Summary
Objectives
Diarrhea is a leading cause of morbidity and mortality for children, although sparse data is available on the etiology of diarrhea in China. This study was conducted to determine main causes that underlie childhood diarrhea and related diseases.
Method
Surveillance data for diarrhea was collected from 213 participating hospitals between 2009 and 2013. These stool specimens, from children aged 0e59 months, were then analyzed for a panel of etiological agents consisting of 5 viruses, 8 bacteria and 3 protozoa. The proportion of children who tested positive for each pathogen was calculated and seasonal patterns for major organisms were determined.
Results
Pathogens were identified in 44.6% of the 32,189 samples from children with diarrhea. The most commonly detected pathogens were rotavirus (29.7% of cases), norovirus (11.8%), Diarrheagenic Escherichia coli (DEC; 5.0%), adenovirus (4.8%), non-typhoidal Salmonella (NTS; 4.3%), and Shigella spp. (3.6%). A strong seasonal pattern was observed for these organisms, including rotavirus (winter), norovirus (autumn), and DEC, NTS, and Shigella (summer).
Conclusion:
A wide range of enteropathogens were detected in this five-year surveillance study; rotavirus and norovirus were most common among children under the age five. These findings should serve as robust evidence for public health entities when planning and developing national intervention programs in China.
Keywords: Etiology, Diarrhea, Outpatients, Children, Sentinel surveillance, China
Introduction
Diarrhea is one of the leading causes of morbidity and mortality among children aged under 5 and estimated at 1.73 billion episodes and 711,800 deaths each year.1 China is one of 15 high-incidence countries1; an estimated 770 million episodes of diarrhea (0.7 episodes per person year) occur annually, of which 194 million are attributed to children aged <5 years (1.9 episodes per person year).2 Although infectious diarrheal incidents have been a mandatory notifiable disease/syndrome since 1989,3 most cases are reported based only on clinical diagnosis. Data regarding trends, etiology and incidence of diarrhea through laboratory-based surveillance study have not been available in China. To address this data gap, a few small scale studies have previously been conducted in China,4–7 but these have generally focused on a single or limited number of enteropathogens in a hospital or local region, or over a short study period (usually one year); thus these studies did not capture long-term trends or permit robust extrapolation to the entire population of China.
Beginning in 2009, the Chinese Ministry of Science and Technology and Ministry of Health launched a nationwide, multicenter, prospective study: the National Key Science and Technology Project on Infectious Disease Surveillance Technique Platform. This platform was intended to enhance nationwide etiology identification, uncover the major pathogenic agents of epidemic infectious diseases, and inform policy makers on future vaccine and intervention development and enhance recommendations in China. This study presents microbiological results from the first 5 years (2009–2013) of surveillance of diarrheal illness data among children aged <5 years who presented to a hospital outpatient setting in China.
Materials and methods
Case recruitment
From Jan. 1st 2009 to Dec. 31st 2013, ongoing surveillance of diarrhea for all age groups was conducted in 213 participating hospitals (‘sentinel’ hospitals) throughout the country (Fig. 1). Sentinel hospitals were selected based on their catchment areas, population served, and interest in participating in the study. Three guidelines were adhered to when selecting sentinel hospitals: 1. each of the 31 provinces of mainland China would include a minimum of one hospital; 2. various types of hospitals (e.g., children’s, general, urban and rural community health service centers) would be included; and 3. hospitals would have the capability and resources to conduct ongoing surveillance.
Figure 1.
Location of 213 sentinel hospitals and 92 network laboratories participating in diarrheal illnesses surveillance of China, 2009–2013.
Patients visiting outpatient clinics (primarily enteric, pediatric and internal medicine) of sentinel hospitals were registered, and a standard case definition was used to determine eligibility. Diarrhea was defined as ≥3 passages of watery, loose, mucus-, or bloody-stools within a 24-h period. Patients referred from other hospitals or patients not initially diagnosed in sentinel hospitals were excluded from this study. A total sample size of ~10,000 persons per year was selected for the whole country. The total sample size was then allocated and assigned to each sentinel hospital (median = 54 cases/hospital/year), after weighting using the recorded outpatient numbers from the previous year. A convenient sampling method was used in sentinels to recruit that number of participants among eligible cases. To account for seasonal variations of diarrheal illness onset, it was determined that each hospital was to recruit ≥5% of the allocated number on a monthly basis. Information regarding demographics (e.g., sex, date of birth, and address) and clinical characteristics (e.g., signs/symptoms, date of symptom onset, date of diagnosis) were collected using a standardized case reporting form (CRF) at the time of recruitment. Verbal consent was acquired from parents/guardians and recorded by practitioner on CRF. Fecal specimens were subsequently collected and transferred to a network laboratory for microbiological testing.
Collection and transport of specimens
For each case-patient, three aliquots of feces were collected by his/her parents under the instruction of a trained nurse at the hospital. For virological testing, 5 g of fresh whole stool was collected in sterilized containers without preservatives and stored at -20 °C for processing.For bacteriological testing, fresh whole stool was collected using 5 sterilized cotton swabs and immediately placed in Cary–Blair Medium at 4 °C for transporting. For parasitological testing, 5 g of fresh whole stool was collected in sterilized containers without adding any preservatives. Collected specimens were packed and transported in ice boxes to network laboratories in accordance with UN3373 transportation requirements within 24 h for bacteria tests, and 48 h for viruses and protozoa tests. Upon arrival, network laboratory staff inspected and recorded the quality of specimens (e.g., temperature, volume and consistency). Unqualified specimens (specimen volume <5 g, swabs not preserved in Cary–Blair Medium) were rejected and resubmission requested.
Microbiological testing
A total of 92 network laboratories participated in the study. Selection of network laboratories was primarily based on their ability and interest in performing ongoing surveillance testing. Public health laboratories, clinical laboratories and laboratories in academic institutions were included. Each laboratory served a specified number of sentinel hospitals in the same catchment area (Fig. 1).
Following a literature review and discussions with expert consultants, a panel of proven and/or plausible enteropathogens was assayed in this study. Viruses included adenovirus, astrovirus, norovirus, rotavirus and sapovirus; bacterial pathogens included Aeromonas hydrophila, Campylobacter spp., diarrheagenic Escherichia coli (DEC), Plesiomonas shigelloides, non-typhoidal Salmonella spp. (NTS), Shigella spp., Vibrio spp., and Yersinia spp.; parasitic pathogens included Entamoeba histolytica, Cryptosporidium spp. and Giardia lamblia. A uniform study protocol,8 including standardized test methods and operation procedures (Supplementary Table 1 & Fig. 1), was accepted by all network laboratories and implemented before initiating surveillance. As laboratories in China were classified by their functions (e.g. viral, bacterial, and protozoal laboratories) and generally lacked the capacity to perform the full-range of assays for all 16 pathogens, not all specimens underwent complete assay at the expected testing items.
For rotavirus testing, fecal specimens were directly examined using enzyme-linked immunosorbent assay (ProSpecT™ rotavirus kit, Oxoid Ltd, Basingstoke, UK) to confirm presence of Group A rotavirus antigens according to manufacturer’s instruction; RT-PCR was used for further genotyping of ELISA-positive samples (Supplementary Table 2-1). For the remaining viruses, multiplex RT-PCR was used (Supplementary Table 2-2). Bacterial testing employed two conventional culture approaches to isolate bacterial organisms from stool. The first was a direct isolation procedure; swabs from a Cary–Blair tube were plated directly onto Salmonella–Shigella agar (SS), MacConkey (MAC) agar, xylose lysine desoxycholate (XLD) agar, and Campylobacter-selective agar plates (Karmali selective medium and Skirrow blood agar). Campylobacter-selective plates were incubated at 42 °C under microaerophilic conditions; the remaining agar plates were incubated aerobically at 37 °C. The second approach used enrichment procedures. Swabs were inoculated in selenite brilliant green sulfa enrichment broth (SBG), alkaline peptone water (AWP), m E. coli broth (mEC) and phosphate buffered saline (PBS). For Yersinia proliferation, inoculated PBS was incubated at 4 ° C for 10 days; all other enrichment broths were incubated aerobically at 37 °C. Suspicious colonies formed on selective agar plates were further identified and characterized by morphological, biochemical, serological and genetic assays following standardized laboratory procedures (Supplementary Appendix 1); For protozoa, wet mount microscopy examination and commercially available ELISA kits (EIA3467 & EIA4453, DRG Instruments GmbH, Marburg, Germany) were used to examine stool specimens directly according to manufacturer’s instruction.
Data analysis
In this study, children aged 0–59 months were included due to the high disease incidence of this age group.1,9 Children were further divided into five age groups: neonatal (0–28 days), post-neonatal (29 dayse5 months), infant (6–11 months), toddler (12–23 months), and pre-school children (24–59 months). Negative assay results were termed pathogen unspecified. The onset date of cases was divided into four seasons, spring (March–May), summer (June–August), autumn (SeptembereNovember), and winter (DecembereFebruary). As not all case-patient samples underwent a full range assay of 16 enteropathogens, the prevalence of a specific pathogen (proportion of cases tested positive for each organism) was calculated by dividing the number of positive samples by the total number tested for that pathogen. Chi-square tests were used for the comparison of proportions. Seasonal patterns of major enteropathogens were also studied by fitting average monthly positive percentage of pathogens in stool specimens to a linear regression model containing harmonic terms according to the method of Naumova et al.10 All analyses were conducted in R version 2.13.0 (R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria).
Ethics
This study was approved by the ethical review committee at the Chinese Center for Disease Control and Prevention (China CDC, Beijing, China).
Results
Characteristics of case-patients
During the data collection (2009–2013), a total of 32,189 children aged 0–59 months who visited a hospital outpatient setting were recruited in this investigation, of which 21,025 (65.3%) were male, and 11,164 (34.7%) female. Infants constituted the largest age group (n = 12,784, 39.7%), followed by post-neonatal (n = 7208, 22.4%), toddlers (n = 7184, 22.3%), pre-school children (n = 4432, 13.8%), and neonatal (n = 581, 1.8%). Besides diarrhea, vomiting was the most common clinical manifestation with 32.8% of cases (9959/30,395) presenting with concomitant vomiting. Other common symptoms included fever (22.4%, 2528/11,288), respiratory symptoms (11.7%, 3776/32,189) and dehydration (3.6%, 405/11,288). Median duration from illness onset to seeing a doctor was 2 days (range: 0–14 days). Of the 32,189 stool specimens submitted to network laboratories, watery stool (n = 17,035, 52.9%) were the most common type, followed by loose stool (n = 8419, 26.2%), mucus-stool (n = 5561, 17.3%), and bloody-stool (n = 1174, 3.6%).
Prevalence of enteropathogens
Among viral agents, rotavirus was the most commonly identified organism (29.7%, 6325/21,319), followed by norovirus (11.8%, 2161/18,266), adenovirus (4.8%, 879/18,266), Astrovirus (3.2%, 583/18,266), and sapovirus (1.8%, 377/18,266). Among bacterial agents, DEC was the most commonly identified bacteria (5.0%, 795/15,962), followed by NTS (4.3%, 789/18,261), Shigella (3.6%, 614/17,140), and A. hydrophila (1.0%, 162/15,921). Other bacteria generally had a lower prevalence of <1.0%. Among protozoal agents, E. histolytica had the highest positive percentage of 0.7% (14/1926). In total, 55.4% of samples had no specific pathogens identified (Table 1).
Table 1.
Microbiological testing results of children aged 0–59 months who submitted a stool specimen in diarrheal illnesses surveillance of China, 2009–2013.
| Enteropathogens | No. tested | No. identified | Positive percentage (%) |
|---|---|---|---|
| Viruses | |||
| Rotavirus (groups A, B and C) | 21,319 | 6325 | 29.7 |
| Rotavirus A | 20,848 | 6194 | 29.7 |
| Rotavirus B | 18,266 | 75 | 0.4 |
| Rotavirus C | 18,266 | 80 | 0.4 |
| Norovirus | 18,266 | 2161 | 11.8 |
| G I | 18,266 | 215 | 1.2 |
| G II | 18,266 | 1946 | 10.7 |
| Sapovirus | 18,266 | 337 | 1.8 |
| Adenovirus | 18,266 | 879 | 4.8 |
| Astrovirus | 18,266 | 583 | 3.2 |
| Bacteria | |||
| Diarrhea-genic Escherichia coli | 15,962 | 795 | 5.0 |
| ETEC | 15,962 | 159 | 1.0 |
| EPEC | 15,962 | 254 | 1.6 |
| EHEC | 15,962 | 54 | 0.3 |
| EAEC | 15,962 | 253 | 1.6 |
| EIEC | 15,962 | 75 | 0.5 |
| Shigella spp | 17,140 | 614 | 3.6 |
| Non-typhoidal Salmonella spp | 18,261 | 789 | 4.3 |
| Campylobacter spp | 16,257 | 100 | 0.6 |
| C. jejuni | 16,257 | 83 | 0.5 |
| C. coli | 16,257 | 18 | 0.1 |
| Vibrio spp | 17,675 | 48 | 0.3 |
| V. cholerae (serogroups O1 and O139) | 17,675 | 1 | 0 |
| V. parahaemolyticus | 17,675 | 33 | 0.2 |
| V. flurialis | 17,675 | 13 | 0.1 |
| V. mimicus | 17,675 | 1 | 0.0 |
| Yersinia spp | 17,117 | 61 | 0.4 |
| Y. enterocolitica, | 17,117 | 60 | 0.4 |
| Y. pseudotuberculosia | 17,117 | 1 | 0 |
| Aeromonas hydrophila | 15,921 | 162 | 1.0 |
| Plesiomonas shigelloides | 15,922 | 22 | 0.1 |
| Protozoa | |||
| Entamoeba histolytica | 1926 | 14 | 0.7 |
| Cryptosporidium spp | 1919 | 8 | 0.4 |
| Giardia lamblia | 1898 | 2 | 0.1 |
| Pathogen unspecifieda | 469 | 260 | 55.4 |
Abbreviation: ETEC, Enterotoxigenic E. coli; EAEC, Enteroaggregative E. coli; EHEC, Enterohaemorrhagic E. coli; EIEC, Enteroinvasive E. coli; EPEC, Enteropathogenic E. coli.
Full range 16 enteropathogens tested (n = 469 samples) were used to calculate prevalence of pathogen unspecified.
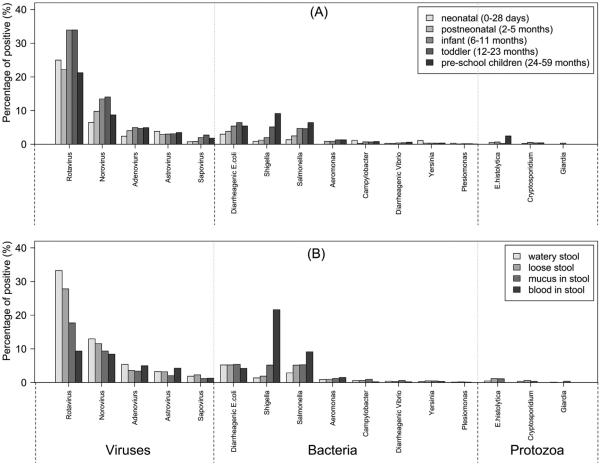
The prevalence of enteropathogens among age groups was compared. Viral agents (e.g., rotavirus and norovirus) were more likely to be identified in infants and toddlers (p < 0.01), while bacterial agents (e.g., DEC, NTS and Shigella) were more likely to be present in the stool of older children (p < 0.01); the prevalence increased with age (Fig. 2, Panel A). Shigella and NTS were more frequently associated with bloody stools (p < 0.01), while rotavirus and norovirus were more frequently associated with watery diarrhea (p < 0.01, Fig. 2, Panel B).
Figure 2.
Positive percentage of enteropathogens among children aged 0–59 months in diarrheal illnesses surveillance of China, 2009–2013. Panel (A): by age groups; Panel (B): by stool specimen types.
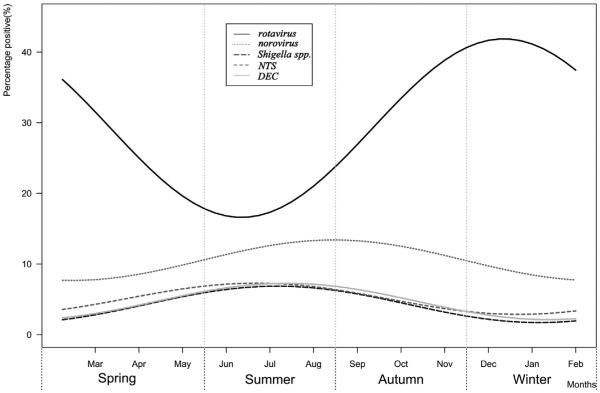
The prevalence of rotavirus, norovirus, Shigella, NTS, and DEC showed a strong seasonal pattern (Fig. 3). The seasonal curve of rotavirus had a peak in winter and trough in summer. In contrast, bacterial agents had a peak in summer and trough in winter. The prevalence of norovirus peaked in autumn.
Figure 3.
Seasonal pattern of major enteropathogens among children aged 0–59 months in diarrheal illnesses surveillance of China, 2009–2013.
Serotyping and genotyping
Among 1404 genotyped Group A rotavirus strains, G3P[8], G9P[8], G1P[8], G1P[4], and G2P[4] were the leading genotypes identified, accounting for 23.2%, 15.0%, 13.0%, 5.1%, and 4.8%, respectively (Table 2); GII was the predominant genogroup of norovirus, accounting for 90.0% (n = 1946) of positive samples. Among 795 DEC isolates, EPEC was the most frequently detected group (n = 254, 31.9%), followed by EAEC (n = 253, 31.8%), ETEC (n = 159, 20.0%), EIEC (n = 75, 9.4%), and EHEC (n = 54, 6.8%). Of the 614 Shigella isolates, the predominant serotype was Shigella flexneri 2a (n = 218, 35.5%) and Shigella sonnei (n = 181, 29.5%). Of 789 identified NTS isolates, 346 were serotyped. Salmonella Enteritidis and Salmonella Typhimurium was the most common accounting for 33.5% (n = 116) and 30.9% (n = 107), respectively. Only one isolate of Vibrio cholerae (serogroup O139), from a boy aged 2 years, was identified.
Table 2.
Frequency of Group A Rotavirus genotypes identified among children aged 0–59 months in diarrheal illnesses surveillance of China, 2009–2013.
| Genotypes | P[4] | P[6] | P[8] | P[9] | P[10] | P[11] | P non-typable | Total |
|---|---|---|---|---|---|---|---|---|
| G1 | 71 | 20 | 182 | 1 | 10 | 19 | 23 | 326 |
| G2 | 68 | 9 | 26 | 0 | 15 | 3 | 9 | 130 |
| G3 | 44 | 26 | 326 | 10 | 31 | 14 | 27 | 478 |
| G4 | 10 | 11 | 3 | 0 | 6 | 0 | 11 | 41 |
| G8 | 1 | 3 | 4 | 1 | 1 | 0 | 2 | 12 |
| G9 | 24 | 16 | 211 | 2 | 9 | 37 | 20 | 319 |
| G non-typable | 2 | 1 | 7 | 0 | 2 | 83 | 3 | 98 |
| Total | 220 | 86 | 759 | 14 | 74 | 156 | 95 | 1404 |
Discussion
This study was the first nationwide, multicenter, laboratory-based surveillance on diarrheal illnesses in China, with the prevalence of a wide range of enteropathogens studied prospectively. Over a period of five consecutive years, 32,189 children aged 0–59 months visiting a hospital outpatient clinic provided samples, of which 44.6% had at least one enteropathogen identified. rotavirus and norovirus were the predominant viral agents, and DEC, Shigella and NTS were the most common bacterial agents; protozoa were rarely identified in diarrhea samples from these children.
In this study, rotavirus was most frequently associated with diarrhea in children, while norovirus was the second most common enteropathogen. This result was similar to other studies conducted previously in China4,5 as well as other countries prior to the introduction of rotavirus vaccination.11,12 However, a different pattern was observed in USA, which introduced a rotavirus vaccination program in 2006, resulting in an expected sharp decline in prevalence of rotavirus, consequentially allowing norovirus to become the most predominant enteropathogen in children aged <5 years.13 With respect to global diarrheal disease, China has been considered a high incidence country. To effect substantial decline in diarrheal illnesses, inhibiting the rotavirus disease is a national and global priority. As hygienic measures, such as provision of clean water and improved sanitation, are less likely to impact the prevalence of rotavirus,14 inclusion of a rotavirus vaccine in the national immunization program is strongly recommended.
DEC has been one of the most common bacteria associated with diarrhea in developing countries.15,16 It has been estimated that about 14–17% of symptomatic patients would have a positive DEC identification in stool specimens.16 In this five year surveillance program, DEC was identified in 5.0% of children. This figure was slightly lower than this stated prediction, but higher than previously observed in China at 1.6%.7 However, DEC are not routinely screened for in most countries,17 including in China. With advances in the area of molecular technology and development of rapid assay methods, routine detection of DEC becomes more available, which has allowed investigators to better determine the role of DEC as a cause of diarrhea in children. Similarly, several other leading bacterial causes of diarrheal illnesses in developed countries, such as Campylobacter,16 may have been underappreciated in this study, potentially as a result of culturally-based laboratory approaches. Other factors that potentially affected laboratory yields in this study include use of antibiotics prior to visiting a health-care provider, quality of samples submitted by patients, and time span between illness onset and specimen collection. To help improve diarrheal illness surveillance data and understanding of results, such factors require a standardized approach in future collection.
Interestingly, a distinctive diversity of age and seasonal distribution of enteropathogens was observed between viruses and bacteria in children with diarrhea. For rotavirus, norovirus and sapovirus, children aged 6–23 months had the highest prevalence, but in DEC, NTS and Shigella older children had a higher prevalence. This diversity of age distribution might reflect a natural change in host immunity to different organisms. Using rotavirus as an example, most children acquire 2 or more infections of rotavirus by the age of 2, and therefore immunity has been established resulting in a steadily decreasing prevalence with older children.18,19 However, for bacteria, the situation appears different. Compared with viruses, older children had a higher prevalence for DEC, NTS and Shigella, which may reflect a different pattern of host immunity and transmissibility of infectious agents.17 The seasonal pattern was also different between viruses and bacteria. Viruses were prevalent in winter and autumn months, while bacteria were prevalent in summer months. This seasonal pattern was also observed in several other studies and strongly associated with environmental factors,10,20 such as ambient temperature, precipitation and humidity, which may have influence on exposure frequency, host immunity and pathogen. This information of age and seasonal distribution for enteropathogens is essential when planning the timing and targeted population for intervention programs in China.
This study had several limitations. Firstly, most specimens submitted to laboratories did not receive the full range assay of enteropathogens. Testing for protozoa was notably incomplete as only 2 laboratories had capacity and resources for these pathogens. Nevertheless, this was the most comprehensive data collection on the etiology of diarrhea in children in China. The large sample size, extensive coverage area and catchment population over 31 provinces, mean these results are reasonably representative, to date. Secondly, bias in data collection methods may have occurred between different geographic regions. Patients from eastern China may be overly represented, comparatively, as most sentinel hospitals were located in these provinces. Thirdly, the seasonal patterns of major enteropathogens were analyzed on aggregated nationwide data. Therefore, results may not be representative of distinct regions of China where factors such as climate, temperature, rainfall and humidity vary and subsequently influence the prevalence of enteropathogens. Fourthly, long-term trends were not present in this study as the five year period was too short to obtain this data. It is anticipated that further long-term continuation and collection of surveillance data will be able to document these trends.
Conclusion
In summary, this study has, for the first time, described the spectrum of major enteropathogens causing diarrhea in children aged 0–59 months who attended a hospital outpatient setting in China. rotavirus has caused considerable discomfort among children, and remains a public health priority for China. Age and seasonal distribution of major enteropathogens in children with diarrhea were also described. This data can inform development and implementation of diarrhea control and prevention programs in China. To provide a better understanding of diarrheal illnesses and assess any intervention program effectively, long-term trends and population-based disease effect measurements should be documented by future studies.
Supplementary Material
Acknowledgments
Thanks to all the patients, nurses, clinicians, and laboratory, research, and administrative staff who took part, or contributed to, this surveillance study.
Funding
This work was a Ministry of Science and Technology of the People’s Republic of China funded program under the National Key Science and Technology Project on Infectious Disease Surveillance Technique Platform of China[2009ZX10004-201, 2009ZX10004-202, 2009ZX10004-203, 2009ZX10004-204, 2009ZX10004-205, 2009ZX10004-207, 2009ZX10004-208, 2009ZX10004-209, 2009ZX10004-210, 2009ZX10004-211, 2009ZX10004-212, 2009ZX10004-213, 2012ZX10004-201, 2013ZX10004-202, 2012ZX10004-206, 2012ZX10004-207, 2012ZX10004-208, 2012ZX10004-209, 2012ZX10004-210, 2012ZX10004-211, 2012ZX10004-212, 2012ZX10004-213].
Footnotes
Disclaimer
The findings and conclusions of this report are those of the authors and do not necessarily represent the views of the funding agency, the official position of US CDC and the policy of the China CDC.
Conflict of interest statements
The authors have no conflicts of interest to disclose.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jinf.2015.03.001.
References
- 1.Walker CLF, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, et al. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381(9875):1405–16. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu Weili. A cross-sectional study of diarrheal illnesses in parts of China. Chin J Epidemiol. 1989;10(5):257–60. [Google Scholar]
- 3.National Health and Family Planning Commission of PRC Law of the People’s Republic of China on the prevention and treatment of infectious diseases. 2004 [in Chinese] [Google Scholar]
- 4.Chen Y, Li Z, Han D, Cui D, Chen X, Zheng S, et al. Viral agents associated with acute diarrhea among outpatient children in Southeastern China. Pediatr Infect Dis J. 2013;32(7):e285–90. doi: 10.1097/INF.0b013e31828c3de4. [DOI] [PubMed] [Google Scholar]
- 5.Lou JT, Xu XJ, Wu YD, Tao R, Tong MQ. Epidemiology and burden of rotavirus infection among children in Hangzhou, China. J Clin Virol. 2011 Jan;50(1):84–7. doi: 10.1016/j.jcv.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Von Seidlein L, Kim DR, Ali M, Lee H, Wang X, Thiem VD, et al. A multicentre study of Shigella diarrhoea in six Asian countries: disease burden, clinical manifestations, and microbiology. PLoS Med. 2006 Sep;3(9):e353. doi: 10.1371/journal.pmed.0030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qu M, Deng Y, Zhang X, Liu G, Huang Y, Lin C, et al. Etiology of acute diarrhea due to enteropathogenic bacteria in Beijing, China. J Infect. 2012;65(3):214–22. doi: 10.1016/j.jinf.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Management Office of National Science and Technology Major Project of China, Chinese Center for Disease Control and Prevention Diarrheal syndrome surveillance protocol (2012 version) 2012 [in Chinese] [Google Scholar]
- 9.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naumova E, Jagai J, Matyas B, DeMaria A, MacNeill I, Griffiths J. Seasonality in six enterically transmitted diseases and ambient temperature. Epidemiol Infect. 2007;135(02):281–92. doi: 10.1017/S0950268806006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IID Study Team. A report of the study of infectious intestinal disease in England Commun Dis Rep Wkly. 2000 Dec;10(51):457. [PubMed PMID: 11191030] [PubMed] [Google Scholar]
- 12.Podkolzin AT, Fenske EB, Abramycheva NY, Shipulin GA, Sagalova OI, Mazepa VN, et al. Hospital-based surveillance of rotavirus and other viral agents of diarrhea in children and adults in Russia, 2005–2007. J Infect Dis. 2009;200(Suppl. 1):S228–33. doi: 10.1086/605054. [DOI] [PubMed] [Google Scholar]
- 13.Payne DC, Vinjé J, Szilagyi PG, Edwards KM, Staat MA, Weinberg GA, et al. Norovirus and medically attended gastroenteritis in US children. N Engl J Med. 2013;368(12):1121–30. doi: 10.1056/NEJMsa1206589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mrukowicz J, Szajewska H, Vesikari T. Options for the prevention of rotavirus disease other than vaccination. J Pediatr Gastroenterol Nutr. 2008 May;46(Suppl. 2):S32–7. doi: 10.1097/MPG.0b013e31816f79b0. [DOI] [PubMed] [Google Scholar]
- 15.Thapar N, Sanderson IR. Diarrhoea in children: an interface between developing and developed countries. Lancet. 2004;363(9409):641–53. doi: 10.1016/S0140-6736(04)15599-2. [PubMed PMID: 14987892] [DOI] [PubMed] [Google Scholar]
- 16.Davidson G, Barnes G, Bass D, Cohen M, Fasano A, Fontaine O, et al. Infectious diarrhea in children: working group report of the First World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2002;35:S143–50. doi: 10.1097/00005176-200208002-00012. [DOI] [PubMed] [Google Scholar]
- 17.Nic Fhogartaigh C, Dance DAB. Bacterial gastroenteritis. Medicine. 2013;41(12):693–9. [Google Scholar]
- 18.Velazquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter-Campbell S, et al. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med. 1996 Oct 3;335(14):1022–8. doi: 10.1056/NEJM199610033351404. [DOI] [PubMed] [Google Scholar]
- 19.Velazquez FR, Matson DO, Guerrero ML, Shults J, Calva JJ, Morrow AL, et al. Serum antibody as a marker of protection against natural rotavirus infection and disease. J Infect Dis. 2000 Dec;182(6):1602–9. doi: 10.1086/317619. [DOI] [PubMed] [Google Scholar]
- 20.Jagai JS, Sarkar R, Castronovo D, Kattula D, McEntee J, Ward H, et al. Seasonality of rotavirus in South Asia: a meta-analysis approach assessing associations with temperature, precipitation, and vegetation index. PLoS One. 2012;7(5):e38168. doi: 10.1371/journal.pone.0038168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.