Abstract
The nucleus accumbens (NAc) is important for the ability of motivationally-significant stimuli to guide behavior. To further delineate its role in appetitive Pavlovian conditioning, we tested the hypothesis that the NAc contributes to memory consolidation and expression for a goal-tracking version of Pavlovian conditioned approach (PCA) in rats. We found that neither post-training reversible inactivation with the GABA receptor agonists muscimol and baclofen nor inhibition of protein synthesis with anisomycin in either the core or shell regions of the NAc had an effect on approach to a reward port in response to a reward-predictive cue (CS+). In contrast, pre-test reversible inactivation of both the core and shell decreased conditioned responding during the CS+. Unlike inactivation of the core, however, reversible inactivation of the shell also produced an increase in responding during the CS− and the inter-trial interval. This suggests that the NAc is not involved in the consolidation of goal-tracking PCA, but that once the memory is formed, the core is required for expression of the CS-US association and the shell is required to inhibit conditioned approach behavior at times when the CS+ is not presented.
Keywords: nucleus accumbens core, nucleus accumbens shell, Pavlovian conditioning, appetitive conditioning, learning, memory, rat
Introduction
Historically, the nucleus accumbens (NAc) has been considered a site of “limbic-motor integration” important for the ability of motivationally-significant stimuli to guide goal-directed behavior [1–3]. The NAc and its subnuclei, the core and shell, are involved in a variety of appetitive behaviors [4–10]. In a type of appetitive Pavlovian conditioning called Pavlovian conditioned approach (PCA), repeated pairing of a conditioned stimulus (CS) with the delivery of a reward can induce multiple conditioned responses during CS presentation, including approach to both the CS and the site of US delivery [3, 11]. Conditioned approaches to the site of US delivery are known as goal-tracking [12].
Memory consolidation refers to the biological stabilization of long-term memories, and in rodents, this process is generally thought to occur during several hours immediately following memory acquisition [13–15]. Experimentally, memory consolidation can be differentiated from memory acquisition through the timing of experimental manipulations; pre-training manipulations are thought to affect memory acquisition while post-training manipulations are thought to affect memory consolidation [13, 14]. We demonstrated previously that inhibition of protein synthesis impairs consolidation of the goal-tracking form of PCA [16], but the location of this consolidation-related protein synthesis is unknown.
There is evidence that the NAc shell, but not the core, is involved in the consolidation, but not the acquisition, of goal-tracking PCA. Post-training infusions of the psychostimulant d-amphetamine in the full NAc as well as the NAc shell enhanced goal-tracking PCA, suggesting that the shell is involved in consolidation, but infusions of d-amphetamine in the core had no effect [17]. In addition, pre-training lesions of the NAc core and NAc shell had no effect on acquisition of goal-tracking PCA, and pre-training lesions that contralaterally disconnected the NAc and basolateral amygdala had no effect on the acquisition of first order conditioning (although second order conditioning was impaired), suggesting that the NAc is not necessary for PCA acquisition [18, 19]. However, there is evidence that the NAc is necessary for the expression of goal-tracking PCA as pre-test lesions of the NAc core (but not the shell) have been shown to impair the expression of this behavioral task [20].
To further elucidate the role of the NAcc core and shell in goal-tracking PCA, we have examined the effect of reversible inactivation of these brain regions on the consolidation and expression of a goal-tracking PCA task. Additionally, we have used the protein synthesis inhibitor anisomycin to investigate whether protein synthesis-dependent plasticity is necessary within these brain regions for the consolidation of the PCA memory.
Materials and Methods
Subjects
Male Long-Evans rats (Harlan, Indianapolis, IN) weighing between 250–280g were individually housed on ventilated racks in polycarbonate cages, and subjects were kept on a 12-h light: 12-h dark cycle (lights on at 7 a.m.). The day before the start of behavioral training in the Pavlovian Conditioned Approach (PCA) task, rats were water restricted, such that they were allowed free access to water for two hours per day, immediately after behavioral sessions. All rats received food ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Ernest Gallo Clinic and Research Center at the University of California, San Francisco, and are in accordance with “PHS Policy on Humane Care and Use of Laboratory Animals,” Office of Laboratory Animal Welfare, National Institutes of Health, USA, revised 2002.
Surgical Procedures
Rats were anesthetized with isoflurane (Baxter, Deerfield, IL) at an initial dose of 5% w/v for induction; the dose was gradually decreased to approximately 2% w/v for maintenance. Guide cannulae (26 gauge, Plastics One, Roanoke, VA) were implanted bilaterally into the nucleus accumbens (NAc) core or shell using standard stereotaxic procedures. Stereotaxic coordinates for the NAc core were 1.5 mm anterior to bregma, 1.5 mm from the midline, and 5.4 mm ventral (measured from dura); stereotaxic coordinates for the NAc shell were 1.4 mm anterior to bregma, .75 mm from the midline, and 5.0 mm ventral (measured from dura). As an analgesic, all rats were given ad lib access to acetaminophen (.4 mg/mL, oral, McNeil Consumer and Specialty Pharmaceuticals, Fort Washington, PA) for several days following surgery, and they were allowed at least 5 days to recover before the start of handling and behavioral procedures.
Drugs and Infusion Procedures
Internal cannulae (33 gauge, Plastics One, Roanoke, VA) were attached to 25 μL syringes (Hamilton, Reno, NV) via polyethylene tubing filled with distilled water. Drug solutions were separated from the distilled water by a small air bubble, and infusions were controlled by a syringe pump (Harvard Apparatus, Holliston, MA). For infusions into the NAc core, the internal cannulae extended 1.0 mm past the end of the guide cannulae, and for infusions into the NAc shell, the internal cannulae extended 2.0 mm past the end of the guide cannulae. Rats were held gently by the experimenter during the infusion.
Anisomycin (Sigma, St. Louis, MO) was dissolved in HCl, adjusted to a pH of 7.2, and diluted in phosphate-buffered saline [21, 22]. Anisomycin was infused at a dose of 62.5 μg per .5 μL per hemisphere. This dose (at this injection volume) was chosen because it impairs the consolidation of instrumental conditioning when infused into the NAc core [6]. In addition, a similar dose results in >90% inhibition of protein synthesis in the hippocampus within 10 min of infusion into the lateral ventricle [23] and >90% inhibition of protein synthesis for >2 hr in the cortex when infused locally [24]. The drug was infused at a rate of .25 μL/min, and the internal cannulae were left in place for 1 additional minute to allow for diffusion.
The GABAA agonist, muscimol, and the GABAB agonist, baclofen, (Sigma, St. Louis, MO) were both dissolved in phosphate-buffered saline at concentrations of .1 mM for muscimol and 1.0 mM for baclofen, and the muscimol/baclofen combination was infused at a total volume of .3 μL per hemisphere. Infusions of this dose (at this injection volume) into the NAc impairs responding in an operant model of relapse [25]. The muscimol/baclofen combination (M/B) was infused at a rate of .3 μL/min, and the internal cannulae were left in place for 2 additional minutes to allow for diffusion. Although ANI and M/B were infused at different injection volumes, we felt it was important to choose injection volumes that were directly comparable to the other studies that have used these drugs [6, 23–25].
Histology
Rats were deeply anesthetized with pentobarbital (190 mg/kg, IP, Virbac AH, Fort Worth, TX) and transcardially perfused with phosphate-buffered saline followed by 10% formalin (Fisher Scientific, Fair Lawn, NJ). Brains were extracted and sliced on a microtome at a thickness of 50 μm. Slices were stained with thionin and examined under a light microscope for accurate cannula placements. Cannula placement was determined through comparison with an atlas of the rat brain [26], and rats with inaccurate cannula placements were discarded from the study. More subjects were lost due to inaccurate cannula placements in the NAc shell than in the NAc core, resulting in fewer numbers of animals for experiments involving infusions in the NAc shell.
Behavioral Procedures
General Behavioral Apparatus
All behavioral experiments were conducted in conditioning chambers (MedAssociates, Georgia, VT) that were housed in sound-attenuating chambers. Syringe pumps delivered sucrose into a rectangular recess (sucrose port) located on the right wall of the chamber, and photobeams detected a rat’s entrance into the sucrose port. A stimulus light was located on the far left side of the right wall; a houselight was located in the top center of the left wall; and a 2.9 kHz tone could be played from speakers. A computer with MedAssociates software controlled all the equipment in the conditioning chambers, and entries into the sucrose port were recorded by the software.
Pavlovian Conditioned Approach (PCA)
The Pavlovian conditioned approach (PCA) task was conducted as described previously [16]. To teach rats to consume sucrose from the sucrose port, rats were first given a single magazine training session that lasted approximately 1 hr. After a 5-min habituation period, 30 deliveries of a 10% sucrose solution (w/v, .2 mL) were given on a VI-120 s schedule, and no conditioned stimuli were presented. All subsequent PCA sessions were training sessions that lasted 90 min, and training sessions were separated by 24 h. The training sessions began with a 5-min habituation period, and then presentations of a CS+ and a CS− (15 presentations of each) were given in random order on a VI-150 s schedule. The stimuli used were a 2.9 kHz auditory tone and a lighting change (the stimulus light was turned on; the houselight was turned off), and the choice of stimulus to be used as the CS+ was counterbalanced across subjects. Stimulus presentations lasted for 10 s, and a 10% sucrose solution (w/v, .2 mL) was delivered immediately after the offset of the CS+.
The number of sucrose port entries during the 10 sec CS+ presentations, the 10 sec CS− presentations, and the 10 sec preceding each CS+ presentation (the pre-CS+ period) was recorded by computer. To use a normalized measure of conditioning that compared behavior during the CS+ and CS−, we subtracted the total number of port entries during all CS− presentations from the total number of port entries during all CS+ presentations, and we termed this measure of behavior the difference score. We also used two additional measures of conditioning. First, we calculated the percent of the total CS presentation time that the rat spent in the sucrose port. In addition, we calculated the total number of CS presentations during which a rat made a CS response. On any given trial, a rat was defined as making a CS response if the rat was present in the sucrose port at any time during the CS presentation. To examine whether the rats exhibited motivation to consume the sucrose solution, we also determined whether the rats responded to the US delivery on each trial. On any given trial, a rat was defined as making a US response if the rat was present in the sucrose port within 30 s of the onset of the syringe pump that delivered the reward.
Statistical Analysis
The data were initially analyzed using a 2-way or 3-way ANOVA, and, depending on the experiment, CS+ Type (light or tone), Treatment, and/or Treatment Order were used as between-subjects factors, and Session or Treatment was used as a within-subjects factor. If there were no main effects of CS+ Type (either light or tone) and no differences between treatment groups depending on CS+ Type, statistical analyses (1-way ANOVA or 2-way repeated measures ANOVA) were collapsed across CS+ type. Significant main effects and/or interactions were further analyzed through planned comparisons. Three-way ANOVA analyses were conducted using Statistica software (StatSoft, Inc., Tulsa, OK), and all other statistical analyses were conducted using SigmaStat software (SPSS, Inc, Chicago, IL).
Results
Reversible Inactivation During the Consolidation of Pavlovian Conditioned Approach
To investigate whether the NAc is involved in the consolidation of the goal-tracking version of PCA, we used two approaches: post-training local reversible inactivation using GABA-receptor agonists and post-training local protein synthesis inhibition using anisomycin. Although the former approach identifies an involvement of a given region in consolidation, it could impair consolidation through two different mechanisms. First, GABA agonists could impair consolidation through GABA-receptor mediated suppression of neuronal activity necessary to modulate plasticity occurring in other brain regions. Alternately, GABA-receptor mediated decreases in membrane potential could blunt the activity of other receptor-activated second-messenger pathways and suppress plasticity in the region itself. In contrast, the use of protein synthesis inhibitors tests whether changes in protein synthesis, a step considered necessary for neuronal plasticity underlying long-term memory formation, is required for the consolidation of this task, and therefore identifies a site of memory formation [14].
We first examined whether reversible inactivation of the NAc core or shell would impair the consolidation of PCA. After one day of magazine training to familiarize subjects with the location and mode of reward delivery (see Methods), rats were given 4 PCA training sessions, each separated by 24 h. A cocktail of muscimol, a GABAA agonist, and baclofen, a GABAB agonist, was used to reversibly inactivate the NAc core or shell, and either vehicle or the muscimol/baclofen cocktail (M/B) was infused into the core or the shell immediately after each of the first three PCA training sessions (see Figure 2a for the experimental design). Schematic representations of the cannula placements in core and shell are shown in Figure 1.
Figure 2. Reversible inactivation of the NAc does not affect the consolidation of PCA behavior.
a) Experimental design.
b) Mean difference score ± SEM during PCA training sessions for rats that received infusions into the NAc core (Vehicle: n=6; M/B: n=7). The difference score reported in this and the following figures represents the number of port entries during the CS+ minus the number of port entries during the CS−.
c) Mean difference score ± SEM during PCA training sessions for rats that received infusions into the NAc shell (Vehicle: n=7; M/B: n=8).
d) Mean number of port entries (± SEM) exhibited during stimulus presentations in the 4th PCA training session for rats that received infusions in the NAc core. “Pre-CS+” = the 10 sec preceding the onset of the CS+.
e) Mean number of port entries (± SEM) exhibited during stimulus presentations in the 4th PCA training session for rats that received infusions in the NAc shell. “Pre-CS+” = the 10 sec preceding the onset of the CS+.
Figure 1. Schematic representation of cannula placements for rats that received post-training reversible inactivation.
a) Cannula placements for rats that received post-training infusions of vehicle or M/B in the NAc core. Coordinates are A/P from Bregma.
b) Cannula placements for rats that received post-training infusions of vehicle of M/B in the NAc shell. Coordinates are A/P from Bregma.
NAcc Core Infusions
Post-training infusions of M/B into the NAc core had no effect on conditioned responding, as indicated by our measure of stimulus discrimination, the mean difference score (Figure 2b). A 2-way repeated measures ANOVA did find a significant main effect of Session [F(3,33)=2.01, P<.001], suggesting that all rats learned across training sessions, but there was neither a significant main effect of Treatment nor a significant interaction between factors [Treatment: F(1,11)=.20, P=.67; Interaction: F(3,33)=.07, P=.98].
Because the difference score is a normalized behavioral measure, however, it is possible that, even though there was no change in the mean difference score after intra-core M/B infusions, there could have been identical changes in the raw number of port entries during both the CS+ and CS− presentations. Therefore, to confirm that there was no difference between treatment groups, we investigated the port entry behavior in more detail and examined the number of port entries made by subjects during the 10 second CS+ presentation, the 10 second CS− presentation, and the 10 seconds preceding the onset of the CS+ (the pre-CS+ period). Similar to the difference score measure, all rats showed an increase across training sessions in the number of port entries made during the CS+ [2-way repeated measures ANOVA; Session: F(3,33)=24.672, P<.001], but there was no difference between rats that received M/B infusions in the core and those that received vehicle [see Figure 2d; Treatment: F(1,11)=.20, P=.66, Interaction: F(3,33)=.18, P=.91]. Likewise, there was no difference between treatment groups in the number of port entries made during the pre-CS+ period [2-way repeated measures ANOVA; Treatment: F(1,11)=.26, P=.62; Session: F(3,33)=.24, P=.87; Interaction: F(3,33)=.12, P=.95]. There was also no effect of M/B infusions in the core on the number of port entries made during the CS− presentations, and although rats trained with a light as a CS− exhibited a higher number of port entries during the CS− than those trained with a tone as a CS−, the effect of the M/B infusions did not depend on the CS+ configuration [3-way repeated measures ANOVA; CS+ Type: F(1,9)=14.54, P<.005; Treatment: F(1,9)=.61, P=.45; Session: F(3,27)=.38, P=.77; CS+ Type × Treatment Interaction: F(1,9)=.002, P=.96; CS+ Type × Session Interaction: F(3,27)=.21, P=.89; CS+ Type × Treatment × Session Interaction: F(3,27)=1.04, P=.39]. Therefore, post-training infusions of M/B into the NAc core had no effect on the number of port entries made during training,
We also examined two additional measures of conditioning. Like the mean difference score measure, post-training intra-core M/B infusions had no effect on the average number CS+ presentations during which rats made a CS response [2-way repeated measures ANOVA; Treatment: F(1,11)=1.17, P=.30; Session: F(3,33)=52.82, P<.001; Interaction: F(3,33)=.38, P=.77]. We also examined the percent of time that the rats spent in the sucrose port during the CS+ presentations, and a 2-way repeated measures ANOVA found a significant statistical interaction between Treatment and Session [Treatment: F(1,11)=2.03, P=.18; Session: F(3,33)=27.34; P<.001; Interaction: F(3,33)=3.36, P<.03]. Further analysis found that rats that received post-training infusions of M/B into the NAc core spent a lower percent of their time in the sucrose port during CS+ presentations in the fourth training session [Vehicle group = 43.79% ± 7.94% (mean ± SEM); M/B group = 23.09% ± 7.42%; Student-Newman-Keuls planned pairwise comparison: P<.01], suggesting that these rats exhibited a slight impairment in consolidation of later learning using this behavioral measure. However, there was no difference between the two groups in the first three training sessions [Student-Newman-Keuls planned pairwise comparisons, veh vs. M/B; Train 1: P=1.00; Train 2: P=.81; Train 3: P=.22]. In addition, across training sessions, rats in both treatment groups significantly increased the percent of time they spent in the sucrose port during CS+ presentations [1-way repeated measures ANOVAs; Vehicle group, Session: F(3,15)=19.54, P<.0011; M/B Group, Session: F(3,18)=7.55, P<.002], suggesting that post-training intra-core M/B infusions did not prevent successful learning of the task over the four training sessions. Therefore, post-training infusions of M/B in the NAc core resulted in impaired conditioned responding in one behavioral measure of conditioning and had no effect on two other behavioral measures of conditioning.
NAc Shell Infusions
Post-training infusions of M/B into the NAc shell also had no effect on PCA consolidation. The mean difference scores during PCA training sessions for rats that received infusions into the NAc shell are shown in Figure 2c. A 3-way repeated measures ANOVA found no significant main effect of Treatment, nor a significant Treatment × Session interaction [Treatment: F(1,11)=.03, P=.86; Treatment × Session Interaction: F(3,33)=.16, P=.92]. However, there was a significant main effect of Session [Session: F(3,33)=15.78, P<.001], suggesting that all animals successfully learned the task. In general, the rats with a light as the CS+ did exhibit higher difference scores that the rats with a tone as the CS+ [CS+ Type: F(1,11)=5.28, P<.05; CS+ Type × Session Interaction: F(3,33)=5.89, P<.003], but there was no statistical interaction between CS+ Type and Treatment [CS+ Type × Treatment Interaction: F(1,11)=.07, P=.80; CS+ Type × Treatment × Session Interaction: F(3,33)=.83, P=.49], suggesting that the effect of M/B infusions did not depend on the CS configuration.
To confirm that there was no difference between treatment groups, we investigated the port entry behavior in more detail. Post-training infusions of M/B in the NAc shell had no effect on the number of port entries made during the pre-CS+ period [see Figure 2e; 2-way repeated measures ANOVA; Treatment: F(1,13)=3.64, P=.08; Session: F(3,39)=.58, P=.63; Interaction: F(3,39)=.18, P=.91], the CS− [2-way repeated measures ANOVA; Treatment: F(1,13)=2.61, P=.13; Session: F(3,39)=.86, P=.47; Interaction: F(3,39)=.79, P=.51], or the CS+ [3-way repeated measures ANOVA; CS+ Type: F(1,11)=5.5.3, P<.04; Treatment: F(1,11)=.35, P=.57; Session: F(3,33)=14.34, P<.001; Treatment × Session Interaction: F(3,33)=.07, P=.97]. In general, the rats with a light as the CS+ did make more port entries during the CS+ compared to the rats with a tone as the CS+ [CS+ Type: F(1,11)=5.5.3, P<.04; CS+ Type × Session Interaction: F(3,33)=3.57, P<.03], but there was no statistical interaction between CS+ Type and Treatment [CS+ Type × Treatment Interaction: F(1,11)=.04, P=.85; CS+ Type × Treatment × Session Interaction: F(3,33)=1.17, P=.33], suggesting that the effect of M/B infusions did not depend on the CS configuration.
We also examined two additional behavioral measures of conditioning. Post-training M/B infusions in the NAc shell had no effect on the average number CS+ presentations during which rats made a CS response [3-way repeated measures ANOVA; Treatment: F(1,11)=.62, P=.45; Session: F(3,33)=14.22, P<.001; Treatment × Session Interaction: F(3,33)=2.48, P=.08]. As with the difference score measure and the raw number of port entries during the CS+, the rats with a light as the CS+ exhibited CS responses during more of the CS+ presentations compared to the rats with a tone as the CS+ [CS+ Type: F(1,11)=7.05, P<.03; CS+ Type × Session Interaction: F(3,33)=2.93, P<.05], but there was no statistical interaction between CS+ Type and Treatment [CS+ Type × Treatment Interaction: F(1,11)=1.12, P=.31; CS+ Type × Treatment × Session Interaction: F(3,33)=2.35, P=.09], suggesting that the effect of M/B infusions did not depend on the CS configuration. Post-training M/B infusions in the NAc shell also had no effect on the percent of time that the rats spent in the sucrose port during the CS+ presentations [2-way repeated measures ANOVA; Treatment: F(1,13)=.005, P=.95; Session: F(3,39)=17.52, P<.001; Interaction: F(3,39)=1.41, P=.26]. Therefore, post-training M/B infusions in the NAc shell had no effect on multiple behavioral measures of conditioning.
Inhibition of Protein Synthesis During the Consolidation of Pavlovian Conditioned Approach
To examine further if changes within the NAc cells are necessary for the consolidation of this task, we investigated whether protein synthesis is necessary in either the NAc core or shell for the consolidation of Pavlovian conditioned approach (PCA). After one day of magazine training, rats were given 4 daily PCA training sessions. Either vehicle or the protein synthesis inhibitor anisomycin (ANI) was infused into the NAc core or shell immediately after each of the first three training sessions (see Figure 4a for experimental design). Schematic representations of the cannula placements are shown in Figure 3.
Figure 4. Protein synthesis inhibition within the NAc does not affect the consolidation of PCA behavior.
a) Experimental design.
b) Mean difference score ± SEM during PCA training sessions for rats that received infusions into the NAc core (Vehicle: n=12; Ani: n=11).
c) Mean difference score ± SEM during PCA training sessions for rats that received infusions into the NAc shell (Vehicle: n=6; Ani: n=6).
d) Mean number of port entries (± SEM) exhibited during stimulus presentations in the 4th PCA training session for rats that received infusions in the NAc core. “Pre-CS+” = the 10 sec preceding the onset of the CS+.
e) Mean number of port entries (± SEM) exhibited during stimulus presentations in the 4th PCA training session for rats that received infusions in the NAc shell. “Pre-CS+” = the 10 sec preceding the onset of the CS+.
Figure 3. Schematic representation of cannula placements for rats that received post-training inhibition of protein synthesis.
a) Cannula placements for rats that received post-training infusions of vehicle or ANI in the NAc core. Coordinates are A/P from Bregma.
b) Cannula placements for rats that received post-training infusions of vehicle or ANI in the NAcc shell. Coordinates are A/P from Bregma.
NAc Core Infusions
Infusions of ANI into the NAc core had no effect on the consolidation of conditioned approach to the reward port (Figure 4b). A 2-way repeated measures ANOVA analyzing the difference scores across training sessions found a significant main effect of Session [F(3,63)=52.81; P<.001], but there was neither a significant main effect of Treatment [F(1,21)=0.00; P=.98] nor a significant interaction between the Treatment and Session [F(3,63)=1.25; P=.30].
To confirm that there was no difference between treatment groups, we investigated the port entry behavior in more detail. As with the difference score, post-training infusions of ANI in the NAc core had no effect on the number of port entries during the CS+ [see Figure 4d; 2-way repeated measures ANOVA; Treatment: F(1,21)=.13, P=.73; Session: F(3,63)=54.92, P<.001; Interaction: F(3,63)=.23, P=.87] or during the pre-CS+ period [2-way repeated measures ANOVA; Treatment: F(1,21)=1.88, P=.19; Session: F(3,62)=.85, P=.47; Interaction: F(3,63)=.45, P=.72]. A 3-way repeated measures ANOVA analyzing the number of port entries during the CS− across training session did not find a main effect of Treatment, but there was a significant statistical interaction between Treatment and Session [Treatment: F(1,19)=3.57, P=.07; Session: F(3,57)=.95, P=.42; Treatment × Session Interaction: F(3,57)=2.99, P<.04]. However, further analyses found that there was no consistent difference between treatment groups; there was a difference between the vehicle and ANI groups during the third training session [Student-Newman-Keuls planned pairwise comparison; Train 3: P<.03], but there was no difference between groups during any of the other training sessions [Student-Newman-Keuls planned pairwise comparisons; Train 1: P=.21; Train 2: P=.85; Train 4: P=.11]. In general, rats with a light as the CS+ did make more port entries during the CS− compared to the rats with a tone as the CS+ [CS+ Type: F(1,19)=18.04, P<.001; CS+ Type × Session Interaction: F(3,57)=.11, P=.95], but there was no statistical interaction between CS+ Type and Treatment [CS+ Type × Treatment Interaction: F(1,19)=.50, P=.49; CS+ Type × Treatment × Session: F(3,57)=.57, P=.64], suggesting that the effect of M/B infusions did not depend on the CS configuration.
When we examined two additional measures of conditioning, we found that post-training administration of ANI in the NAc core had no effect on either the average number of CS+ presentations during which rats made a CS response [2-way repeated measures ANOVA; Treatment: F(1,21)=.07, P=.80; Session: F(3,62)=59.14, P<.001; Interaction: F(3,63)=.83, P=.48] or the percent of time spent in the sucrose port during the CS+ [2-way repeated measures ANOVA; Treatment: F(1,21)=.66, P=.42; Session: F(3,63)=45.63, P<.001; Interaction: F(3,63)=.20, P=.89]. Therefore, even when multiple behavioral measures of conditioning were examined, post-training infusions of ANI in the NAc core had little effect on PCA behavior.
NAc Shell Infusions
Like infusions into the core, post-training infusions of ANI into the NAc shell also had no effect on the consolidation of PCA behavior (see Figure 4c). A 2-way repeated measures ANOVA analyzing the mean difference score across PCA training sessions found a main effect of Session, suggesting that all subjects successfully learned the task [Session: F(3,30)=30.23; P<.001], but there was no difference between rats that received ANI infusions in the NAc shell and those that received vehicle infusions [Treatment: F(1,10)=2.19, P=.17]; Treatment × Session interaction: F(3,30)=.33; P=.81].
To confirm that there was no difference between treatment groups, we examined the number of port entries that were made during the CS+ presentations, CS− presentations, and pre-CS+ periods. Rats that received post-training infusions of ANI in the NAc shell made similar numbers of port entries during the CS+ presentations [see Figure 4e; 2-way repeated measures ANOVA; Treatment: F(1,10)=2.07; P=.18; Session: F(3,30)=19.81, P=<.001; Interaction: F(3,30)=.22, P=.88], CS′ presentations [2-way repeated measures ANOVA; Treatment: F(1,10)=.02, P=.89; Session: F(3,30)=4.34, P<.02; Interaction: F(3,30)=.89, P=.46], and pre-CS+ period [2-way repeated measures ANOVA; Treatment: F(1,10)=.32, P=.58; Session: F(3,30)=.88, P=.46; Interaction: F(3,30)=.07, P=.98] compared rats that received vehicle. Therefore, post-training infusions of ANI into the NAc shell had no effect on the number of port entries made during training,
When we examined two additional measures of conditioning, we found that post-training administration of ANI in the NAc shell had no effect on either the average number of CS+ presentations during which rats made a CS response [2-way repeated measures ANOVA; Treatment: F(1,10)=1.43, P=.26; Session: F(3,30)=21.52, P<.001; Interaction: F(3,30)=.63, P=.60] or the percent of time spent in the sucrose port during the CS+ [2-way repeated measures ANOVA; Treatment: F(1,10)=2.44, P=.15; Session: F(3,30)=21.82, P<.001; Interaction: F(3,30)=.52, P=.67]. Therefore, even when multiple behavioral measures of conditioning were examined, post-training infusions of ANI in the NAc shell did not impair the consolidation of PCA behavior.
In summary, neither post-training reversible inactivation of the NAc core and shell nor post-training inhibition of protein synthesis in the NAc core and shell appears to affect consolidation of PCA. These data suggest that NAc is not required for successful consolidation of the associations underlying goal-tracking PCA, under the conditions of our study.
Reversible Inactivation During the Expression of Pavlovian Conditioned Approach
To determine whether the NAc is involved in the expression of PCA once it has been acquired, we investigated whether reversible inactivation of the NAc core and shell immediately before testing would impair PCA behavior. After one session of magazine training, rats were given a series of 4 PCA training sessions, each separated by 24 h. The fifth and sixth training sessions served as test sessions, and infusions of either vehicle or M/B were administered 5 min before each of these sessions. This experiment used a within-subjects design, and the subjects were counterbalanced for treatment order. Therefore, half of the rats received an infusion of vehicle before the first test session and an infusion of M/B before the second test session; the conditions were reversed for the other half of the rats (see Figure 6a for experimental design). Figure 5 shows the cannula placements for these subjects.
Figure 6. Reversible inactivation of the NAc impairs the expression of PCA behavior.
a) Experimental design.
b) Mean difference score (± SEM) during PCA training and test sessions for rats that received infusions into the NAc core (n=15). **P<.01.
c) Mean difference score (± SEM) during PCA training and test sessions for rats that received infusions into the NAc shell (n=8). **P<.01.
d) and e) Port entries exhibited by representative rats. The top half of each graph is a perievent raster in which each horizontal row of tick marks represents a separate trial during the training/test session, each individual tick mark represents a single port entry, black circles represent the onset of the CS+, and black triangles represent the offset of the CS+ and onset of the pump that delivered the sucrose reward. The bottom half of each graph is a perievent histogram showing the total number of port entries (during 2 sec bins) made during all trials of the training/test session. The zero time point on the histogram represents the onset of the CS+. The panels on the left depict port entries made during the first training session; the middle panels depict port entries made during the test session immediately after vehicle infusion; and, the panels on the right depict port entries made during the test session immediately after M/B infusion.
d) Port entries by a representative rat that received infusions in the NAc core.
e) Port entries by a representative rat that received infusions in the NAc shell.
f) Mean number of port entries (± SEM) during PCA test session stimulus presentations for rats that received infusions in the NAc core. “Pre-CS+” = the 10 sec preceding the onset of the CS+. *P<.05.
g) Mean number of port entries (± SEM) during PCA test session stimulus presentations for rats that received infusions in the NAc shell. “Pre-CS+” = the 10 sec preceding the onset of the CS+. *P<.05, **P<.01.
h) Mean number of port entries (± SEM) during the entire 90 min PCA test session. **P<.01.
i) Mean number of trials (± SEM) in the PCA test session for which there was a response to US (sucrose) delivery.
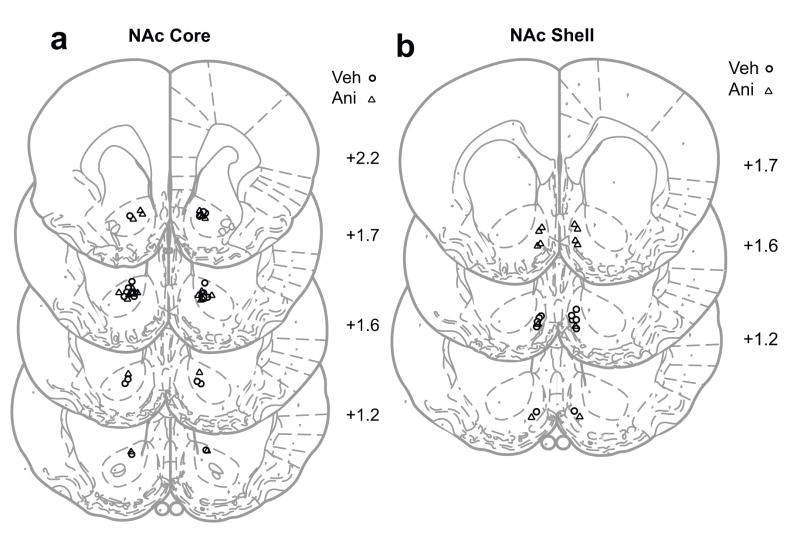
Figure 5. Schematic representation of cannula placements for rats that received pre-test reversible inactivation.
a) Cannula placements for rats that received pre-test infusions of vehicle and M/B in the NAc core. Note that there is only one group of rats because this was a within-subjects study. Coordinates are A/P from Bregma.
b) Cannula placements for rats that received pre-test infusions of vehicle and M/B in the NAc shell. Note that there is only one group of rats because this was a within-subjects study. Coordinates are A/P from Bregma.
NAc Core Infusions
Figure 6b shows the learning curve for conditioned responding as well as the effects of pre-test M/B infusion for rats that received infusions into the NAc core. There was a significant increase in the mean difference score across the four training sessions [1-way repeated measures ANOVA; F(3,42)=31.17, P<.001], indicating that rats successfully acquired the CS-US association before experiencing the test sessions. During the test sessions, rats exhibited an impaired ability to discriminate between the CS+ and CS− when M/B was infused into the NAc core before the test session. A 2-way repeated measures ANOVA analyzing the difference scores during the test sessions found a significant main effect of Treatment [F(1,13)=14.09, P<.002], but not a significant effect of Treatment Order [F(1,13)=1.11, P=.31] nor a significant interaction between the two factors [F(1,13)=1.44, P=.25]. Detailed examination of port entry behavior by an individual subject (Figure 6d) suggests that the decrease in difference scores seen after intra-NAc core M/B administration was due to a markedly impaired conditioned response to the CS+. Data from the group as a whole supports this view; when rats were given M/B infusions, they made fewer port entries during the CS+ compared to vehicle infusions [Figure 6f; 2-way repeated measures ANOVA; Treatment: F(1,13)=5.83, P<.031; Order: F(1,13)=1.86, P=.20; Interaction: F(1,13)=1.49, P=.24]. However, the number of port entries that the rats made during the 10 sec preceding the onset of the CS+ remained unchanged after M/B infusions, even though, in general, rats with a tone as a CS+ made more port entries during this period than rats with a light as a CS+ [Figure 6f; 3-way repeated measures ANOVA; Treatment: F(1,11)=.01, P=.93; Order: F(1,11)=4.54, P=.06; CS+ Type: F(1,11)=12.10, P<.01; Treatment × Order Interaction: F(1,13)=1.07, P=.32; CS+ Type × Order Interaction: F(1,11)=1.00, P=.34; CS+ Type × Treatment Interaction: F(1,11)=.20, P=.66; CS+ Type × Order × Treatment Interaction: F(1,11)=.79, P=.39]. In addition, a 2-way repeated measures ANOVA analyzing the number of port entries that the rats made during the CS− found no main effect of Treatment (Figure 6f; 2-way repeated measures ANOVA; Treatment: F(1,13)=2.47, P=.14; Interaction: F(1,13)=.56, P=.47). Although there was no significant effect of Treatment and no significant interaction between Treatment and Order, there was a main effect of Order (Order: F(1,13)=5.95, P<.030). However, pairwise comparisons found that there was no effect of Treatment regardless of whether subjects received the infusion of M/B during the 1st test (Student-Newman-Keuls planned pairwise comparison, P=.15) or during the 2nd test (Student-Newman-Keuls planned pairwise comparison, P=.56). Therefore, the significant main effect of Order is more likely due to an overall decrease (regardless of treatment) in the mean number of CS− port entries between the first and second test sessions.
We also examined two additional behavioral measures of conditioned responding. First, we examined the number of CS+ presentations during which rats made a CS response. All rats showed an increase across training sessions in the average number of CS+ presentations with a CS response [1-way repeated measures ANOVA; Session: F(3,42)=95.36, P<.001]. During the test sessions, rats made a CS response during fewer of the CS+ presentations after infusions of M/B in the NAc core compared to vehicle infusions [2-way repeated measures ANOVA; Treatment: F(1,13)=7.14, P<.02], and M/B infusions had the same effect on behavior regardless of whether the drugs were administered before the 1st or 2nd test [Order: F(1,13)=.14, P=.72; Interaction: F(1,13)=.15, P=.71]. Next, we examined the percent of time spent in the sucrose port during CS+ presentations. Again, all rats showed an increase across training sessions in the percent of time spent in the sucrose port during CS+ presentations [1-way repeated measures ANOVA; Session: F(3,42)=27.10, P<.001], suggesting that all rats successfully learned the task. A 2-way repeated measures ANOVA analyzing this behavioral measure during the test sessions found a statistical trend toward an effect of Treatment [F(1,13)=3.81, P=.07], and there was neither a significant effect of Treatment Order [F(1,13)=.01, P=.94] nor a significant interaction between the two factors [F(1,13)=.07, P=.79]. Therefore, pre-test reversible inactivation of the NAc core resulted in impaired conditioned responding to the CS+ in two behavioral measures of conditioning, and there was a statistical trend toward impaired responding in a third behavioral measure of conditioning.
It is possible that the impaired behavior during the CS+ presentation could be explained by a decrease in the rats’ general activity level or motivation to consume the US rather than a specific effect of the drugs on PCA expression. We therefore examined two additional measures of behavior during the test sessions. To assess the rats’ general level of activity and exploration during the test sessions, we determined the total amount of port entries made throughout the 90 min sessions. As shown in Figure 6h, the total number of port entries was unchanged after the infusion of M/B when compared to the total number of port entries after the infusion of vehicle [1-way repeated measures ANOVA; F(1,14)=.19, P=.67], indicating that the impairment in the PCA task seen after the infusion of M/B into the core was not due to reduced general levels of activity. To assess the rats’ level of motivation to consume the US, we determined the total number of trials during which the rats made a response to consume the US (see Methods). As shown in Figure 6i, infusions of M/B into the NAc core did not affect the number of trials during which the rats made responses to consume the US [1-way repeated measures ANOVA; F(1,14)=2.17; P=.16], indicating that the M/B-induced impairment in PCA was not due to a reduction in motivation to consume the US. This combination of data suggests that pre-test infusions of M/B in the NAc core specifically impaired the expression of the PCA association.
NAc Shell Infusions
Figure 6c shows the difference scores during all PCA sessions for rats that received infusions into the NAc shell. A 1-way repeated measures ANOVA analyzing the difference scores of all rats across the four training sessions found a significant increase in the mean difference score [F(3,21)=17.16, P<.001], indicating that the rats had successfully learned the CS-US association before the first test session. A 2-way repeated measures ANOVA analyzing the difference scores during the test sessions found a significant main effect of Treatment [F(1,6)=30.43, P<.001], indicating that infusion of M/B into the NAc shell impaired expression of PCA. This analysis also showed that the impairing effect of M/B on PCA could not be explained by the order of infusions [2-way repeated measures ANOVA; Treatment Order: F(1,6)=.20, P=.67; Interaction: F(1,6)=.65, P=.45]. Although infusions of M/B into the NAc shell and core both decreased test session performance, the behavioral impairments seen after infusions in the shell (Figure 6e) differed from those seen after infusions in the core (Figure 6d). Like rats that received M/B infusions in the core, rats that received M/B infusions in the shell also made significantly fewer port entries during the CS+ (Figure 3g; 2-way repeated measures ANOVA; Treatment: F(1,6)=16.20, P<.007; Order: F(1,6)=.30, P=.60; Interaction: F(1,6)=.10, P=.76). However, these rats also exhibited a significantly greater number of port entries during the 10 sec preceding the onset of the CS+ (Figure 6g; 2-way repeated measures ANOVA; Treatment: F(1,6)=7.27, P<.036; Order: F(1,6)=.05, P=.83; Interaction: F(1,6)=1.05, P=.35) and during the CS− (Figure 6g; 2-way repeated measures ANOVA; Treatment: F(1,6)=32.25, P<.001; Order: F(1,6)=.24, P=.64; Interaction: F(1,6)=3.32, P=.12). Therefore, M/B infusions in the shell may have impaired the normal expression of PCA by affecting the rats’ ability to inhibit unnecessary and extraneous behaviors.
We also examined two additional behavioral measures of conditioned responding. First, we examined the number of CS+ presentations during which rats made a CS response. All rats showed an increase across training sessions in the average number of CS+ presentations with a CS response [1-way repeated measures ANOVA; Session: F(3,21)=24.23, P<.001]. Subjects showed impaired performance during the test sessions after infusion of M/B in the NAc shell compared to vehicle infusions. After infusion of M/B in the NAc shell, rats made a CS response during fewer of the CS+ presentations compared to vehicle infusions [2-way repeated measures ANOVA; Treatment: F(1,6)=20.52, P<.01], and M/B infusions had the same effect on behavior regardless of whether the drugs were administered before the 1st or 2nd test [Order: F(1,6)=1.87, P=.22; Interaction: F(1,6)=.12, P=.76]. Next, we examined the percent of time spent in the sucrose port during CS+ presentations. Again, all rats showed an increase across training sessions in the percent of time spent in the sucrose port during CS+ presentations [1-way repeated measures ANOVA; Session: F(3,21)=47.94, P<.001], suggesting that all rats successfully learned the task. During the test sessions, rats spent a smaller percent of time in the sucrose port during CS+ presentations after infusions of M/B in the NAc shell compared to vehicle infusions [2-way repeated measures ANOVA; Treatment: F(1,6)=10.60, P<.02], and M/B infusions had the same effect on behavior regardless of whether the drugs were administered before the 1st or 2nd test [Order: F(1,6)=.01, P=.91; Interaction: F(1,6)=1.12, P=.33]. Therefore, pre-test reversible inactivation of the NAc shell resulted in impaired PCA behavior using multiple different behavioral measures of conditioning.
As before, we examined additional measures of behavior to determine if the impairing effect of M/B in the shell could be explained by alterations in general activity levels or motivation to consume the US. As shown in Figure 6h, rats exhibited a greater number of port entries during the test session after infusion of M/B in the NAc shell when compared to the test session after infusion of vehicle [1-way repeated measures ANOVA; F(1,7)=12.61, P<.009]; however, this result is the opposite of what would be expected if the impairing effect of M/B on difference scores was due to a decrease in activity level or locomotor activity. Indeed, this result supports the view that rats’ behavior after intra-NAc shell M/B infusions became less specific and efficient. As shown in Figure 6i, infusions of M/B into the NAc shell did not affect the number of trials during which the rats made responses to consume the US [1-way repeated measures ANOVA; F(1,7)=4.20; P=.08], indicating that the M/B-induced impairment in PCA was not attributable to decreased motivation to consume the US. Together, these data suggest that pre-test infusions of M/B in the NAc shell specifically impaired normal expression of behavior in the PCA task, but the behavioral impairments seen after infusions in the NAc shell were different than the impairments seen after infusions in the NAc core.
Discussion
These studies revealed two main results. First, we found that the NAc is not critical for the consolidation of goal-tracking PCA, but once the memory is formed, the NAc is necessary for the normal expression of this behavior. Second, our results suggest that the NAc core is required for expression of the CS-US association while the NAc shell is also required for the ability to inhibit conditioned approach behavior at times when the reward is unavailable.
The consolidation of Pavlovian conditioned approach
The results of our experiments suggest that, at least with our behavioral procedure, neither the NAc core nor shell is critical for the consolidation of goal-tracking PCA. We found that post-training infusions of ANI into the NAc core and shell had no effect on three different behavioral measures of conditioning. This suggests that protein synthesis is not required for the consolidation of goal-tracking PCA and implies that the associational memories necessary for normal performance in this behavioral task are not stored in the NAc. We also found that post-training infusions of M/B in the NAc shell had no effect on three different behavioral measures of conditioning. Post-training infusions of M/B in the NAc core had no effect on two behavioral measures of conditioning, but they did cause an impairment in the percent of time spent in the sucrose port during CS+ presentations. Since each separate behavioral measure of conditioning describes a slightly different aspect of conditioned responding, these data raise the possibility that the NAc core might be more involved in the learning of one aspect of conditioned responding compared to the others. Even in the case of the percent time measure, however, rats were still able to successfully learn the behavioral task. Together, the findings suggest that the NAc core does not play a critical role in consolidation of conditioned responding in goal-tracking PCA.
However, there are several alternative interpretations of our data. It is possible that the doses of M/B and ANI used in these experiments were simply too low to have an effect on the consolidation of PCA, and that higher doses of these drugs are required. We think this is unlikely because the same dose of M/B had an effect on the expression of PCA, and the same dose of ANI (infused into the NAc) was shown previously to successfully impair the consolidation of instrumental conditioning [6]. A second possibility is that, the cellular cascades underlying formation and stabilization of the PCA memory had already begun before the end of the training session, and our post-training infusions might have occurred too late to modulate consolidation, as all PCA training sessions were 90 min long. Thus, it is possible that M/B and ANI could have had a strong effect on behavior if they were administered at a different time point. However, we demonstrated previously that learning in this behavioral procedure can still be modulated by post-training drug administration [16]. In fact, this previous study demonstrated that systemic administration of ANI (which would take significantly longer to reach the NAc than intracranial infusions) impaired the consolidation of this behavioral task. Hence it is likely our drug treatments were effective, allowing us to conclude that the NAc does not appear to contribute to consolidation of a goal-tracking PCA response.
Our results support previous studies suggesting the NAc core is not involved in learning of the goal-tracking version of PCA [17, 18], but our results do contrast with a previous study showing that infusions of d-amphetamine in the NAcc shell positively modulate the consolidation of the goal-tracking version of PCA [17]. However, the discrepancy between this study and ours could be explained by the different experimental manipulations used. While our data suggest that the NAc is not necessary for PCA consolidation, they do not rule out the possibility that the NAc shell (or the core for that matter) is involved in PCA consolidation in a non-critical modulatory manner such as that seen with enhancement of learning.
In addition, our results contrast with studies investigating the role of the NAc in the acquisition of a behavioral task that is similar to but distinct from our goal-tracking PCA task. In the sign-tracking version of PCA, repeated pairings of a CS with the delivery of a reward induce conditioned approaches to CS [2]. Studies examining the role of the NAc in the sign-tracking version of PCA have consistently found that the NAc core contributes to acquisition of this task [27–29]. Therefore, even though both of these behavioral tasks involve learning to associate a CS presentation with the delivery of a reward, different neural circuitries likely mediate learning of distinct motor outputs constituting different conditioned responses [30].
It is also possible that only very specific subregions of the NAc are involved in PCA consolidation. Our infusions were limited to the rostral medial portions of the NAc core and shell, but NAc afferents and efferents are known to vary along the medial/lateral, dorsal/ventral, and rostral/caudal axes [31–34]. Notably, in some of the studies mentioned above that investigated the role of the NAc in PCA learning and found results contrasting with ours, the locations of the drug infusions were slightly more caudal and/or more lateral than the locations of our infusions [17, 27, 28]. Further study is needed to determine if and how subregions within the NAc core and shell contribute differentially to PCA consolidation.
If the NAc is not required for goal-tracking PCA consolidation, what brain regions are necessary? The amygdala is one candidate as it receives inputs from a variety of brain regions that could convey information about the CS and US [35–37]. Although acquisition and expression of goal-tracking PCA is not altered by amygdalar lesions [19, 38–40], intra-amygdala pharmacological manipulations enhance goal-tracking PCA consolidation [41, 42]. Other candidate brain regions include dorsal striatal and limbic cortical regions.
The expression of Pavlovian conditioned approach
Although we found no effect of post-training infusions of M/B, pre-test infusions of M/B into the NAc core and shell impaired the expression of goal-tracking PCA. Inactivation of the NAc core decreased responding during the CS+, suggesting that functional integrity of core is critical for the ability to express the CS-US association. This finding agrees with previous studies implicating the NAc core in the expression of PCA behavior [20, 27, 28, 43]. Hence the role of the NAc core in the expression of appetitive Pavlovian responses is broader than its role in the formation of memory for these associations.
Reversible inactivation of the medial aspect of the NAc shell resulted in a decrease in CS+ responding along with an increase in responding during the CS− and the inter-stimulus interval. Unlike reversible inactivation of the core, functional impairment of the shell rendered subjects less able to inhibit inappropriate responding during periods of reward unavailability, suggesting that the shell is necessary for the behavioral efficiency observed after training. This agrees with other findings suggesting that the NAc shell contributes to the learned ability to ignore stimuli that signal reward unavailability [44–49]. In studies of operant behavior in rats, for example, reversible inactivation or administration of glutamate receptor antagonists in the NAc shell caused an increase in the number of lever presses on an inactive (control) lever [44, 45]. Our results also agree with other studies suggesting that the NAc core and shell can have opposing functions in multiple different behavioral tasks. Recent studies from different labs have found that reversible inactivation of the NAc shell increased cocaine- and food-seeking behavior in a cue-induced reinstatement paradigm, while reversible inactivation of the NAc core had the opposite effect [50, 51]. In addition, multiple studies from the same group have shown that lesions of the NAc shell disrupt latent inhibition, but this behavior is not impaired after NAc core lesions [47–49]
It is worth noting that our results contrast with a previous study showing that excitotoxic NAc shell lesions do not affect expression of a goal-tracking PCA [20], but this discrepancy could be due to the increased possibility of compensatory changes in other brain regions after permanent lesions compared to acute inactivation.
Although it is unclear why the NAc is involved in the expression but not the consolidation of PCA, this finding could fit in with classical thought on the role of the NAc as a site of “limbic-motor integration” that is involved in sensorimotor gating [1–3]. It is possible that the CS-US association in the PCA task is stored and maintained in a brain region upstream of the NAc. After the memory is formed, however, information about the association might need to be processed in the NAc subnuclei in order to select (or inhibit) the most appropriate behavioral motor responses.
Conclusions
These studies indicate that the NAc is not involved in the consolidation of the goal-tracking version of PCA, but once the memory is formed, the NAc is necessary for the normal expression of PCA behavior. In addition, we demonstrated that the NAc core is needed for expression of the CS-US association while the NAc shell is also required for the ability to inhibit conditioned approach behavior during times when reward is unavailable. These data suggest that the NAc core and shell are involved in differenct aspects of PCA expression. When our findings are synthesized with current evidence in the literature, it suggests that the NAc is differentially involved in the learning of goal-tracking and sign-tracking PCA but plays a broader role in the expression of both types of PCA behavior.
Acknowledgments
We would like to thank L.L. Sahuque, R. Van Bautista, and Dr. N. Chaudhri for technical assistance and many helpful discussions. This work was supported by funds from the State of California for Medical Research on Alcohol and Substance Abuse through the University of California at San Francisco and NIDA Grant# F31 DA016881 to CAB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mogenson GJ. Limbic-motor integration. In: Epstein AN, editor. Progress in Psychobiology and Physiological Psychology. New York: Academic Press; 1987. pp. 117–170. [Google Scholar]
- 2.Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Progress in Neurobiology. 1980;14(2–3):69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- 3.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience and Biobehavioral Reviews. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 4.Balleine BW. Neural bases of food-seeking: affect, arousal and reward in corticostriatolimbic circuits. Physiology and Behavior. 2005;86:717–730. doi: 10.1016/j.physbeh.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 5.Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward: the role of amygdala-ventral striatal subsystems. Annals of the New York Academy of Sciences. 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez PJ, Sadeghian K, Kelley AE. Early consolidation of instrumental learning requires protein synthesis in the nucleus accumbens. Nature Neuroscience. 2002;5(2):1327–1331. doi: 10.1038/nn973. [DOI] [PubMed] [Google Scholar]
- 7.Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;2929:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- 8.Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. European Journal of Neuroscience. 2001;13:1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- 9.Corbit LH, Muir JL, Balleine BW. The role of the nucleus accumbens in instrumental conditoning: evidence of a functional dissociation between accumbens core and shell. Journal of Neuroscience. 2001;21(9):3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neuroscience and Biobehavioral Reviews. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Holland PC. Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. Journal of Experimental Psychology: Animal Behavior Processes. 1977;3(1):77–104. doi: 10.1037//0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- 12.Boakes RA. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz HMB, editors. Operant-Pavlovian Interactions. Hillsdale, NJ: Erlbaum; 1977. pp. 67–97. [Google Scholar]
- 13.Dudai Y. Molecular bases of long-term meories: a question of persistence. Current Opinion in Neurobiology. 2002;12:211–216. doi: 10.1016/s0959-4388(02)00305-7. [DOI] [PubMed] [Google Scholar]
- 14.Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- 15.McGaugh JL. Memory -- a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 16.Blaiss CA, Janak PH. Post-training, but not post-reactivation, administration of amphetamine and anisomycin modulates Pavlovian conditioned approach. Neurobiology of learning and memory. 2007;87:644–658. doi: 10.1016/j.nlm.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips GD, Setzu E, Hitchcott PK. Facilitation of appetitive Pavlovian conditioning by d-amphetamine in the shell, but not the core, of the nucleus accumbens. Behavioral Neuroscience. 2003;117(4):675–684. doi: 10.1037/0735-7044.117.4.675. [DOI] [PubMed] [Google Scholar]
- 18.Cassaday HJ, Horsley RR, Norman C. Electrolytic lesions to nucleus accumbens core and shell have dissociable effects on conditioning to discrete and contextual cues in aversive and appetitive procedures respectively. Behavioural Brain Research. 2005;160:222–235. doi: 10.1016/j.bbr.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Setlow B, Holland PC, Gallagher M. Disconnection of the basolateral amygdala complex and nucleus accumbens impairs appetitive pavlovian second-order conditioned response. Behavioral Neuroscience. 2002;116(2):267–275. doi: 10.1037//0735-7044.116.2.267. [DOI] [PubMed] [Google Scholar]
- 20.Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive Pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by d-amphetamine. J Neurosci. 1999;19(6):2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nader K, Schafe GE, LeDoux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 22.Schafe GE, Nadel NV, Sullivan GM, Harris A, LeDoux JE. Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learning and Memory. 1999;6:97–110. [PMC free article] [PubMed] [Google Scholar]
- 23.Meiri N, Rosenblum K. Lateral ventricle injection of the protein synthesis inhibitor anisomycin impairs long-term memory in a spatial memory task. Brain Research. 1998;789:48–55. doi: 10.1016/s0006-8993(97)01528-x. [DOI] [PubMed] [Google Scholar]
- 24.Rosenblum K, Meiri N, Dudai Y. Taste memory: the role of protein synthesis in gustatory cortex. Behavioral and Neural Biology. 1993;59:49–56. doi: 10.1016/0163-1047(93)91145-d. [DOI] [PubMed] [Google Scholar]
- 25.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. Journal of Neuroscience. 2001;21(21):8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. New York: Academic Press; 1998. [Google Scholar]
- 27.Dalley JW, Laane K, Theobald DEH, Armstrong HC, Corlett PR, Chudasama Y, Robbins TW. Time-limited modulation of appetitive Pavlovian memory by D1 and NMDA receptors in the nucleus accumbens. Proc Natl Acad Sci USA. 2005;102(17):6189–6194. doi: 10.1073/pnas.0502080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Differential involvement of NMDA, AMPA/Kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of Pavlovian approach behavior. J Neurosci. 2001;21(23):9471–9477. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parkinson JA, Willoughby PJ, Robbins TW, Everitt BJ. Disconnection of the anterior cingulate cortex and nucleus accumbens core impairs Pavlovian approach behavior: further evidence for limbic cortical-ventral striatopallidal systems. Behavioral Neuroscience. 2000;114(1):42–63. [PubMed] [Google Scholar]
- 30.Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward. Annals of the New York Academy of Sciences. 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- 31.Groenewegen HJ, Wright CI, Beijer AVJ, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Annals of the New York Academy of Sciences. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- 32.Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Research Reviews. 2007 doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Usuda I, Tanaka K, Chiba T. Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Research. 1998;797:73–93. doi: 10.1016/s0006-8993(98)00359-x. [DOI] [PubMed] [Google Scholar]
- 34.Voorn P, Vanderschuren LJMJ, Groenewegen HJ, Robbins TW, Pennartz CMA. Putting a spin on the dorsal-ventral divide of the striatum. Trends in Neurosciences. 2004;27(8):468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto T. Neural substrates for the processing of cognitive and affective aspects of taste in the brain. Arch Histol Cytol. 2006;69(4):243–255. doi: 10.1679/aohc.69.243. [DOI] [PubMed] [Google Scholar]
- 36.McDonald AJ. Cortical pathways to the mammalian amygdala. Progress in Neurobiology. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 37.LeDoux JE. Emotion circuits in the brain. Annual Reviews in Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 38.Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland PC, Schoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. J Neurosci. 2003;23(35):11078–84. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hatfield T, Han JS, Conley M, Gallagher M, Holland P. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. J Neurosci. 1996;16(16):5256–65. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive Pavlovian conditioning: lesions impair one class of conditioned behavior. J Neurosci. 1990;10(6):1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hitchcott PK, Bonardi CMT, Phillips GD. Enhanced stimulus-reward learning by intra-amygdala administration of a D3 dopamine receptor agonist. Pyschopharmacology. 1997;133:240–248. doi: 10.1007/s002130050397. [DOI] [PubMed] [Google Scholar]
- 42.Hitchcott PK, Harmer CJ, Phillips GD. Enhanced acquisition of discriminative approach following intra-amygdala d-amphetamine. Psychopharmacology. 1997;132:237–246. doi: 10.1007/s002130050341. [DOI] [PubMed] [Google Scholar]
- 43.Cardinal RN, Parkinson JA, Lachenal G, Halkerston KM, Rudarakanchana N, Hall J, Morrison CH, Howes SR, Robbins TW, Everitt BJ. Effects of selective excitotoxic lesions of the nucleus accumbens core, anterior cingulate cortex, and central nucleus of the amygdala on autoshaping performance in rats. Behavioral Neuroscience. 2002;116(4):553–567. doi: 10.1037//0735-7044.116.4.553. [DOI] [PubMed] [Google Scholar]
- 44.Yun IA, Nicola SM, Fields HL. Contrasting effects of dopamine and glutamate receptor antagonist injection in the nucleus accumbens suggest a neural mechanism underlying cue-evoked goal-directed behavior. European Journal of Neuroscience. 2004;20:249–263. doi: 10.1111/j.1460-9568.2004.03476.x. [DOI] [PubMed] [Google Scholar]
- 45.See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology. 2007;194:321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- 46.Floresco SB, Ghods-Sharifi S, Vexelman C, Magyar O. Dissociable roles for the nucleus accumbens core and shell in regulating set shifting. Journal of Neuroscience. 2006;26(9):2449–2457. doi: 10.1523/JNEUROSCI.4431-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gal G, Schiller D, Weiner I. Latent inhibition is disrupted by nucleus accumbens shell lesion but is abnormally persistent following entire nucleus accumbens lesion: The neural site controlling the expression and disruption of the stimulus preexposure effect. Behav Brain Res. 2005;162(2):246–55. doi: 10.1016/j.bbr.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 48.Weiner I, Gal G, Feldon J. Disrupted and undisruptable latent inhibition following shell and core lesions. Ann N Y Acad Sci. 1999;877:723–7. doi: 10.1111/j.1749-6632.1999.tb09310.x. [DOI] [PubMed] [Google Scholar]
- 49.Weiner I, Gal G, Rawlins JN, Feldon J. Differential involvement of the shell and core subterritories of the nucleus accumbens in latent inhibition and amphetamine-induced activity. Behav Brain Res. 1996;81(1–2):123–33. doi: 10.1016/s0166-4328(96)00051-4. [DOI] [PubMed] [Google Scholar]
- 50.Di Ciano P, Robbins TW, Everitt BJ. Differential effects of nucleus accumbens core, shell, or dorsal striatal inactivations on the persistence, reacquisition, or reinstatement of responding for a drug-paired conditioned reinforcer. Neuropsychopharmacology. 2008;33(6):1413–25. doi: 10.1038/sj.npp.1301522. [DOI] [PubMed] [Google Scholar]
- 51.Floresco SB, McLaughlin RJ, Haluk DM. Opposing roles for the nucleus accumbens core and shell in cue-induced reinstatement of food-seeking behavior. Neuroscience. 2008;154(3):877–84. doi: 10.1016/j.neuroscience.2008.04.004. [DOI] [PubMed] [Google Scholar]