Abstract
Background
Previous comparisons of gastric cancer between the West and the East have focused predominantly on Japan and Korea, where early gastric cancer is prevalent, and have not included the Chinese experience, which accounts for approximately half the world’s gastric cancer.
Methods
Patient characteristics, surgical procedures, pathologic information, and survival were compared among gastric cancer patients who underwent curative intent gastrectomy at two large volume cancer centers in China and the US between 1995 and 2005.
Results
Median age and body mass index were significantly higher in US patients. The proportion of proximal gastric cancer was comparable. Gastric cancer patients in China had larger tumors and a later stage at presentation. The median number of positive lymph nodes was higher (5 vs 4, p<0.02) despite a lower lymph node retrieval (16 vs 22, p<0.001) in Chinese patients. The probability of death due to gastric cancer in Chinese patients was 1.7 fold of that in the US (p<0.0001) after adjusting for important prognostic factors.
Conclusions
Even after adjusting for important prognostic factors Chinese gastric cancer patients have a worse outcome than US gastric cancer patients. The differences between Chinese and US gastric cancer are a potential resource for understanding the disease.
Keywords: Gastric Cancer, Gastrectomy
INTRODUCTION
Despite a worldwide decline in the incidence of gastric cancer, Chinese incidence (29.9 new diagnoses per 100,000 people) remains high. Every year there are an estimated 221,478 deaths due to gastric cancer in China, nearly half of the world’s gastric cancer deaths.[1] Though Japan and Korea have overall gastric cancer outcomes that are superior to those in the United States, when early stage cancers are compared head to head, survival is similar.[2] The overall difference in survival is due to earlier stage at presentation, fewer proximal and gastroesophageal junction (GEJ) lesions in Asian countries, and histologic and genetic differences.[3–5] The outcome of Chinese gastric cancer has never been compared to Japanese, Korean, or Western gastric cancer.
We present data from two large cohorts of gastric cancer patients following curative intent resection in China and the US. This was a challenge, because, since 2005, it has become standard in most United States centers to treat advanced stage patients (T3 or N+ on pre-operative endoscopic ultrasound) with neoadjuvant chemotherapy, and this is not current practice in China. As a result of these different treatment strategies, we compared patients from 1995 – 2005 who did not receive neoadjuvant therapy with at least five years of survival followup (through 2010). To our knowledge is the only such large scale comparison between Chinese and US gastric cancer patients.
PATIENTS AND METHODS
Patients
Following IRB approval at both centers, gastric cancer patients diagnosed and treated in Memorial Sloan-Kettering Cancer Center (MSKCC) and Beijing Cancer Hospital (BCH) from 1995 to 2005 were compared. Patients were included if they underwent curative intent gastrectomy without pre-operative chemotherapy or radiation. Curative intent resection was defined as an R0 resection (microscopically negative margin) of the gastric tumor and associated lymph nodes. Those who underwent wedge resection or endoscopic resection were excluded. Both institutions maintain prospective gastric cancer databases that collect variables including age, sex, height, weight, family history, tumor size, location, differentiation, Lauren classification, extent of gastrectomy, extent of lymph node dissection, depth of invasion, T stage, number of positive nodes and retrieved lymph nodes, N stage, and status at follow-up. TNM stage was reconfirmed with original information according to the sixth edition of the TNM staging system (AJCC/UICC, 2002) to facilitate comparison with previous reports.[3] Disease status at last follow-up was based on a recent updates of institutional prospective database with review of medical records, telephone interviews, and mail correspondence. At MSKCC, review of the Social Security Death Index was done. Disease status was categorized as DOD (death of disease), DOC (death of other causes), NED (no evidence of disease), and LOF (loss of follow-up). DOD and DOC are determined prospectively by clinicians and researchers at each institution, and overall survival (OS) was defined as the time from resection to death from any cause or last follow-up.
Surgical procedures and pathological examination
Curative resection was performed with a negative margin, generally 4cm or more. Frozen section pathology was selectively utilized. Intraoperative finding of peritoneal metastasis indicated palliative resection. Lymph nodes along named perigastric arteries were removed as required for D2 lymphadenectomy. At both centers pathological review is performed by dedicated gastric cancer pathologists.
There were some differences in surgical approach. At MSKCC the extent of lymphadenectomy was documented at the time op operation. Patients with proximal gastric cancer received either a total gastrectomy with Roux-en-Y reconstruction or a proximal gastrectomy. Generally, distal gastrectomy patients in whom >50% of the stomach was resected were reconstructed with a Roux-en-Y, while patients with a lesser resection received a Billroth II reconstruction. Surgical specimens were examined by specialized pathologists for primary tumor analysis and lymph node analysis.
In BCH, a non-touch maneuver was utilized. Proximal gastrectomy was attempted if a histologically negative distal margin could be achieved; otherwise total gastrectomy was performed; in all cases a histologically negative esophageal margin was required to be included in this study. When distal gastrectomy was performed, reconstruction was generally a Billroth I gastroduodenostomy. Gastrojejunostomy was adopted instead of gastroduodenostomy only when a histologically negative margin could not be achieved. Exposure of main perigastric arteries to dissect associated lymph nodes was done in most cases, but sorting lymph nodes during the operation was not routine. Dissection of lymph nodes from the specimens was done by rotating pathologists or surgeons in the pathology department.
Adjuvant treatment and follow-up
Patients with stage III or more advanced disease, and some stage II patients, were recommended to receive adjuvant chemotherapy with 5-Fluororacil containing regimens for six months. Radiation was only recommended for those with positive surgical margins in China. Most patients completed the full course of chemotherapy, although the complete details of how many patients completed their course was not recorded. At MSKCC, patients had follow-up every 3–6 months for the first 3 years, then annually for at least 5 years. At BCH, patients had 3 month follow-up for the first two years, every six months for the next 3 years, and then annually or as needed. Abdominal ultrasonography was used more often in BCH for routine follow-up while computed tomography was the routine at MSKCC.
Statistical Analysis
The primary end point of our study was death due to gastric cancer, defined as the time from resection to death due to disease or last follow-up. Disease status is recorded prospectively at both centers. Mortality rate was defined as death within 30 days or in-hospital mortality when admitted beyond 30 days. Overall survival (OS) was defined as the time from resection to death from any cause or last follow-up. Patient demographic, surgical and pathological variables were compared using the chi square test or the Wilcoxon rank sum test. OS was estimated using the Kaplan-Meier method and survival curves were compared using the log-rank test. Methods of competing risk survival analysis were used to estimate and compare the cumulative incidence of disease-specific death, with death due to other or unknown causes treated as a competing factor. Gray’s method was used to compare the cumulative incidence curves predicting the probability of death due to gastric cancer.[6] Multivariate competing risk analysis was used to assess the effect of country on probability of death due to gastric cancer after controlling for factors from an internationally validated prognostic gastric cancer nomogram[7], and were used to compare these results to a similar analysis of Korean gastric cancer patients. P values less than 0.05 were considered statistically significant. All analyses were performed using SAS 9.2 (SAS institute, Cary, NC) or the cmprsk package in R 2.11.
RESULTS
Patient characteristics
A total of 1,027 United States and 1,173 Chinese patients who underwent R0 resection between 1995 and 2005 were identified. Of these, 316 US patients (who received neoadjuvant chemotherapy) and 215 Chinese patients (2 with other cancers, 69 neoadjuvant chemotherapy, and 144 with no survival status) were excluded. In total we compared 711 MSKCC patients to 958 BCH patients.
Table 1 shows patient demographics of patients in our study by center. The majority of our patients were male. Patients at MSKCC had a median age of 69 years, eight years older than those at BCH, and more than 25% of MSKCC patients were older than 75. At both centers, only one percent of patients were younger than 30. The median body mass index (BMI) of MSKCC patients was significantly larger that of BCH patients, and the proportion of obese patients (BMI>30kg/m2) at MSKCC was more than tenfold higher than at BCH. More than 20 percent of the patients at BCH had a BMI less than 20kg/m2.
Table 1.
Patient Demographics 1995–2005
| Country
|
P-value | ||
|---|---|---|---|
| China | United States | ||
| No. of Patients | 958 | 711 | |
| Male | 699 (73%) | 425 (60%) | <.0001* |
| Female | 259 (27%) | 286 (40%) | |
| Age (yr) | <.0001* | ||
| <30 | 10 (1%) | 6 (1%) | |
| 30–75 | 899 (94%) | 518 (73%) | |
| >75 | 49 (5%) | 187 (26%) | |
| Median (Range) | 61 (22 – 87) | 69 (22 – 96) | <.0001‡ |
| BMI (kg/m2) | <.0001* | ||
| <25 | 648 (68%) | 303 (43%) | |
| 25–30 | 231 (24%) | 185 (26%) | |
| >30 | 20 (2%) | 146 (21%) | |
| Unknown | 59 (6%) | 77 (11%) | |
| Median (Range) † | 22.8 (14 – 35.1) | 25.4 (14.1 – 47.6) | <.0001‡ |
| Race | |||
| Caucasian | -- | 538 (76%) | |
| Black | -- | 45 (6%) | |
| Asian | 958 (100%) | 74 (10%) | |
| Hispanic | -- | 53 (7%) | |
| Other | -- | 1 (0%) | |
|
| |||
| Family History | 0.0009* | ||
| No | 766 (80%) | 579 (81%) | |
| Yes | 192 (20%) | 92 (13%) | |
| Unknown | -- | 40 (6%) | |
Statistical tests based on available data
Chi-square test used for categorical variables.
Wilcoxon rank sum test used for continuous variables
n=899 (China), n=634 (United States)
Surgical characteristics
Table 2 shows clinical and pathologic characteristics. Total gastrectomy was performed more frequently at MSKCC (22% vs 10%). Proximal gastrectomy was performed in 40 percent of the patients in BCH compared with 30 percent at MSKCC, and the proportion of patients with thoracotomy was comparable between two centers.
Table 2.
Pathologic and surgical characteristics
| Country
|
P-value* | ||
|---|---|---|---|
| China N=958 |
United States N=711 |
||
| Tumor location | 0.0003 | ||
| Upper and GE Junction | 379 (40%) | 277 (39%) | |
| Middle | 194 (20%) | 189 (27%) | |
| Lower | 376 (39%) | 232 (33%) | |
| Total | 5 (1%) | 13 (2%) | |
| Unknown | 4 (0%) | -- | |
| Differentiation | 0.4 | ||
| Differentiated | 506 (53%) | 361 (51%) | |
| Undifferentiated | 441 (46%) | 342 (48%) | |
| Unknown | 11 (1%) | 8 (1%) | |
| Lauren classification | <.0001 | ||
| Intestinal | 708 (74%) | 419 (59%) | |
| Diffuse | 71 (7%) | 203 (29%) | |
| Mixed | -- | 79 (11%) | |
| Undefined | 179 (19%) | 10 (1%) | |
| Tumor size (cm) | <.0001 | ||
| <4 | 350 (37%) | 382 (54%) | |
| 4–7 | 445 (46%) | 222 (31%) | |
| >7 | 123 (13%) | 98 (14%) | |
| Unknown | 40 (4%) | 9 (1%) | |
| Median (Range) | 4.5 (0.1 – 18.5) | 3.5 (0 – 21) | <.0001‡ |
| T stage | <.0001 | ||
| T0/1 | 110 (11%) | 252 (36%) | |
| T2 | 477 (50%) | 235 (33%) | |
| T3 | 326 (34%) | 207 (29%) | |
| T4 | 41 (4%) | 17 (2%) | |
| Unknown | 4 (0%) | -- | |
| N stage | <.0001 | ||
| N0 | 320 (33%) | 367 (52%) | |
| N1 | 351 (37%) | 219 (31%) | |
| N2 | 189 (20%) | 90 (13%) | |
| N3 | 72 (8%) | 35 (5%) | |
| Unknown | 26 (3%) | -- | |
| Stage | <.0001 | ||
| 0/Ia | 95 (10%) | 209 (29%) | |
| Ib | 173 (18%) | 147 (21%) | |
| II | 253 (26%) | 141 (20%) | |
| IIIa | 216 (23%) | 114 (16%) | |
| IIIb | 96 (10%) | 54 (8%) | |
| IV | 102 (11%) | 46 (6%) | |
| Unknown | 23 (2%) | -- | |
| Lymph node retrieval | <.0001 | ||
| 0–14 | 411 (43%) | 156 (22%) | |
| 15–30 | 412 (43%) | 345 (49%) | |
| >30 | 107 (11%) | 209 (29%) | |
| Unknown | 28 (3%) | 1 (0%) | |
| Median (Range) | 16 (1 – 71) | 22 (1 – 72) | <.0001‡ |
| Positive lymph nodes (N+ only) | |||
| Median (Range)§ | 5 (1 – 53) | 4 (1 – 63) | 0.02‡ |
| Operation | <.0001 | ||
| Total gastrectomy | 97(10%) | 155(22%) | |
| Distal gastrectomy | 477 (50%) | 340 (48%) | |
| Proximal gastrectomy | 384(40%) | 216 (30%) | |
| Lymphadenectomy | <.0001 | ||
| D1 | 29 (3%) | 109 (15%) | |
| D2 | 929 (97%) | 597 (84%) | |
| Unknown | - | 5 (1%) | |
Chi-square test used for categorical variables unless otherwise noted.
Wilcoxon rank sum test used for continuous variables
N+ patients only
Major complications occurred in 236 patients at MSKCC (33%) with a 30-day mortality of 2 percent, similar to other reports.[3,8] At BCH, major complications defined as bleeding, leak, obstruction (including ileus, anastomotic obstruction, and gastroparesis), perforation, intra-abdominal abscess, reoperation, and fistula occurred in 87 patients (9.1%). Nine patients died, with the mortality rate of 0.9 percent in this series.
Pathological characteristics
There were significant differences in terms of tumor location, Lauren classification, tumor size, T stage, N stage, TNM stage, and lymph node retrieval between the two sites (Table 2).
While the proportion of proximal gastric cancer was similar at the two centers (39% and 40%), there were more distal lesions at BCH. Intestinal type classification predominated in both groups, but there were many more patients with a diffuse classification at MSK than there were at BCH (29% vs 7%). Early stage gastric cancer was 11 percent at BCH and 36 percent at MSKCC. Patients in BCH had larger tumor size, were thicker, and more frequently node positive. The median size was 4.5cm vs 3.5cm. Fifty-nine percent had tumors larger than 4cm at BCH compared to 45 percent at MSKCC. Nearly 50% of BCH patients were locally advance at presentation (as compared to 30% of MSKCC patients). More patients at BCH (22%) than at MSKCC (43%) had fewer than 15 lymph nodes retrieved. The median number of positive lymph nodes at the two centers was similar (5 and 4), while the proportion of N2 and N3 tumors was higher in BCH (28% vs 18%).
Survival analysis
The median and maximum follow-up among survivors was similar in both groups, and the maximum time of follow-up was 13.1 years. Five year survival at MSKCC was 58%, compared to 46% at BCH. We performed a competing risk analysis to address potential underestimation of the risk of death when death from other causes was censored (Table 3). At MSKCC the probability of death due to other causes was higher than at BCH (10% vs 2%) and the probability of death due to gastric cancer was lower (32% vs 53%).
Table 3.
Overall Survival and Probability of Disease-Specific death
| China
|
United States
|
p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Events | 5-year | 95%CI | N | Events | 5-year | 95%CI | ||
| Overall survival | 958 | 543 | 46% | 42–49 | 711 | 317 | 58% | 54–61 | <.0001* |
| Probability of death | |||||||||
| Due to gastric cancer | 526 | 53% | 50–56 | 219 | 32% | 28–36 | <.0001§ | ||
| Due to other causes | 17 | 2% | 1–3 | 98 | 10% | 8–13 | <.0001§ | ||
| Median follow-up among survivors (Range) | 6.2 yrs (0.34–13.1) | 5.2 yrs (0.04–13.1) | |||||||
Log-rank test
Gray’s test
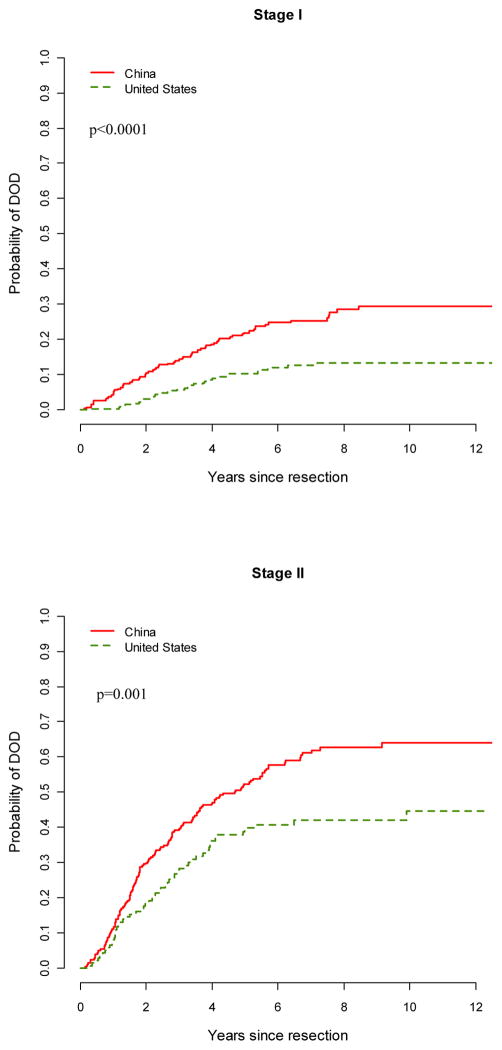
The probability of death due to gastric cancer by subcategories is shown in table 4. Patients with tumors at a later T stage, higher N stage, diffuse type tumors, and undifferentiated histologies had a higher probability of death due to gastric cancer. When compared stage by stage, the probability of death due to gastric cancer at MSKCC was significantly lower than at BCH for stages 0/I to III (Figure 1). This held true for N0 or N1 tumors, Laurent classification, differentiated or undifferentiated tumors, or T2 tumors. At both centers, tumors involving the whole stomach, the upper third and the GEJ had worse outcomes when compared to distal tumors. For tumors at either location, a significantly lower probability of death due to gastric cancer was found at MSKCC. The five year death probability of upper/GEJ tumors at MSKCC was comparable to that of distal lesions in BCH, with proximal lesions at BCH carrying the worst prognosis in our cohort.
Table 4.
Probability of Death Due to Gastric Cancer by subcategories
| Country
|
|||||
|---|---|---|---|---|---|
| China | United States | ||||
| 5-year | 95% CI | 5-year | 95% CI | P-value* | |
| Stage-specific | |||||
| I | 22% | 17–27 | 10% | 7–14 | <.0001 |
| Including T0 | 22% | 17–27 | 10% | 6–13 | <.0001 |
| IA only | 7% | 1–12 | 6% | 2–10 | 0.51 |
| II | 52% | 46–59 | 40% | 31–48 | 0.001 |
| III | 72% | 67–77 | 56% | 48–64 | 0.005 |
| IV | 86% | 78–93 | 79% | 66–92 | 0.42 |
| Lymph node status | |||||
| N0 | 25% | 20–30 | 14% | 11–18 | 0.0002 |
| N1 | 61% | 56–67 | 38% | 31–45 | <.0001 |
| N2 | 77% | 70–83 | 67% | 56–77 | 0.08 |
| N3 | 83% | 74–92 | 83% | 69–97 | 0.7 |
| Tumor location | |||||
| Upper +GE Junction | 70% | 65–75 | 35% | 29–41 | <.0001 |
| Middle | 41% | 34–48 | 36% | 28–43 | 0.1 |
| Lower | 42% | 37–47 | 24% | 18–30 | <.0001 |
| Total | 100% | 61–100 | 54% | 25–83 | 0.02 |
| Lauren classification | |||||
| Intestinal | 49% | 45–53 | 26% | 21–30 | <.0001 |
| Diffuse | 83% | 74–92 | 40% | 33–47 | <.0001 |
| Differentiation | |||||
| Differentiated | 49% | 44–53 | 23% | 19–28 | <.0001 |
| Undifferentiated | 58% | 53–62 | 41% | 35–46 | <.0001 |
| Tumor size | |||||
| <4 | 35% | 30–40 | 21% | 17–25 | <.0001 |
| 4–7 | 62% | 58–67 | 42% | 35–49 | <.0001 |
| >7 | 70% | 62–79 | 52% | 41–63 | 0.006 |
| T stage | |||||
| T1 | 7% | 2–11 | 8% | 4–11 | 0.82 |
| T2 | 52% | 47–56 | 30% | 23–36 | <.0001 |
| T3 | 67% | 62–72 | 60% | 53–67 | 0.39 |
| T4 | 79% | 65–92 | 61% | 35–86 | 0.09 |
| Operation | |||||
| Total Gastrectomy | 78% | 69–86 | 45% | 36–53 | <.0001 |
| Distal Gastrectomy | 37% | 33–42 | 24% | 19–29 | <.0001 |
| Proximal Gastrectomy | 66% | 61–71 | 29% | 16–42 | <.0001 |
| Lymphadenectomy | |||||
| D1 | 52% | 32–72 | 25% | 16–34 | 0.004 |
| D2 | 53% | 49–56 | 33% | 29–37 | <.0001 |
Gray’s test
Figure 1.
Gastric cancer-specific probability of death by stage in China and the US cohorts. P value represents log-rank analysis of survival.
By multivariate analysis, the hazard ratio of death due to gastric cancer in BCH was eighty percent higher than that at MSKCC when controlling for factors from an internationally validated nomogram[7] (HR 1.8, p<.0001) (Table 5). Incorporating additional factors such as BMI, family history, lymphadenectomy, differentiation, and operation into the model revealed similar results (HR 1.7, p<.0001). In contrast, the hazard ratios of death due to gastric cancer in a high-volume gastric cancer hospital in Korea versus MSKCC were 0.8 (p=0.05) and 0.7 (p=0.09) from the nomogram model and the nomogram with additional factors model, respectively.[3]
Table 5.
Comparison of multivariate models and the change in Hazard Ratio (HR) and p value for Country
| Model | Variables in Model | China vs. US Adjusted HR for Country | 95% CI | p-value | Korea vs. US Adjusted HR for Country | 95% CI | p-value |
|---|---|---|---|---|---|---|---|
| Separate models comparing China vs. US and Korea vs. US | |||||||
| Nomogram based model | Country + Age + Sex + Lauren + Tumor location + T-stage + Tumor size + Retrieved LNs + Positive LNs | 1.8 | 1.4–2.3 | <.0001 | 0.8* | 0.6–1.0 | 0.05 |
|
| |||||||
| Nomogram based model & additional factors | Country + Age + Sex + Lauren + Tumor location + T-stage + Tumor size + Retrieved LNs + Positive LNs + Family History + BMI + Lymphadenectomy + Differentiation + Operation | 1.7 | 1.3–2.2 | <.0001 | 0.8* | 0.6–1.0 | 0.09 |
Results from Strong et al. 2010.[3] The reciprocal of hazard ratio and confidence interval are reported to present US as the reference group
DISCUSSION
With a direct comparison of large cohorts from two high-volume gastric cancer hospitals in the United States and China, we found significant differences in gastric cancer outcomes following curative resection. After 2005 the proportion of cases treated at MSKCC with neoadjuvant chemotherapy rises dramatically, while no such treatment change occurs at BCH. As a result cases with neoadjuvant chemotherapy were excluded to provide the least biased comparison and to account for country practice differences, which explains in part the relatively high incidence of early gastric cancer at MSKCC. The proportion of proximal lesions was comparable (40% in both groups) while other East/West comparisons have noted a greater prevalence of proximal or GEJ lesions in the West. Gastric cancer in China was more often advanced with larger tumors, thicker tumors, and more node positive tumors, suggestive of a later diagnosis. The outcome of gastric cancer in China is consistently worse than that in the US with a 70 to 80 percent increase in probability of death due to gastric cancer, even following curative resection.
The large proportion of Chinese patients with proximal gastric cancer is in stark contrast to the Japanese and Korean experience.[3–5] Data from the Gastric Cancer Registry of Japan demonstrated that the proportion of upper third gastric cancers did not exceed 20 percent from 1975 to 1989.[9] A recent review of 12,026 gastric cancer patients in Korea reported an increase of upper third cancer from 5.3 percent in 1980s to 14.0 percent in 2000s [10], and the incidence of GEJ tumors is steadily rising in Europe and America. In our Chinese cohort, the proportion of proximal gastric cancer was extremely high and a selection bias must be considered as proximal gastric cancers with early symptoms more often underwent curative resection. Data from the Chinese National Gastric Cancer Registry demonstrate that primary cardia cancers accounted for 22 percent of all gastric cancers and 39% of the diagnoses made in high incidence areas.[11] The proportions of proximal gastric cancer were approximately one third in several large hospital-based series from China.[12–14]
These tumors tend to be more aggressive subtypes with poorer prognosis, and the factors associated with GEJ adenocarcinoma in Western countries are different from those in China. [15–19] Recent genome-association studies also provided new hints of genetic susceptibility to proximal gastric cancer [20,21], upon which in China are superimposed epidemiological risk factors such as spicy or salty food, and dietary deficiency in certain vitamins and minerals [15]. As China is in a period of socioeconomic transformation, risk factors of GEJ adenocarcinoma are steadily increasing in urban areas [22] while those for primary cardia cancer in rural areas remain unchanged. Further studies regarding prevention of both types of proximal gastric cancer are needed.
The combination of later stage and diagnosis and a high rate of proximal tumors likely accounts for the majority of poor outcomes in our China cohort. Furthermore, unlike our prior comparison with Korean gastric cancer patients, in China there were fewer distal gastric cancers. This subtype of gastric cancer is known to have a better prognosis and with fewer of such gastric cancer subtypes in the Chinese patients, survival also more closely mirrored Western statistics. In addition to the 30% of Chinese gastric cancer patients who present with metastatic disease and were excluded from this study, approximately 44% of patients in our Chinese cohort presented with stage III disease. In the US cohort, early stage gastric cancers accounted for 36 percent of patients, much higher than 11 percent in Chinese patients. Excluding patients who received neo-adjuvant chemotherapy enriched our sample for early stage cancer and partially explains this disparity. The high proportion of early gastric cancers generally attributed to Eastern disease reflects the Korean and Japanese experience, but is not reflective of China.
Early stage gastric cancer harbors a probability of death of less than 10 percent in both sites. A recent comparison of Filipino-Americans and the Philippine resident population highlighted the importance of the access to, and utilization of, diagnostic and therapeutic facilities in developing countries.[23] A socioeconomic analysis among Japanese patients suggests that improved survival is associated with more gastric cancer detection.[24] Unfortunately, Chinese, especially those in rural areas, are reluctant to see the doctor until the presence of severe symptoms.
Surgical technique did not contribute significantly to the differences in survival between the two groups once curative resection was achieved. More than 84 percent of the patients underwent D2 lymphadenectomy. Major changes in surgical and pathologic procedures at BCH have implemented, but they began after 2005 and were not captured in this series. While there were more total gastrectomies at MSKCC (22% vs 10%), this is unlikely to account for a difference in survival, as at total gastrectomy is not associated with a survival benefit compared to proximal gastrectomy.[25,26] About one quarter of the patients underwent thoracotomy for proximal lesions. Most studies do not support a survival benefit between thoracotomy or laparotomy, [27,28] but there is less morbidity with a subdiaphragmatic resection.[29]
We did not include adjuvant chemotherapy in our survival analysis as only 10% of US patients received adjuvant therapy, compared to 86.4% of Chinese patients. This is partially due to the exclusion of patients who received neoadjuvant therapy (who also usually received adjuvant treatment)[3], the fact adjuvant treatment was not included in the international nomogram we used, [7] and that many MSKCC patients receive adjuvant therapy at other institutions after receiving their definitive surgical resection at MSKCC, and as a result the details of their chemotherapy treatment are not always captured.
Overall, the probability of death due to gastric cancer in Chinese patients within the first five post-operative years is higher than that for the American or Korean patients. This observation raises an important question: are there biological differences between US and Chinese gastric cancer not currently explained? While some previous studies do not support this idea, [30–32] the data is conflicting. When compared to British gastric cancer samples, Japanese samples have more nm23, more x-erb-B2 expression, and a higher PCNA index.[33] Immigration epidemiological studies shown decreasing incidence of gastric cancer in Chinese Americans.[34] In Japanese Americans, gastric cancer presented with only some of the characteristics seen in the West.[35] These changes suggest that environmental risk factors influence a background of ethnic and genetic risk factors to define different biological behavior.
In addition to similar prevalence of H. pylori infection, a known carcinogen[36], Korea and China share similar dietary habits with high amounts of salty foods, carbohydrates, and higher intake of cooked than fresh vegetables. Interestingly, Korean patients with gastric cancer have a much better outcome than Chinese patients (Table 5), a phenomenon likely explained by the high proportion of early gastric cancer, predominantly distal disease,2 and easy access to medical service in highly urbanized Korea.
There are distinct differences in outcome of gastric cancer following curative resection between the US and China. To account for the differences between our two populations we adjusted for important prognostic factors from an internationally validated nomogram, and even after this adjustment the survival differences remain. Understanding these differences will not only shed light on the biology of gastric cancer but may also provide clues to treating Chinese gastric cancer, which accounts for half of the world’s gastric cancer.
Synopsis.
Chinese gastric cancer patients presented with different characteristics and poorer outcome compared to the US. The different gastric cancer behaviors and presentations between the US and China warrants further study.
Acknowledgments
Sources of Funding: Soudavar Memorial Fellowship in Memorial Sloan-Kettering Cancer Center, Natural Science Foundation of China (81071983), Beijing Municipal Science & Technology Commission NOVA program (2007B-057), and National 863 High Tech R&D Program of China (2006AA02A402).
The authors thank Mrs. Marianne Beninati for her work in management of the database.
Abbreviations
- GEJ
Gastroesophageal junction
- BMI
Body Mass Index
- DOD
Dead of Disease
- DOC
Dead Unknown Cause
- NED
No Evidence of Disease
- LOF
Lost to followup
- MSKCC
Memorial Sloan Kettering Cancer Center
- BCH
Beijing Cancer Hospital
- Hp
Helicobacter pylori
- WHO
World Health Organization
- GERD
Gastroesophageal reflux disease
Footnotes
Author Contributions: Strong, Wu, Selby, Coit, Ji, and Brennan participated in conception and design of this project; Strong and Wu participated in data collection; Strong, Wu, Gonen, and Hsu participated in data analysis; all authors participated in manuscript drafting and critical revision and all authors gave their approval for the final draft of the manuscript.
References
- 1.World Health Organization. Estimated Cancer Incidence, Mortality, and Prevalence Worldwide in 2012. [Google Scholar]
- 2.Strong VE, Song KY, Park CH, et al. Comparison of disease-specific survival in the United States and Korea after resection for early-stage node-negative gastric carcinoma. J Surg Oncol. 2013;107:634–640. doi: 10.1002/jso.23288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strong VE, Song KY, Park CH, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Annals of surgery. 2010;251:640–646. doi: 10.1097/SLA.0b013e3181d3d29b. [DOI] [PubMed] [Google Scholar]
- 4.Noguchi Y, Yoshikawa T, Tsuburaya A, et al. Is gastric carcinoma different between Japan and the United States? Cancer. 2000;89:2237–2246. [PubMed] [Google Scholar]
- 5.Bollschweiler E, Boettcher K, Hoelscher AH, et al. Is the prognosis for Japanese and German patients with gastric cancer really different? Cancer. 1993;71:2918–2925. doi: 10.1002/1097-0142(19930515)71:10<2918::aid-cncr2820711006>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 6.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. The Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 7.Kattan MW, Karpeh MS, Mazumdar M, Brennan MF. Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:3647–3650. doi: 10.1200/JCO.2003.01.240. [DOI] [PubMed] [Google Scholar]
- 8.Selby LV, Vertosick EA, Sjoberg DD, et al. Morbidity after Total Gastrectomy: Analysis of 238 Patients. Journal of the American College of Surgeons. 2015;220:863–871. e862. doi: 10.1016/j.jamcollsurg.2015.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Kaneko S, Sobue T. Trends in reported incidences of gastric cancer by tumour location, from 1975 to 1989 in Japan. International journal of epidemiology. 2004;33:808–815. doi: 10.1093/ije/dyh053. [DOI] [PubMed] [Google Scholar]
- 10.Ahn HS, Lee HJ, Yoo MW, et al. Changes in clinicopathological features and survival after gastrectomy for gastric cancer over a 20-year period. The British journal of surgery. 2011;98:255–260. doi: 10.1002/bjs.7310. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Zhang S, Chen Z. An epidemiologic trend analysis on cardiac cancer in high incidence areas of esophageal cancer and gastric cancer in China. China Cancer. 2008;17:998–1000. [Google Scholar]
- 12.Zhang X, Huang C, Lu H. Clinical observation of surgical treatment and prognosis in gastric cancer. Chin Med J. 2002;82:1142–1143. [Google Scholar]
- 13.Chen L, Zhang Y, Wei B, et al. Surgical treatment for patients with gastric cancer: report of 2335 cases] Zhonghua wei chang wai ke za zhi. Chinese journal of gastrointestinal surgery. 2007;10:421–424. [PubMed] [Google Scholar]
- 14.Wu A-w, Ji J-f, Yang H, et al. Long-term outcome of a large series of gastric cancer patients in China. Chinese Journal of Cancer Research. 2010;22:167–175. [Google Scholar]
- 15.Qiao YL, Dawsey SM, Kamangar F, et al. Total and cancer mortality after supplementation with vitamins and minerals: follow-up of the Linxian General Population Nutrition Intervention Trial. Journal of the National Cancer Institute. 2009;101:507–518. doi: 10.1093/jnci/djp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blot WJ, Devesa SS, Kneller RW, Fraumeni JF., Jr Rising incidence of adenocarcinoma of the esophagus and gastric cardia. Jama. 1991;265:1287–1289. [PubMed] [Google Scholar]
- 17.Lagergren J, Bergstrom R, Nyren O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Annals of Internal Medicine. 1999;130:883. doi: 10.7326/0003-4819-130-11-199906010-00003. [DOI] [PubMed] [Google Scholar]
- 18.Pera M, Cameron AJ, Trastek VF, et al. Increasing incidence of adenocarcinoma of the esophagus and esophagogastric junction. Gastroenterology. 1993;104:510–513. doi: 10.1016/0016-5085(93)90420-h. [DOI] [PubMed] [Google Scholar]
- 19.Souza RF, Spechler SJ. Concepts in the prevention of adenocarcinoma of the distal esophagus and proximal stomach. CA a cancer journal for clinicians. 2005;55:334–351. doi: 10.3322/canjclin.55.6.334. [DOI] [PubMed] [Google Scholar]
- 20.Abnet CC, Freedman ND, Hu N, et al. A shared susceptibility locus in PLCE1 at 10q23 for gastric adenocarcinoma and esophageal squamous cell carcinoma. Nature genetics. 2010;42:764–767. doi: 10.1038/ng.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang LD, Zhou FY, Li XM, et al. Genome-wide association study of esophageal squamous cell carcinoma in Chinese subjects identifies susceptibility loci at PLCE1 and C20orf54. Nature genetics. 2010;42:759–763. doi: 10.1038/ng.648. [DOI] [PubMed] [Google Scholar]
- 22.Ji CY, Sun JL, Chen TJ. Dynamic analysis on the prevalence of obesity and overweight school-age children and adolescents in recent 15 years in China. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi. 2004;25:103–108. [PubMed] [Google Scholar]
- 23.Redaniel MT, Laudico A, Mirasol-Lumague MR, et al. Cancer survival discrepancies in developed and developing countries comparisons between the Philippines and the United States. British journal of cancer. 2009;100:858–862. doi: 10.1038/sj.bjc.6604945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuwahara A, Takachi R, Tsubono Y, et al. Socioeconomic status and gastric cancer survival in Japan. Gastric Cancer. 2010;13:222–230. doi: 10.1007/s10120-010-0561-4. [DOI] [PubMed] [Google Scholar]
- 25.Shiu MH, Moore E, Sanders M, et al. Influence of the extent of resection on survival after curative treatment of gastric carcinoma. A retrospective multivariate analysis. Arch Surg. 1987;122:1347–1351. doi: 10.1001/archsurg.1987.01400230135024. [DOI] [PubMed] [Google Scholar]
- 26.Harrison LE, Karpeh MS, Brennan MF. Total gastrectomy is not necessary for proximal gastric cancer. Surgery. 1998;123:127–130. [PubMed] [Google Scholar]
- 27.Barbour AP, Rizk NP, Gonen M, et al. Adenocarcinoma of the gastroesophageal junction: influence of esophageal resection margin and operative approach on outcome. Annals of surgery. 2007;246:1–8. doi: 10.1097/01.sla.0000255563.65157.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morales TG. Adenocarcinoma of the gastric cardia. Digestive diseases. 1997;15:346–356. doi: 10.1159/000171610. [DOI] [PubMed] [Google Scholar]
- 29.Sasako M, Sano T, Yamamoto S, et al. Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: a randomised controlled trial. The lancet oncology. 2006;7:644–651. doi: 10.1016/S1470-2045(06)70766-5. [DOI] [PubMed] [Google Scholar]
- 30.McCulloch P, Taggart T, Ochiai A, et al. c-erbB2 and p53 expression are not associated with stage progression of gastric cancer in Britain or Japan. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 1997;23:304–309. doi: 10.1016/s0748-7983(97)90669-7. [DOI] [PubMed] [Google Scholar]
- 31.Davis PA, Sano T. The difference in gastric cancer between Japan, USA and Europe: what are the facts? what are the suggestions? Critical reviews in oncology/hematology. 2001;40:77–94. doi: 10.1016/s1040-8428(00)00131-1. [DOI] [PubMed] [Google Scholar]
- 32.Hundahl SA, Stemmermann GN, Oishi A. Racial factors cannot explain superior Japanese outcomes in stomach cancer. Arch Surg. 1996;131:170–175. doi: 10.1001/archsurg.1996.01430140060016. [DOI] [PubMed] [Google Scholar]
- 33.Livingstone JI, Yasui W, Tahara E, Wastell C. Are Japanese and European gastric cancer the same biological entity? An immunohistochemical study. British journal of cancer. 1995;72:976–980. doi: 10.1038/bjc.1995.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwong SL, Chen MS, Jr, Snipes KP, et al. Asian subgroups and cancer incidence and mortality rates in California. Cancer. 2005;104:2975–2981. doi: 10.1002/cncr.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hundahl S, Phillips J, Menck H. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer. 2000;88:921–932. [PubMed] [Google Scholar]
- 36.You WC, Li JY, Zhang L, et al. Etiology and prevention of gastric cancer: a population study in a high risk area of China. Chinese journal of digestive diseases. 2005;6:149–154. doi: 10.1111/j.1443-9573.2005.00222.x. [DOI] [PubMed] [Google Scholar]