Abstract
Objectives:
Previous studies have demonstrated that intranasal administration of inactivated (fixed) Francisella tularensis (iFt) live vaccine strain (LVS) in conjunction with the mucosal adjuvant, cholera toxin B (CTB), provides full protection against subsequent lethal challenge with Ft LVS and partial protection against the more virulent Ft SchuS4 strain. Understanding the mechanisms of CTB-induced immune stimulation that confer protection against Ft will be valuable to the development of an effective vaccine against this highly virulent fatal pathogen. In this study, an in vitro system was utilized to further elucidate the immunologic adjuvant effect of CTB when administered with the fixed bacterial immunogen iFt.
Methods:
The murine macrophage cell line (RAW264.7) was treated with combinations of iFt and CTB. The treated RAW264.7 cells and their supernatants were collected and assessed for cell surface marker expression and cytokine secretion. In addition, the ability of RAW264.7 cells to present bacterial antigens (iFt or LVS) to an Ft-specific T-cell hybridoma cell line, following exposure to CTB, was analyzed.
Results:
We found that RAW264.7 cells responded to treatment with iFt + CTB by an increased secretion of the proinflammatory cytokines interleukin 6 and tumor necrosis factor α and upregulation of the surface expression of toll-like receptor 4 and the costimulatory molecules CD80 and CD86. Furthermore, the experimental vaccine treatment iFt + CTB enhanced the ability of macrophages to present iFt antigens to an FT-specific T-cell hybridoma cell line, although they failed to do so with LVS.
Conclusion:
The adjuvant CTB administered in conjunction with iFt showed evidence of enhancing an antigen-specific proinflammatory response in vitro. These observations allow us to define, in part, the mechanisms of immune activation conferred by mucosal administration of iFt + CTB against lethal F. tularensis challenge.
Keywords: cholera toxin B, immunologic adjuvants, vaccines
Introduction
Francisella tularensis (Ft) is a Gram-negative intracellular pathogen that results in the potentially lethal disease tularemia. Less than 10 colony-forming units (CFU) of the highly pathogenic type A strain are required to cause life-threatening illness, with mortality rates between 30% and 60% when left untreated [Pechous et al. 2009]. Due to the highly infectious nature and potential for aerosol dissemination, Ft has been classified as a category A biological threat with no US Food and Drug Administration (FDA) approved vaccine available [Oyston et al. 2004]. A major contributor to the bacterium’s pathogenicity is its capacity for intracellular replication and evasion of the host immune system. Several mechanisms of immune subversion have been described, including expression of poorly immunogenic lipopolysaccharide (LPS), downregulation of inflammatory cytokine secretion, and prevention of phagosome acidification and maturation in host macrophages [Hajjar et al. 2006; Telepnev et al. 2003; Clemens et al. 2004; Steiner et al. 2014]. A successful vaccination strategy against Ft will likely rely on both the innate and adaptive response to overcome the immune evading mechanisms that contribute to the highly virulent nature of Francisella. In addition, research has demonstrated intranasal immunization with the Ft live vaccine strain (LVS) confers superior protection against subsequent exposure to the virulent biovar A strain, suggesting that mucosal immunization may provide optimal protection against Francisella [Wu et al. 2005]. Stimulating both a cellular and humoral response through intranasal delivery will likely require the use of a mucosal adjuvant, such as cholera toxin B (CTB), to enhance the immune response against Ft. Administration of CTB has been reported to increase mucosal epithelium permeability, enhance antigen presentation, and drive T-cell proliferation, B-cell differentiation, and B-cell isotype switching [Lavelle et al. 2004; Gagliardi et al. 2002; Kim et al. 1998; Maeyama et al. 2001; Holmgren et al. 2003]. Studies conducted by Bitsaktsis and colleagues have demonstrated CTB does act as an effective adjuvant against lethal Ft challenge when administered with inactivated Ft LVS (iFt) [Bitsaktsis et al. 2009]. To further elucidate the role of CTB as an adjuvant, an in vitro system was utilized to determine the effects of iFt + CTB mixture on murine macrophages, which are the preferred host tissue of Ft. Our results suggest an important role for CTB in modulating the immune response in macrophage by increasing proinflammatory cytokine secretion and upregulating the expression of costimulatory receptors CD80 and CD86 which contribute to robust antigen presentation, leading to T-cell activation and expansion. Indeed, experimental vaccine treatments of iFt and CTB increase the ability of the fixed immunogen, iFt, to be presented to an Ft-specific T-cell hybridoma by the murine macrophage cell line, RAW 264.7. However, despite the CTB-driven macrophage activation observed in vitro, antigen presentation of LVS to the same Ft-specific T-cell hybridoma was not significant, suggesting that cytokine secretion is the contributing factor by macrophages in the protection against Ft challenge following the use of CTB as a mucosal adjuvant.
Materials and methods
Bacteria
The Ft LVS was kindly provided by Dr Edmund Gosselin (Albany Medical College, Albany, NY, USA). The bacterium was cultured at 37°C in Muller–Hinton broth supplemented with 2% IsovitaleX (Becton Dickinson, Franklin Lakes, NJ). Cultures were grown to a density of 2 × 109 CFU/ml and stored in liquid nitrogen. iFt was then washed three times in sterile phosphate-buffered saline (PBS).
Generation of immunogen
To generate iFt LVS, live bacteria grown to a concentration of 2 × 109 CFU/ml were incubated in 25 ml of sterile PBS containing 2% paraformaldehyde (Sigma-Aldrich, St. Louis, MO) for 90 min at room temperature while shaking. iFt was then washed three times in sterile PBS. Inactivation was confirmed by culturing 100 μl of the fixed iFt on chocolate agar plates (Fisher Scientific, Waltham, MA) and monitoring for 7 days. The iFt preparation were stored in PBS at −20°C until use.
Cell culture
The murine myeloid cell line RAW 264.7 was kindly provided by Dr Allan Blake (Seton Hall University, South Orange, NJ, USA). Cells were grown in RPMI 1640 medium with 2 mM L-glutamine (Lonza, Basel, Switzerland) containing 10% fetal bovine serum (Lonza) and 100 U/ml penicillin with 100 μg/ml streptomycin (ATCC, Manasas, VA). The Ft-specific T-cell hybridoma FT256D10 was obtained from Dr Edmund Gosselin (Albany Medical College). These cells were grown in RPMI 1640 medium with 2mM L-glutamine (Lonza) containing 10% fetal bovine serum (Lonza) and 100 U/ml penicillin, 100 µg/ml streptomycin (ATCC) supplemented with 1 mM sodium pyruvate (Sigma-Aldrich), 1% minimal essential medium (MEM) nonessential amino acids (Sigma-Aldrich), 50 μM 2-mercaptoethanol (Sigma-Aldrich), and 0.02 mg/ml Hygromycin B (Sigma-Aldrich). Cells were maintained at 37°C and 5% CO2.
Cytokine measurement
Raw 264.7 (5 × 105 cells/well) were treated with CTB (1 and 5 µg/ml; Sigma-Aldrich), iFt (1 × 105 CFU/well), or a combination of iFt and CTB at the previous concentrations. Supernatants were harvested at 2, 12, 24, and 48 h, and stored at −20°C until cytokine analysis. Supernatants were assayed for interferon γ (IFNγ), tumor necrosis factor α (TNFα), interleukin (IL)-10, IL-4, and IL-6 using enzyme-linked immunosorbent assay (ELISA) Max Kits (Biolegend, San Diego, CA) according to manufacturers’ instructions.
Flow cytometry
Raw 264.7 (5 × 105 cells/well) were treated with CTB (1 or 5 µg/ml), iFt (1 × 105 CFU/well), or a combination of iFt and CTB at the previous concentrations. After 24 h cells were collected and resuspended in flow cytometry staining buffer (FACS) buffer (PBS with 10% fetal bovine serum (FBS) and 0.02% sodium azide). Cells were stained for murine cell surface markers using the following antibodies purchased from Biolegend: CD86 (Pacific Blue), I-A/I-E (Alexa fluor 488), CD80 (PE), CD282 (Alexa fluor 647), CD284 (PE), and CD11b (Pacific Blue, PE, or Alexa Fluor 488). Following staining, cells were washed with PBS and resuspended in 2% paraformaldehyde and analyzed on a MACS Quant flow cytometer (Miltenyi Biotech, San Diego, CA). Cells were gated using FlowJo software according to forward and side scatter properties (FlowJo, Ashland, OR).
Antigen presentation assay
Raw 264.7 cells (5 × 105 cells/well) were pretreated with 1 or 5 µg/ml of CTB, iFt (1 × 105 CFU/well), or a combination of CTB and iFt at the concentrations previously listed. Cells were then incubated for 24 h at 37°C in 5% CO2 and then exposed to Ft LVS or iFt. The Ft LVS or iFt (1 × 106 CFU/well) was added to the pretreated Raw 264.7 cells, which were then cocultured with the Ft256D10 T-cell hybridoma (2.5 × 105) for a period of 24 h. The supernatants were collected and analyzed for IL-5 as an indicator of activation using ELISA (Biolegend).
Statistical analyses
Experimental conditions for cytokine analysis were performed in triplicate and statistical data were generated using Student’s t test to compare treatments with PBS controls. Statistical analysis and graphs were performed using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA). Flow cytometry density plots were generated using FlowJo software.
Results
CTB enhances the proinflammatory cytokine response to the iFt immunogen in RAW 264.7 macrophages
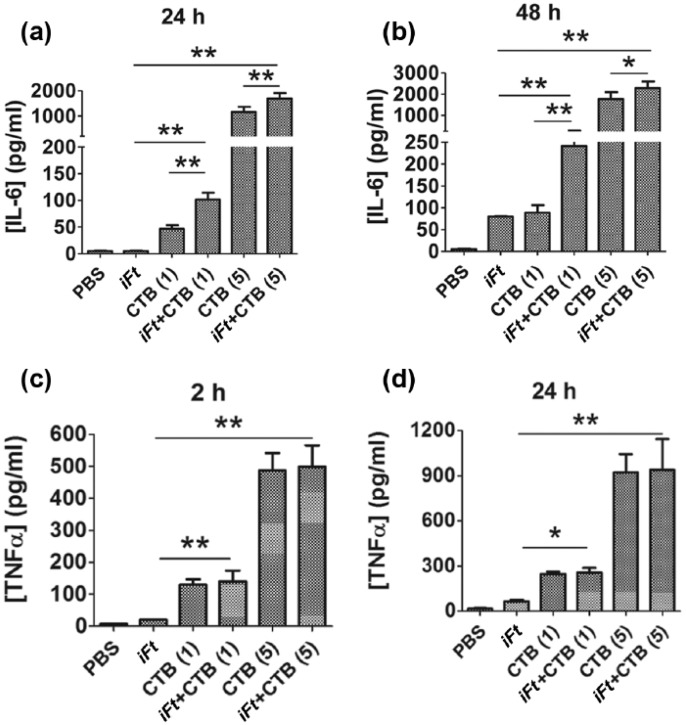
To evaluate the potential of CTB as an immune-stimulating adjuvant in combination with the fixed bacterial immunogen iFt, we assessed the cytokine secretion by the RAW 264.7 cells, treated with CTB or iFt alone, or CTB in combination with iFt (Figure 1). Cells treated with both 1 and 5 µg of CTB in combination with iFt showed increased secretion of IL-6 at both 24 and 48 h compared with cells treated with the adjuvant or immunogen alone [Figure 1(a, b)]. While cells treated with CTB alone produced elevated amounts of IL-6 compared with the control (PBS), the addition of iFt in conjunction with CTB significantly increased IL-6 production by 20-fold, suggesting that the adjuvant enhanced the ability of the iFt immunogen to elicit a proinflammatory response. IL-6 is a proinflammatory cytokine which enhances the innate immune system through the induction of acute phase proteins and stimulation of T and B lymphocytes [Heinrich et al. 2003]. In addition, a 15-fold increase in the proinflammatory cytokine TNFα was observed at 2 and 24 h in cells treated with CTB compared with groups not exposed to the adjuvant [Figure 1(c, d)]. TNFα is primarily produced by activated macrophages and works in concert with IL-6 to orchestrate the local inflammatory response through leukocyte recruitment and activation. While CTB treatment increased the secretion of TNFα, the addition of iFt did not significantly alter the cytokine production, suggesting that CTB has a direct immunogenic effect on murine macrophages in terms of TNFα production. In addition, anti-inflammatory cytokines IL-4 and IL-10 were not detected in any of the treatment groups (data not shown).
Figure 1.
Increased production of proinflammatory cytokines by RAW 264.7 cells treated with CTB and iFt. RAW 264.7 cells were treated with iFt (1 × 105 CFU/well) and CTB (1 or 5 µg) or a combination of CTB and iFt. Supernatants were collected at 2, 24, and 48 h and the levels of IL-6 (a, b) and TNFα (c, d) were measured by ELISA (*p < 0.05 and **p < 0.01, n = 3).
CTB, cholera toxin B; ELISA, enzyme-linked immunosorbent assay; iFt, inactivated (fixed) Francisella tularensis; IL, interleukin; PBS, phosphate-buffered saline; TNF, tumor necrosis factor.
CTB treatment in combination with iFt increases expression of toll-like receptor 4 on RAW 264.7 cells
Pattern recognition receptors, such as toll-like receptors (TLRs), identify conserved pathogen associated molecules and initiate signaling cascades that are essential to the innate immune response. Since TLR4 is the major ligand for LPS, a molecule present in the cell wall of all Gram-negative bacteria, we sought to determine whether treatment with CTB and iFt would increase the expression of this pattern recognition receptor. For this purpose, expression of TLR4 on Raw 264.7 cells following treatment was analyzed by flow cytometry. Cells treated with CTB and iFt had a 37.2% increase in TLR4 expression at 24 h compared with the untreated control [Figure 2(a, c)]. After 48 h of treatment, TLR4 expression was 59.2% greater than the expression observed in the untreated cells [Figure 2(b, c)]. At both time points, the mean fluorescent intensity (MFI) of TLR4 on RAW 264.7 cells treated with iFt + CTB was threefold higher than when treated with iFt alone [Figure 2(c)]. In fact, it is of interest that TLR4 expression decreased following treatment of cells with iFt alone, perhaps suggesting that this may be an alternate mechanism of immune evasion by the bacterium [Figure 2(a–c)]. TLR4 expression was unaffected by CTB alone (data not shown).
Figure 2.
TLR4 expression is increased on RAW 264.7 cells treated with CTB and iFt. RAW 264.7 cells were treated with PBS, iFt or iFt + CTB for 24 or 48 h. The levels of TLR4 were detected by flow cytometry (a, b). The mean fluorescent intensity (MFI) of TLR4 is also presented for each treatment (c) (**p < 0.01). CTB, cholera toxin B; iFt, inactivated (fixed) Francisella tularensis; IL, interleukin; PBS, phosphate-buffered saline; TLR, toll-like receptor; PE, phycoerythrin.
CTB treatment enhances expression of the costimulatory molecules CD80 and CD86 on RAW 264.7 cells
Costimulatory molecules CD80 and CD86 are expressed at low levels on unstimulated macrophages. These ligands provide the necessary secondary signal, along with major histocompatibility complex (MHC) complexed antigenic peptide, to induce T-cell activation allowing for clonal expansion and cytokine production [Crawford et al. 2006). Therefore, the effect of CTB on the expression levels of CD80, CD86 and MHC class II on RAW cells was determined in vitro. For this purpose, RAW 264.7 cells were incubated for 24 and 48 h with the fixed bacterial immunogen iFt in the presence or absence of the CTB adjuvant, and the expression levels of costimulatory molecules was determined by flow cytometry (Figure 3). Cells treated with iFt and CTB had a concentration-dependent increase in CD80 [Figure 3(a)] and CD86 [Figure 3(b)] on their surface. Treatment of cells with iFt or CTB alone did not affect the expression of these costimulatory molecules, suggesting that the increase observed requires the synergism between the bacterial immunogen and CTB (Figure 3 and data not shown).
Figure 3.
Costimulatory molecules CD80 and CD86 are upregulated with iFt + CTB treatment. RAW 264.7 cells were cultured with iFt in the presence or absence of CTB for 24 and 48 h. Expression of MHC class II, CD80 (a) and CD86 (b) was assessed by flow cytometry. Results are also presented as a bar graph (c). These data are representative of three experiments (*p < 0.05 and **p < 0.01, n = 2). CTB, cholera toxin B; iFt, inactivated (fixed) Francisella tularensis; IL, interleukin; MHC, major histocompatibility class; PBS, phosphate-buffered saline.
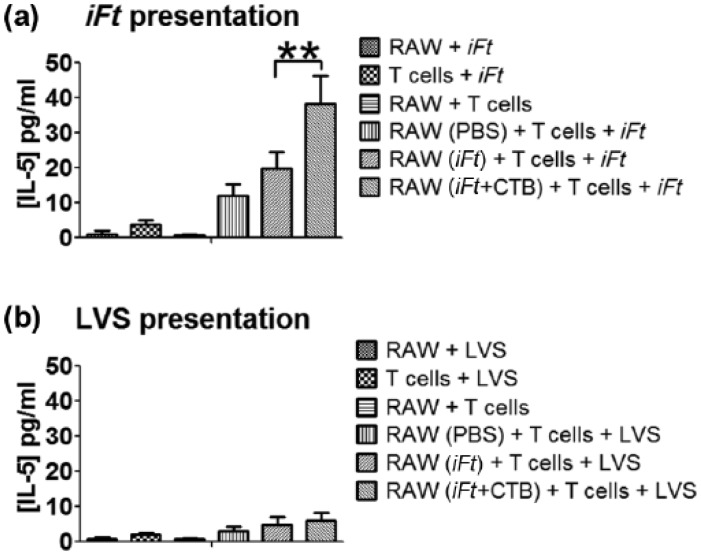
Antigen presentation in vitro is enhanced by pretreatment of RAW 264.7 cells with iFt plus CTB
Unstimulated macrophages express low levels of MHC class II and are generally poor activators of naïve T cells. The enhanced MHC class II and costimulatory molecule expression by RAW 264.7 cells treated with iFt + CTB suggests that the adjuvant may increase the capacity of these cells to present antigen in vitro. In order to assess antigen presentation, the RAW 264.7 cells were primed with iFt + CTB and cultured with either iFt or FT LVS and the Ft-specific T-cell hybridoma (FT256D10). Presentation of Ft antigens and subsequent activation of the T-cell hybridoma was assessed through IL-5 secretion. Cells treated with iFt + CTB and further exposed to iFt antigens had a significant increase in IL-5 secretion compared with cells treated with iFt alone [Figure 4(a)], indicating improved antigen presentation from the macrophage cells. Conversely, when pretreated macrophages were exposed to FT LVS there was little evidence of T-cell activation in all groups [Figure 4(b)]. The difference in IL-5 secretion observed between the live and fixed antigen may be attributed to the ability of live Ft to subvert innate antimicrobial effector functions and proliferate in the intracellular niche of RAW 264.7.
Figure 4.
Experimental vaccine treatment of iFt and CTB enhances antigen presentation of fixed bacterial antigens by RAW 264.7 cells. RAW 264.7 cells were treated as previously described and subsequently cocultured with the Ft-specific T-cell hybridoma, FT256D10, for 24 h together with either iFt (a) or LVS (b) as a source of bacterial antigen. Antigen presentation was assessed by the levels of IL-5 secreted by the activated T-cell hybridoma. IL-5 in the culture supernatants was measured by ELISA (*p < 0.05 and **p < 0.01, n = 3). CTB, cholera toxin B; ELISA, enzyme-linked immunosorbent assay; iFt, inactivated (fixed) Francisella tularensis; IL, interleukin; PBS, phosphate-buffered saline.
Discussion
Studies suggest the primary immune response to Ft infection requires IFNγ- and TNF-mediated activation of macrophages and neutrophil recruitment, followed by a secondary response from CD4 and CD8 T cells required to overcome the infection and produce a long-lasting memory response [Elkins et al. 2003]. In the current study we observed an increase in macrophage mediated proinflammatory cytokines IL-6 and TNFα, suggesting enhancement of the acute inflammatory response when CTB is administered with iFt. Harnessing a healthy acute response through adjuvant addition would provide crucial support in bacterial clearance and antigen presenting cell (APC) recruitment during the initial phase of infection. Following the acute response, activated macrophages and other professional APCs present antigen to T cells which require a costimulatory signal provided by CD80 and CD86 on the APCs binding CD28 on T cells. The increase of costimulatory molecules in response to CTB and iFt could indicate the activated macrophages are more efficient at costimulation, limiting the occurrence of antigen presentation resulting in tolerance or anergy. Moreover, the abundance of costimulatory molecules on macrophages could enhance the proliferation of effector T cells and the generation of an Ft specific response.
In addition to increased costimulatory molecules, higher levels of the pathogen recognition receptor TLR4 were detected by flow cytometry. LPS is found on the surface of Gram-negative bacteria like Ft and is usually a potent activator of TLR4. The LPS expressed by Ft has limited antigenic activity compared with LPS produced by other Gram-negative bacteria such as Escherichia coli and Salmonella. As a result, Ft LPS has been shown to have limited binding to TLR4 and signals primarily through TLR2. While the atypical LPS endotoxin expressed on Ft is a poor activator of TLR4, one study demonstrated that TLR4-deficient mice were more vulnerable to intracutaneous infection by LVS [Macela et al. 1996]. Hence, increases in the TLR4 receptor may be significant if increased receptor density raises the potential for LPS ligation and macrophage activation.
The increase in the costimulatory molecules on RAW cells, following treatment with the fixed bacterial immunogen iFt plus CTB, suggests the potential for enhanced antigen presentation. To confirm that Ft antigens was more readily presented to T lymphocytes, an antigen presentation assay was performed using the Ft-specific T-cell hybridoma FT256D10 cocultured with either iFt or LVS as a source of Ft antigens. Antigen presentation was increased in cells exposed to the fixed antigen while Ft LVS had no effect. These observations may be the byproduct of a hallmark of Ft pathogenesis: intracellular survival and replication in the cytosol of a host macrophage. During the initial infection, Ft is sequestered within the phagasome, resulting in increases in TNFα and nuclear factor κB (NFκB) driven proinflammatory cytokine production [Telepnev et al. 2005]. The bacterium evades initial destruction and subverts the primary host defense through altering acidification and maturation of the phagosome and then escaping to the cytosol to replicate [Fernandes-Alnemri et al. 2010]. In coordination with entrance into the cytosol, Ft inhibits NFκB, thus modulating the host inflammatory reaction to the intracellular bacterium. In response to cytosolic localization of Ft, the mouse macrophage produces type I IFN and absent in melanoma 2 (AIM2) protein, leading to activation of the inflammasome [Asare and Kwaik, 2010; Sjostedt, 2006]. As a result of inflammasome activation, the host macrophages undergo caspase-1 and death-fold containing adaptor protein mediated cell death in an attempt to quell bacterial replication and expansion [Mariathasan et al. 2005]. This cytopathogenic effect has been observed after 24–48 h in the Ft LVS infected murine macrophage cell line J774 [Lai et al. 2001]. These phenomena may have resulted in the limited antigen presentation of LVS we observed in RAW 264.7.
While the Ft LVS strain can present challenges in the context of antigen presentation, the inactivated bacteria administered with CTB significantly increased antigen presentation and thus T-cell activation. This demonstrates the stimulation of cellular immunity invoked by CTB when administered with iFt, which could provide sufficient protection against lethal Ft challenge. In fact, when CTB is administered as an adjuvant with iFt in vivo, increased protection against Ft biovar A and biovar B challenge was noted [Bitsaktsis et al. 2009]. To further correlate our observations of CTB in vitro with protective cellular immunity, future evaluation of CTB coadministered with iFT in a murine model is required. Evidence of adjuvant-enhanced macrophage activation could be established through quantification of proinflammatory cytokines such as TNFα, IL-6, IL-12, and IL-1 in bronchoalveolar lavage fluid and lung homogenates. In addition, in vivo macrophage activation can be assessed through the expression of costimulatory molecules on the surface of peritoneal cells and alveolar macrophages following immunization with CTB and the immunogen. Furthermore, memory T-cell responses can be demonstrated ex vivo by monitoring T-cell expansion and cytokine secretion in isolated splenocytes exposed to iFT. However, the lack of enhanced antigen presentation of LVS by murine macrophages suggests that the role they play in protection observed in vivo is primarily due to enhanced proinflammatory cytokine production rather than providing the bridge between innate and adaptive immunity.
While evidence suggests CTB is a potent immunogen, certain challenges exist which have slowed the potential for FDA approval of CTB as an adjuvant. Foremost among these concerns are reports of side effects ranging from headaches and nasal bleeding to some rare cases of encephalitis [Scerpella et al. 1995]. Many of the side effects observed however may be overcome by optimizing dosing regimens or creating CTB-coupled vaccination strategies to provide a strong immune response while limiting side effects [Guo et al. 2012].
In summary, the adjuvant CTB administered with iFt showed in vitro evidence of enhancing cellular immunity through increases in proinflammatory immune-stimulating cytokines. CTB also appears to activate an antigen-specific response by increasing costimulatory molecules and antigen presentation, therefore generating pathogen-specific T-cell responses. These observations demonstrate mechanisms through which CTB could stimulate protection in an in vivo infectious disease model. Hence, utilizing an adjuvant like CTB may provide the necessary enhancement of the innate and adaptive facets of the immune response required to protect against Ft infection.
Acknowledgments
We would like to thank Dr K. Elkins (US Food and Drug Administration, Bethesda, MD, USA) for providing the F. tularensis LVS (ATCC 29684; American Type Culture Collection).
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by Seton Hall University start-up research funds.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Kari Wiedinger, Seton Hall University, South Orange, NJ, USA.
Heather Romlein, Seton Hall University, South Orange, NJ, USA.
Constantine Bitsaktsis, Department of Biological Sciences, Seton Hall University, South Orange, NJ, USA.
References
- Asare R., Kwaik Y. (2010) Exploitation of host cell biology and evasion of immunity by Francisella tularensis. Front Microbiol 1: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitsaktsis C., Rawool D., Li Y., Kurkure N., Iglesias B., Gosselin E. (2009) Differential requirements for protection against mucosal challenge with Francisella tularensis in the presence versus absence of cholera toxin B and inactivated F. tularensis. J Immunol 182: 4899–4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens D., Lee B., Horwitz M. (2004) Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages. Infect Immun 72: 3204–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A., Macleod M., Schumacher T., Corlett L., Gray D. (2006) Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J Immunol 176: 3498–3506. [DOI] [PubMed] [Google Scholar]
- Elkins K., Cowley S., Bosio C. (2003) Innate and adaptive immune responses to an intracellular bacterium, Francisella tularensis live vaccine strain. Microbes Infect 5: 135–142. [DOI] [PubMed] [Google Scholar]
- Fernandes-Alnemri T., Yu J., Juliana C., Solorzano L., Kang S., Wu J., et al. (2010) The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol 11: 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi M., Sallusto F., Marinaro M., Vendetti S., Riccomi A., De Magistris M. (2002) Effects of the adjuvant cholera toxin on dendritic cells: stimulatory and inhibitory signals that result in the amplification of immune responses. Int J Med Microbiol 291: 571–575. [DOI] [PubMed] [Google Scholar]
- Guo L., Liu K., Xu G., Li X., Tu J., Tang F., et al. (2012) Prophylactic and therapeutic efficacy of the epitope vaccine CTB-UA against Helicobacter pylori infection in a BALB/c mice model. Appl Microbiol Biotechnol 95: 1437–1444. [DOI] [PubMed] [Google Scholar]
- Hajjar A., Harvey M., Shaffer S., Goodlett D., Sjostedt A., Edebro H., et al. (2006) Lack of in vitro and in vivo recognition of Francisella tularensis subspecies lipopolysaccharide by Toll-like receptors. Infect Immun 74: 6730–6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich P., Behrmann I., Haan S., Hermanns H., Muller-Newen G., Schaper F. (2003) Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 374: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Czerkinsky C., Eriksson K., Mharandi A. (2003) Mucosal immunisation and adjuvants: a brief overview of recent advances and challenges. Vaccine 21(Suppl. 2): S89–S95. [DOI] [PubMed] [Google Scholar]
- Kim P., Eckmann L., Lee W., Han W., Kagnoff M. (1998) Cholera toxin and cholera toxin B subunit induce IgA switching through the action of TGF-beta 1. J Immunol 160: 1198–1203. [PubMed] [Google Scholar]
- Lai X., Golovliov I., Sjostedt A. (2001) Francisella tularensis induces cytopathogenicity and apoptosis in murine macrophages via a mechanism that requires intracellular bacterial multiplication. Infect Immun 69: 4691–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelle E., Jarnicki A., McNeela E., Armstrong M., Higgins S., Leavy O., et al. (2004) Effects of cholera toxin on innate and adaptive immunity and its application as an immunomodulatory agent. J Leukoc Biol 75: 756–763. [DOI] [PubMed] [Google Scholar]
- Macela A., Stulik J., Hernychova L., Kroca M., Krocova Z., Kovarova H. (1996) The immune response against Francisella tularensis live vaccine strain in LPS(N) and LPS(D) mice. FEMS Immunol Med Microbiol 13: 235–258. [DOI] [PubMed] [Google Scholar]
- Maeyama J., Isaka M., Yasuda Y., Matano K., Kozuka S., Taniguchi T., et al. (2001) Cytokine responses to recombinant cholera toxin B subunit produced by Bacillus brevis as a mucosal adjuvant. Microbiol Immunol 45: 111–117. [DOI] [PubMed] [Google Scholar]
- Mariathasan S., Weiss D., Dixit V., Monack D. (2005) Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J Exp Med 202: 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyston P., Sjostedt A., Titball R. (2004) Tularaemia: bioterrorism defence renews interest in Francisella tularensis. Nat Rev Microbiol 2: 967–978. [DOI] [PubMed] [Google Scholar]
- Pechous R., McCarthy T., Zahrt T. (2009) Working toward the future: insights into Francisella tularensis pathogenesis and vaccine development. Microbiol Mol Biol Rev 73: 684–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerpella E., Sanchez J., Mathewson I., Torres-Cordero J., Sadoff J., Svennerholm A., et al. (1995) Safety, immunogenicity, and protective efficacy of the whole-cell/recombinant B subunit (WC/rBS) oral cholera vaccine against travelers’ diarrhea. J Travel Med 2: 22–27. [DOI] [PubMed] [Google Scholar]
- Sjostedt A. (2006) Intracellular survival mechanisms of Francisella tularensis, a stealth pathogen. Microbes Infect 8: 561–567. [DOI] [PubMed] [Google Scholar]
- Steiner D., Furuya Y., Metzger D. (2014) Host-pathogen interactions and immune evasion strategies in Francisella tularensis pathogenicity. Infect Drug Resist 7: 239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telepnev M., Golovliov I., Grundstrom T., Tarnvik A., Sjostedt A. (2003) Francisella tularensis inhibits toll-like receptor-mediated activation of intracellular signalling and secretion of TNF-alpha and IL-1 from murine macrophages. Cell Microbiol 5: 41–51. [DOI] [PubMed] [Google Scholar]
- Telepnev M., Golovliov I., Sjostedt A. (2005) Francisella tularensis LVS initially activates but subsequently down-regulates intracellular signaling and cytokine secretion in mouse monocytic and human peripheral blood mononuclear cells. Microb Pathog 38: 239–47. [DOI] [PubMed] [Google Scholar]
- Wu T., Hutt J., Garrison K., Berliba L., Zhou Y., Lyons C. (2005) Intranasal vaccination induces protective immunity against intranasal infection with virulent Francisella tularensis biovar A. Infect Immun 73: 2644–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]