerber et al., 1983; Factor et al., 1994; Preisegger et al., 1999). Although debated, oval cells are proposed to emerge from the Canals of Hering (Fausto and Campbell, 2003; Dorrell and Grompe, 2005; Fig. 2A) and, after injury, are thought to interpose themselves between biliary ductules and so-called “intermediate hepatocytes,” cells which share characteristics of both hepatocytes and oval cells (Factor et al., 1994; Preisegger et al., 1999). This transitory morphology and proximity of the hepatocytes to oval cells has been taken as evidence of differentiation of the latter into the former, and static visualization by light microscopy is consistent with that view (Factor et al., 1994).
Several in vitro experiments performed in the 1980s demonstrated that cultured oval cells could adopt either hepatocyte of biliary features depending upon culture conditions, a phenomenon similar to that observed with culture of fetal rat hepatocytes that have bona fide multipotent properties (Germain et al., 1985, 1988a,b). Similar results were obtained by investigators who derived cell lines with “oval” cell properties (Tsao et al., 1984) and subsequently many such lines have been derived which are referred to as “liver epithelial cell lines” or BMEL cells (“bipotential mouse embryonic liver”; Strick-Marchand et al., 2004).
Nevertheless, there is limited lineage-based evidence to confirm such ontological conclusions about bipotentiality (Taub, 2004; Zaret and Grompe, 2008). One of the difficulties in doing lineage tracing experiments with oval cells has been the lack of oval cell-specific markers. Although several features that distinguish oval cells from normal biliary epithelial cells have been reported (e.g., Sirica et al., 1990), immunohistologic studies with most antibodies are unable to distinguish between oval cells and BECs (Fausto and Campbell, 2003). Importantly, oval cells represent a heterogeneous population that includes both epithelial-derived and bone marrow-derived cells that exhibit distinct cell surface profiles (Dorrell et al., 2008; Okabe et al., 2009). Recent efforts have sought to identify novel cell-surface antibodies that are oval cell specific (Dorrell et al., 2008), but these and other studies have often yielded antibodies that also recognize normal BECs (Yanger and Stanger, unpublished data). Thus, at least based on immunostaining data, there is no compelling evidence that oval cells represent a distinct cell type that is not present in the normal liver.
One lineage tracing study which supports the view that oval cells can exhibit bipotentiality used Fox1l-Cre mice to label putative progenitor cells (Sackett et al., 2009). Foxl1 is a mesenchymal marker, and mice in which the Foxl1 lineage was labeled (using a lacZ lineage “reporter”) exhibited beta-galactosidase activity in both hepatocytes and biliary cells following injury. This result does not distinguish between the possibility that a single bipotential progenitor cell was labeled vs. the alternative explanation that hepatocytes and biliary cells both independently activated the Foxl1 promoter (leading to cell labeling) upon injury, and additional experiments will be needed to resolve these issues.
Despite the paucity of direct evidence of progenitor cell activity in vivo, several experiments do support the notion that oval cells represent a distinct population of cells not present in the normal liver. In particular, electron microscopy studies reveal that following injury with the hepatotoxin Dipin, a series of “transitional” cells—cells with ultrastructural features of both ductular cells and hepatocytes—were found (Factor et al., 1994). These cells were larger than oval cells but smaller than hepatocytes, had variable nucleocytoplasmic ratios, mitochondrial content, and glycogen rosettes (Factor et al., 1994). Thus, at the ultrastructural level, oval cells do seem to comprise a heterogenous population of cells, with some exhibiting intermediate features “between” hepatocytes and BECs.
Cell transplantation experiments provide another line of evidence in support of oval cell bipotentiality. Again, because of a paucity of markers, oval cell isolation for such studies have been forced to rely on cell fractionation techniques based on the smaller size of the oval cells (Fausto and Campbell, 2003). Cells isolated in this fashion, although contaminated with others cells such as hematopoietic cells, can rescue fumarylacetoacetate hydrolase mutant (FAH−/−) mice as effectively as hepatocytes, indicating that this population has robust hepatocyte differentiation–reconstitution capacity (Wang et al., 2003a). This study, however, does not specifically address whether new biliary cells emerged from these transplanted cells. Lineage tracing studies also give support to the notion that nonhepatocytes can give rise to hepatocytes during toxin-mediated injury (Pichard et al., 2009).
During the late 1990s, it was proposed that bone-marrow (BM) derived cells might serve as facultative stem cells for liver regeneration and might even give rise to oval cells (Petersen et al., 1999). However, subsequent studies showed that this phenomenon was not due to transdifferentiation but cell fusion (Alvarez-Dolado et al., 2003; Wang et al., 2003b). These cell fusion events occur with low frequency, but in the appropriate selective environment, hepatocytes generated by a fusion event are capable of significant expansion through simple replication. Thus, while there may be a small degree of cell-fusion occurring with bone marrow cells, the frequency and extent of this phenomenon is considered to be of nonphysiological significance (Wang et al., 2003a; Oertel and Shafritz, 2008).
In summary, the liver is believed to have two mechanisms for regeneration depending upon the mechanism of injury (Fig. 1B). Following partial extirpation (PHx), remaining hepatocytes re-enter the cell cycle, undergoing one or two rounds of cell division resulting in a complete recovery of liver size. This mechanism for regrowth is well established, and several of the signaling pathways that mediate this growth response have been identified (although the identity of the size “sensor” that informs the liver that regrowth is needed remains elusive). By contrast, injury with hepatotoxins results in the emergence of numerous small cells—most commonly referred to as oval cells—that arise in the portal tracts and make their way into the lobules. Cell transplantation studies and in vitro differentiation experiments suggest that these cells exhibit multipotency under these experimental conditions, but definitive evidence that these cells act as multipotent progenitor cells remains deficient. Likewise, although these cells are commonly believed to arise from biliary cells within the Canals of Hering, their precise origins also remain uncertain.
FACULTATIVE STEM CELLS IN THE PANCREAS
The mammalian pancreas is responsible for coordinating the organism’s response to food intake and can be divided into two compartments: exocrine and endocrine. The exocrine compartment (which is composed of acinar cells and duct cells) produces digestive enzymes that cleave peptides, lipids, and large carbohydrates, which are secreted in a “pro-form” into the intestine by means of the ductal system. The endocrine compartment is composed of several different hormone-producing cells, the most important of which are the insulin-producing beta cells and is contained within clusters of cells known as the islets of Langerhans. The cellular basis of normal tissue homeostasis has not been carefully examined in the pancreas, but it is believed that—akin to the liver—replication of existing cells is able to compensate for the low rate of cell death that occurs as part of normal “wear and tear” on the pancreas throughout life.
During embryonic development, the pancreas arises from a patch of endoderm immediately adjacent to the liver through the differential action of adjacent signals from the mesenchyme (Zaret, 2008). Despite this close embryonic relationship, the liver and pancreas differ in their regenerative capacity. Specifically, the pancreas exhibits minimal regrowth following partial pancreatectomy (PPx), in contrast to the liver’s robust response following PHx. This difference is apparent both in the embryo (Stanger et al., 2007) and in the adult (Dor and Stanger, 2007). Nevertheless, several parallels between regeneration in the liver pancreas do exist.
One similarity is that the nature of the regenerative response depends upon the nature of the injury. Under physiologic conditions, pancreatic injury does not occur through en bloc loss of pancreatic tissue, as occurs with partial extirpation like PPx, but rather through direct (and specific) cellular injury. The most dramatic manifestation of pancreatic injury is acute pancreatitis, which is caused by direct injury to acinar cells or obstruction of the pancreatic duct leading to backflow of pancreatic juice and acinar cell death. This results in the auto-activation of digestive enzymes, leading to marked inflammation and death of pancreatic cells. Despite what can sometimes be a remarkably severe injury, however, the pancreas is typically able to recover from such an insult and return to normal histology and function. The cellular mechanism underlying this recovery is not known in detail, but a process of “de-differentiation” and “re-differentiation” that depends upon Notch signaling has been proposed (Jensen et al., 2005). Importantly, the possibility that acinar cell regeneration following pancreatitis occurs through a (facultative) stem/progenitor cell mechanism—potentially the pancreatic “centroacinar cell”—has not been ruled out (Rovira et al., 2010).
Similarly, the endocrine compartment has a rather profound capacity for regrowth following the loss of beta cells. Using a pulse-chase lineage labeling approach, Dor et al. (2004) showed that the beta cells which replace those lost during PPx are largely derived from the replication of remaining beta cells (a similar mechanism accounts for the expansion of beta cell mass that occurs during growth from newborn to adult). Similarly, targeted ablation of beta cells through the expression of “toxic genes” leads to the recovery of beta cell mass through the replication of existing cells (Nir et al., 2007; Cano et al., 2008). Furthermore, even within the islet compartment, there is no evidence that beta cells differ dramatically with respect to their replicative potential (Teta et al., 2007). Thus, simple replication seems to be the major mechanism for regeneration of the beta cell compartment after injury (Fig. 1C, top panel). Nevertheless, it appears that under different injury conditions new beta cells can come from other sources (Fig. 1C, bottom panel). For example, under conditions of “extreme” beta cell loss, alpha cells undergo a process of reprogramming (transdifferentiation) to become beta cells (Thorel et al., 2010).
Studies initiated in the late 1990s raised the possibility that FSCs residing in the pancreatic ducts could serve as an alternative source for new beta cells (Fig. 2B). Among these were studies showing that ductal adenocarcinomas could arise from oncogene misexpression in islet cells (Yoshida and Hanahan, 1994). Molecular evidence supporting the idea that duct cells might give rise to beta cells came from the finding that following PPx pancreatic duct cells experienced a burst of proliferative activity that was accompanied by up-regulation of the transcription factor Pdx1 (Bonner-Weir et al., 1993; Sharma et al., 1999). Pdx1 is expressed in both pancreatic progenitor cells and mature beta cells, but it is expressed at only low levels in pancreatic ducts. Hence, the induction of Pdx1 was taken as a sign that cells within the ductal compartment might adopt a progenitor-like identity during injury. In parallel, numerous reports appeared in the literature consistent with this hypothesis (Ramiya et al., 2000; Heremans et al., 2002; Gasa et al., 2004; Hao et al., 2006; Yatoh et al., 2007). In addition, “pancreatic stem cells,” which exhibited the ability to give rise to all pancreatic lineages, were described (Seaberg et al., 2004). Together, these reports of plasticity and progenitor activity (albeit largely in vitro) gave momentum to the notion that the pancreas harbors FSCs that could become activated upon injury and reconstitute damaged tissue.
In humans, pancreatic injury most commonly occurs following blockage of the main pancreatic duct, often as a result of an obstructing gallstone, leading to diffuse pancreatitis. Injury is accompanied in many cases by beta cell dysfunction and hyperglycemia. Of note, elevated blood glucose constitutes one of “Ranson’s Criteria,” a classic measure of mortality risk from acute pancreatitis. In experimental models, ligation of the main pancreatic duct (pancreatic duct ligation; PDL) has long been known to result in islet hyperplasia (Rosenberg et al., 1983) and therefore Xu et al. (2008) sought to determine the source of new beta cells following this specific (and “physiologic”) injury. Following PDL, Xu et al. found that a subset of ductal cells began to express Neurogenin3 (Ngn3), a pro-endocrine transcription factor whose expression decreases significantly following embryonic islet development (when new beta cells most clearly arise from progenitor cells). These Ngn3-expressing cells closely resembled embryonic endocrine progenitor cells and, when transplanted into a permissive environment, were capable of differentiating into beta cells. Although Xu et al. did not use lineage tracing to prospectively label the cells that would become beta cells, a study published later in the same year provides additional support for a lineage relationship between ductal cells and beta cells (Inada et al., 2008). In the latter study, a transgenic strain of mice expressing a tamoxifen-inducible form of the Cre recombinase (CreERT2) was used to specifically label pancreatic ductal cells. When mice labeled in this manner were subjected to PDL, the lineage label appeared in nearly half of the islets (as opposed to less than 15% of islets in the absence of PDL). Similar studies using other markers for ductal cells, however, failed to show this exocrine to endocrine trans-differentiation relationship (Solar et al., 2009). Thus, the ability of ductal cells to adopt a progenitor phenotype remains controversial.
Taken together, these results raise the possibility that the progeny of cells from the ductal compartment can become beta cells, at least in the context of an obstructing injury. Although these results await replication, these studies represent prima facie evidence that pancreatic ductal cells are FSCs, because ductal cells do not normally give rise to beta cells. If confirmed, these findings raise several important questions: Do all cells within the ductal compartment have the capacity to serve as FSCs, or is this property limited to a subset of ductal cells? What path do the cells follow en route to becoming beta cells? Do they directly transition into endocrine progenitor cells, or do they follow a more circuitous route? And finally, what molecular mechanisms underlie these transitions?
PERSPECTIVE AND FUTURE DIRECTIONS
The notion that a differentiated cell can be—under certain circumstances—recruited to function as a stem cell is related to the larger concept of cellular plasticity. In many organisms, terminally differentiated cells are able to undergo a process of dedifferentiation to reconstitute the stem cell state. In the Drosophila male germline, for example, cells that have undergone the first step in spermatogonial differentiation (to become “transient amplifying” cells) can reverse direction and take on a stem cell identity (Brawley and Matunis, 2004). In urodele amphibians (newts and salamanders), stem cell reversion is even more dramatic. These organisms are able to regenerate a complete limb following amputation at any site along the proximodistal axis, a type of regrowth known as “epimorphic regeneration.” During epimorphic regeneration, differentiated mesenchymal cells at the wound site become incorporated into a structure known as the “wound blastema.” Cells within the blastema are de-differentiated and function as mesenchymal stem cells that are capable of reconstituting the limb (Brockes and Kumar, 2005). However, even this de-differentiation is associated with heterogeneity, as many of the de-differentiated cells manage to maintain features of their original identity and position within the regenerating limb (Kragl et al., 2009).
In mammals, terminally differentiated cells can be converted into pluri-potent cells through the action of defined factors (Takahashi and Yamanaka, 2006), but under physiologic conditions such reversions do not occur readily. The best example of mammalian epimorphic regeneration is the successive renewal of deer antlers. Following the shedding of old antlers, new antlers regenerate from body pedicles in the skull, a structure that is reminiscent of the wound blastema in amphibians. However, cellular and molecular analyses have revealed that antler regeneration occurs by a completely distinct mechanism (Li et al., 2009). Rather than relying on a process of de-differentiation, antler renewal is driven by stem cells that remain present within the pedicles.
Thus, the FSC hypothesis is of particular interest because it might constitute a bridge between mammalian and amphibian modes of regeneration. However, there remains considerable controversy regarding the origins, potential, and even existence of FSCs in the liver and pancreas. The FSC model originated from static morphologic/histologic studies in the liver before many of the concepts and tools of mammalian stem cell biology were developed and thus has not been rigorously challenged in that system. In the pancreas, several features of the ductal FSC model remain unproven, including the direct demonstration (particularly in a clonal manner) of bipotentiality of the putative progenitor cells. In both systems, the relative contribution of FSCs to tissue repair—whether dedifferentiation–redifferentiation is rare or commonplace compared with replication of existing cells—is poorly characterized.
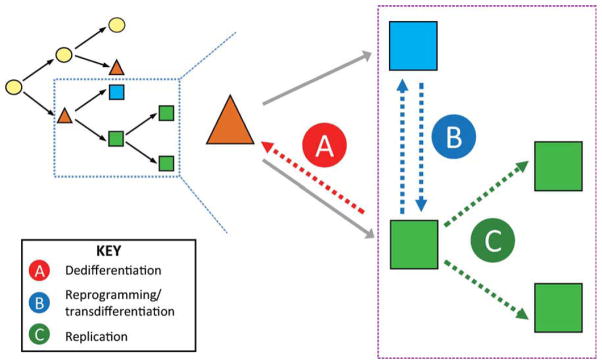
In addition, there are alternative explanations for the reported changes in cell behavior following injury to the liver or pancreas (Fig. 3). For example, cellular plasticity allowing for the interconversion of differentiated cell types (transdifferentiation or cellular “reprogramming”) could mimic an FSC mechanism of repair. Indeed, such a mechanism would be hard to distinguish from stem cell-mediated regeneration, because the same lineage output would occur in either scenario (see Fig. 3A,B), unless clonal analysis or careful lineage tracing were used to distinguish between the two. Support for this model comes from limited evidence that hepatocytes can differentiate into biliary cells in vivo (Michalopoulos et al., 2005) and the finding that liver cells can be converted into pancreatic cells under experimental conditions (Horb et al., 2003).
Fig. 3.
Schematic of alternative cellular mechanisms of regeneration. As opposed to a unidirectional hierarchy resulting in mature cells through stem-cell differentiation or replication (black arrows), other mechanisms could account for tissue restoration (blue box expanded). These putative mechanisms include both the FSC model and other alternatives. A: A mature, differentiated cell (green square) could dedifferentiate and acquire a progenitor identity (orange triangle), thus functioning as a FSC. B: Mature cells could undergo reprogramming, which would allow them to interchange/transdifferentiate into other differentiated cells. Such a mechanism could be difficult to distinguish from the dedifferentiation–redifferentiation model in A unless the fate of the differentiated cells were followed with precision. C: Finally, simple replication of existing differentiated cells could account for restoration of tissue mass. In this scenario, putative FSCs could simply be “bystanders” and not formally contribute to the regenerated tissue.
A recent study by (Furuyama et al., 2010) highlights the difficulty inherent in resolving between transdifferentiation and stem-cell models for regeneration but also provides important insights into the nature of normal cellular homeostasis in the pancreas in liver. In this impressive piece of work, the authors specifically labeled the ductal compartment in the pancreas and liver (using a strain of mice in which CreER was expressed under the control of the duct specific Sox9-promoter). Following administration of tamoxifen to the animals, BECs were labeled with high efficiency and over time could be seen giving rise to hepatocytes in a portal-to-central distribution across the lobule. This indicates that at least a subset of BEC-like cells have the potential to give rise to hepatocytes, although whether this result represents a self-renewing stem cell population within the BEC compartment (Fig. 2A) or transdifferentiation from BECs to hepatocytes over time cannot be determined from these experiments. These findings also raise the question of whether oval cells, if they do in fact represent a bipotential precursor population, are truly facultative stem cells, or whether they represent an expansion of stem cells that normally reside in the liver. In the pancreas, labeled Sox9 ductal cells rapidly gave rise to acinar cells but never gave rise to endocrine cells. This result likewise does not distinguish between a stem/progenitor mechanism and transdifferentiation in homeostasis of the exocrine compartment, but it does suggest that ductal lineage cells do not give rise to endocrine cells under conditions of normal homeostasis. Additional lineage tracing experiments will be needed to more fully define cellular potential under injury conditions, but the reagents created by (Furuyama et al., 2010) are tools that will prove critical to determining the potential of cells in the ductal compartment of the pancreas and liver.
Finally, simple replication of existing cell types remains a viable possibility for normal tissue homeostasis and recovery from injury (Fig. 3C). Importantly, these distinct regenerative mechanisms are not mutually exclusive, and it remains possible that dedifferentiation, cellular reprogramming, and replication all contribute to the mammalian regenerative response. The challenge going forward is to devise tools and techniques to track and quantify these cellular events and—if multipotent FSCs truly do arise from differentiated cells in the liver and pancreas—to understand the signals and changes in chromatin that lead to the (re)acquisition of the stem cell state in vivo. Such an understanding may provide opportunities for greater control over cell fate in a manner that could augment recovery and regeneration following organ damage.
Acknowledgments
We apologize to the many investigators whose work we were unable to cite because of space constraints.
References
- Alvarez-Dolado M, Pardal R, Garcia-Ver-dugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE. A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes. 1993;42:1715–1720. doi: 10.2337/diab.42.12.1715. [DOI] [PubMed] [Google Scholar]
- Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science. 2004;304:1331–1334. doi: 10.1126/science.1097676. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Kumar A. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science. 2005;310:1919–1923. doi: 10.1126/science.1115200. [DOI] [PubMed] [Google Scholar]
- Cano DA, Rulifson IC, Heiser PW, Swigart LB, Pelengaris S, German M, Evan GI, Bluestone JA, Hebrok M. Regulated beta-cell regeneration in the adult mouse pancreas. Diabetes. 2008;57:958–966. doi: 10.2337/db07-0913. [DOI] [PubMed] [Google Scholar]
- de Rooij DG. Proliferation and differentiation of spermatogonial stem cells. Reproduction. 2001;121:347–354. doi: 10.1530/rep.0.1210347. [DOI] [PubMed] [Google Scholar]
- Dor Y, Stanger BZ. Regeneration in liver and pancreas: time to cut the umbilical cord? Sci STKE. 2007;2007:pe66. doi: 10.1126/stke.4142007pe66. [DOI] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- Dorrell C, Erker L, Lanxon-Cookson KM, Abraham SL, Victoroff T, Ro S, Canaday PS, Streeter PR, Grompe M. Surface markers for the murine oval cell response. Hepatology. 2008;48:1282–1291. doi: 10.1002/hep.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell C, Grompe M. Liver repair by intra- and extrahepatic progenitors. Stem Cell Rev. 2005;1:61–64. doi: 10.1385/SCR:1:1:061. [DOI] [PubMed] [Google Scholar]
- Eckfeldt CE, Mendenhall EM, Verfaillie CM. The molecular repertoire of the ‘almighty’ stem cell. Nat Rev Mol Cell Biol. 2005;6:726–737. doi: 10.1038/nrm1713. [DOI] [PubMed] [Google Scholar]
- Factor VM, Radaeva RS, Thorgeirsson SS. Origin and fate of oval cells in dipin-induced hepatocarcinogenesis in the mouse. Am J Pathol. 1994;145:409–422. [PMC free article] [PubMed] [Google Scholar]
- Farber E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3′-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956;16:142–148. [PubMed] [Google Scholar]
- Fausto N, Campbell JS. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev. 2003;120:117–130. doi: 10.1016/s0925-4773(02)00338-6. [DOI] [PubMed] [Google Scholar]
- Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, Masui T, Kawaguchi M, Takaori K, Doi R, Nishi E, Kakinoki R, Deng JM, Behringer RR, Nakamura T, Uemoto S. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2010 doi: 10.1038/ng.722. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Gasa R, Mrejen C, Leachman N, Otten M, Barnes M, Wang J, Chakrabarti S, Mirmira R, German M. Proendocrine genes coordinate the pancreatic islet differentiation program in vitro. Proc Natl Acad Sci U S A. 2004;101:13245–13250. doi: 10.1073/pnas.0405301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber MA, Thung SN, Shen S, Stromeyer FW, Ishak KG. Phenotypic characterization of hepatic proliferation. Antigenic expression by proliferating epithelial cells in fetal liver, massive hepatic necrosis, and nodular transformation of the liver. Am J Pathol. 1983;110:70–74. [PMC free article] [PubMed] [Google Scholar]
- Germain L, Goyette R, Marceau N. Differential cytokeratin and alpha-fetoprotein expression in morphologically distinct epithelial cells emerging at the early stage of rat hepatocarcinogenesis. Cancer Res. 1985;45:673–681. [PubMed] [Google Scholar]
- Germain L, Blouin MJ, Marceau N. Biliary epithelial and hepatocytic cell lineage relationships in embryonic rat liver as determined by the differential expression of cytokeratins, alpha-fetoprotein, albumin, and cell surface-exposed components. Cancer Res. 1988a;48:4909–4918. [PubMed] [Google Scholar]
- Germain L, Noël M, Gourdeau H, Marceau N. Promotion of growth and differentiation of rat ductular oval cells in primary culture. Cancer Res. 1988b;48:368–378. [PubMed] [Google Scholar]
- Ghoshal AK, Mullen B, Medline A, Farber E. Sequential analysis of hepatic carcinogenesis. Regeneration of liver after carbon tetrachloride-induced liver necrosis when hepatocyte proliferation is inhibited by 2-acetylaminofluorene. Lab Invest. 1983;48:224–230. [PubMed] [Google Scholar]
- Hao E, Tyrberg B, Itkin-Ansari P, Lakey JR, Geron I, Monosov EZ, Barcova M, Mercola M, Levine F. Beta-cell differentiation from nonendocrine epithelial cells of the adult human pancreas. Nat Med. 2006;12:310–316. doi: 10.1038/nm1367. [DOI] [PubMed] [Google Scholar]
- Heremans Y, Van De Casteele M, in’t Veld P, Gradwohl G, Serup P, Madsen O, Pipeleers D, Heimberg H. Recapitulation of embryonic neuroendocrine differentiation in adult human pancreatic duct cells expressing neurogenin 3. J Cell Biol. 2002;159:303–312. doi: 10.1083/jcb.200203074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GM. Experimental pathology of the liver: 1. Restoration of liver of white rat following surgical removal. Arch Pathol Lab Med. 1931;12:186–202. [Google Scholar]
- Horb ME, Shen CN, Tosh D, Slack JM. Experimental conversion of liver to pancreas. Curr Biol. 2003;13:105–115. doi: 10.1016/s0960-9822(02)01434-3. [DOI] [PubMed] [Google Scholar]
- Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, Sharma A, Bonner-Weir S. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci U S A. 2008;105:19915–19919. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JN, Cameron E, Garay MV, Starkey TW, Gianani R, Jensen J. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology. 2005;128:728–741. doi: 10.1053/j.gastro.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Kragl M, Knapp D, Nacu E, Khattak S, Maden M, Epperlein HH, Tanaka EM. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature. 2009;460:60–65. doi: 10.1038/nature08152. [DOI] [PubMed] [Google Scholar]
- Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, Mikaelyan A, Roberts LR, Demetris AJ, Sun Z, Nevens F, Roskams T, Thorgeirsson SS. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12:410–416. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- Li C, Yang F, Sheppard A. Adult stem cells and mammalian epimorphic regeneration-insights from studying annual renewal of deer antlers. Curr Stem Cell Res Ther. 2009;4:237–251. doi: 10.2174/157488809789057446. [DOI] [PubMed] [Google Scholar]
- Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos GK, Barua L, Bowen WC. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology. 2005;41:535–544. doi: 10.1002/hep.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest. 2007;117:2553–2561. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel M, Shafritz DA. Stem cells, cell transplantation and liver repopulation. Biochim Biophys Acta. 2008;1782:61–74. doi: 10.1016/j.bbadis.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe M, Tsukahara Y, Tanaka M, Suzuki K, Saito S, Kamiya Y, Tsujimura T, Nakamura K, Miyajima A. Potential hepatic stem cells reside in EpCAM+ cells of normal and injured mouse liver. Development. 2009;136:1951–1960. doi: 10.1242/dev.031369. [DOI] [PubMed] [Google Scholar]
- Overturf K, al-Dhalimy M, Ou CN, Fine-gold M, Grompe M. Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am J Pathol. 1997;151:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- Pichard V, Aubert D, Ferry N. Direct in vivo cell lineage analysis in the retrorsine and 2AAF models of liver injury after genetic labeling in adult and newborn rats. PLoS One. 2009;4:e7267. doi: 10.1371/journal.pone.0007267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- Preisegger KH, Factor VM, Fuchsbichler A, Stumptner C, Denk H, Thorgeirsson SS. Atypical ductular proliferation and its inhibition by transforming growth factor beta1 in the 3,5-diethoxy-carbonyl-1,4-dihydrocollidine mouse model for chronic alcoholic liver disease. Lab Invest. 1999;79:103–109. [PubMed] [Google Scholar]
- Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB, Cornelius JG. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med. 2000;6:278–282. doi: 10.1038/73128. [DOI] [PubMed] [Google Scholar]
- Rosenberg L, Brown RA, Duguid WP. A new approach to the induction of duct epithelial hyperplasia and nesidioblastosis by cellophane wrapping of the hamster pancreas. J Surg Res. 1983;35:63–72. doi: 10.1016/0022-4804(83)90127-0. [DOI] [PubMed] [Google Scholar]
- Roskams TA, Libbrecht L, Desmet VJ. Progenitor cells in diseased human liver. Semin Liver Dis. 2003;23:385–396. doi: 10.1055/s-2004-815564. [DOI] [PubMed] [Google Scholar]
- Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP, Leach SD. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc Natl Acad Sci U S A. 2010;107:75–80. doi: 10.1073/pnas.0912589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackett SD, Li Z, Hurtt R, Gao Y, Wells RG, Brondell K, Kaestner KH, Greenbaum LE. Foxl1 is a marker of bipotential hepatic progenitor cells in mice. Hepatology. 2009;49:920–929. doi: 10.1002/hep.22705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaberg RM, Smukler SR, Kieffer TJ, Enikolopov G, Asghar Z, Wheeler MB, Korbutt G, van der Kooy D. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat Biotechnol. 2004;22:1115–1124. doi: 10.1038/nbt1004. [DOI] [PubMed] [Google Scholar]
- Sharma A, Zangen DH, Reitz P, Taneja M, Lissauer ME, Miller CP, Weir GC, Habener JF, Bonner-Weir S. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes. 1999;48:507–513. doi: 10.2337/diabetes.48.3.507. [DOI] [PubMed] [Google Scholar]
- Sirica AE, Mathis GA, Sano N, Elmore LW. Isolation, culture, and transplantation of intrahepatic biliary epithelial cells and oval cells. Pathobiology. 1990;58:44–64. doi: 10.1159/000163564. [DOI] [PubMed] [Google Scholar]
- Slack JM. Essential developmental biology. 2. Bath: Blackwell Publishing; 2006. [Google Scholar]
- Slack JM. Origin of stem cells in organogenesis. Science. 2008;322:1498–1501. doi: 10.1126/science.1162782. [DOI] [PubMed] [Google Scholar]
- Solar M, Cardalda C, Houbracken I, Martín M, Maestro MA, De Medts N, Xu X, Grau V, Heimberg H, Bouwens L, Ferrer J. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell. 2009;17:849–860. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Stanger BZ, Tanaka AJ, Melton DA. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature. 2007;445:886–891. doi: 10.1038/nature05537. [DOI] [PubMed] [Google Scholar]
- Strick-Marchand H, Morosan S, Charneau P, Kremsdorf D, Weiss MC. Bipotential mouse embryonic liver stem cell lines contribute to liver regeneration and differentiate as bile ducts and hepatocytes. Proc Natl Acad Sci U S A. 2004;101:8360–8365. doi: 10.1073/pnas.0401092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell. 2007;12:817–826. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Thorel F, Népote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464:1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao MS, Smith JD, Nelson KG, Grisham JW. A diploid epithelial cell line from normal adult rat liver with phenotypic properties of ‘oval’ cells. Exp Cell Res. 1984;154:38–52. doi: 10.1016/0014-4827(84)90666-9. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- Wang X, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. The origin and liver repopulating capacity of murine oval cells. Proc Natl Acad Sci U S A. 2003a;100:11881–11888. doi: 10.1073/pnas.1734199100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003b;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- Xu X, D’Hoker J, Stangé G, Bonné S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, Bouwens L, Scharfmann R, Gradwohl G, Heimberg H. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Yatoh S, Dodge R, Akashi T, Omer A, Sharma A, Weir GC, Bonner-Weir S. Differentiation of affinity-purified human pancreatic duct cells to beta-cells. Diabetes. 2007;56:1802–1809. doi: 10.2337/db06-1670. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Hanahan D. Murine pancreatic ductal adenocarcinoma produced by in vitro transduction of polyoma middle T oncogene into the islets of Langerhans. Am J Pathol. 1994;145:671–684. [PMC free article] [PubMed] [Google Scholar]
- Zaret KS. Genetic programming of liver and pancreas progenitors: lessons for stem-cell differentiation. Nat Rev Genet. 2008;9:329–340. doi: 10.1038/nrg2318. [DOI] [PubMed] [Google Scholar]
- Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–1494. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Rogler LE, Teperman L, Morgan G, Rogler CE. Identification of hepatocytic and bile ductular cell lineages and candidate stem cells in bipolar ductular reactions in cirrhotic human liver. Hepatology. 2007;45:716–724. doi: 10.1002/hep.21557. [DOI] [PubMed] [Google Scholar]
- Zipori D. The nature of stem cells: state rather than entity. Nat Rev Genet. 2004;5:873–878. doi: 10.1038/nrg1475. [DOI] [PubMed] [Google Scholar]