Abstract
TRPA1 receptor is activated by endogenous inflammatory mediators and exogenous pollutant molecules relevant to respiratory diseases. Previous studies have implicated TRPA1 as a drug target for antitussive therapy. Here we evaluated the relative efficacy of TRPA1 activation to evoke cough. In conscious guinea pigs the TRPA1 agonist allyl-isothiocyanate (AITC) evoked cough with a maximally effective concentration of 10mM that was abolished by the selective TRPA1 antagonist AP-18. AITC (10mM) was approximately 3-times less effective in inducing cough than capsaicin (50 μM). Ex vivo single fiber extracellular recordings revealed that, similarly to capsaicin, AITC evoked activation in airway jugular C-fibers, but not in airway nodose Aδ-fibers. Consistent with the cough studies, AITC was approximately 3-times less effective than capsaicin in evoking sustained activation of the jugular C-fibers. Another TRPA1 agonist, cinnamaldehyde, was approximately twofold more effective than AITC in inducing cough. However, the cinnamaldehyde (10mM)-induced cough was only partially inhibited by the TRPA1 antagonist AP-18, and was abolished by combination of AP-18 and the TRPV1 antagonist I-RTX. We conclude that in naïve guinea pigs, TRPA1 activation initiates cough that is relatively modest compared to the cough initiated by TRPV1, likely due to lower efficacy of TRPA1 stimulation to induce sustained activation of airway C-fibers.
Keywords: cough, TRPA1, TRPV1, allyl-isothiocyanate, cinnamaldehyde, capsaicin, airway C-fiber, vagus nerve
1. Introduction
TRPA1 is a nonselective cationic TRP channel expressed in nociceptors. TRPA1 is present in the vast majority of vagal nociceptive C-fibers in the respiratory tract (Hu et al., 2010; Nassenstein et al., 2008). Activation of TRPA1 in the terminals of these fibers evokes action potential discharge (Nassenstein et al., 2008). The relevance of TRPA1 expression in respiratory afferents nerves is heightened by the fact that many environmental airborne irritants are effective at activating TRPA1(Bautista et al., 2006; Bessac and Jordt, 2010). These irritants include acrolein found in car exhausts and cigarette smoke (Bautista et al., 2006), industrial pollutant toluene diisocyanate (TDI) (Taylor-Clark et al., 2009b) and ozone (Taylor-Clark and Undem, 2010). In addition, a large number of endogenous molecules produced in inflammation are effective activators of the TRPA1 receptor (Andersson et al., 2008). These molecules are exemplified by endogenously occurring alkenyl aldehydes 4-hydroxynonenal (Trevisani et al., 2007) and 4-oxononenal (Taylor-Clark et al., 2008a), cyclopentenone prostaglandin, 15-deoxydelta(12,14)-prostaglandin J2 (15dPGJ2) (Taylor-Clark et al., 2008b) and nitrooleic acid (Taylor-Clark et al., 2009a). The wide range of TRPA1 sensitivity has been attributed to the unique conserved mechanisms of TRPA1 activation by reactive electrophiles (Hinman et al., 2006; Kang et al., 2010; Macpherson et al., 2007).
Based on the observations that TRPA1 activation leads to activation of the respiratory vagal C-fibers and the knowledge that many of the environmental irritants known to cause coughing activates TRPA1, it was speculated that TRPA1 may be rational target for novel anti-tussive therapy (Grace and Belvisi, 2010; Nassini et al., 2010; Taylor-Clark et al., 2009c). In support of this notion, two recent studies in guinea pigs have provided direct and compelling evidence that TRPA1 agonists evoke coughing in a manner that can be antagonized by TRPA1 antagonists (Andre et al., 2009; Birrell et al., 2009). In the present study we have focused on the effectiveness of TRPA1 agonists in inducing cough as compared to the TRPV1 agonist capsaicin that is an effective and thoroughly evaluated reference tussive stimulant. Our studies support the conclusions that TRPA1 activation, presumably in jugular C-fibers, leads to coughing but show that it is a relatively modest tussive stimulant. This is likely explained by a differential pattern of action potential discharge in the cough-triggering jugular C-fibers when activated via TRPA1 vs. TRPV1. In addition we provided data indicating that whereas the TRPA1 activator cinnamaldehyde may be a useful pharmacological tool in vitro to stimulate TRPA1, this activator may also lead to TRPV1 activation in vivo. Some data from this study have been previously presented as abstract (Brozmanova et al., 2010)
2. Materials and methods
2.1. Cough studies
The cough studies were approved by the Jessenius Medical School Ethic Committee. Male Hartley Dunkin guinea pigs (n=57, 250-350g) were purchased from Department of Experimental Pharmacology, Slovak Academy of Science, Dobra Voda, Slovakia. The method for evaluation of cough has been validated previously (Brozmanova et al., 2006; Brozmanova et al., 2002; Brozmanova et al., 2008; Plevkova et al., 2004). Briefly, the animals were individually placed in a double-chamber body plethysmoghraph (type 855, Hugo Sachs Electronik, March-Hugstetten, Germany). The head chamber was connected to the nebulizer (Pari Provokation Test I, Menzel, Germany, manufacturer's specification: output 5 l.min-1, particle mass median aerodynamic diameter 1.2μm). A suction device set to balance the nebulizer output was connected to the head chamber to maintain constant airflow through the head chamber. Respiratory changes in the airflow were recorded using pneumotachograph (Godart, Germany) with Fleisch pneumotachograph head connected to the head chamber. Respiratory sounds including cough were recorded with a microphone from the head chamber. Cough was detected as the expiratory airflow accompanied by the cough sound by an investigator not familiar with the purpose of the experiment.
The aerosols of vehicles (PBS, 1% DMSO in PBS or 2% ethanol/2% Tween80 in PBS, see below), AITC (0.3-10mM), cinnamaldehyde (1-10mM), capsaicin (50μM) and citric acid (0.4M) were nebulized for periods indicated for each experiment (5 or 10 min). In the studies with the TRPA1 antagonists, AP-18 (1mM) or HC-030031(1mM) (Eid et al., 2008; McNamara et al., 2007; Petrus et al., 2007) was nebulized for 5 min followed by the TRPA1 agonist (AITC or cinnamaldehyde) (10mM) together with the TRPA1 antagonist for 5 min. Following 5 min, the same TRPA1 agonist (10mM) was nebulized for 5 min to assess the reversibility of the TRPA1 antagonist effect. Paired control experiments were carried out with the vehicle for AP-18 (1% DMSO). The studies with the TRPV1 selective antagonist iodo-resiniferatoxin (I-RTX, 30μM) and combination of AP-18 (1mM) and I-RTX (30μM) were carried out in an identical manner except that the reversibility was not assessed. The concentrations of TRPA1 and TRPV1 agonists were selected based on their reported in vitro potency (Brozmanova et al., 2011; Eid et al., 2008; McNamara et al., 2007; Petrus et al., 2007; Undem and Kollarik, 2002), and the selectivity of AP-18 (1mM) for TRPA1 vs. TRPV1, as well as the selectivity of I-RTX (30μM) for TRPV1 vs. TRPA1 was evaluated (see discussion for details). The cough challenges in animals were separated by at least 7 days.
Several types of vehicles were used in the study in order to assess the influence of the solvent on the efficacy of TRPA1 agonists. The type of vehicle is indicated for each experiment. In most experiments the chemicals were dissolved in DMSO and further diluted in phosphate buffered saline (PBS) so that the vehicle was 1% DMSO in PBS. In one set of experiments for comparison with previously study (Andre et al., 2009), AITC and cinnamaldehyde were dissolved in 50% ethanol/50% Tween80 to 250mM and further diluted in PBS so that the vehicle was 2% ethanol/2% Tween80 in PBS. In one set of experiments AITC was dissolved just prior to use directly in PBS (for ≈30 min with constant stirring) to final concentrations ≤10mM that is lower than its reported water solubility (2g/L that is ≈20mM, (SRC, 2009)). In our guinea pig cough model PBS (10 min) induced a modest, linearly time-dependent cough (Fig. 1A). Vehicle 2% ethanol/2% Tween80 in PBS also induced modest linearly time-dependent cough (0.26 coughs/min, R2=0.94, n=14). Vehicle DMSO (1% in PBS, 10 min) induced more prominent coughing that was also linearly time-dependent (0.62 coughs/min, R2=0.99, n=17). For TRPA1 antagonist studies, a TRPA1 antagonist (AP-18 or HC-030031) and a TRPA1 agonist (AITC or cinnamaldehyde) were together dissolved in DMSO to concentrations 100mM and 1M, respectively, and further diluted in PBS to final solutions containing the TRPA1 antagonist (1mM), the TRPA1 agonist (10mM). I-RTX was dissolved in DMSO to stock solution 10mM. Capsaicin was dissolved in ethanol to stock concentration 0.1M and further diluted in PBS to 50μM (containing 0.05% ethanol, ethanol in concentrations <1% has no effect on cough in guinea pigs (Gatti et al., 2009)). Citric acid was dissolved in saline to final concentration 0.4M. Two guinea pigs (out of 59) were excluded from the study because of excessive coughing (number of coughs >mean+3SD of the number of coughs evoked by the same stimulus in the remaining tested animals).
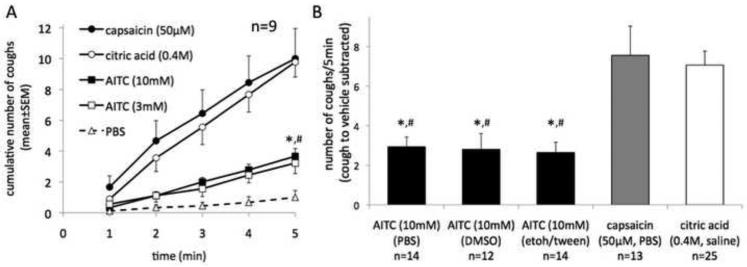
Figure 1. The TRPA1 agonist allyl isothiocyanate (AITC) is less effective in inducing cough than the TRPV1 activators capsaicin and citric acid.
(A) Time course of the cough induced by inhalation of allyl isothiocyanate (AITC), capsaicin and citric acid (paired study, n=9). Note that increasing the concentration of AITC from 3mM to 10mM did not further increase coughing indicating that the maximally effective concentration of AITC was attained. AITC (10mM) was less effective in inducing cough than capsaicin (50μM) and citric acid (0.4M). (Friedman test at 5 min time point, P<0.01, followed by Dunn's Test: *P<0.05 AITC vs. capsaicin, #P<0.01 AITC vs. citric acid). (B) The efficacy of AITC in inducing cough was not influenced by the type of vehicle used. The vehicles were PBS, DMSO (1%) in PBS, ethanol (2%)/Tween80 (2%) in PBS and in paired control experiments caused 0.2, 0.6 and 0.3 coughs/min, respectively. The cough to vehicle was subtracted from the cough to AITC. Kruskal-Wallis test (P<0.01) followed by Dunn's Test: *P<0.01 AITC vs. capsaicin, #P<0.01 AITC vs. citric acid.
2.2. Extracellular single unit recordings
Extracellular recordings were described previously (Kollarik and Undem, 2002; Undem et al., 2004). Briefly, guinea pigs were killed by CO2 inhalation and exsanguination. The trachea, larynx and right mainstem bronchus with intact right-side extrinsic vagal innervation were dissected and the tissue was pinned in a small Sylgard-lined Perspex chamber filled with Krebs bicarbonate buffer (KBS, composed of: NaCl, 118 mM; KCl, 5.4 mM; NaH2PO4, 1.0 mM; MgSO4, 1.2 mM; CaCl2, 1.9 mM; NaHCO3, 25.0 mM; dextrose, 11.1 mM, and gassed with 95% O2-5% CO2, pH=7.4, 35°C) containing indomethacin (3 μM). The chamber had two compartments: tissue compartment containing the airways, and recording compartment containing rostral vagus with the nodose and jugular ganglia. The hole between compartments was sealed with petroleum jelly. The airways were cut along ventral surface and pinned open mucosal side up. The tissue and recording chambers were separately superfused with KBS (pH=7.4, 37°C, 4 ml min−1.
Extracellular recordings were performed using an aluminosilicate glass microelectrode (pulled with Flaming-Brown micropipette puller, Sutter Instrument Company, Novato, CA, USA) and filled with 3 M sodium chloride (electrode resistance ~ 2 MΩ). The electrode in an electrode holder was connected directly to a headstage (A-M Systems, Everett, WA, USA). A return electrode of silver-silver chloride wire and an earthed silver-silver chloride pellet were placed in recording compartment. The signal was amplified (Microelectrode AC amplifier 1800, A-M Systems), filtered, and the resultant activity was displayed on oscilloscope (TDS 340, Tektronix, Beaverton, OR, USA) and TA240 chart recorder (Gould, Valley View, OH, USA). The data were stored and analyzed on a Mac computer using TheNerveOfIt software (sampling frequency 33 kHz, PHOCIS, Baltimore, MD, USA) and processed by Microsoft Excel. The recording electrode was micromanipulated into the vagal ganglion. A mechanosensitive receptive field was identified when the mechanical stimulus (Von Frey hair, 1.8-3N) bluntly applied to mucosal surface of the airway evoked action potentials. Conduction velocity was calculated by dividing the distance along the nerve pathway by the time between the stimulation pulse and the action potential. In most experiments one fiber per animal was recorded. AITC (stock 1M in DMSO) and capsaicin (stock 0.01M in ethanol) were diluted in KBS and delivered by superfusion of the tissue compartment. The response was quantified as a maximum action potential discharge in any 1s interval (peak frequency, Hz) and a maximum action potential discharge in any 30s interval (expressed as number of action potentials in 30s).
2.3. Statistical analysis
For cough studies non-parametric paired, non-paired and multiple comparisons tests were used as appropriate. Electrophysiological data were analyzed by paired and non-paired T-test. P<0.05 was considered significant. The time course of cough was fitted by linear regression.
3. Results
3.1. Characterization of the TRPA1-mediated cough in the guinea pig
Consistent with the previous report (Andre et al., 2009) inhalation of AITC evoked cough in our guinea pig model (Fig. 1). Compared to cough induced by AITC at a concentration of 3mM, increasing the concentration to 10mM did not further increase the cough indicating that the maximally effective concentration was attained (Fig.1A). AITC in the concentration of 10mM was therefore used in subsequent experiments. We tested several types of vehicles (PBS, DMSO 1%/PBS and ethanol 2%/Tween80 2%/PBS) and found that the vehicle did not influence the magnitude of the AITC-induced cough (Fig. 1B). Lower concentrations (0.3-1mM) of AITC failed to consistently evoke cough (n=4).
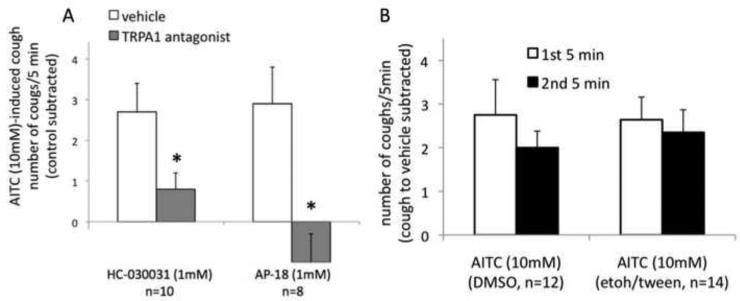
The studies using TRPA1 antagonists confirmed that the cough induced by inhalation of AITC (10mM) is mediated by the TRPA1 in our model. The TRPA1 selective antagonists HC-030031 (1mM) and AP-18 (1mM) substantially inhibited and abolished, respectively, the cough induced by AITC (10mM) (Fig. 2A). The inhibitory effect of AP-18 was nearly completely reversible (see legend for Fig. 2A). In control experiments AP-18 (1mM) did not have any effect on the citric acid-induced cough. Citric acid (0.4M) evoked 7.2±0.4 and 7.4±1.4 coughs/5min in the presence of vehicle and AP-18 (1mM), respectively (n=5, P=0.6, Wilcoxon signed rank test). Inhalation of AP-18 (1mM, 7 min) alone did not induce cough (data not shown).
Figure 2. Characterization of the TRPA1-mediated cough in the guinea pig.
(A) Inhibition of AITC-induced cough by TRPA1 antagonists HC-030031 and AP-18 (paired studies). Note that AP-18 abolished the cough induced by AITC. The inhibition of AITC-induced cough by AP-18 was reversible. After 5 min of recovery following the AP-18 study, AITC evoked 2.3±1.0 coughs /5min (control subtracted). The magnitude of cough is expressed as the number of coughs/5min above the appropriate control. For AITC alone the control is DMSO (1% in PBS), for AITC with a TRPA1 antagonist, the control is the TRPA1 antagonist with DMSO (1% in PBS). See METHODS for details. HC-030031 and AP-18 were tested in different groups of guinea pigs. *P<0.05 Wilcoxon signed rank test. (B) Extending AITC inhalation to 10 min did not reveal a more robust response to AITC (tested with two different vehicles, DMSO (1%) in PBS and ethanol (2%)/Tween80 (2%) in PBS, see Fig. 1 legend for more details).
Extending the inhalation of AITC (10mM) to 10 min did not reveal more robust coughing (Fig. 2B). Further extending the inhalation of AITC (10mM) to 20 min showed a modest desensitization. The number of coughs during the second 10 min interval was approximately 70% of the number of coughs during the first 10 min interval (71±14%, n=12, P<0.05, Wilcoxon signed rank test). The cough to AITC (10mM) was reproducible after 7 days. The number of coughs evoked by the repeated inhalation of AITC (10mM, 5 min) was 104±9% of the number of coughs evoked by the first challenge (n=11, P=0.9, Wilcoxon signed rank test).
3.2. The TRPA1 agonist AITC is less effective in inducing cough than the TRPV1 activators capsaicin and citric acid
The TRPV1 receptor selective agonist capsaicin (50μM) was approximately 3-times more effective in inducing cough than AITC (10mM) (Fig. 1A paired, Fig. 1B unpaired comparison). These data suggest that the activation of TRPV1 in the airway by inhaled TRPV1 agonist is substantially more effective to trigger cough than the activation of TRPA1. As expected the cough to capsaicin (50μM) was completely abolished by the selective TRPV1 antagonist I-RTX confirming that the action of capsaicin in the concentration of 50μM was selective for TRPV1 in our model. In an unpaired study, the number of coughs above control evoked by capsaicin (50μM, 5 min) in the absence and presence of I-RTX (30μM) was 8.2±2.1 (n=9) and −0.8±0.5 (n=6), respectively, (P<0.01, Mann Whitney test). We also evaluated another commonly used tussigen, citric acid that partially utilizes the TRPV1 receptor to evoke cough in cough models (Lalloo et al., 1995; Trevisani et al., 2004). Similarly to capsaicin, citric acid in the concentration of 0.4M, that is lower than its maximally effective concentration for evoking cough in our system, was also approximately 3-times more effective than AITC (10mM) (Fig. 1A paired, Fig. 1B unpaired comparison).
3.3. TRPA1 stimulation is less effective than TRPV1 stimulation in inducing sustained action potential discharge in the cough-triggering afferent nerves (jugular C-fibers) in the guinea pig trachea
One explanation for the lower efficacy of TRPA1 (compared to TRPV1) stimulation in triggering cough is that it is less effective in activating the airway afferent nerves that mediate cough. The cough in the guinea pig can be induced by stimulation of two afferent nerve fiber types: 1) the capsaicin-insensitive (TRPV1-negative) low threshold mechanosensitive nodose Aδ-fibers, and 2) the capsaicin-sensitive (TRPV1-positive) afferent jugular nerve fibers (mostly C-fibers) (Canning and Chou, 2009; Canning et al., 2004). Because the TRPA1 expression is often restricted to TRPV1-positive neurons (Nassenstein et al., 2008) we hypothesized that TRPA1 agonists activate jugular nerve fibers, but not nodose Aδ-fibers. Indeed, we found that AITC does not activate nodose Aδ-fibers (n=6). AITC (100μM, 20 min) evoked no activation in 5 nodose Aδ-fibers, and only a trivial response (frequency averaging <0.2Hz) in the remaining one nodose Aδ-fiber. All tested nodose Aδ-fibers were readily responsive to control mechanical stimulus (von Frey filament), but none responded to capsaicin (1μM, n=4)(Riccio et al., 1996). We therefore hypothesized that the TRPA1 stimulation is less effective than the TRPV1 stimulation in evoking action potential discharge in the tracheal jugular C-fibers. The concentrations of AITC and capsaicin were selected based on our previous studies in the vagal nerve terminals (Brozmanova et al., 2011; Kollarik and Undem, 2002).
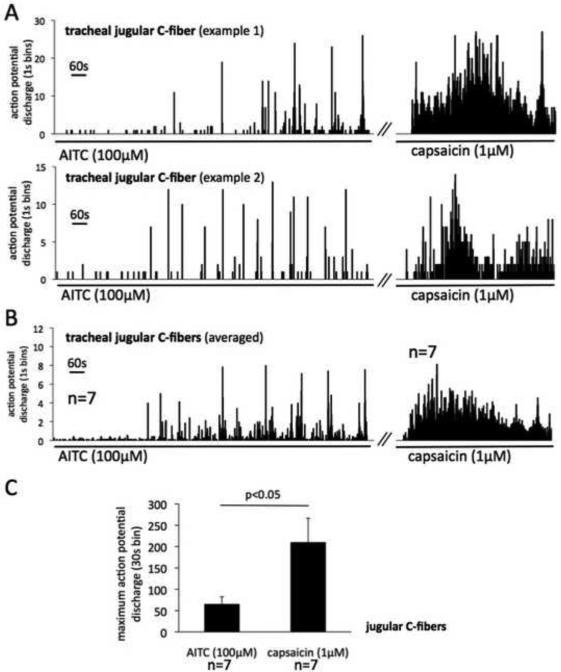
The majority (7/9) of the jugular C-fibers fibers responded to AITC (100μM, 20 min) with overt action potential discharge (the fibers studied had virtually no baseline activity) (Fig. 3). The two AITC-insensitive fibers responded to capsaicin (1μM). The time course of the AITC-induced action potential discharge showed an interesting pattern. Individual jugular C-fibers responded to AITC by episodic brief (1-3s) high frequency action potential bursts (peak frequency 23±7Hz, n=7 similar to that induced by capsaicin 1μM, 20±3Hz, n=7, P=0.7) with little discharge between the bursts. Two examples of such pattern are shown in figure 3A. This pattern of discharge resulted in relatively modest total activity over longer intervals. To assess the response over longer time intervals we analyzed the response in 30s intervals (maximum 30s bins, Fig. 3C). In contrast to AITC, capsaicin (1μM, 10min) evoked a robust more sustained action potential discharge (Fig. 3A) in all fibers tested (n=7). The sustained AITC-induced discharge was substantially (3-fold) smaller than that induced by capsaicin (Fig. 3C).
Figure 3. TRPA1 stimulation is less effective than TRPV1 stimulation in inducing sustained action potential discharge in the cough-triggering afferent nerves (jugular C-fibers) in the guinea pig trachea.
Extracellular recordings were made from the jugular neurons projecting C-fibers into the trachea in the isolated vagally-innervated guinea pig airway preparation. (A) Two representative examples of the jugular C-fiber response to AITC and capsaicin. Histograms show the time course of the AITC- and capsaicin-induced action potential discharge. Note that individual jugular C-fibers responded to AITC in episodic brief (1-3s) high frequency action potential bursts. In contrast, capsaicin evoked robust more sustained action potential discharge. The delay in the onset of the response is attributable to the method of the drug delivery (see discussion). (B) Averaged responses of jugular C-fibers to AITC (n=7) and capsaicin (n=7). Only the C-fibers responsive to AITC (7/9) and capsaicin (7/7) were included in analysis. Error bars are omitted for clarity. (C) Capsaicin is more effective than AITC to evoke sustained activation of the jugular C-fibers (P<0.05, unpaired T-test).
3.4. In addition to TRPA1, TRPV1 contributes to cough induced by inhaled cinnamaldehyde in naïve conscious guinea pigs
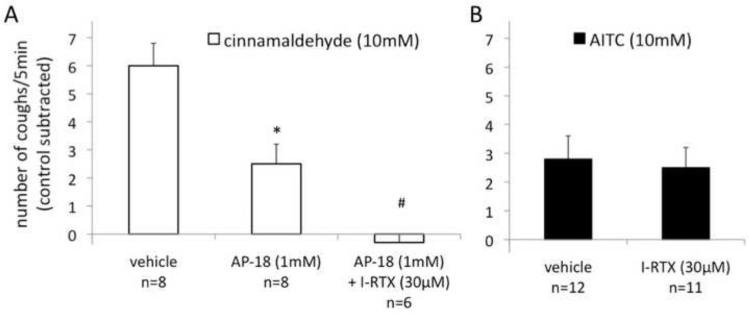
Another commonly used TRPA1 agonist cinnamaldehyde was also effective in evoking cough in our guinea pig model (Fig. 4). Surprisingly, we observed that cinnamaldehyde was approximately twofold more effective in inducing cough than AITC in the equimolar concentration of 10mM. We carried out two paired studies, each with different vehicle. In one study the coughing evoked by AITC (10mM) and cinnamaldehyde (10mM) was 2.8±0.8 and 6.0±0.9 coughs /5min (cough to vehicle 1% DMSO subtracted), respectively (n=12, P<0.01, Wilcoxon signed rank test). In another study the coughing was 2.6±0.5 and 4.3±0.5 coughs/5 min (cough to vehicle 2% ethanol/2% Tween80 subtracted), respectively (n=14, P<0.05, Wilcoxon signed rank test). This was an unexpected observation since cinnamaldehyde has been reported to have slightly lower potency and similar efficacy as AITC on cloned TRPA1 (Bandell et al., 2004). However, in contrast to AITC (10mM)-induced cough that was completely abolished by AP-18 (1mM), the cinnamaldehyde (10mM)-induced cough was only partially inhibited by AP-18 (1mM) (Fig. 4A). The AP-18 inhibitable component of the cinnamaldehyde-induced cough was quantitatively similar to cough induced by AITC (Fig. 1). Also, in contrast to AITC that was inhibited by HC-030031 (1mM) by approximately 70% (Fig. 1B), HC-030031 (1mM) inhibited the cinnamaldehyde (10mM)-induced cough only by approximately 40% (n=7, P<0.05, Wilcoxon signed rank test). Thus the antagonist data indicate that unlike AITC, cinnamaldehyde triggers cough through TRPA1 as well as another TRPA1-independent pathway.
Figure 4. In addition to TRPA1, TRPV1 contributes to cough induced by inhaled cinnamaldehyde in naïve conscious guinea pigs.
(A) Cough evoked by cinnamaldehyde was only partially inhibited by the selective TRPA1 antagonist AP-18, but was abolished by the combination of AP-18 and the selective TRPV1 antagonist I-RTX. (P<0.01 Kruskal-Wallis test, *P<0.05 vs. vehicle, #P<0.05 vs. AP-18, Dunn's test). The inhibition of cinnamaldehyde-induced cough by AP-18 was partially reversible (after 5 min of recovery following the antagonist study cinnamaldehyde evoked 5.4+1.2 coughs/5min, control subtracted). Vehicle, DMSO (1%) in PBS. (B) In control experiments I-RTX had no effect on AITC-induced cough (unpaired study, P>0.5, Mann Whitney test). Vehicle, DMSO (1%) in PBS.
Based on the consistent observation that the most effective known ion channel receptor mediating activation of jugular C-fibers is the multimodal TRPV1, we speculated that cinnamaldehyde may in some way lead to stimulation of TRPV1 in a complex organ such as the airway. We indeed found that the cinnamaldehyde (10mM)-induced cough was completely abolished by combination of AP-18 (1mM) and the TRPV1 antagonist I-RTX (30μM) (Fig. 4A). In control experiments I-RTX (30μM) had no effect on AITC (10mM)-induced cough (Fig. 4B).
We next evaluated activation of jugular nerve fibers by cinnamaldehyde. In preliminary experiment we noted that 100μM cinnamaldehyde (10min) caused only marginal activation of jugular fibers (n=5, data not shown). Therefore, we used cinnamaldehyde in the concentration of 300μM. Cinnamaldehyde (300μM) evoked overt action potential discharge in 5 of 7 jugular C-fibers tested. However, even in the concentration of 300μM cinnamaldehyde was not more effective than AITC (100μM) to induce sustained activation. The maximum action potential discharge in 30s bin evoked by cinnamaldehyde (300μM) and AITC (100μM) was 77±22 (n=5) and 65±18 (n=7, data from Fig. 3A), respectively (P>0.5, unpaired T-test). The peak frequency (the maximum action potential discharge in 1s bin) of the cinnamaldehyde (300μM)- and AITC (100μM)-induced discharge was also similar (19±6Hz vs. 23±6Hz, P>0.5, unpaired T-test). The activation induced by cinnamaldehyde (300μM) was inhibited by AP-18 by >80%. The maximum action potential discharge in 30s bin evoked by cinnamaldehyde (300μM) in the continuous presence of AP-18 (30μM) was 17±8 (n=6, P<0.05, unpaired T-test, compared to cinnamaldehyde alone, 77±22, n=5). Therefore, unlike the in vivo environment where TRPA1 and TRPV1 are involved in the cinnamaldehyde-evoked cough, when cinnamaldehyde is evaluated in a more isolated system it activates C-fibers mostly (perhaps entirely) via the TRPA1 receptor.
4. Discussion
We show that the stimulation of TRPA1 is notably (≈3-fold) less effective than the stimulation of TRPV1 in evoking cough in guinea pigs. We also show that similar to the TRPV1 agonists, the TRPA1 agonists activate jugular C-fibers, but not low threshold mechanosensitive nodose Aδ-fibers in the trachea. However, we found that the stimulation of TRPA1 is ≈3-fold less effective than the stimulation of TRPV1 in evoking sustained action potential discharge in tracheal C-fibers. These data are consistent with the conclusion that the mechanism underlying the lower tussive efficacy of TRPA1 agonists is their lower efficacy to induce sustained activation in the tracheal C-fibers. Our data also indicate that while the TRPA1 agonist AITC is selective for TRPA1 in vivo, another commonly used TRPA1 agonist cinnamaldehyde can induce cough in guinea pig not only by stimulating TRPA1 but also via a TRPA1-independent mechanism that involves TRPV1.
Our conclusion that TRPA1 is a modest activator of cough relative to TRPV1 in the guinea pig is based on pharmacological analysis by using two TRPA1 agonists (AITC and cinnamaldehyde), two TRPV1 agonists (capsaicin and citric acid) as well as selective TRPA1 and TRPV1 antagonists. We used the maximally effective concentration 10mM of AITC (Fig. 1A) which was selective for TRPA1(Fig. 1B). The efficacy of AITC in inducing cough was independent of the vehicle used (Fig. 1B). Consistent with previous studies, capsaicin caused cough by selectively stimulating TRPV1.
In order to induce cough, the concentration of an agonist in the solution nebulized in vivo needs to be relatively high in comparison to in vitro EC50. This can be attributed to dilution during the deposition of aerosol in the airways and diffusion in the tissue. Here we compared cough induced by capsaicin in the concentration of 50μM that is 300-1000-times its in vitro EC50 at TRPV1 receptor (0.05-0.2μM) and by AITC in the concentration of 10mM that is also about 300-1000-times its in vitro EC50 at TRPA1 receptor (10-30μM)(Bandell et al., 2004; Savidge et al., 2002). Thus, AITC and capsaicin concentrations were comparable in the terms of the potency on their respective receptors.
Cinnamaldehyde was more effective than AITC. This was surprising inasmuch as this was not observed previously in vivo (Andre et al., 2009), at the cloned TRPA1 receptors (see for example (Bandell et al., 2004; Bessac and Jordt, 2010)) or vagal nerve terminals (Brozmanova et al., 2011). Indeed we found that cinnamaldehyde, even in the concentration 3-fold higher (300μM) was similarly effective as AITC(100μM) to activate tracheal jugular C-fibers in the isolated tissue. Moreover, the activation of C-fibers by cinnamaldehyde was nearly abolished by AP-18 indicating that in the isolated airway preparation, cinnamaldehyde activates jugular fibers exclusively via the TRPA1 receptor. This is in agreement with the observation that cinnamaldehyde does not activate TRPV1 in expression systems (Bandell et al., 2004).
Unlike the cough to AITC, however, the cinnamaldehyde-evoked cough was only partially inhibited by AP-18. The portion of the cough response inhibited by AP-18 (i.e. the TRPA1-mediated portion) was relatively modest (Fig. 4) and similar to AITC-induced cough. Based on the knowledge that TRPV1 integrates multiple chemical stimuli and effectively triggers cough, we speculated that TRPV1 contributes to the non-TRPA1 component of cinnamaldehyde-induced cough in our model. We found that combination of AP-18 and the TRPV1 antagonist I-RTX(30μM) abolished the cough induced by cinnamaldehyde (Fig. 4A). I-RTX(30μM) had no effect on AITC-induced cough (Fig. 4B) showing that I-RTX did not influence TRPA1. Thus, it appears that unlike in the ex vivo isolated systems cinnamaldehyde can lead to activation of TRPV1 when inhaled in vivo in our guinea pig model.
It is unclear how cinnamaldehyde leads to stimulation of TRPV1 in our model. Perhaps conditions present in the airways in vivo (but not in vitro), such as factors from blood or air-liquid interface are required for the modification of cinnamaldehyde into a TRPV1 activator, or to allow cinnamaldehyde to cause release of TRPV1 activators. Previous study did not report TRPA1-independent effects of cinnamaldehyde (Andre et al., 2009). In that study, cinnamaldehyde was less effective than AITC to evoke cough (Fig. 3 in (Andre et al., 2009)) which is contrary to our observations. We addressed this issue by two separate randomized paired studies in which we used different batches of AITC and cinnamaldehyde and tested two different vehicles, and found cinnamaldehyde more effective than AITC. The reason for the discrepancy between our data and those in (Andre et al., 2009) is unclear.
Previous studies provide hints that TRPA1 activation is less effective in inducing cough than TRPV1 activation. Andre and colleagues (2009) reported the cough evoked by AITC and the selective TRPV1 agonist resiniferatoxin in the same system (Andre et al., 2009). The concentration of resiniferatoxin used in this study (0.5μM) was about 10-50-times of its reported EC50 at the guinea pig TRPV1 (0.01-0.06μM) (Savidge et al., 2002). However, the concentration of AITC required to evoke the same number of coughs was 10mM, i.e. 300-1000-times of its reported EC50 at TRPA1 receptor (10-30μM). AITC (1mM) was ineffective. Similarly, Birrell and colleagues (2009) reported that the concentration of the TRPA1 agonist acrolein required to consistently evoke cough in the guinea pig was 100mM which is ≥10,000 of the EC50 of acrolein at TRPA1 receptor (1-10μM)(Andre et al., 2008; Bautista et al., 2006; Birrell et al., 2009). Thus, high concentrations of TRPA1 agonists relative to their in vitro potency are required to evoke cough.
We selected AITC and cinnamaldehyde as the most widely used and, arguably, the best characterized effective agonists of TRPA1. To our knowledge, TRPA1 agonists with outstanding efficacy relative to AITC and cinnamaldehyde have not been reported although TRPA1 activators have been studied extensively. While it is impossible to exclude that a more efficient agonist will be identified in the future, this appears unlikely because of the unique mechanisms of TRPA1 activation that appears common to all TRPA1 agonists.
Cough in the guinea pig can be triggered by the activation of two vagal afferent nerve types in large airways: capsaicin-insensitive nodose Aδ-fibers and capsaicin-sensitive jugular afferent nerve fibers (mostly C-fibers). (Canning and Chou, 2009; Canning et al., 2004). We show that the nodose Aδ-fibers were not activated via TRPA1. In contrast, the TRPA1 agonists stimulated tracheal capsaicin-sensitive jugular C-fibers (Fig. 3). This finding is consistent with the conclusion that both capsaicin and AITC evoke cough via the activation of jugular afferent fibers. It is also in agreement with a recent observation that repeated capsaicin inhalations (predicted to desensitize the capsaicin-sensitive afferent nerves) nearly abolished cough to TRPA1 agonists (Andre et al., 2009).
Although both capsaicin and AITC activated tracheal jugular C-fibers, the action potential discharge pattern and the magnitude of overall activation were substantially different. AITC-induced activation consisted of episodic brief high frequency action potential bursts with little discharge between the bursts. This resulted in relatively modest total activity over longer intervals (Fig. 3C). In contrast, capsaicin evoked a more sustained action potential discharge that resulted in approximately 3-fold higher overall activity (Fig.3C). These data indicate that the mechanism underlying the difference between the TRPA1- and TRPV1-mediated cough is the lower efficacy of the TRPA1 stimulation than the TRPV1 stimulation to evoke sustained action potential discharge in the cough-triggering tracheal C-fibers (Fig. 3). The delay in the onset of activation is attributable to the time required to achieve desired concentration at receptive field (3-5min depending on location). This was more pronounced with AITC(100μM) that is in lower concentration ineffective to activate vagal nerves (Brozmanova et al., 2011), but less with capsaicin(1μM) that is effective at lower concentrations (Undem and Kollarik, 2002)
The information on the relative efficacy of TRPA1 vs. TRPV1 should aid those studying cough, inasmuch as capsaicin is the most common tool for evaluation of cough. That the stimulation of TRPA1 is less effective in inducing cough than the stimulation of TRPV1 has implication for testing of novel antitussive drugs that are TRPA1 inhibitors. For example, the TRPA1 antagonists may appear less effective in inhibiting cough in physiological models (i.e. naïve healthy animals) than in the models of inflammation in which TRPA1-mediated activation is likely potentiated. We have also demonstrated that of the cough-mediating airway afferent nerve subtypes it is the tracheal capsaicin-sensitive jugular subtype that is activated via TRPA1. Thus the drug strategies aimed at inhibiting this afferent nerve subtype (for example selective inhibition of jugular subtype excitability or neural plasticity) is predicted to be effective to inhibit both TRPV1- and TRPA1-mediated coughing. Finally, our data also indicate that caution is required when cinnamaldehyde is used as a selective TRPA1 activator in complex systems since it may have a non-TRPA1-mediated effects. In summary, our data force the conclusion that the TRPA1 activation is a modest cough trigger. It may be speculated, however, that the efficacy of TRPA1 stimulation to trigger cough is increased in inflammation.
Acknowledgements
This work was supported by CEVYPET (EU) and CEKR (EU). M.T. is supported by the Department of Health (Slovakia) Grant 2007/54-UK-15. M.K. is supported by DK074480 and HL062296.
Footnotes
Statement of conflicts of interest: None
The authors have no conflicts of interests.
References
- Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M, Baraldi PG, Poole DP, Bunnett NW, Geppetti P, Patacchini R. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest. 2008;118:2574–2582. doi: 10.1172/JCI34886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre E, Gatti R, Trevisani M, Preti D, Baraldi PG, Patacchini R, Geppetti P. Transient receptor potential ankyrin receptor 1 is a novel target for protussive agents. Br J Pharmacol. 2009;158:1621–1628. doi: 10.1111/j.1476-5381.2009.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bessac BF, Jordt SE. Sensory detection and responses to toxic gases: mechanisms, health effects, and countermeasures. Proc Am Thorac Soc. 2010;7:269–277. doi: 10.1513/pats.201001-004SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell MA, Belvisi MG, Grace M, Sadofsky L, Faruqi S, Hele DJ, Maher SA, Freund-Michel V, Morice AH. TRPA1 Agonists Evoke Coughing in Guinea- pig and Human Volunteers. Am J Respir Crit Care Med. 2009 doi: 10.1164/rccm.200905-0665OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozmanova M, Calkovsky V, Plevkova J, Bartos V, Plank L, Tatar M. Early and late allergic phase related cough response in sensitized guinea pigs with experimental allergic rhinitis. Physiol Res. 2006;55:577–584. doi: 10.33549/physiolres.930840. [DOI] [PubMed] [Google Scholar]
- Brozmanova M, Hanacek J, Tatar M, Strapkova A, Szepe P. Effects of hyperoxia and allergic airway inflammation on cough reflex intensity in guinea pigs. Physiol Res. 2002;51:529–536. [PubMed] [Google Scholar]
- Brozmanova M, Mazurova L, Ru F, Tatar M, Kollarik M. Abstract Book, SIXTH INTERNATIONAL COUGH SYMPOSIUM 2010. London: 2010. TRPA1 RECEPTOR-MEDIATED COUGH IN THE GUINEA PIG. [Google Scholar]
- Brozmanova M, Plevkova J, Tatar M, Kollarik M. Cough reflex sensitivity is increased in the guinea pig model of allergic rhinitis. J Physiol Pharmacol 59 Suppl. 2008;6:153–161. [PubMed] [Google Scholar]
- Brozmanova M, Ru F, Surdenikova L, Mazurova L, Taylor-Clark T, Kollarik M. Preferential activation of the vagal nodose nociceptive subtype by TRPA1 agonists in the guinea pig esophagus. Neurogastroenterol Motil. 2011;23:e437–445. doi: 10.1111/j.1365-2982.2011.01768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Chou YL. Cough sensors. I. Physiological and pharmacological properties of the afferent nerves regulating cough. Handb Exp Pharmacol. 2009;187:23–47. doi: 10.1007/978-3-540-79842-2_2. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol. 2004;557:543–558. doi: 10.1113/jphysiol.2003.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid SR, Crown ED, Moore EL, Liang HA, Choong KC, Dima S, Henze DA, Kane SA, Urban MO. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol Pain. 2008;4:48. doi: 10.1186/1744-8069-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti R, Andre E, Barbara C, Dinh TQ, Fontana G, Fischer A, Geppetti P, Trevisani M. Ethanol potentiates the TRPV1-mediated cough in the guinea pig. Pulm Pharmacol Ther. 2009;22:33–36. doi: 10.1016/j.pupt.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Grace MS, Belvisi MG. TRPA1 receptors in cough. Pulm Pharmacol Ther. 2010 doi: 10.1016/j.pupt.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Gu Q, Lin RL, Kryscio R, Lee LY. Calcium transient evoked by TRPV1 activators is enhanced by tumor necrosis factor-{alpha} in rat pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol. 2010;299:L483–492. doi: 10.1152/ajplung.00111.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, Theobald DL, Garrity PA. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 2010;464:597–600. doi: 10.1038/nature08848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollarik M, Undem BJ. Mechanisms of acid-induced activation of airway afferent nerve fibres in guinea-pig. J Physiol. 2002;543:591–600. doi: 10.1113/jphysiol.2002.022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalloo UG, Fox AJ, Belvisi MG, Chung KF, Barnes PJ. Capsazepine inhibits cough induced by capsaicin and citric acid but not by hypertonic saline in guinea pigs. J Appl Physiol. 1995;79:1082–1087. doi: 10.1152/jappl.1995.79.4.1082. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassenstein C, Kwong K, Taylor-Clark T, Kollarik M, Macglashan DM, Braun A, Undem BJ. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol. 2008;586:1595–1604. doi: 10.1113/jphysiol.2007.148379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassini R, Materazzi S, De Siena G, De Cesaris F, Geppetti P. Transient receptor potential channels as novel drug targets in respiratory diseases. Curr Opin Investig Drugs. 2010;11:535–542. [PubMed] [Google Scholar]
- Petrus M, Peier AM, Bandell M, Hwang SW, Huynh T, Olney N, Jegla T, Patapoutian A. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain. 2007;3:40. doi: 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plevkova J, Kollarik M, Brozmanova M, Revallo M, Varechova S, Tatar M. Modulation of experimentally-induced cough by stimulation of nasal mucosa in cats and guinea pigs. Respir Physiol Neurobiol. 2004;142:225–235. doi: 10.1016/j.resp.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Riccio MM, Kummer W, Biglari B, Myers AC, Undem BJ. Interganglionic segregation of distinct vagal afferent fibre phenotypes in guinea-pig airways. J Physiol. 1996;496(Pt 2):521–530. doi: 10.1113/jphysiol.1996.sp021703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savidge J, Davis C, Shah K, Colley S, Phillips E, Ranasinghe S, Winter J, Kotsonis P, Rang H, McIntyre P. Cloning and functional characterization of the guinea pig vanilloid receptor 1. Neuropharmacology. 2002;43:450–456. doi: 10.1016/s0028-3908(02)00122-3. [DOI] [PubMed] [Google Scholar]
- SRC . PhysProp Database. SCR; 2009. http://www.srcinc.com/what-we-do/databaseforms.aspx?id=386, http://www.srcinc.com/ [Google Scholar]
- Taylor-Clark TE, Ghatta S, Bettner W, Undem BJ. Nitrooleic acid, an endogenous product of nitrative stress, activates nociceptive sensory nerves via the direct activation of TRPA1. Mol Pharmacol. 2009a;75:820–829. doi: 10.1124/mol.108.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark TE, Kiros F, Carr MJ, McAlexander MA. Transient receptor potential ankyrin 1 mediates toluene diisocyanate-evoked respiratory irritation. Am J Respir Cell Mol Biol. 2009b;40:756–762. doi: 10.1165/rcmb.2008-0292OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark TE, McAlexander MA, Nassenstein C, Sheardown SA, Wilson S, Thornton J, Carr MJ, Undem BJ. Relative contributions of TRPA1 and TRPV1 channels in the activation of vagal bronchopulmonary C-fibres by the endogenous autacoid 4-oxononenal. J Physiol. 2008a;586:3447–3459. doi: 10.1113/jphysiol.2008.153585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark TE, Nassenstein C, McAlexander MA, Undem BJ. TRPA1: A potential target for anti-tussive therapy. Pulm Pharmacol Ther. 2009c;22:71–74. doi: 10.1016/j.pupt.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark TE, Undem BJ. Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. J Physiol. 2010;588:423–433. doi: 10.1113/jphysiol.2009.183301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark TE, Undem BJ, Macglashan DW, Jr., Ghatta S, Carr MJ, McAlexander MA. Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1). Mol Pharmacol. 2008b;73:274–281. doi: 10.1124/mol.107.040832. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Milan A, Gatti R, Zanasi A, Harrison S, Fontana G, Morice AH, Geppetti P. Antitussive activity of iodo-resiniferatoxin in guinea pigs. Thorax. 2004;59:769–772. doi: 10.1136/thx.2003.012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andre E, Patacchini R, Cottrell GS, Gatti R, Basbaum AI, Bunnett NW, Julius D, Geppetti P. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undem BJ, Chuaychoo B, Lee MG, Weinreich D, Myers AC, Kollarik M. Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol. 2004;556:905–917. doi: 10.1113/jphysiol.2003.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undem BJ, Kollarik M. Characterization of the vanilloid receptor 1 antagonist iodo-resiniferatoxin on the afferent and efferent function of vagal sensory C-fibers. J Pharmacol Exp Ther. 2002;303:716–722. doi: 10.1124/jpet.102.039727. [DOI] [PubMed] [Google Scholar]