Abstract
Developmental exposure to endocrine disruptors is suspected to be one of the main factors responsible for the increased incidence of breast cancer in industrialized countries. New data published in the Journal of Clinical Endocrinology and Metabolism show that exposure to dichlorodiphenyltrichloroethane during fetal life is associated with an increased risk of breast cancer.
The increase in the incidence of breast cancer that has occurred during the past 50 years can neither be accounted for by the introduction of mammography for screening nor by known risk factors. Instead, the increased incidence has been hypothesized to be due to exposure to endocrine disruptors such as dichlorodiphenyltri-chloroethane (DDT), which were widely used from the 1940s and thus introduced into the environment.1 This increase in breast cancer incidence should be addressed by two distinct yet complementary endeavours, namely, improved medical care and public health policies. A new study by Cohn and colleagues shows that in utero exposure to DDT is linked to an increased risk of breast cancer later in life and will undoubtedly re-initiate the debate about the safety of DDT and other insecticides.2
Epidemiological and experimental studies have revealed that the breast is particularly sensitive to carcinogenic insult during morphogenesis and remodelling.3 Use of the synthetic estrogen diethylstilbestrol to prevent miscarriages is an example of this feature of breast tissue. Fetal exposure to diethylstilbestrol results in multiple deleterious effects in the genital tract and breasts, which range from malformations to cancer.1 These outcomes are a consequence of altered morphogenesis.
The finding that exposure to nonmutagenic, hormonally active agents during development could increase the risk of developing cancer was unexpected. However, ecological developmental biology4 and the tissue organization field theory of carcinogenesis5 provide the theoretical foundations for such an outcome. The tissue organization field theory of carcinogenesis proposes that carcinogenesis is morphogenesis gone awry, and posits that exposure to hormonally active agents at the wrong time during development interferes with key mediators of morphogenesis, such as the reciprocal interactions between the mesenchyme and epithelium. These effects involve massive changes in the pattern of gene expression in both compartments, altered composition of the extracellular matrix and altered anatomy of the mammary gland. Furthermore, these effects are exacerbated by the action of ovarian and pituitary hormones during puberty and adulthood, which leads to neoplastic development (Figure 1).6
Figure 1.
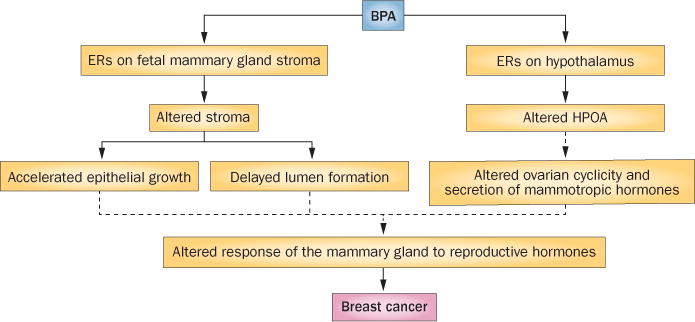
Model of xenoestrogen induction of mammary gland carcinogenesis. BPA binds to the fetal mammary gland mesenchymal ERs and, in turn, affects the composition of the extracellular matrix, which increases tissue rigidity and delays lumen formation. BPA also induces precocious adipocyte differentiation, which in turn accelerates duct elongation and branching. These changes lead to an increased sensitivity to mammotropic hormones in adulthood. BPA also binds to ERs in the hypothalamus, where it alters the control of ovarian cyclicity and secretion of mammotropic hormones. Solid arrows link effects of in utero exposure to BPA in rodents and nonhuman primates. Dashed arrows indicate hypothesized links between effects during fetal mammary gland development and mammary carcinogenesis. Abbreviations: BPA, bisphenol A; ERs, estrogen receptors; HPOA, hypophyseal–pituitary–ovarian axis. Modified with permission from Elsevier Ltd © Paulose, T. et al. Reprod. Toxicol. 54, 58–65 (2014).
Owing to the long interval between exposure and clinical manifestation of breast cancer (the median age at diagnosis in women is 61 years7), the actual effects of fetal exposure to environmental endocrine disruptors in humans are difficult to assess. This difficulty motivated the development of surrogate rodent models. Rodents exposed during gestation to low doses of xenoestrogens such as bisphenol A, a chemical to which humans are widely exposed, show altered mammary gland morphogenesis that manifests during the period of exposure and beyond.6 During adulthood, functional and anatomical alterations are observed, encompassing increased sensitivity to estrogens and progesterone, increased lateral branching, induction of intraductal hyperplasias, carcinoma in situ and palpable tumours.3
Epidemiological studies such as that recently reported by Cohn and colleagues2 are especially important and relevant to the issue of whether a strong correlation exists between experimental carcinogenesis in rodents and the aetiopathogenesis of breast cancer in women. This one-of-a-kind case–control study involved a 54 year follow-up of >20,000 pregnancies and the resulting live-born daughters.2 In this study, the levels of DDT, other chemicals present in the technical grade formulation and DDT metabolites were measured in the serum of mothers either during pregnancy or immediately after delivery. The study found that high maternal serum levels of o,p′-DDT (an isomer of DDT) predicted a fourfold increase in the daughter’s risk of developing breast cancer. Most cases were estrogen-receptor and progesterone-receptor positive and receptor tyrosine-protein kinase erbB-2 (also known as HER2)-negative.
“…high maternal serum levels of o,p′-DDT … predicted a fourfold increase in the daughter’s risk of developing breast cancer”
An additional relevant fact is that a separate study of breast cancer incidence in the mothers of the same cohort revealed that exposure before the age of 14 years resulted in a fivefold increase in the risk of breast cancer,8 and that the risk was greatest in women who were exposed to DDT before 4 years of age. Together, these two studies2,8 show that exposure to the estrogenic insecticide DDT increases the risk of breast cancer when women are exposed during the main events of mammogenesis, which occur from fetal to pubertal development. As already mentioned, these early exposures alter de novo breast morphogenesis and endogenous hormone levels; environmental endocrine disruptors continue to contribute to the carcinogenic outcome by altering tissue remodelling during adulthood. In this regard, another case–control study showed that the combined effect of environmental estrogens (that is, the ‘total xenoestrogen burden’) at the time of diagnosis is an important risk factor for breast cancer.9
The clinical and epidemiological relevance of the study by Cohn and colleagues2 is manifold. Firstly, their study suggests that cases that are currently being diagnosed in places where the use of DDT has been discontinued might have been initiated by developmental exposure to DDT. Secondly, their study predicts that exposure to DDT is the main culprit in the risk of breast cancer in countries where this pesticide is still used to control malaria, thus, inviting a re-evaluation of this already controversial decision. Thirdly, this study confirms in humans what has already been shown in rodent models, thus indicating that we must consider the results of animal studies not only for understanding the carcinogenic process, but also for developing public health policy. Fourthly, this study demonstrates that future studies on the effect of endocrine disruptors in breast cancer will be extremely difficult to perform as the delay between exposure and outcome spans 50 years. Moreover, multiple exposures to endocrine disruptors evidently work additively,8 thus making it necessary to address the joint effects of these exposures. Finally, from a public health perspective, the study by Cohn and co-workers indicates that exposure to these hormonally active agents should be curtailed; in this regard, physicians should have a central role in educating patients to reduce their exposure.
In conclusion, we concur with the Endocrine Society public statement on endocrine-disrupting chemicals (EDCs) that there is a need “to increase understanding of effects of EDCs, including enhancing increased basic and clinical research, invoking the precautionary principle, and advocating involvement of individual and scientific society stakeholders in communicating and implementing changes in public policy and awareness.”10
Acknowledgments
The authors would like to acknowledge the support of the National Institute of Environmental Health Sciences, Award Number R01ES08314. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health. The authors also acknowledge Cheryl Schaeberle for her excellent editorial assistance.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Soto AM, Sonnenschein C. Environmental causes of cancer: endocrine disruptors as carcinogens. Nat Rev Endocrinol. 2010;6:363–370. doi: 10.1038/nrendo.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohn BA, et al. DDT exposure in utero and breast cancer. J Clin Endocrinol Metab. doi: 10.1210/jc.2015-1841. http://dx.doi.org/10.1210/jc.2015-1841. [DOI] [PMC free article] [PubMed]
- 3.Paulose T, Speroni L, Sonnenschein C, Soto AM. Estrogens in the wrong place at the wrong time: fetal BPA exposure and mammary cancer. Reprod Toxicol. 2015;54:58–65. doi: 10.1016/j.reprotox.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert SF, Epel D. Ecological developmental biology: the environmental regulation of development, health, and evolution. Sinauer Associates; 2015. [Google Scholar]
- 5.Soto AM, Sonnenschein C. The tissue organization field theory of cancer: a testable replacement for the somatic mutation theory. BioEssays. 2011;33:332–340. doi: 10.1002/bies.201100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soto AM, Brisken C, Schaeberle CM, Sonnenschein C. Does cancer start in the womb? Altered mammary gland development and predisposition to breast cancer due to in utero exposure to endocrine disruptors. J Mammary Gland Biol Neoplasia. 2013;18:199–208. doi: 10.1007/s10911-013-9293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Cancer Institute. SEER cancer statistics factsheets: breast cancer. 2015 [online], http://seer.cancer.gov/statfacts/html/breast.html.
- 8.Cohn BA, Wolff MS, Cirillo PM, Sholtz RI. DDT and breast cancer in young women: new data on the significance of age at exposure. Environ Health Perspect. 2007;115:1406–1414. doi: 10.1289/ehp.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibarluzea JM, et al. Breast cancer risk in the combined effect of environmental estrogens. Cancer Causes Control. 2004;15:591–600. doi: 10.1023/B:CACO.0000036167.51236.86. [DOI] [PubMed] [Google Scholar]
- 10.Diamanti-Kandarakis E, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]


