Abstract
It has been about 30 years since the first plant engineering technology was established. Although the concept of plant-based pharmaceuticals or vaccines motivates us to develop practicable commercial products using plant engineering, there are some difficulties in reaching the final goal: to manufacture an approved product. At present, the only plant-made vaccine approved by the United States Department of Agriculture is a Newcastle disease vaccine for poultry that is produced in suspension-cultured tobacco cells. The progress toward commercialization of plant-based vaccines takes much effort and time, but several candidate vaccines for use in humans and animals are in clinical trials. This review discusses plant engineering technologies and regulations relevant to the development of plant-based vaccines and provides an overview of human and animal vaccines currently under clinical trials.
Keywords: GMP-compliant, human vaccine, plant-based vaccine, plant transformation, veterinary vaccine
Introduction
In the past quarter century, plant genetic engineering technologies have progressed dramatically. Barta and colleagues were the first to transcribe a chimeric gene of nopaline synthase and human growth hormone in sunflower and tobacco plants using the Ti plasmid [Barta et al.1986]. Shortly thereafter, mouse monoclonal antibody was produced and functionally assembled in tobacco leaf segments [Hiatt et al. 1989]. As bioreactors, plants may yield high amounts of recombinant proteins; these proteins are not contaminated with pathogens of animals or humans and can be stored without refrigeration at low cost. A number of recombinant proteins have been produced in plants, and the production of protein-based pharmaceuticals has partially shifted from bacterial, fungal, and mammalian cell cultures to plants and plant cell cultures [Lico et al. 2012; Merlin et al. 2014; Twyman et al. 2005]. Commercialized enzymes and reagents produced in plants are available. For instance, human type I collagen, which can self-assemble into fine homogenous fibrils, is manufactured in tobacco plants [Shoseyov et al. 2014]. Bovine trypsin produced in maize, TrypZean (Sigma-Aldrich), has been on the market since 2002. TrypZean is particularly useful in animal cell cultures because it has no contaminants of animal origin. Rice has been used to manufacture human lysozyme and lactoferrin [Hennegan et al. 2005; Yang et al. 2002]. Protalix, an Israeli company, has developed a method to produce plant-based biopharmaceuticals in cultured transgenic carrot or tobacco cells [van Dussen et al. 2013; Zimran et al. 2011]. In 2012, Protalix and its partner Pfizer received approval from the United States Food and Drug Administration (FDA) of the United States for taliglucerase alfa for Gaucher’s Disease.
On the other hand, plant-based human vaccines are not yet commercialized, although production of dozens of viral and bacterial subunit vaccines is attempted in transgenic plants. Recombinant subunit vaccines are safer than traditional vaccines, because they contain no live pathogens. Various plants such as tobacco, rice, maize, potato, alfalfa, lettuce, tomato, carrot, peanut, and soybean are used as hosts for gene introduction, which is achieved in vitro by using protoplast or cell culture, or hairy root culture. Nuclear or chloroplast genome recombination is routinely used to obtain transgenic plants. The choice of the plant species and technology determines the vaccine administration route because some plants can be consumed only when processed, whereas heat or pressure treatments may destroy the antigen. Cereal crops are attractive for subunit vaccine production because vaccines produced in seeds are stable over long storage periods [Hefferon, 2013].
There are two options for vaccine administration: injection (intramuscular or subcutaneous) and mucosal (oral or nasal) administration. Injection-type vaccines elicit strong protective immunity by preferentially inducing IgG production. They are most suitable against pathogens that infect via a systemic or respiratory route; however, the antigens have to be purified before administration. These vaccines are often produced in tobacco plants using transient expression.
Oral- or nasal-type vaccines induce mucosal and systemic immunity [Azegami et al. 2014; Lamichhane et al. 2014]. In a conceptual sense, oral plant-based vaccines are ideal because the manufacturing process is simple; no additional medical devices are needed for injection; and the antigen immunogenicity and biological activities are preserved in the gastrointestinal tract due to their natural bioencapsulation in a plant cell organelle. Oral plant-based vaccines have been developed in edible plants, including rice, maize, potato, lettuce, and carrot. Once these vaccines pass through the gastric environment and reach the small intestine, antigens are incorporated into M cells in the follicle-associated epithelium (FAE) for the induction of mucosal and systemic immune responses [Azegami et al. 2014; Holmgren and Czerkinsky, 2005].
This review discusses technologies and regulations in the development of plant-based vaccines and recent achievements in the production of vaccines that are already or expected to be under clinical trials and are intended for worldwide distribution in the near future.
Recombinant technologies
To use plants as bioreactors for commercial vaccines, one needs to (a) attain a high expression level of recombinant genes, (b) be able to quickly and easily design and produce new antigens in response to new pathogen subtypes, and (c) identify the genes to be transfected and ensure the safety of produced proteins for use in humans or animals.
Agrobacterium-based nucleus transformation
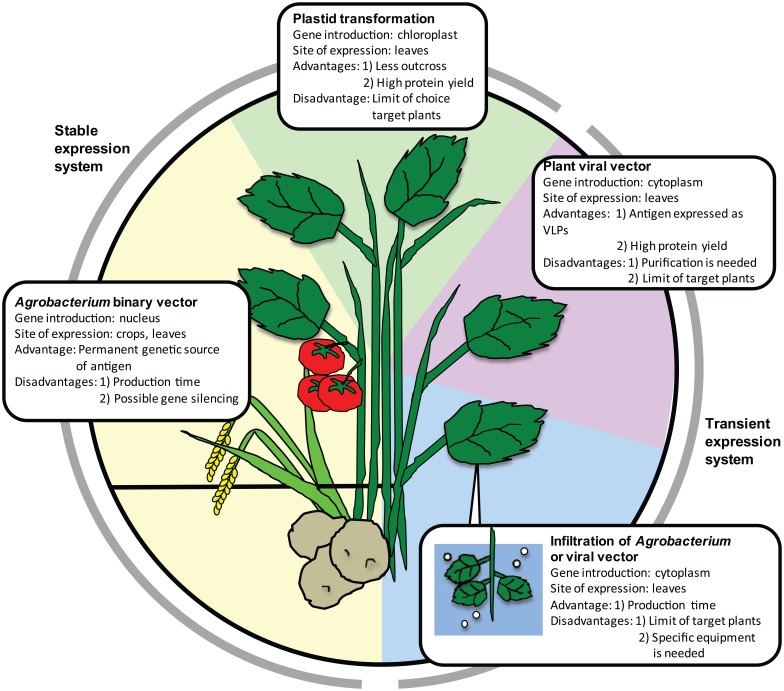
Plant recombinant technologies are being constantly improved and diversified [Hefferon, 2014] (Figure 1). Genes can be introduced into plants either directly or by using the gram-negative bacterium Agrobacterium tumefaciens. Plant transformation with polyethyleneglycol or by electroporation usually includes protoplast preparation by removing the cell wall, which requires time and skill. Almost half of plant transformation technologies use A. tumefaciens, which infects plants naturally. The T-DNA region between the left and right borders of the A. tumefaciens Ti plasmid is introduced into plant genome and transcribed in the plant cell; this process induces abnormal plant hormone production, resulting in crown gall disease. The T-DNA region can be replaced with a gene of interest, and the Ti plasmid has been modified into a binary vector that can be manipulated in Escherichia coli [Bevan, 1984]. Selection pressure is used to establish stable integration of the gene of interest in the nuclear genome. Once the transgenic line stably producing the target protein is established, it can be used as a permanent source of vaccine and established as a master seed bank. A stable and characterized line can be adopted as Good Manufacturing Practice (GMP)-compliant production. Yet, the development of such lines is time-consuming and can be complicated by gene silencing, host genome damage, or hybridization with non-transgenic crops cultivated without strict regulations.
Figure 1.
Plant transgenic technologies and their advantages and disadvantages.
VLP, virus-like particle.
Plastid transformation
A new technology, which targets the chloroplast genome instead of the nuclear genome, is now available. Chloroplasts originate from cyanobacteria that were incorporated into algae. Chloroplast and nuclear genomes coevolved, and now chloroplast genomes possess only 100–250 genes, which is smaller than nuclear genomes. The chloroplast genome is maternally inherited, and plants can stably produce protein without transgene outcrossing via pollen. Multiple chloroplasts with a high transgene copy number can accumulate large amounts of recombinant protein (as much as 70% of total leaf protein [Oey et al. 2009]). Foreign genes are usually transformed into chloroplast DNA by biolistic process or polyethylene glycol treatment of protoplasts [Cardi et al. 2010]. For plastid transformation, a target gene and selectable marker genes are placed between the two flanking sequences originated from the chloroplast genome to induce homologous recombination between the vector and plastid genome [Verma and Daniell, 2007; Scotti et al. 2012]. The chloroplasts of tobacco and other leafy plants such as carrot, petunia, and lettuce have mainly been used; nonphotosynthetic plant organs are less efficient in producing target proteins [Rigano and Walsley, 2005; Verma and Daniell, 2007].
Transient expression with plant virus expression vectors
The benefit of transient expression using plant viral vectors is their high replication ability in the target plant, resulting in high vaccine yield. Several plant viruses are used for this purpose: tobacco mosaic virus (TMV), cowpea mosaic virus (CPMV), potato virus (PVX), alfalfa mosaic virus, and plum pox virus [Salazar-González et al. 2015]. First-generation plant viral expression vectors encode almost all viral complements; the gene encoding the protein or peptide of interest is placed downstream of viral polymerase, movement protein, and coat protein genes. This technology has been employed to produce various plant-made vaccines, such as those against human papilloma virus [Cerovska et al. 2012; Noris et al. 2011] and influenza virus [Ravin et al. 2012; Shoji et al. 2011], by modifying PVX or TMV and a vaccine against norovirus [Lai and Chen, 2012] by modifying TMV. These recombinant viruses retain infectivity to plants and then shed the transgene, which may spread to other plants, thus prompting safety concerns. Second-generation viral vectors, which are safe in natural environments, have been developed. These vectors rely on an integrated system that has the minimal number of viral elements required for vector replication, whereas other functions, such as DNA delivery, are provided by nonviral elements. These ‘deconstructed’ expression vectors usually provide higher yields than those attained with full-virus vectors [Peyret and Lomonossoff, 2013; Salazar-González et al. 2015].
Infiltration technologies for transient expression
New time-saving technologies to introduce recombinant genes into plants have also been developed. Protocols called ‘agroinfiltration’ and ‘magnifection’ use vacuum or syringe to infiltrate leaves of 6-week-old plants such as Nicotiana benthamiana or Arabidopsis with Agrobacterium containing either binary vectors or deconstructed viral vectors [Leuzinger et al. 2013]. The use of agroinfiltration in vaccine production was pioneered by the Canadian biotechnology company Medicago, which developed virus-like particle (VLP) vaccines for influenza HA antigens, and these vaccines were used in a clinical trial [Landry et al. 2010]. Antigenicity of human and animal viruses is often determined by the conformation of their surface proteins, and to acquire protective immunity, it is desirable to express antigens in a VLP form [Kushnir et al. 2012; Vacher et al. 2013]. Agroinfiltration uses suspensions of A. tumefaciens; this method allows the production of large amounts of vaccine proteins within a few days to a couple of weeks, which is much faster than in stable expression systems [Leuzinger et al. 2013]. Another advantage of this protocol is that a variety of T-DNA vectors can be used. Agroinfiltration has also been applied to other leafy plants, such as lettuce [Chen et al. 2013].
‘Magnifection’ has been developed to address various safety concerns, namely the use of intact viral expression vectors and possible transgene loss during systemic spreading. It combines the agroinfiltration method with the delivery of cDNA encoding a ‘deconstructed’ TMV-based vector [Gleba et al. 2004, 2005, 2014; Marillonnet et al. 2004]. The magnifection system is restricted to N. benthamiana. Icon Genetics, a German plant biotechnology company, has developed and adapted this technology as MagnICONTM for the manufacturing of various plant-based vaccines, including high amounts of hepatitis B virus (HBV) surface antigen (HBsAg; up to 300 mg/kg N. benthamiana fresh leaves) in the form of VLP [Huang et al. 2006], norovirus capsid proteins [Scotti and Rybicki, 2013; Rybicki, 2014], and non-Hodgkin lymphoma vaccines, which proceeded to a phase I clinical trial (Table 1) [McCormick et al. 2008].
Table 1.
GMP-compliant plant factories for biopharmaceuticals.
| Company | Location | Plant | Bioproduct |
|---|---|---|---|
| Kentucky BioProcessing LLC (KBP) | Owensboro, KY, USA | Tobacco, potato | Norovirus VP1 Ebola virus antibody (ZMapp) |
| Sigma-Aldrich Fine Chemicals | St. Louis, MO, USA | Maize | Trypsin |
| Medicago Inc. | Quebec, Canada | Nicotiana benthamiana | Influenza HA-VLP |
| Protalix | Carmiel, Israel | Carrot cells, tobacco cells | Alphataliglicerase |
| Caliber Biotherapeutics LLC | Byran, TX, USA | Tobacco | Influenza HA |
| Fraunhofer CMB USA | Newark, DE, USA | Nicotiana benthamiana | Influenza HA |
| Fraunhofer IME | Aachen, Germany | Tobacco | Antibody (for HIV) |
| National Institute of Advanced Industrial Science and Technology | Hokkaido, Japan | Strawberry | Canine interferon alpha |
| Institute of Medical Science, The University of Tokyo | Tokyo, Japan | Rice | Cholera toxin B subunit |
A group from Fraunhofer USA Center for Molecular Biotechnology (CMB) has developed ‘launch vector’, an advanced gene infiltration system that combines the elements of TMV vector and A. tumefaciens binary plasmids [Musiychuk et al. 2007]. The hybrid launch vector pBID4 contains the 35S promoter from cauliflower mosaic virus (35S CaMV) that drives transcription of the viral genome, the nopaline synthase (nos) terminator, genes for virus replication and cell-to-cell movement proteins, and the target gene cloned under the transcriptional control of the coat protein subgenomic mRNA promoter. Following infiltration, primary transcripts produced in the nucleus are transported into the cytoplasm, resulting in robust protein production [Musiychuk et al. 2007].
The pEAQ system is based on full-length or truncated versions of CPMV RNA-2 and permits efficient and rapid protein production without viral replication [Peyret and Lomonossoff, 2013; Sainsbury et al. 2009]. In pEAQ system, a series of small binary vectors, which may be used for production of a wide variety of proteins in both transient and stable expression systems, was engineered. These vectors contain the 35S CaMV promoter, nos terminator, the P19 sequence encoding a suppressor of silencing, and 5′- and 3′-UTRs from CPMV RNA-2. The gene of interest is inserted between the UTRs. A new-generation vector, pCPMV-HT, provides extremely high translational efficiency and, consequently, a high level of the recombinant protein [Peyret and Lomonossoff, 2013].
Other methods with improved performance of transfection
Engineering of genetically modified plants without the use of antibiotic resistance genes as selection markers eliminates the potential risk of transfer of these genes to gut microbes when the vaccine is orally administered or to the environment. Using Agrobacterium-mediated nucleus transformation, Mejima and colleagues cotransformed the selection marker hygromycin phosphotransferase gene and cholera toxin B subunit gene encoded by separate T-DNA vectors and selected marker-free candidate plants by segregation in the seed progeny [Mejima et al. 2015]. Daniell and colleagues used betaine aldehyde dehydrogenase (BADH) gene from spinach as a selectable marker for plastid transformation [Daniell et al. 2001]. This enzyme is naturally produced in the chloroplast and converts toxic betaine aldehyde into non-toxic glycine betaine. BADH transgenic tobacco plant showed higher BADH activity than did nontransgenic plants, indicating that transgenic plants could be selected by the level of enzyme activity.
Knocking down mRNAs for rice storage proteins, glutelin and 13K prolamin, by RNA interference (RNAi) using the same T-DNA vector enhanced the production of botulinum neurotoxin A or cholera toxin B subunit in rice seeds [Kuroda et al. 2010; Kurokawa et al. 2013; Yuki et al. 2012]. Other options to increase protein production in plants include modification of codon usage from that of the host to that of the plant [Hiwasa-Tanase et al. 2011; Jackson et al. 2014], intron introduction in TMV before the target gene [Chakravarthi et al. 2015], and co-expression of a suppressor of gene silencing [Garabagi et al. 2012; de Ronde et al. 2014].
N-glycosylation is relatively well-conserved in eukaryotes; however, there are several sugar-modification enzymes specific to plants. Knockout of genes for plant-specific N-glycan-processing enzymes and the introduction of the enzymatic machinery catalyzing synthesis, transport, and addition of mammalian sugars have been reported [Gomord et al. 2010]. Whereas this strategy is effective for producing native forms of viral antigens, unexpected N-glycosylation of vaccine antigens of bacterial origin produced in plants may result in molecular heterogeneity and difficulties in recognition by immune cells. Because N-glycosylation occurs at asparagine residues, the substitution of asparagine with aspartic acid or another amino acid can solve this problem [Yuki et al. 2013].
Risk analysis and regulations
Potential risks
Several considerable risks are associated with plant-based pharmaceuticals [Kirk et al. 2005]. Plant-made vaccines, particularly the oral ones, might induce either allergenicity or oral tolerance, which are two conflicting phenomena. Post-translational modifications such as N-glycosylation might induce allergic responses, whereas co-administration with oral adjuvants to broadly stimulate the mucosal immune system might induce hypersensitive responses to other proteins in daily food [Guan et al. 2013]. Frequent administration of plant-made vaccines via the oral route can enhance regulatory T-cell activation against vaccine antigen [Fujihashi et al. 1999], as seen in successful hyposensitization therapy by oral antigen intake in cases of pollen allergy or food allergy [Cox et al. 2012; Sato et al. 2014].
Another potential risk of the use of plant-made vaccines is their influence on the environment. Small-scale production of genetically modified plants (including their production for research purposes) must be managed. Even manufacturing in regulated facilities to control the quality and safety of the products may pose difficulties in the use of genetically modified plants. Another aspect is that open-field production of stably transfected plants increases the possibility of contamination of nontransgenic crops intended for human or animal consumption [Bawa and Anilakumar, 2013; Fischer et al. 2012]. Recombinant genes can spread to field crops via pollen and accidentally result in genetic modifications in nontarget plants. Genetically modified plants might be eaten by wild animals or accidentally harvested by humans. Contact with insects and the release of contaminated water to the environment are also possible mechanisms for DNA or antigen escape. The probability and severity of each risk will depend on the plant species and the antigen and has to be determined on a case-by-case basis for each plant-based vaccine.
Government regulations
In the United States, the FDA ensures the safety for both manufacturing and clinical use of plant-based biopharmaceuticals and vaccines; it also approves and licenses them. Similar to other biopharmaceuticals, plant-based vaccines should be free of impurities, including other transgenes and resistance marker products, which must all be evaluated under the same criteria. The FDA also approves GMP facilities for plant manufacturing. In 2005, the World Health Organization (WHO) conducted an ‘informal consultation on scientific basis for regulatory evaluation of candidate human vaccines from plants’ [van der Laan et al. 2006]. In its report, the WHO recommended that the guidelines on Good Agricultural and Collection Practices (GACP), which are typically applied to herbal plants, should also be applied to plants producing biopharmaceuticals. A report of quality-control methods for medicinal plant materials recommended tests to assess the identity, purity, and content of biopharmaceutical plant materials. The United States Department of Agriculture (USDA) also plays a key role in the introduction of plant-made pharmaceuticals. The USDA approves veterinary biologics such as vaccines. The USDA considers the nature of the plant, the probability of cross-contamination, and the genetic background of stably transfected plants. The USDA also considers the risk-management strategies, taking into account physical and geographical aspects and plant reproduction.
The European Medicines Agency (EMA) published draft guidance notes on ‘the quality of biological active substances produced by stable transgene expression in higher plants’ [EMA, 2008], which complement the FDA and USDA regulations. However, these notes cover only plants with stable expression of transgenes and exclude transiently transfected plants and plant cell cultures. The use of several transient expression technologies, which rely on plant viral vectors or agroinfiltration (or both), requires advanced regulation applicable to all plant transgenic technologies. An interesting point in the EMA guidelines is a statement of the importance of the establishment of master and working seed banks from the final transformant. Both banks should be well-characterized in respect to the transgene (sequence, integrity, site of insertion, copy number, and the fate of the marker sequence), recombinant protein expression (tissue and organ specificity, regulation, and expression level), and unintended changes in the levels of endogenous plant proteins. The storage properties (conditions, shelf-life, and closure criterion) of the master transgenic bank should be defined also. The authorities of European Food Safety Authority (EFSA) and EMA partially overlap. The former cares for the cultivation of transgenic plants, and the latter cares for biopharmaceutical products from transgenic plants. Importantly, EMA regulatory guidelines [EMA, 2008] indicate that all biopharmaceutical products intended for phase I trials should be manufactured according to GMP. Thus, GMP compliance appears to be a key point in developing plant-based vaccines for clinical use.
GMP facilities for plant bioreactors
Production of recombinant proteins used in pharmaceutical applications requires certain quality standards. The GMP grade is compulsory for clinical applications. At least two large biomanufacturing facilities are capable of producing plant-derived HA proteins under GMP conditions, Fraunhofer CMB [Shoji et al. 2011, 2015] in the United States and Medicago Inc. in Canada [Yusibov et al. 2014]. Fraunhofer CMB is situated in Delaware. It possesses a GMP pilot plant that is capable of dealing with regulatory and clinical affairs and technology transfer. The key processing areas in the GMP plant are equipped with complete processing cycles for transient expression in plants, such as plant and bacterium cultivation, infiltration, plant harvest, and protein purification. The major product in this facility is influenza HA antigen, which is produced by using plant-based Proficia™ technology, VLPs, and the VLPExpress™ platform for transient expression in N. benthamiana. Medicago Inc., located in Québec, is now proceeding to phase II clinical trial with influenza VLP (H5) produced in N. benthamiana by using the agroinfiltration method (see http://www.medicago.com). Rabies and rotavirus antigens produced by Medicago Inc. are at a preclinical stage. Some university-launched plant-made vaccines that reached phase I clinical trials have been produced in collaboration with GMP facilities. Norovirus capsid protein subunit vaccine produced in potato tubers was developed in Arizona State University and manufactured at Kentucky BioProcessing GMP plant [Tacket et al. 2000]. In Japan, rice-based cholera vaccine has been developed at the Institute of Medical Science, The University of Tokyo (IMSUT); test vaccine produced at the GMP facility at IMSUT is now in a phase I clinical trial [Yuki et al. 2013; Mejima et al. 2015] (Table 1).
Human vaccines in clinical trials
Only a few plant-based human vaccines have reached clinical trials (Table 2). Non-toxic B subunit of heat-labile enterotoxin (LTB) of enterotoxigenic E. coli (ETEC) produced either in potato or maize was administered orally to healthy volunteers to examine its safety and immunogenicity. Raw, diced, transgenic potato tubers containing LTB (0.4 or 1.1 mg) were given to volunteers on days 0, 7, and 21 [Tacket et al. 1998, 2007; Yusibov et al. 2011]. For maize-derived LTB, the clinical study was placebo-controlled, and each group received 2.1 g of either transgenic or wild-type maize germ meal suspended in water on days 0, 7, and 21 [Tacket et al. 2004, 2007]. No adverse effects of vaccination were noticed in comparison with the placebo control. LTB-specific IgA-secreting cells were detected in peripheral blood 1 week after the first vaccination. Serological survey revealed that vaccinated volunteers had increased levels of LTB-specific serum IgG (91% of the volunteers) and IgA (55%) on day 59.
Table 2.
Plant-based human vaccines in clinical trials.
| Pathogen or disease | Antigen | Plant | Expression system | Administration route | Clinical trial | Reference |
|---|---|---|---|---|---|---|
| Enterotoxigenic E. coli | LTB | Potato | Transgenic | Oral | Phase I | Tacket et al. [1998] |
| Enterotoxigenic E. coli | LTB | Maize | Transgenic | Oral | Phase I | Tacket et al. [2004] |
| Norovirus | Capsid protein | Potato | Transgenic | Oral | Phase I | Tacket et al. [2000] |
| Hepatitis B virus | Viral major surface protein | Lettuce | Transgenic | Oral | Phase I | Kapusta et al. [1999] |
| Hepatitis B virus | Viral major surface protein | Potato | Transgenic | Oral | Phase I | Thanavala et al. [2005] |
| Rabies virus | Glycoprotein and nucleoprotein (fusion) | Spinach | Viral vector (transient) | Oral | Phase I | Yusibov et al. [2002] |
| Influenza virus (H5N1) | HA | Nicotiana benthamiana | Launch vector (transient) | Intramuscular | Phase I | Chichester et al. [2012] |
| Influenza virus (H1N1; 2009 pandemic) | HA | Nicotiana benthamiana | Launch vector (transient) | Intramuscular | Phase I | Cummings et al. [2014] |
| Influenza virus (H5N1) | HA (H5; VLP) | Nicotiana benthamiana | Agrobacterial binary vector (transient) | Intramuscular | Phase IPhase II | D’Aoust et al. [2008] Landry et al. [2010] |
| Influenza virus (H7N9) | HA (H7; VLP) | Nicotiana benthamiana | Agrobacterial binary vector (transient) | Intramuscular | Phase I | Medicago Inc. (http://www.medicago.com) |
| Influenza virus | HA (VLP) (seasonal; quadrivalent) | Nicotiana benthamiana | Agrobacterial binary vector (transient) | Intramuscular | Phase I | Medicago Inc. (http://www.medicago.com) |
| Cholera | CTB | Rice | Transgenic | Oral | Phase I | Nochi et al. [2009] |
| Yuki et al. [2013] |
Norovirus capsid protein VP1 was produced in potato tubers in the same way as LTB. As norovirus is a nonenveloped virus, VP1 is the only protein of the capsid. Vaccination with approximately 500 µg of recombinant VP1 was done on days 0, 7, and 21 (three doses) or on days 0 and 21 (two doses). Some volunteers had VP1-specific antibodies before the study because norovirus is highly infectious, and repeated epidemics are frequent, especially in winter [Campos and Lees, 2014; Teunis et al. 2008]. However, 20% of vaccinated volunteers developed VP1-specific serum IgG titers. The geometric mean of IgG titers (four responders) was 1:67 before vaccination and 1:757 after vaccination [Tacket et al. 2000].
According to the WHO, almost 780 000 people die every year from HBV infections worldwide. In different regions, 1–10% of the adult population are chronically infected with HBV, whereas 80–90% of infants infected under the age of 6 months develop chronic infections that lead to cirrhosis or liver cancer [MacLachlan and Cowie, 2015; Norkrans, 1990]. HBV surface antigen (HBsAg) was produced in plants. HBsAg transgenic lettuce leaves (0.1–0.5 µg of HBsAg per 100 g of fresh tissue) were given to adult volunteers (initially 200 g, then 150 g within 2 months). Two of three vaccinated volunteers showed transient protective levels of HBsAg-specific IgG (above 10 IU/l) 2 weeks after the second vaccination, but no HBsAg-specific serum IgA was detected [Kapusta et al. 1999].
Another clinical study was conducted with oral HBsAg produced in transgenic potato [Thanavala et al. 2005]. All volunteers enrolled in this study had received three doses of HBV injection-type vaccine within 15 years. The placebo group was given nontransgenic potato, the two-dose group was vaccinated at 0 and 28 days with 100 g of transgenic potato (850 ± 210 µg of antigen), and the three-dose group was vaccinated with the same doses at 0, 14, and 28 days. The authors found that 52.9% of participants in the two-dose group and 62.5% of participants in the three-dose group had elevated serum HBsAg antibody titers over the 70-day follow-up period after the first immunization.
Current human rabies vaccines are efficacious both pre- and post-exposure to rabies virus [Toovey, 2007]. Endemic rabies is spreading all over the world except in a few countries. To reduce mortality caused by rabies, a regular stock of safe rabies vaccine in endemic areas is needed. Antigenic determinants of rabies virus G and N proteins have been mapped, and a synthetic chimeric peptide (G5–24-N31D) containing a linear epitope of G protein and an epitope of N protein was found to be immunogenic in mice [Dietzschold et al. 1990]. Yusibov et al. [2002] fused fragments encoding a chimeric protein of G protein (amino acids 253–275) and N protein (amino acids 414–418) with that of alfalfa mosaic virus coat protein and introduced this fusion construct into TMV lacking native coat protein. Spinach was infected with recombinant virus to obtain transient expression of the chimeric rabies peptide. Three of 5 volunteers previously vaccinated with a commercial injection-type vaccine had elevated rabies-specific IgG after having ingested 3 doses of spinach (20 g; 84 µg of chimeric rabies peptide each) at 2-week intervals. Another protocol involved volunteers with no history of rabies vaccination; five of nine participants responded to the rabies antigen. An additional single dose of commercial vaccine enhanced rabies virus-neutralizing antibody production (three out of nine participants). Regardless of the vaccination order, spinach oral rabies vaccine in combination with currently available vaccines might enhance immunity against rabies virus [Yusibov et al. 2002].
Influenza virus has 16 hemagglutinin (HA) subtypes, and even in the strains with the same subtype, antigenic shift often occurs to abolish cross-protective immunity of the host. For example, in 2009, H1N1-type influenza virus became pandemic [Itoh et al. 2009; Neumann et al. 2009]. To control its spread by vaccination, fast production of a new HA antigen was required. As mentioned above, Medicago Inc. developed the technology to produce VLPs of influenza virus HA in N. benthamiana with the A. tumefaciens-based transient expression system [D’Aoust et al. 2008; Yusibov et al. 2011; US patent application number 20130183341]. VLPs of the expected size were found between the plasma membrane and the cell wall of N. benthamiana cells [D’Aoust et al. 2008]. Phase I and II clinical trial of the VLP composed of HA protein of H5N1 influenza virus (A/Indonesia/5/05) (H5-VLP) has been completed. In a phase I clinical trial, 5, 10, or 20 µg of H5-VLP was subcutaneously injected twice with alum adjuvant [Landry et al. 2010]. The vaccine-induced hemagglutinin inhibition titer at all tested doses. In addition, a phase II clinical trial of H5-VLP was conducted as a randomized, placebo-controlled, dose-ranging study that used 20, 30, or 45 µg of H5-VLP [Landry et al. 2014]. After 6 months of vaccination with H5-VLP, the volunteer group showed cross-protective CD4+ T-cell responses, which were not observed in the placebo group, indicating strong induction of long-term cell-mediated immunity by plant-made H5-VLP. The immunogenicity of plant-specific glycans has also been studied in this clinical trial; some vaccine recipients developed plant N-glycan-specific allergic or hypersensitivity symptoms. Some volunteers (34%) developed transient IgG and, in some cases, IgE to plant glyco-epitopes, but no IgE responses to mannose residues (MMXF motifs) were observed. The levels of antibodies returned to baseline by 6 months in most participants [Ward et al. 2014]. Medicago Inc. also completed phase I clinical trial using 5, 13, or 28 µg of H1N1 influenza (A/California/7/09) VLP (H1-VLP) vaccine [Landry et al. 2010]. All doses tested were safe and well-tolerated and induced immune response to the virus, including cell-mediated immunity. The company intends to proceed phase IIa trial of its seasonal trivalent vaccine with antigens from the recommended pandemic H1N1, H3N2, and B influenza strains [Redkiewicz et al. 2014].
Another plant-based influenza vaccine that has completed phase I clinical trial is in production at Fraunhofer CMB USA. HA from A/California/04/2009 H1N1 (HAC1) and A/Indonesia/05/05 H5N1 (HAI-05) has been produced in N. benthamiana by using an infiltration method of A. tumefaciens in which genes are regulated by the ‘launch vector’ [Shoji et al. 2011, 2015]. Pre-clinical studies using mice and rabbits were conducted by the injection of HAC1 and HAI-05 twice with 3-week-intervals. Seropositive rate of serum HA antibody responses were 100% in mice with the dose of 5 µg for HAC1, and 45 µg for HAI-05 in a dose-dependence test with alum-adjuvant. Rabbits also showed seropositive for HA with the dose of 90 µg in both HAC1 and HAI-05 [Shoji et al. 2011]. A phase I clinical trial for HAC1 and HAI-05 was conducted as a randomized, double-blind, placebo-controlled study with healthy 18–49-year-old volunteers [Chichester et al. 2012; Cummings et al. 2014]. In both cases, three doses (15, 45, and 90 μg) of purified antigen with or without Alhydrogel® (as adjuvant) were administered twice intramuscularly. Nearly all adverse events were mild to moderate; the highest responses were detected by hemagglutining inhibition (HI) and viral-neutralizing (VN) antibody titers and were observed in the group immunized with the highest dose (90 μg) without adjuvant.
Development of veterinary vaccines
Antigens of farm-animal pathogens have been expressed in plants, and a few of them have been tested in host species. Plant-made vaccines for veterinary use are listed in Table 3. The first USDA-approved plant-made vaccine was for veterinary use: Newcastle disease vaccine for poultry from the USDA Center for Veterinary Biologics was approved in 2006 [reviewed in Floss et al. 2007; Kolotilin et al. 2014; Rybicki 2010]. Dow AgroSciences LCC (Indianapolis, IN, USA) produces hemagglutinin and neuraminidase of Newcastle disease virus in suspension-cultured tobacco cells [reviewed in Yusibov et al. 2011]. Two subcutaneous doses of this vaccine at a 2-week interval administered to neonatal chicks ensure 90% protection against Newcastle disease virus challenge. For farm animals, the cost of immunization tends to limit profit from selling products such as meat, milk, and eggs. Therefore, plant-based vaccines are an asset for the animal use if they can be manufactured at low cost. Moreover, edible vaccines require little effort for administration. In poultry, in addition to the approved vaccine mentioned above, glycoprotein of Newcastle disease virus has been expressed in potato, tobacco, maize, and rice [Guerrero-Andrade et al. 2006; Kolotilin et al. 2014; Zhou et al. 2004]. S1 glycoprotein gene of chicken infectious bronchitis virus (IBV) has been introduced into potato [Zhou et al. 2004]. Oral immunization with transgenic potato tubers (5 g; 12.45 µg of S protein) three times or intramuscular immunization with transgenic potato extracts two or three times elicited high neutralizing antibody titers against IBV in chicken serum. These levels were similar to those in chickens immunized with live attenuated intranasal vaccine and conferred 60–80% protection from a virulent IBV strain. Lymphocyte proliferation and IL-2 production by spleen cells were confirmed in vitro, indicating that the potato-based vaccine induced protective immunity. To protect chicken against infectious bursal disease virus (IBDV), the protective antigen VP2 was expressed in rice and tobacco [Gómez et al., 2013; Wu et al. 2007], and efficiently protected chickens from a highly virulent IBDV strain.
Table 3.
Plant-based vaccines for veterinary use.
| Host | Pathogen | Antigen | Plant | Administration route | Treated animal | Reference |
|---|---|---|---|---|---|---|
| Chicken | Newcastle disease | Hemagglutinin-neuraminidase | Tobacco suspension cells | Subcutaneous | Chicken | Vermij et al. [2006] |
| Approved by USDA | ||||||
| Chicken | Newcastle disease | F protein | Maize | Oral | Chicken | Guerrero-Andrade et al. [2006] |
| Chicken | Newcastle disease | F protein | Rice | Oral | Mice | Yang et al. [2007] |
| Chicken | IBV | S1 glycoprotein | Potato | Oral | Chicken | Zhou et al. [2004] |
| Chicken | IBDV | VP2 | Rice | Oral | Chicken | Wu et al. [2007] |
| Pig | ETEC | Fimbriae (F4) | Tobacco (chloroplast) | N/D | Pig (in vitro assay in intestines) | Kolotilin et al. [2012] |
| Pig | ETEC | Fimbriae (F4) | Alfalfa | Oral | Piglet | Joensuu et al. [2006] |
| Pig | ETEC | Cholera toxin B subunit | Rice | Oral | Pig | Takeyama et.al. [2015] |
| Pig | ETEC | Fimbriae (F4) | Barley | Subcutaneous | Mice | Joensuu et al. [2006] |
| Pig | Foot and mouth disease virus | VP1 | Nicotiana bentamiana | Intramuscular | Pig | Yang et al. [2007] |
| Pig | TGEV | S protein | Tobacco | Intramuscular | Pig | Tuboly et al. [2000] |
| Cattle | Bovine Herpesvirus | gD protein | Tobacco | Intramuscular and subcutaneous | Cattle | Pérez Filgueira et al. [2003] |
| Cattle | Bovine Viral Diarrhea Virus | E2 protein | Alfalfa | Intramuscular | Cattle | Peréz Aguirreburualde et al. [2013] |
| Cattle | Rinderpest virus | Hemagglutinin | Peanut | Oral | Cattle | Khandelwal et al. [2003] |
Plant-based vaccines for pig protection from ETEC and foot and mouth disease virus (FMDV) have been well characterized. ETEC fimbriae (of type F4) were produced in alfalfa chloroplasts and remained stable for 2 years when alfalfa was dried and stored at room temperature [Joensuu et al. 2006]. Recombinant proteins in combination with cholera toxin as an adjuvant were introduced intragastrically into piglets, resulting in a reduction in ETEC excretion in their feces. Kolotilin and colleagues stably expressed F4 fimbrial adhesin FaeG in tobacco chloroplasts (1% of dry leaf weight or 11.3% of total soluble leaf protein) [Kolotilin et al. 2012]. Although the authors did not challenge vaccinated animals, they showed that recombinant F4 competitively inhibited the attachment of F4-positive ETEC to pig small-intestinal villi in vitro [Kolotilin et al. 2012]. We have produced rice-based cholera toxin B subunit (CTB) vaccine, which is efficient against pig ETEC heat-labile toxin because the amino acid sequence and conformation of this toxin are similar to those of cholera toxin. The use of CTB-producing rice, named MucoRice-CTB, ensured high antigen stability in the gastrointestinal tract and mucosal immune induction in a mouse model [Nochi et al. 2007; Tokuhara et al. 2010]. Pregnant sows and weaned minipigs produced antigen-specific IgG and IgA in their sera upon MucoRice-CTB immunization. MucoRice-CTB also induced maternal CTB-specific IgG and IgA in the colostrum and milk of sows after farrowing. These antigen-specific maternal antibodies offer newborn piglets passive immunization with ingested milk. CTB-specific antibodies also were secreted into the gut lumen of weaned piglets and reduced intestinal loop fluid accumulation upon ETEC challenge, indicating a protective effect of MucoRice-CTB against ETEC diarrhea. Therefore, MucoRice-CTB could be a candidate oral vaccine for inducing both passive and active immunity to protect both suckling and weaned piglets from ETEC diarrhea [Takeyama et al. 2015].
FMDV infects pigs and cows but not humans and causes a major animal disease, the prevention of which requires international cooperation. Yang and colleagues expressed VP1 of FMDV on the surface of bamboo mosaic virus in N. benthamiana and Chenopodium quinoa [Yang et al. 2007]. Purified VP1-expressing bamboo mosaic virus was intramuscularly injected with an oil adjuvant into 2-month-old piglets twice with a 6-week interval. The neutralization titer was elevated in all piglets that received 0.5, 1, or 5 mg of purified antigen even after single vaccination. FMDV VP1 and the structural polyprotein P1 produced in tobacco, potato, alfalfa, or tomato protected mice and guinea pigs from FMDV challenge [Carrillo et al. 2001; Dus Santos et al. 2005].
Rabies virus causes a zoonotic disease transmitted from wild animals such as bats, raccoons, and foxes to pet animals and humans. To control rabies in humans, vaccination of wildlife and pets is needed [Fooks et al. 2014]. Capturing large numbers of wild animals for vaccine injection is almost impossible, and distribution of bait mixed with oral vaccine in the risk regions could be a major solution for rabies control [Yang et al. 2013]. Rabies G protein has been expressed in several species, including tobacco, tomato, spinach, carrot, and maize [Loza-Rubio et al. 2012; McGarvey et al. 1995; Rojas-Anaya et al. 2009]. Oral immunization with rabies G protein produced in maize (0.5–2 mg) protected sheep from rabies strain CASS-88; the mortality rate from virus challenge 120 days after vaccination was reduced in a dose-dependent manner [Loza-Rubio et al. 2012]. Plant-made rabies antigens may be used at different doses both in humans and animals.
Aquafarming relies on ocean water, and excessive use of antibiotics contaminates the environment. The use of oral vaccines for disease prevention in fisheries and aquaculture may ameliorate this problem [Clarke et al. 2013].
Conclusions
Several key points are essential for the development of a broadly effective GMP-compliant regulatory framework for clinical application of plant-based vaccines in humans and animals. First, we need to determine the eligible combinations of target plants and transgenic protocols. Unlike open-air farming, the production of transgenic plants for biotherapeutic use is strictly regulated. Plant selection would affect the whole procedure throughout commercialization. Transient expression systems enable the rapid production of high amounts of target proteins, but their implementation is complex because it requires infiltration and the large-scale use of A. tumefaciens or viral vectors. Second, we need to determine the most suitable cultivation system for transgenic plants (open-field or in-house cultivation). Open-field cultivation is less expense than greenhouse or in-house cultivation, but plant factories offer controllable, reproducible cultivation conditions suitable for GMP manufacturing. Finally, we need to define the procedures for manufacturing and processing of plant-based pharmaceuticals. The challenge is to facilitate the procedures without compromising quality, which is a prerequisite for manufacturing plant-based human and animal vaccines.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Natsumi Takeyama, Division of Mucosal Immunology, The Institute of Medical Science, The University of Tokyo, Tokyo, Japan; Research Department, Nippon Institute for Biological Science, Ome, Tokyo, Japan.
Hiroshi Kiyono, Division of Mucosal Immunology, The Institute of Medical Science, The University of Tokyo, Tokyo, Japan International Research and Development Center for Mucosal Vaccines, The Institute of Medical Science, The University of Tokyo, Tokyo, Japan.
Yoshikazu Yuki, Division of Mucosal Immunology, Department of Microbiology and Immunology, The Institute of Medical Science, The University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo 108-8639, Japan.
References
- Azegami T., Yuki Y., Kiyono H. (2014) Challenges in mucosal vaccines for the control of infectious diseases. Int Immunol 26: 517–528. [DOI] [PubMed] [Google Scholar]
- Barta A., Sommergruber K., Thompson D., Hartmuth K., Matzke M., Matzke A. (1986) The expression of a nopaline synthase–human growth hormone chimaeric gene in transformed tobacco and sunflower callus tissue. Plant Mol Biol 6: 347–357. [DOI] [PubMed] [Google Scholar]
- Bawa A., Anilakumar K. (2013) Genetically modified foods: safety, risks and public concerns – a review. J Food Sci Technol 50: 1035–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. (1984) Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res 12: 8711–8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos C., Lees D. (2014) Environmental transmission of human noroviruses in shellfish waters. Appl Environ Microbiol 80: 3552–3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardi T., Lenzi P., Maliga P. (2010) Chloroplasts as expression platforms for plant-produced vaccines. Expert Rev Vaccines 9: 893–911. [DOI] [PubMed] [Google Scholar]
- Carrillo C., Wigdorovitz A., Trono K., Dus Santos M., Castañón S., Sadir A., et al. (2001) Induction of a virus-specific antibody response to foot and mouth disease virus using the structural protein VP1 expressed in transgenic potato plants. Viral Immunol 14: 49–57. [DOI] [PubMed] [Google Scholar]
- Cerovska N., Hoffmeisterova H., Moravec T., Plchova H., Folwarczna J., Synkova H., et al. (2012) Transient expression of Human papillomavirus type 16 L2 epitope fused to N- and C-terminus of coat protein of potato virus X in plants. J Biosci 37: 125–133. [DOI] [PubMed] [Google Scholar]
- Chakravarthi M., Philip A., Subramonian N. (2015) Truncated ubiquitin 5’ regulatory region from Erianthus arundinaceus drives enhanced transgene expression in heterologous systems. Mol Biotechnol 19: 820–835 [DOI] [PubMed] [Google Scholar]
- Chen Q., Lai H., Hurtado J., Stahnke J., Leuzinger K., Dent M. (2013) Agroinfiltration as an effective and scalable strategy of gene delivery for production of pharmaceutical proteins. Adv Tech Biol Med 1: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichester J., Jones R., Green B., Stow M., Miao F., Moonsammy G., et al. (2012) Safety and immunogenicity of a plant-produced recombinant hemagglutinin-based influenza vaccine (HAI-05) derived from A/Indonesia/05/2005 (H5N1) influenza virus: a phase 1 randomized, double-blind, placebo-controlled, dose-escalation study in healthy adults. Viruses 4: 3227–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J., Waheed M., Lössl A., Martinussen I., Daniell H. (2013) How can plant genetic engineering contribute to cost-effective fish vaccine development for promoting sustainable aquaculture? Plant Mol Biol 83: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox L., Casale T., Nayak A., Bernstein D., Creticos P., Ambroisine L., et al. (2012) Clinical efficacy of 300IR 5-grass pollen sublingual tablet in a US study: the importance of allergen-specific serum IgE. J Allergy Clin Immunol 130: 1327–1334. [DOI] [PubMed] [Google Scholar]
- Cummings J., Guerrero M., Moon J., Waterman P., Nielsen R., Jefferson S., et al. (2014) Safety and immunogenicity of a plant-produced recombinant monomer hemagglutinin-based influenza vaccine derived from influenza A (H1N1)pdm09 virus: a phase 1 dose-escalation study in healthy adults. Vaccine 32: 2251–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H., Muthukumar B., Lee S. (2001) Marker free transgenic plants: engineering the chloroplast genome without the use of antibiotic selection. Curr Genet 39: 109–116. [DOI] [PubMed] [Google Scholar]
- D’Aoust M., Lavoie P., Couture M., Trépanier S., Guay J., Dargis M., et al. (2008) Influenza virus-like particles produced by transient expression in Nicotiana benthamiana induce a protective immune response against a lethal viral challenge in mice. Plant Biotechnol J 6: 930–940. [DOI] [PubMed] [Google Scholar]
- de Ronde D., Pasquier A., Ying S., Butterbach P., Lohuis D., Kormelink R. (2014) Analysis of Tomato spotted wilt virus NSs protein indicates the importance of the N-terminal domain for avirulence and RNA silencing suppression. Mol Plant Pathol 15: 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzschold B., Gore M., Marchadier D., Niu H., Bunschoten H., Otvos L., Jr., et al. (1990) Structural and immunological characterization of a linear virus-neutralizing epitope of the rabies virus glycoprotein and its possible use in a synthetic vaccine. J Virol 64: 3804–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dus Santos M., Carrillo C., Ardila F., Ríos R., Franzone P., Piccone M., et al. (2005) Development of transgenic alfalfa plants containing the foot and mouth disease virus structural polyprotein gene P1 and its utilization as an experimental immunogen. Vaccine 23: 1838–1843. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency (EMA) (2008) Guideline on the Quality of Biological Active Substances Produced by Stable Transgene Expression in Higher Plants (EMEA/CHMP/BWP/48316/2006). London, UK: European Medicines Agency. [Google Scholar]
- Fischer R., Schillberg S., Hellwig S., Twyman M., Drossard J. (2012) GMP issues for recombinant plant-derived pharmaceutical proteins. Biotech Adv 30: 434–439. [DOI] [PubMed] [Google Scholar]
- Floss D., Falkenburg D., Conrad U. (2007) Production of vaccines and therapeutic antibodies for veterinary applications in transgenic plants: an overview. Transgenic Res 16: 315–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fooks A., Banyard A., Horton D., Johnson N., McElhinney L., Jackson A. (2014) Current status of rabies and prospects for elimination. Lancet 384: 1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihashi K., Dohi T., Kweon M., McGhee J., Koga T., Cooper M., et al. (1999) Gammadelta T cells regulate mucosally induced tolerance in a dose-dependent fashion. Int Immunol 11: 1907–1916. [DOI] [PubMed] [Google Scholar]
- Garabagi F., Gilbert E., Loos A., McLean M., Hall J. (2012) Utility of the P19 suppressor of gene-silencing protein for production of therapeutic antibodies in Nicotiana expression hosts. Plant Biotechnol J 10: 1118–1128. [DOI] [PubMed] [Google Scholar]
- Gleba Y., Klimyuk V., Marillonnet S. (2005) Magnifection – a new platform for expressing recombinant vaccines in plants. Vaccine 23: 2042–2048. [DOI] [PubMed] [Google Scholar]
- Gleba Y., Marillonnet S., Klimyuk V. (2004) Engineering viral expression vectors for plants: the ‘full virus’ and the ‘deconstructed virus’ strategies. Curr Opin Plant Biol 7: 182–188. [DOI] [PubMed] [Google Scholar]
- Gleba Y., Tusé D., Giritch A. (2014) Plant viral vectors for delivery by Agrobacterium. Curr Top Microbiol Immunol 375: 155–192. [DOI] [PubMed] [Google Scholar]
- Gómez E., Lucero M., Chimeno Zoth S., Carballeda J., Gravisaco M., Berinstein A. (2013) Transient expression of VP2 in Nicotiana benthamiana and its use as a plant-based vaccine against infectious bursal disease virus. Vaccine 31: 2623–2637. [DOI] [PubMed] [Google Scholar]
- Gomord V., Fitchette A., Menu-Bouaouiche L., Saint-Jore-Dupas C., Michaud D., Faye L. (2010) Plant-specific glycoprotein patterns in the context of therapeutic protein production. Plant Biotechnol J 8: 564–587. [DOI] [PubMed] [Google Scholar]
- Guan Z., Guo B., Huo Y., Guan Z., Dai J., Wei Y. (2013) Recent advances and safety issues of transgenic plant-derived vaccines. Appl Microbiol Biotechnol 97: 2817–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Andrade O., Loza-Rubio E., Olivera-Flores T., Fehérvári-Bone T., Gómez-Lim M. (2006) Expression of the Newcastle disease virus fusion protein in transgenic maize and immunological studies. Transgenic Res 15: 455–463. [DOI] [PubMed] [Google Scholar]
- Hefferon K. (2013) Plant-derived pharmaceuticals for the developing world. Biotechnol J 8: 1193–1202. [DOI] [PubMed] [Google Scholar]
- Hefferon K. (2014) Plant virus expression vector development: new perspectives. BioMed Res Int 2014: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennegan K., Yang D., Nguyen D., Wu L., Goding J., Huang J., et al. (2005) Improvement of human lysozyme expression in transgenic rice grain by combining wheat (Triticum aestivum) puroindoline b and rice (Oryza sativa) Gt1 promoters and signal peptides. Transgenic Res 14: 583–592. [DOI] [PubMed] [Google Scholar]
- Hiatt A., Cafferkey R., Bowdish K. (1989) Production of antibodies in transgenic plants. Nature 342: 76–78. [DOI] [PubMed] [Google Scholar]
- Hiwasa-Tanase K., Nyarubona M., Hirai T., Kato K., Ichikawa T., Ezura H. (2011) High-level accumulation of recombinant miraculin protein in transgenic tomatoes expressing a synthetic miraculin gene with optimized codon usage terminated by the native miraculin terminator. Plant Cell Rep 30: 113–24. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Czerkinsky C. (2005) Mucosal immunity and vaccines. Nat Med 11: S45–S53. [DOI] [PubMed] [Google Scholar]
- Huang Z., Santi L., LePore K., Kilbourne J., Arntzen C., Mason H. (2006) Rapid, high-level production of hepatitis B core antigen in plant leaf and its immunogenicity in mice. Vaccine 24: 2506–2513. [DOI] [PubMed] [Google Scholar]
- Itoh Y., Shinya K., Kiso M., Watanabe T., Sakoda Y., Hatta M., et al. (2009) In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature 460: 1021–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M., Sternes P., Mudge S., Graham M., Birch R. (2014) Design rules for efficient transgene expression in plants. Plant Biotechnol J 12: 925–933. [DOI] [PubMed] [Google Scholar]
- Joensuu J., Verdonck F., Ehrström A., Peltola M., Siljander-Rasi H., Nuutila A., et al. (2006) F4 (K88) fimbrial adhesin FaeG expressed in alfalfa reduces F4+ enterotoxigenic Escherichia coli excretion in weaned piglets. Vaccine 24: 2387–2394. [DOI] [PubMed] [Google Scholar]
- Kapusta J., Modelska A., Figlerowicz M., Pniewski T., Letellier M., Lisowa O., et al. (1999) A plant-derived edible vaccine against hepatitis B virus. FASEB J 13: 1796–1799. [DOI] [PubMed] [Google Scholar]
- Khandelwal A., Lakshmi S., Shaila M. (2003) Oral immunization of cattle with hemagglutinin protein of rinderpest virus expressed in transgenic peanut induces specific immune responses. Vaccine 21: 3282–3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk D., McIntosh K., Walmsley A., Peterson R. (2005) Risk analysis for plant-made vaccines. Transgenic Res 14: 449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolotilin I., Kaldis A., Devriendt B., Joensuu J., Cox E., Menassa R. (2012) Production of a subunit vaccine candidate against porcine post-weaning diarrhea in high-biomass transplastomic tobacco. PLoS One 7: e42405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolotilin I., Topp E., Cox E., Devriendt B., Conrad U., Joensuu J. (2014) Plant-based solutions for veterinary immunotherapeutics and prophylactics. Vet Res 45: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda M., Kimizu M., Mikami C. (2010) A simple set of plasmids for the production of transgenic plants. Biosci Biotechnol Biochem 74: 2348–2351. [DOI] [PubMed] [Google Scholar]
- Kurokawa S., Nakamura R., Mejima M., Kozuka-Hata H., Kuroda M., Takeyama N., et al. (2013) MucoRice-cholera toxin B-subunit, a rice-based oral cholera vaccine, down-regulates the expression of a-amylase/trypsin inhibitor-like protein family as major rice allergens. J Proteome Res 12: 3372–3382. [DOI] [PubMed] [Google Scholar]
- Kushnir N., Streatfield S., Yusibov V. (2012) Virus-like particles as a highly efficient vaccine platform: diversity of targets and production systems and advances in clinical development. Vaccine 31: 58–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai H., Chen Q. (2012) Bioprocessing of plant-derived virus-like particles of Norwalk virus capsid protein under current Good Manufacture Practice regulations. Plant Cell Rep 31: 573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane A., Azegami T., Kiyono H. (2014) The mucosal immune system for vaccine development. Vaccine 32: 6711–6723. [DOI] [PubMed] [Google Scholar]
- Landry N., Pillet S., Favre D., Poulin J., Trépanier S., Yassine-Diab B., et al. (2014) Influenza virus-like particle vaccines made in Nicotiana benthamiana elicit durable, poly-functional and cross-reactive T cell responses to influenza HA antigens. Clin Immunol 154: 164–177. [DOI] [PubMed] [Google Scholar]
- Landry N., Ward B., Trépanier S., Montomoli E., Dargis M., Lapini G., et al. (2010) Preclinical and clinical development of plant-made virus-like particle vaccine against avian H5N1 influenza. PLoS One 5: e15559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuzinger K., Dent M., Hurtado J., Stahnke J., Lai H., Zhou X., et al. (2013) Efficient agroinfiltration of plants for high-level transient expression of recombinant proteins. J Vis Exp 77: e50521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lico C., Santi L., Twyman R., Pezzotti M., Avesani Tywa L. (2012) The use of plants for the production of therapeutic human peptides. Plant Cell Rep 31: 439–451. [DOI] [PubMed] [Google Scholar]
- Loza-Rubio E., Rojas-Anaya E., López J., Olivera-Flores M., Gómez-Lim M., Tapia-Pérez G. (2012) Induction of a protective immune response to rabies virus in sheep after oral immunization with transgenic maize, expressing the rabies virus glycoprotein. Vaccine 30: 5551–5556. [DOI] [PubMed] [Google Scholar]
- MacLachlan J., Cowie B. (2015) Hepatitis B virus epidemiology. Cold Spring Harb Perspect Med 5: a021410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marillonnet S., Giritch A., Gils M., Kandzia R., Klimyuk V., Gleba Y. (2004) In planta engineering of viral RNA replicons: efficient assembly by recombination of DNA modules delivered by Agrobacterium. Proc Natl Acad Sci USA 101: 6852–6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick A., Reddy S., Reinl S., Cameron T., Czerwinkski D., Vojdani F., et al. (2008) Plant-produced idiotype vaccines for the treatment of non-Hodgkin’s lymphoma: safety and immunogenicity in a phase I clinical study. Proc Natl Acad Sci USA 105: 10131–10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarvey P., Hammond J., Dienelt M., Hooper D., Fu Z., Dietzschold B., et al. (1995) Expression of the rabies virus glycoprotein in transgenic tomatoes. Biotechnology 13: 1484–1487. [DOI] [PubMed] [Google Scholar]
- Mejima M., Kashima K., Kuroda M., Takeyama N., Kurokawa S., Fukuyama Y., et al. (2015) Determination of genomic location and structure of the transgenes in marker-free rice-based cholera vaccine by using whole genome resequencing approach. Plant Cell, Tissue Organ Cult 120: 35–48 [Google Scholar]
- Merlin M., Gecchele E., Capaldi S., Pezzotti M., Avesani L. (2014) Comparative evaluation of recombinant protein production in different biofactories: the green perspective. Biomed Res Int 2014: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiychuk K., Stephenson N., Bi H., Farrance C., Orozovic G., Brodelius M., et al. (2007) A launch vector for the production of vaccine antigens in plants. Influenza Other Respir Viruses 1: 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann G., Noda T., Kawaoka Y. (2009) Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 459: 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nochi T., Takagi H., Yuki Y., Yang L., Masumura T., Mejima M., et al. (2007) Rice-based mucosal vaccine as a global strategy for cold-chain- and needle-free vaccination. Proc Natl Acad Sci USA 104: 10986–10991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nochi T., Yuki Y., Katakai Y., Shibata H., Tokuhara D., Mejima M., et al. (2009) A rice-based oral cholera vaccine induces macaque-specific systemic neutralizing antibodies but does not influence pre-existing intestinal immunity. J Immunol 183: 6538–6544. [DOI] [PubMed] [Google Scholar]
- Noris E., Poli A., Cojoca R., Rittà M., Cavallo F., Vaglio S., et al. (2011) A human papillomavirus 8 E7 protein produced in plants is able to trigger the mouse immune system and delay the development of skin lesions. Arch Virol 156: 587–595. [DOI] [PubMed] [Google Scholar]
- Norkrans G. (1990) Epidemiology of hepatitis B virus (HBV) infections with particular regard to current routes of transmission and development of cirrhosis and malignancy. Scand J Infect Dis Suppl 69: 43–47. [PubMed] [Google Scholar]
- Oey M., Lohse M., Kreikemeyer B., Bock R. (2009) Exhaustion of the chloroplast protein synthesis capacity by massive expression of a highly stable protein antibiotic. Plant J 7: 436–445. [DOI] [PubMed] [Google Scholar]
- Peréz Aguirreburualde M., Gómez M., Ostachuk A., Wolman F., Albanesi G., Pecora A., et al. (2013) Efficacy of a BVDV subunit vaccine produced in alfalfa transgenic plants. Vet Immunol Immunopathol 151: 315–324. [DOI] [PubMed] [Google Scholar]
- Pérez Filgueira D., Zamorano P., Domínguez M., Taboga O., Del Médico Zajac M., Puntel M., et al. (2003) Bovine herpes virus gD protein produced in plants using a recombinant tobacco mosaic virus (TMV) vector possesses authentic antigenicity. Vaccine 21: 4201–4209. [DOI] [PubMed] [Google Scholar]
- Peyret H., Lomonossoff G. (2013) The pEAQ vector series: the easy and quick way to produce recombinant proteins in plants. Plant Mol Biol 83: 51–58. [DOI] [PubMed] [Google Scholar]
- Ravin N., Kotlyarov R., Mardanova E., Kuprianov V., Migunov A., Stepanova L., et al. (2012) Plant-produced recombinant influenza vaccine based on virus-like HBc particles carrying an extracellular domain of M2 protein. Biochemistry 77: 33–40. [DOI] [PubMed] [Google Scholar]
- Redkiewicz P., Sirko A., Kamel K., Góra-Sochacka A. (2014) Plant expression systems for production of hemagglutinin as a vaccine against influenza virus. Acta Biochim Pol 61: 551–560. [PubMed] [Google Scholar]
- Rigano M., Walsley A. (2005) Expression systems and developments in plant-made vaccines. Immunol Cell Biol 83: 271–277. [DOI] [PubMed] [Google Scholar]
- Rojas-Anaya E., Loza-Rubio E., Olivera-Flores M., Gomez-Lim M. (2009) Expression of rabies virus G protein in carrots (Daucus carota). Transgenic Res 18: 911–919. [DOI] [PubMed] [Google Scholar]
- Rybicki E. (2010) Plant-made vaccines for humans and animals. Plant Biotechnol J 8: 620–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybicki E. (2014) Plant-based vaccines against viruses. Virol J 11: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainsbury F., Thuenemann E., Lomonossoff G. (2009) pEAQ: versatile expression vectors for easy and quick transient expression of heterologous proteins in plants. Plant Biotechnol J 7: 682–693. [DOI] [PubMed] [Google Scholar]
- Salazar-González J., Bañuelos-Hernández B., Rosales-Mendoza S. (2015) Current status of viral expression systems in plants and perspectives for oral vaccines development. Plant Mol Biol 87: 203–217. [DOI] [PubMed] [Google Scholar]
- Sato S., Yanagida N., Ogura K., Asaumi T., Okada Y., Koike Y., et al. (2014) Immunotherapy in food allergy: towards new strategies. Asian Pac J Allergy Immunol 32: 195–202. [PubMed] [Google Scholar]
- Scotti N., Rigano M., Cardi T. (2012) Production of foreign proteins using plastid transformation. Biotech Adv 30: 387–397. [DOI] [PubMed] [Google Scholar]
- Scotti N., Rybicki E. (2013) Virus-like particles produced in plants as potential vaccines. Expert Rev Vaccines 12: 211–224. [DOI] [PubMed] [Google Scholar]
- Shoji Y., Chichester J., Jones M., Manceva S., Damon E., Mett V., et al. (2011) Plant-based rapid production of recombinant subunit hemagglutinin vaccines targeting H1N1 and H5N1 influenza. Hum Vaccin 7: 41–50. [DOI] [PubMed] [Google Scholar]
- Shoji Y., Prokhnevsky A., Leffet B., Vetter N., Tottey S., Satinover S., et al. (2015) Immunogenicity of H1N1 influenza virus-like particles produced in Nicotiana benthamiana. Hum Vaccin Immunother 11: 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoseyov O., Posen Y., Grynspan F. (2014) Human collagen produced in plants, More than just another molecule. Bioengineered 5: 49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacket C. (2007) Plant-based vaccines against diarrheal diseases. Trans Am Clin Climatol Assoc 118: 79–87. [PMC free article] [PubMed] [Google Scholar]
- Tacket C., Mason H., Losonsky G., Clements J., Levine M., Arntzen C. (1998) Immunogenicity in humans of a recombinant bacterial antigen delivered in a transgenic potato. Nat Med 4: 607–609. [DOI] [PubMed] [Google Scholar]
- Tacket C., Mason H., Losonsky G., Estes M., Levine M., Arntzen C. (2000) Human immune responses to a novel norwalk virus vaccine delivered in transgenic potatoes. J Infect Dis 182: 302–305. [DOI] [PubMed] [Google Scholar]
- Tacket C., Pasetti M., Edelman R., Howard J., Streatfield S. (2004) Immunogenicity of recombinant LT-B delivered orally to humans in transgenic corn. Vaccine 22: 4385–4389. [DOI] [PubMed] [Google Scholar]
- Takeyama N., Yuki Y., Tokuhara D., Oroku K., Mejima M., Kurokawa S., et al. (2015) Oral rice-based vaccine induces passive and active immunity against enterotoxigenic E. coli-mediated diarrhea in pigs. Vaccine 33: 5204–5211. [DOI] [PubMed] [Google Scholar]
- Teunis P., Moe C., Liu P., Miller S., Lindesmith L., Baric R., et al. (2008) Norwalk virus: how infectious is it? J Med Virol 80: 1468–1476. [DOI] [PubMed] [Google Scholar]
- Thanavala Y., Mahoney M., Pal S., Scott A., Richter L., Natarajan N., et al. (2005) Immunogenicity in humans of an edible vaccine for hepatitis B. Proc Natl Acad Sci USA 102: 3378–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuhara D., Yuki Y., Nochi T., Kodama T., Mejima M., Kurokawa S., et al. (2010) Secretory IgA-mediated protection against V. cholerae and heat-labile enterotoxin-producing enterotoxigenic Escherichia coli by rice-based vaccine. Proc Natl Acad Sci USA 107: 8794–8799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toovey S. (2007) Preventing rabies with the Verorab vaccine: 1985–2005 twenty years of clinical experience. Travel Med Infect Dis 5: 327–348. [DOI] [PubMed] [Google Scholar]
- Tuboly T., Yu W., Bailey A., Degrandis S., Du S., Erickson L., et al. (2000) Immunogenicity of porcine transmissible gastroenteritis virus spike protein expressed in plants. Vaccine 18: 2023–2028. [DOI] [PubMed] [Google Scholar]
- Twyman R., Schillberg S., Fischer R. (2005) Transgenic plants in the biopharmaceutical market. Expert Opin Emerg Drugs 10: 185–218. [DOI] [PubMed] [Google Scholar]
- Vacher G., Kaeser M., Moser C., Gurny R., Borchard G. (2013) Recent advances in mucosal immunization using virus-like particles. Mol Pharm 10: 1596–1609. [DOI] [PubMed] [Google Scholar]
- van der Laan J., Minor P., Mahoney R., Arntzen C., Shin J., Wood D., et al. (2006) WHO informal consultation on scientific basis for regulatory evaluation of candidate human vaccines from plants, Geneva, Switzerland, 24–25 January 2005. Vaccine 24: 4271–4278. [DOI] [PubMed] [Google Scholar]
- van Dussen L., Zimran A., Akkerman E., Aerts J., Petakov M., Elstein D., et al. (2013) Taliglucerase alfa leads to favorable bone marrow responses in patients with type I Gaucher disease. Blood Cells Mol Dis 50: 206–211. [DOI] [PubMed] [Google Scholar]
- Verma D., Daniell H. (2007) Chloroplast vector systems for biotechnology applications. Plant Physiol 145: 1129–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermij P. (2006) USDA approves the first plant-based vaccine (News In Brief). Nature Biotechnol 24: 233–234. [Google Scholar]
- Ward B., Landry N., Trépanier S., Mercier G., Dargis M., Couture M., et al. (2014) Human antibody response to N-glycans present on plant-made influenza virus-like particle (VLP) vaccines. Vaccine 32: 6098–6106. [DOI] [PubMed] [Google Scholar]
- Wu J., Yu L., Li L., Hu J., Zhou J., Zhou X. (2007) Oral immunization with transgenic rice seeds expressing VP2 protein of infectious bursal disease virus induces protective immune responses in chickens. Plant Biotechnol J 5: 570–8. [DOI] [PubMed] [Google Scholar]
- Yang D., Guo F., Liu B., Huang N., Watkins S. (2002) Expression and localization of human lysozyme in the endosperm of transgenic rice. Planta 216: 597–603. [DOI] [PubMed] [Google Scholar]
- Yang D., Kim H., Lee K., Song J. (2013) The present and future of rabies vaccine in animals. Clin Exp Vaccine Res 2: 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Liao J., Lai C., Jong M., Liang C., Lin Y., et al. (2007) Induction of protective immunity in swine by recombinant bamboo mosaic virus expressing foot-and-mouth disease virus epitopes. BMC Biotechnol 7: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki Y., Mejima M., Kurokawa S., Hiroiwa T., Kong I., Kuroda M., et al. (2012) RNAi suppression of rice endogenous storage proteins enhances the production of rice-based Botulinum neutrotoxin type A vaccine. Vaccine 30: 4160–4166. [DOI] [PubMed] [Google Scholar]
- Yuki Y., Mejima M., Kurokawa S., Hiroiwa T., Takahashi Y., Tokuhara D., et al. (2013) Induction of toxin-specific neutralizing immunity by molecularly uniform rice-based oral cholera toxin B subunit vaccine without plant-associated sugar modification. Plant Biotechnol J 11: 799–808. [DOI] [PubMed] [Google Scholar]
- Yusibov V., Hooper D., Spitsin S., Fleysh N., Kean R., Mikheeva T., et al. (2002) Expression in plants and immunogenicity of plant virus-based experimental rabies vaccine. Vaccine 20: 3155–3164. [DOI] [PubMed] [Google Scholar]
- Yusibov V., Kushnir N., Streatfield S. (2014) Advances and challenges in the development and production of effective plant-based influenza vaccines. Expert Rev Vaccines 9: 1–17. [DOI] [PubMed] [Google Scholar]
- Yusibov V., Stephen J., Streatfield S., Kushnir N. (2011) Clinical development of plant-produced recombinant pharmaceuticals: vaccines, antibodies and beyond. Human Vaccin 7: 313–321. [DOI] [PubMed] [Google Scholar]
- Zhou J., Cheng L., Zheng X., Wu J., Shang S., Wang J., et al. (2004) Generation of the transgenic potato expressing full-length spike protein of infectious bronchitis virus. J Biotechnol 111: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimran A., Brill-Almon E., Chertkoff R., Petakov M., Blanco-Favela F., Muñoz E., et al. (2011) Pivotal trial with plant cell-expressed recombinant glucocerebrosidase, taliglucerase alfa, a novel enzyme replacement therapy for Gaucher disease. Blood 118: 5767–5773. [DOI] [PubMed] [Google Scholar]