Abstract
At birth, the mechanical environment of valves changes radically as fetal shunts close and pulmonary and systemic vascular resistances change. Given that valves are reported to be mechanosensitive, we investigated remodeling induced by perinatal changes by examining compositional and structural differences of aortic and mitral valves (AVs, MVs) between 2-day-old and 3rd fetal trimester porcine valves using immunohistochemistry and Movat pentachrome staining. Aortic valve composition changed more with birth than the MV, consistent with a greater change in AV hemodynamics. At 2 days, AV demonstrated a trend of greater versican and elastin (P = 0.055), as well as greater hyaluronan turnover (hyaluronan receptor for endocytosis, P = 0.049) compared with the 3rd-trimester samples. The AVs also demonstrated decreases in proteins related to collagen synthesis and fibrillogenesis with birth, including procollagen I, prolyl 4-hydroxylase, biglycan (all P ≤ 0.005), and decorin (P = 0.059, trend). Both AVs and MVs demonstrated greater delineation between the leaflet layers in 2-day-old compared with 3rd-trimester samples, and AVs demonstrated greater saffron-staining collagen intensity, suggesting more mature collagen in 2-day-old compared with 3rd-trimester samples (each P < 0.05). The proportion of saffron-staining collagen also increased in AV with birth (P < 0.05). The compositional and structural changes that occur with birth, as noted in this study, likely are important to proper neonatal valve function. Furthermore, normal perinatal changes in hemodynamics often do not occur in congenital valve disease; the corresponding perinatal matrix maturation may also be lacking and could contribute to poor function of congenitally malformed valves.
Keywords: aortic valve, extracellular matrix, hemodynamics, mitral valve, perinatal
Introduction
At birth, the body undergoes a number of profound changes, 1 of which includes a dramatic restructuring of the circulation. No longer able to rely on the placenta for oxygenation, circulation in the neonate is redirected: fetal shunts, such as the ductus venosus, foramen ovale, and ductus arteriosus, begin to close and blood flow to the nascent lungs greatly increases [1]. The systemic vascular resistance also significantly increases, along with the pressures of the left side of the heart [1,2]. Although the right side of the heart dominates during fetal life, with greater cardiac output than that in the left side of the heart [3], with birth the left side of the heart becomes predominant because it is responsible for the blood flow to the rest of the body. Such a substantial and abrupt change in hemodynamics undoubtedly affects the heart valves, which are essential components of the heart and are responsible for the propagation of blood flow, but this topic has not been widely studied.
Heart valves are essential to proper heart function. Once thought to be passive flaps, heart valves are now known to be complex structures, complete with their own vasculature [4] and nerves [5], that actively participate in heart function and cardiac pathologies [6,7]. Additionally, heart valves dynamically respond to their mechanical environment. Studies have shown that extracellular matrix production and valve cell phenotype in adult valves changes in response to altered mechanical stimulation [8–10]. Although investigation of valve composition and valve cell phenotype in developing valves has been limited, it has been demonstrated that valve composition changes during fetal development [11,12] and continues to mature after birth [13,14]. Fetal valves also demonstrate altered composition that correlates with changes in their mechanical environment [15]. However, how this dramatic change in hemodynamics that occurs with birth affects valve structure and composition has not been studied in detail. Given the mechanosensitive nature of valves that has been extensively studied in adult valves [16–19], as well as evidence that valve composition changes during fetal and postnatal development, we hypothesized that these perinatal events drive changes in mitral and aortic valve (MV, AV) composition and valve cell phenotype.
Further understanding of normal valve changes around birth not only would add to fundamental knowledge of developmental cardiac physiology but also would contribute to the field of congenital heart disease. Heart valves are malformed in the majority of congenital heart disease cases [20], posing a considerable burden for pediatric patients. With current valve replacements and repair options inadequate for many patients [21,22], research is under way to develop tissue-engineered heart valves. Given that pediatric heart valves have unique, age-specific tissue and cell phenotypes compared with older valves [23,24], tissue-engineered heart valves for neonates will require knowledge of valve compositional changes surrounding the birth event. Additionally, understanding the normal perinatal development of valves could lead to other types of interventions for this vulnerable population.
To this end, AVs and MVs from 3rd-trimester pigs and 2-day-old pigs were analyzed to assess differences in leaflet structure and extracellular matrix composition, namely elastin, collagen types I and III, regulatory proteins related to collagen synthesis and turnover, proteoglycans, and glycosaminoglycans.
Methods
Tissue procurement and sample preparation
Hearts from 3rd-trimester pigs and 2-day-old pigs were obtained within 24 hours of death (3rd trimester obtained from Animal Technologies [Tyler, TX, USA] and 2-day-old obtained from Baylor College of Medicine [Houston, TX, USA]); all were conventional cross-bred pigs. The specific time point within the 3rd trimester from which those valves were taken is unknown. The animal sample set included AVs from 4 3rd-trimester pigs and MVs from 3 3rd-trimester pigs and AVs and MVs from four 2-day-old pigs. Multiple tissue strips were taken from the valve of each animal. The study protocol was approved by the Animal Care and Use Committee of Baylor College of Medicine and was conducted in accordance with National Institutes of Health guidelines. The AV leaflets and the anterior leaflet of the MV, along with accompanying myocardium, were dissected from the hearts (Fig. 1A). Tissues were fixed overnight in 10% formalin, embedded in paraffin, sectioned to a thickness of 5 μm, and mounted on slides according to standard procedures. Samples of human congenitally diseased valves were also obtained at the time of surgery and similarly prepared. The research use of these tissues was approved by the Institutional Review Boards at Rice University and Texas Children’s Hospital in Houston, TX.
Figure 1.

A. Photographs of 3rd-trimester mitral valve anterior leaflet (top) and aortic valve (bottom). Scale bar indicates 1 mm. B. Representative Movat-stained 3rd-trimester fetal and 2-day-old aortic and mitral valves. Magnification ×5 objective, scale bar indicates 200 μm.
Histology and immunohistochemistry
Each sample was stained histologically with Movat pentachrome to demonstrate the relative distribution of collagen, glycosaminoglycans, proteoglycans, and elastic fibers. Each sample was also stained immunohistochemically for markers involved in the synthesis and turnover of collagen, including procollagen I (Col I) [25] and procollagen III (Col III) [25] (antibodies LF-41 and LF-69, gifts of Dr Larry Fisher, National Institutes of Health [NIH], Bethesda, MD, USA), prolyl 4-hydroxylase (P4H, MAB2701, Chemicon, Temecula, CA, USA), matrix metalloproteinase 13 (MMP13, MAB3321, Chemicon), and periostin (MAB3548, R&D Systems, Minneapolis, MN, USA). Collagen I is the predominant collagen found in adult valves [26] and is responsible for the valve’s tensile strength, whereas collagen III is a reticular collagen. Prolyl 4-hydroxylase is an enzyme located in the rough endoplasmic reticulum that is required for the critical step of proline hydroxylation in collagen fiber synthesis [27]. Matrix metalloproteinase 13 is a major enzyme responsible for collagen degradation. The quantity of elastin, the major component of elastic fibers that are necessary for valve recoil, was also assessed via immunohistochemistry (ab9519, Abcam, Cambridge, MA, USA). Periostin is an adhesion molecule of the fasciclin family [28] that appears to modulate collagen fibrillogenesis [29] and has been shown to play a role in valve development [28], as well as cardiac remodeling [30,31]. Immunohistochemistry was also performed for the proteoglycans decorin (DCN) and biglycan (BGN) (antibodies LF-122 and LF-104, gifts of Dr Larry Fisher, National Institutes of Health [NIH], Bethesda, MD, USA), both involved in collagen fibrillogenesis [32]. Immunohistochemistry was performed for the large proteoglycan versican (VC, antibody 2-B-1, Associates of Cape Cod, Falmouth, MA, USA), which is involved in elastic fiber formation [33] and is also necessary for several early events in valvulogenesis [34]. The abundance of the glycosaminoglycan hyaluronan (HA), which like VC is necessary for valvulogenesis [35], was assessed using HA-binding protein [36] (400763-1, Associates of Cape Cod). Hyaluronan turnover was assessed by immunohistochemistry for HA receptor for endocytosis (HARE) [37] (antibody mab159, gift of Dr Paul Weigel, University Oklahoma Health Science Center, Oklahoma City, OK, USA). Representative sections were also stained for CD44 (F10-44-2, Abcam), a cell-surface receptor for HA, lysyl oxidase (LOX, IMX-5121, Imgenex, San Diego, CA, USA), which is involved in both elastic and collagen fiber cross-linking, and transforming growth factor-beta (TGFβ, 5559-100, Biovision, Mountain View, CA, USA), a growth factor involved in valve cell activation.
Immunohistochemistry staining intensity was quantified within each histologic layer (ventricularis, atrialis [MV only], spongiosa, and fibrosa) and leaflet region (annulus and midleaflet [spongiosa evaluated in midleaflet only]) using ImageJ software (NIH). Semiquantitative grading (on a scale from 0 to 4, in which 0 was minimum) was also performed on blinded Movat-stained sections to evaluate delineation between leaflet layers and intensity of saffron-staining collagen. The fraction of leaflet composed of saffron-staining collagen was also estimated from blinded Movat-stained sections. Percent change in protein intensity was calculated as the difference in intensities between the 2 groups relative to 3rd-trimester intensities. For purposes of comparisons between AV and MV, the ventricularis of the AV was considered analogous to the elastin-rich atrialis of the MV.
Statistical analysis
Multifactorial analysis of variance was performed using SigmaStat (SPSS, Chicago, IL, USA). When the data were normally distributed, an analysis of variance test was used. When the data set was not normally distributed, a rank transform was performed before the analysis of variance test. In both cases, the level of significance was set at 0.05. Correlations between staining intensities of different proteins within individual leaflet layers, regions, and segments (to assess protein colocalization) were calculated using a Pearson rank order test if the data were normally distributed and a Spearman rank order correlation test if they were not. For correlations between intensities of different proteins, the level of significance was reduced to account for the large number of proteins being considered, and, thus, P ≤ 0.015 was considered a trend and P ≤ 0.0056 considered statistically significant.
Results
Perinatal changes in valve structure
Movat-stained sections demonstrated, to varying degrees, the layered structure of the valve leaflets, namely, the collagen-rich fibrosa layer, the glycosaminoglycan-rich spongiosa in the middle, and a layer rich in elastic fibers on the leaflets’ inflow surface. These sections also revealed substantial changes in valve structure with birth (Fig. 1B), including increases in leaflet layer delineation, the intensity of saffron-staining collagen, and the proportion of the valve leaflet thickness composed of saffron-staining collagen (Fig. 2). In the AV, these structural changes with birth were all statistically significantly different (delineation P < 0.001 [221% increase], saffron-collagen intensity P = 0.029 [106% increase], fraction saffron-staining collagen P = 0.019 [57% increase]). In the MV, however, only the increase in leaflet layer delineation was statistically significant (P = 0.027, 127% increase).
Figure 2.

Delineation and saffron-staining collagen in aortic valve (A) and mitral valve (B), *P < 0.05. Error bars indicate standard error of the mean.
Perinatal changes in valve composition
Elastin and VC in the AV midleaflet tended to be greater in the 2-day-old age group than in the 3rd-trimester age group (36% and 61% increases, respectively, overall P = 0.055) (Fig. 3). There was no significant change in the annular region. Hyaluronan receptor for endocytosis, involved in HA turnover, was greater in the AV annulus and midleaflet of the 3rd-trimester group than in the 2-day-old group (47% decrease, P = 0.049) (Fig. 4), although no significant difference between age groups was noted for HA. The midleaflet of the AV in the 3rd-trimester group demonstrated greater expression of Col I (52% decrease, P < 0.001) (Fig. 5), P4H (33% decrease, P = 0.005), BGN (43% decrease, P < 0.001), and DCN (36% decrease, P = 0.059, trend), which are involved in collagen fibrillogenesis, among other biologic and mechanical roles [32]. The ratio of Col III to Col I was also found to increase with birth for both the midleaflet and annular regions of the AV (P = 0.037 and P = 0.002); no significant change was found in the MV. However, MMP13 in the midleaflet and annulus of 2-day-old MVs was greater than MMP13 in the midleaflet and annulus of the 3rd-trimester MVs (27% increase, P = 0.023). Procollagen III displayed no significant difference between age groups for either MVs or AVs. Although periostin appeared to increase with birth in both MVs and AVs, the change was not statistically significant. Unlike in the AVs, which demonstrated significant perinatal changes in a number of matrix components, in the MVs only MMP13 showed evidence of significant perinatal change in expression.
Figure 3.

Elastin and versican in aortic valve midleaflet, overall P = 0.055. Error bars indicate standard error of the mean.
Figure 4.

Hyaluronan and hyaluronan receptor for endocytosis in aortic valve annulus and midleaflet, *P < 0.05. Error bars indicate standard error of the mean.
Figure 5.
Collagen-related proteins in aortic valve midleaflet, *P < 0.05, ^P = 0.059. Error bars indicate standard error of the mean.
Perinatal changes in relative composition of aortic and mitral valves
The degree of expression of multiple matrix components, such as BGN, HARE, and elastin, was similar between AVs and MVs within the 3rd-trimester group but then became different within the 2-day-old group (Fig. 6). Similarly, although the expression of VC in AVs and MVs were different within the 3rd-trimester age group, the difference between VC expression in AVs and MVs became greater within the 2-day-old age group.
Figure 6.
Differences between aortic and mitral valves in the 3rd-trimester and 2-day-old age groups for biglycan (A) and hyaluronan receptor for endocytosis (B), *P < 0.05. Error bars indicate standard error of the mean.
Location and colocalization of matrix components
As expected from adult heart valves, BGN and DCN demonstrated similar localization, predominantly to the fibrosa, although BGN staining was generally more diffuse. Versican was generally enhanced in the spongiosa layer, as well as the elastin-rich layers (ventricularis and atrialis of MVs and ventricularis of AVs). Elastin showed very distinct, often nearly linear localization to the ventricularis of the AVs and atrialis of the MVs, along with a much thinner line of distribution along the ventricularis of the MV and subendothelial outflow (aortic side) of the AVs. Elastin distribution was spread throughout more of the thickness of the leaflet in the midleaflet and free edge. Procollagen III demonstrated a similar distribution as Col I, although Col III staining was more diffuse. Prolyl 4-hydroxylase was generally more diffuse than both Col I and Col III. Hyaluronan receptor for endocytosis was often localized to the fibrosa in both MVs and AVs (Fig. 7A). Periostin, which appeared to colocalize with Col I, was also concentrated in the fibrosa in both MVs and AVs (Fig. 7A). Matrix metalloproteinase 13 expression was also colocalized with Col I (Fig. 7B). Although before birth HARE tended to be more diffusely expressed throughout the layers of the leaflet, HARE expression after birth appeared to be localized to the fibrosa (Fig. 8). This increased localization of staining with birth was commonly seen with a number of matrix components. Lysyl oxidase, which is involved in crosslinking of collagen and elastic fibers, demonstrated relatively diffuse staining in both valves and age groups (Fig. 7B). In both valves and age groups, TGFβ and CD44 demonstrated diffuse staining, without notable layer or region-specific localization (Fig. 9).
Figure 7.

A. Localization of both periostin and hyaluronan receptor for endocytosis to the fibrosa shown here in a 2-day-old aortic valve sample. Magnification ×5 objective, scale bar indicates 200 μm. B. Colocalization of matrix metalloproteinase 13 with procollagen I in the fibrosa, while lysyl oxidase staining was diffuse; shown here in a 2-day-old mitral valve sample. Magnification ×5 objective, scale bar indicates 200 μm.
Figure 8.

Hyaluronan receptor for endocytosis showed more diffuse expression in 3rd-trimester valves (A) compared with 2-day-old valves (B), as illustrated here with aortic valve samples. Magnification ×5 objective, scale bar indicates 200 μm.
Figure 9.
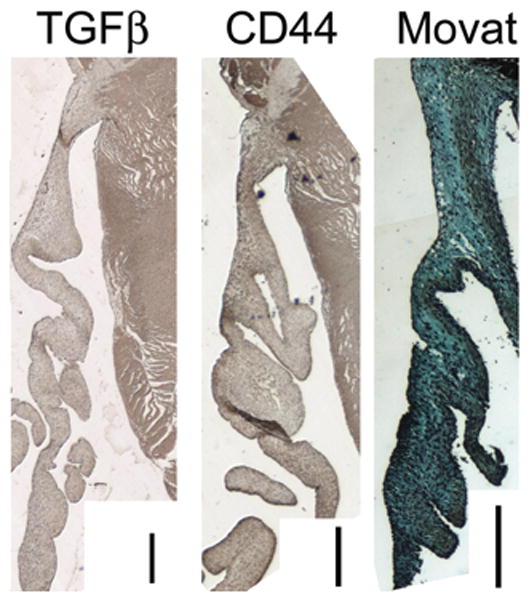
CD44 and transforming growth factor-beta demonstrated diffuse staining in both mitral and aortic valves of both age groups that did not localize to individual layers or regions. Shown here is a 2-day-old mitral valve sample. Magnification ×5 objective, scale bar indicates 200 μm.
Statistical correlations between the intensities of different matrix components within certain layers and regions of the MVs and AVs gave further insight into the interrelated roles of these components in perinatal valves. For instance, consistent with the common functions of BGN and DCN, in the fibrosa of the AVs, BGN was correlated with DCN (annulus, r = 0.864, P = 0.0057; midleaflet, r = 0.794, P = 0.0038). Similarly, consistent with the common roles of BGN and P4H in collagen fibrillogenesis, BGN was correlated with P4H in the midleaflet fibrosa of the AVs (r = 0.794, P = 0.0038). In the ventricularis layer of the AVs, Col I was correlated with HARE in the annular region (r = 0.907, P = 0.0019) and with P4H in the midleaflet region (r = 0.83, P = 0.0056). In the annular fibrosa of the MVs, elastin was correlated with Col III (r = 0.894, P = 0.0066).
Discussion
This histologic and immunohistochemical study showed that the composition and structure of the aortic and mitral valves change with birth, although these changes were more evident in the AVs than in the MVs. In general, perinatal changes in valve structure included increased delineation of leaflet layers and abundance of saffron-stained collagen. Perinatal changes in valve composition, found solely in the AVs, included increases in proteins related to turnover of elastic fibers and decreases in proteins and receptors related to collagen and HA turnover. These changes could have important functional consequences, as well as clinical implications.
Perinatal changes in aortic and mitral valve structure and composition
The AVs and MVs appeared relatively similar before birth and became more distinct with birth. Although the AVs demonstrated numerous structural and compositional changes perinatally, including an increase in leaflet layer delineation, an increase in intensity of saffron-staining collagen, and an increase in the proportion of leaflet thickness comproed of saffron-staining collagen, all were evident in the AVs; only an increase in delineation with birth was evident in the MVs. Additionally, compositional changes with birth were almost exclusively only evident in the AVs, not the MVs. This greater change in AV composition and structure with birth compared with MV composition and structure could relate to greater change in the hemodynamic environment of the AVs, as opposed to the MVs, with birth. The hemodynamic environments (ie, blood flow velocities, after load pressures) of the 2 valves are relatively similar in the fetal circulation [1,38]. At birth, however, the diastolic pressure borne by the AV increases by 51%, whereas the MV must bear a 23% greater diastolic pressure [2]. Thus, the mechanosensitive valves may be demonstrating levels of remodeling that are consistent with their changing mechanical environments.
Indeed, a large body of research in adult valves has examined the roles of specific mechanical factors in valve iology and remodeling. The role of pressure in AV composition has been studied in the ex vivo setting, where it was demonstrated that elevated static pressure caused an increase in collagen synthesis [18] whereas cyclic pressure evoked a magnitude and frequency dependent response in valve composition [17]. The effect of pressure on valve cells has also been implicated in modulation of cell phenotype and matrix synthesis based on differences in transvalvular pressures across the AV and pulmonic valve. Aortic valvular interstitial cells were found to be stiffer and contain more smooth muscle alpha-actin and heat shock protein 47 (a marker of collagen synthesis) than valvular interstitial cells from the pulmonic valve [8]. In addition, Platt and colleagues [19] found that the application of shear stress to intact AV leaflets decreased the expression of cathepsins but increased the expression of MMPs. The effect of strain on adult AV leaflets and valvular interstitial cells has been extensively studied and found to affect collagen, MMP, and glycosaminoglycan production in a magnitude and frequency dependent manner [16,39–43].
Although collagen synthesis (as shown by P4H expression) and Coll I content decreased in the AV midleaflet with birth, it is interesting to note that the collagen in the fibrosa of 2-day-old valves appeared more aligned and mature, as evidenced by greater intensity and proportion of Movat saffron-staining collagen. Additionally, the collagen-remodeling enzyme MMP13 increased with birth in the MVs and was localized to the fibrosa layer. Taken together, these results suggest that although less collagen is being produced immediately after birth, the collagen undergoes significant remodeling with birth as it becomes more mature and aligned. Ex vivo studies in which the AV was subjected to mechanical stimulation have demonstrated greater MMP expression with cyclic strain [41], as well as thicker, more aligned collagen fibers [9]. Alterations in the ratio of Col III to Col I have also been noted in cardiac remodeling, such as dilated cardiomyopathy [44–46], although a consensus has not been reached as to the exact nature of these changes and their implications.
Regional heterogeneity in perinatal changes in valve composition
Perinatal changes in valve composition were predominantly found in the midleaflet, as opposed to in the annular region of the valve. This regional heterogeneity could be attributed to the greater impact of the perinatal hemodynamic changes on the midleaflet region. More specifically, the myocardial anchorage of the annular region of the leaflet may partly shield that region from hemodynamic changes. In addition, the annular region can transduce forces to the myocardium, whereas the midleaflet does not have this anchorage. Greater compositional changes in the midleaflet, as opposed to the annular region of the leaflet, have also been observed in other animal models of altered hemodynamics [7].
Localization of matrix components within aortic and mitral valves
Hyaluronan receptor for endocytosis, also known as stabilin-2, was localized to the fibrosa of the 2-day-old valves. Although HARE has been previously demonstrated in adult mouse valves [47,48] and human adult normal and myxomatous mitral valves [49], its function in valves remains enigmatic. In 1 relevant study in mice, HARE was reported to be absent during development but present in the postnatal murine heart; in these murine valves, HARE was found limited to the valvular endothelium [48]. Another study, however, demonstrated HARE throughout the valve thickness in adult mice [47]. In general, the predominant role of HARE is considered to be HA metabolism, that is, the removal of HA from the bloodstream or extracellular space. Given the involvement of HA in valvulogenesis [35] and the observed decrease in HA during atrioventricular endocardial cushion development in chicks [15], it is not surprising that HA metabolism would change during valve development and that the abundance of HARE would decrease with birth. However, the localization of HARE to the fibrosa layer and its colocalization with collagen in postnatal valves may be more related to other binding sites within the HARE molecule; HARE is known to bind to procollagen [50], although the purpose of this binding in the context of valves is unclear. Hyaluronan receptor for endocytosis can also mediate both heterophilic [51] and homophilic cell-cell interactions [52], and HA binding to HARE triggers intracellular extracellular signal-regulated kinase activation [53]; these results suggest that HARE enables cells to sense matrix turnover. Furthermore, considering the many binding sites for diverse molecules along its length [54], HARE could potentially act as a scaffolding protein providing structure for and promoting interaction between these molecules. Clearly, the roles of HARE are diverse, meaning broader than HA metabolism alone, and many of these roles have yet to be elucidated, especially in the context of heart valves. Although CD44, one of the cellular receptors for HA, did not demonstrate a change in abundance with birth, the receptor activity of CD44 could have changed while expression levels remained relatively constant. There are also a number of other cell surface receptors for HA and other proteins involved in HA metabolism that could be changing during this period.
The colocalization of periostin with collagen in the fibrosa seen in this study is consistent with studies demonstrating that periostin directly binds Coll I and is involved in collagen fibrillogenesis [29]. Several other studies have also demonstrated the location of periostin in AVs and MVs, both during development and in mature valves. In a report of murine embryologic development, periostin showed widespread expression in the AV but greater expression in the atrial aspect (elastic layer) of the MV [55]. In adult mice, however, 2 studies have demonstrated periostin expression localized within the collagenous fibrosa layer of the AV and MV [55,56]. During chick embryologic development, periostin was found in the ventricular aspect of the MV (the fibrosa), but the AV showed lower expression relative to the MV [57]. In general, an increase in periostin with birth would be consistent with the role of periostin in cardiac remodeling in response to increased mechanical stimulation [31].
The localization of collagens and proteoglycans corroborate previous studies of different aged human and porcine MVs and AVs. Procollagen III, a reticular collagen [58], demonstrated more diffuse staining than Col I, as previously reported in human MVs and AVs [59]. The finding that P4H, a marker of collagen synthesis, was more diffusely present than either collagen and was even found outside the fibrosa layer is consistent with previous evidence that collagen turnover is not limited to the fibrosa layer in porcine AVs and MVs [13]. The enhanced staining of BGN and DCN in the fibrosa is also consistent with previous studies [14] and supports the role of these proteoglycans in collagen integrity [60,61]. The change in DCN expression could have affected collagen fiber diameter, given the known role that DCN plays in regulating collagen fiber diameter during collagen fibrillogenesis [60,62]. The localization of VC in the spongiosa and elastin-rich regions also corroborates previous reports in porcine valves [14] and supports a role for VC in elastic fiber homeostasis [33]. Lysyl oxidase staining, which was relatively diffuse in the valves, suggested ongoing maturation of nascent matrix through-out the leaflet in both age groups.
Proposed functional consequences of valve structural and compositional changes
These observed valve compositional and structural changes likely affected the leaflet tissue and overall function. The increase in leaflet layer delineation, which is consistent with previous studies [13], would reduce flexural stress [63]. The increase in elastin would aid in valve flexion and elastic recoil, as well as maintain proper collagen fiber configuration [64]. The increase in collagen maturation would result in greater tensile strength, allowing the valve to withstand the perinatal increase in pressures. A recent study by Balguid and colleagues [65] elegantly demonstrated a positive correlation between collagen cross-linking and tensile elastic modulus in the adult AV. Additionally, our lab has recently demonstrated that an increase in AV and MV tensile strength with postnatal age was associated with an increase in the amount of Movat saffron-staining collagen [66]. The role of decreased HA turnover with birth, as suggested by HARE expression, is less clear. Hyaluronan has numerous roles, both biologic and mechanical [67,68], and the derangement of its regulation has been implicated in several pathologies in a variety of tissues, including valves [49]. Therefore, alterations in HA metabolism observed with birth could have important biologic and mechanical consequences, although the exact nature of those remain to be determined. Similarly, perinatal changes in abundance and localization of proteoglycans could affect tissue mechanics, collagen homeostasis, cell signaling, growth factor regulation, and cell phenotype regulation. For example, DCN sequesters TGFβ [32], a growth factor that modulates valve cell phenotype [69], is implicated in a number of valve diseases, including myxomatous mitral valve disease [70]. Indeed, previous studies have demonstrated the colocalization of DCN with TGFβ in porcine valves [14], although in this set of valves TGFβ staining was found to be relatively diffuse. The proteoglycan VC is commonly found to be upregulated in disease states, as well as development [32], and given its role in elastic fibrillogenesis, the perinatal increase in VC could directly relate to the increase in elastin content with birth found in this study [33]. Greater elastin could result in functional changes in leaflet bending properties as the leaflet adapts to the altered hemodynamics. Clearly, further study is required to better understand the functional consequences of these perinatal compositional and structural changes.
Implications
These data regarding the perinatal development of heart valves have several implications. First, as work continues to develop tissue-engineered heart valves, a broad characterization of valve composition will be necessary to provide design criteria for valves for neonates. This age group is of particular interest given the large number of congenital heart disease patients, who often undergo neonatal surgical repair procedures. Additionally, if the perinatal hemodynamic changes do not occur normally, such as is the case in congenital heart disease, valve composition may be affected, which could in turn affect valvular cell phenotype and subsequent valvular maturation. Therefore, the results of this study suggest a potential mechanism for causing or contributing to the compositional and structural abnormalities observed in congenital valve disease. As illustrated by a representative example in Figure 10, these congenitally diseased valves typically lack a layered leaflet structure and a mature collagenous fibrosa layer and tend to be enriched in glycosaminoglycans. Further characterization of these compositional abnormalities in congenitally diseased valves is currently under way in this laboratory. Lastly, these results could also have implications for the timing of surgical intervention in this patient population, in that early restoration of normal neonatal hemodynamics may promote normal development of valve composition and function.
Figure 10.

Congenitally diseased aortic valve from a 3 month-old female demonstrating lack of layered leaflet structure and enriched glycosaminoglycan composition. Scale bar indicates 200 μm.
Study limitations
Although there were numerous statistically significant findings within this work, certain limitations must be mentioned. Samples were analyzed using immunohistochemistry, a powerful and appropriate method for examining both the abundance and localization of valve matrix components. However, this method is limited by some inherent variability in the staining technique, which in our hands has been quantified to be approximately 14% [7]. To diminish the effect of this variability on our results, multiple samples were taken from each valve and multiple sections cut from each sample were stained; the results were averaged. In addition, there are numerous other aspects of extracellular matrix maturation that should be addressed in future studies, such as collagen cross-linking and fiber diameter, that will further elucidate the nature of remodeling within the perinatal period. Furthermore, it will also be important to translate experimental mechanobiology studies, examining the effect of specific mechanical factors on the observed valve remodeling, from the time frame of adult valves to the perinatal and pediatric periods. This study also used an animal model; however, porcine valves are considered to be anatomically excellent animal models for human valves [71] and indeed are commonly used as animal models for human valve biology and mechanics [72]. Lastly, some of the observed perinatal changes in the tissues may have been due to changes in the biologic milieu, such as circulating growth factors, cytokines, and even inflammatory mediators. Further study is warranted to understand the contribution of these other factors on valve remodeling with birth. In the future, it will also be necessary to elucidate the functional consequences of this perinatal valve remodeling and investigate how altered hemodynamics may relate to the altered valve structure and composition observed in congenital valve disease.
Conclusions
This study demonstrated that AVs and MVs undergo significant remodeling with birth, including increased leaflet layer delineation and alignment of collagen, as well as changes in the amount of collagen turnover, HA metabolism, elastin, and proteoglycans. These perinatal changes were more pronounced in AVs than in MVs, consistent with greater hemodynamic changes experienced by AVs. These results contribute to a growing body of evidence that the valve dynamically adapts to its mechanical environment. Future study is warranted to examine the functional consequences of these changes, especially for patients with congenital valve disease.
Acknowledgments
We appreciate the assistance of the members of the Grande-Allen laboratory, especially Joyce Kuo. We also appreciate guidance from Dr Scott Baggett regarding statistics. This research was supported by a research grant from the March of Dimes, a Hertz Foundation Graduate Fellowship (E.H.S.), a Kirschstein NRSA Fellowship (HL094019, E.H.S.), and a Rice Century Scholar Award (A.D.P.). We offer special thanks to Dr Douglas Burrin and Dr David Miller for assistance in the acquisition of the 2-day-old pig hearts (Children’s Nutrition Research Center, Baylor College of Medicine, Houston, TX, USA) and Dr Charles D. Fraser, Kathleen Carberry, and Karol Arrington (Texas Children’s Hospital, Houston, TX, USA) for assistance in the procurement of human congenitally diseased valves.
References
- 1.Friedman AH, Fahey JT. The transition from fetal to neonatal circulation: normal responses and implications for infants with heart disease. Semin Perinatol. 1993;17:106–121. [PubMed] [Google Scholar]
- 2.Anderson PA, Glick KL, Manring A, Crenshaw C., Jr Developmental changes in cardiac contractility in fetal and postnatal sheep: in vitro and in vivo. Am J Physiol. 1984;247:H371–H379. doi: 10.1152/ajpheart.1984.247.3.H371. [DOI] [PubMed] [Google Scholar]
- 3.Sansoucie DA, Cavaliere TA. Transition from fetal to extrauterine circulation. Neonatal Netw. 1997;16:5–12. [PubMed] [Google Scholar]
- 4.Cooper T, Napolitano LM, Fitzgerald MJ, et al. Structural basis of cardiac valvar function. Arch Surg. 1966;93:767–771. doi: 10.1001/archsurg.1966.01330050071010. [DOI] [PubMed] [Google Scholar]
- 5.Borin C, Vanhercke D, Weyns A. Innervation of the atrioventricular and semi-lunar heart valves: a review. Acta Cardiol. 2006;61:463–469. doi: 10.2143/AC.61.4.2017309. [DOI] [PubMed] [Google Scholar]
- 6.Grande-Allen KJ, Borowski AG, Troughton RW, et al. Apparently normal mitral valves in patients with heart failure demonstrate biochemical and structural derangements: an extracellular matrix and echocardiographic study. J Am Coll Cardiol. 2005;45:54–61. doi: 10.1016/j.jacc.2004.06.079. [DOI] [PubMed] [Google Scholar]
- 7.Stephens EH, Timek TA, Daughters GT, et al. Significant changes in mitral valve leaflet matrix composition and turnover with tachycardia-induced cardiomyopathy. Circulation. 2009;120(11 suppl):S112–S119. doi: 10.1161/CIRCULATIONAHA.108.844159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merryman WD, Youn I, Lukoff HD, et al. Correlation between heart valve interstitial cell stiffness and transvalvular pressure: implications for collagen biosynthesis. Am J Physiol Heart Circ Physiol. 2006;290:H224–H231. doi: 10.1152/ajpheart.00521.2005. [DOI] [PubMed] [Google Scholar]
- 9.Balachandran K, Konduri S, Sucosky P, Jo H, Yoganathan A. An ex vivo study of the biological properties of porcine aortic valves in response to circumferential cyclic stretch. Ann Biomed Eng. 2006;34:1655–1665. doi: 10.1007/s10439-006-9167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta V, Werdenberg JA, Mendez JS, Jane Grande-Allen K. Influence of strain on proteoglycan synthesis by valvular interstitial cells in three-dimensional culture. Acta Biomater. 2008;4:88–96. doi: 10.1016/j.actbio.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Aikawa E, Whittaker P, Farber M, et al. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006;113:1344–1352. doi: 10.1161/CIRCULATIONAHA.105.591768. [DOI] [PubMed] [Google Scholar]
- 12.Hinton RBJ, Lincoln J, Deutsch GH, et al. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res. 2006;98:1431–1438. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- 13.Stephens EH, Grande-Allen KJ. Age-related changes in collagen synthesis and turnover in porcine heart valves. J Heart Valve Dis. 2007;16:672–682. [PubMed] [Google Scholar]
- 14.Stephens EH, Chu CK, Grande-Allen KJ. Valve proteoglycan content and glycosaminoglycan fine structure are unique to microstructure, mechanical load and age: relevance to an age-specific tissue-engineered heart valve. Acta Biomater. 2008;4:1148–1160. doi: 10.1016/j.actbio.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butcher J, McQuinn T, Sedmera D, Turner D, Markwald R. Transitions in early embryonic atrioventricular valvular function correspond with changes in cushion biomechanics that are predictable by tissue composition. Circ Res. 2007;100:1503–1511. doi: 10.1161/CIRCRESAHA.107.148684. [DOI] [PubMed] [Google Scholar]
- 16.Ku CH, Johnson PH, Batten P, et al. Collagen synthesis by mesenchymal stem cells and aortic valve interstitial cells in response to mechanical stretch. Cardiovasc Res. 2006;71:548–556. doi: 10.1016/j.cardiores.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Xing Y, Warnock JN, He Z, Hilbert SL, Yoganathan AP. Cyclic pressure affects the biological properties of porcine aortic valve leaflets in a magnitude and frequency dependent manner. Ann Biomed Eng. 2004;32:1461–1470. doi: 10.1114/b:abme.0000049031.07512.11. [DOI] [PubMed] [Google Scholar]
- 18.Xing Y, He Z, Warnock JN, Hilbert SL, Yoganathan AP. Effects of constant static pressure on the biological properties of porcine aortic valve leaflets. Ann Biomed Eng. 2004;32:555–562. doi: 10.1023/b:abme.0000019175.12013.8f. [DOI] [PubMed] [Google Scholar]
- 19.Platt MO, Xing Y, Jo H, Yoganathan AP. Cyclic pressure and shear stress regulate matrix metalloproteinases and cathepsin activity in porcine aortic valves. J Heart Valve Dis. 2006;15:622–629. [PubMed] [Google Scholar]
- 20.Hoffman J, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 21.Dunn JM. Porcine valve durability in children. Ann Thorac Surg. 1981;32:357–368. doi: 10.1016/s0003-4975(10)61757-2. [DOI] [PubMed] [Google Scholar]
- 22.Solymar L, Rao PS, Mardini MK, Fawzy ME, Guinn G. Prosthetic valves in children and adolescents. Am Heart J. 1991;121:557–568. doi: 10.1016/0002-8703(91)90726-x. [DOI] [PubMed] [Google Scholar]
- 23.Stephens E, Chu C-K, Grande-Allen KJ. Valve proteoglycan content and glycosaminoglycan fine structure are unique to microstructure, mechanical load and age: relevance to an age-specific tissue-engineered heart valve. Acta Biomaterialia. 2008;4:1148–1160. doi: 10.1016/j.actbio.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stephens E, Grande-Allen K. Age-related changes in collagen synthesis and turnover in porcine heart valves. J Heart Valve Dis. 2007;16:672–682. [PubMed] [Google Scholar]
- 25.Fisher LW, Stubbs JT, 3rd, Young MF. Antisera and cDNA probes to human and certain animal model bone matrix noncollagenous proteins. Acta Orthop Scand Suppl. 1995;266:61–65. [PubMed] [Google Scholar]
- 26.Cole WG, Chan D, Hickey AJ, Wilcken DE. Collagen composition of normal and myxomatous human mitral heart valves. Biochem J. 1984;219:451–460. doi: 10.1042/bj2190451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kivirikko K, Myllyla R. Post-translational processing of procollagens. Ann NY Acad Sci. 1985;460:187–201. doi: 10.1111/j.1749-6632.1985.tb51167.x. [DOI] [PubMed] [Google Scholar]
- 28.Butcher JT, Norris RA, Hoffman S, Mjaatvedt CH, Markwald RR. Periostin promotes atrioventricular mesenchyme matrix invasion and remodeling mediated by integrin signaling through Rho/PI 3-kinase. Dev Biol. 2007;302:256–266. doi: 10.1016/j.ydbio.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norris RA, Damon B, Mironov V, et al. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem. 2007;101:695–711. doi: 10.1002/jcb.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oka T, Xu J, Kaiser RA, Melendez J, et al. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res. 2007;101:313–321. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borg TK, Markwald R. Periostin: more than just an adhesion molecule. Circ Res. 2007;101:230–231. doi: 10.1161/CIRCRESAHA.107.159103. [DOI] [PubMed] [Google Scholar]
- 32.Kinsella MG, Bressler SL, Wight TN. The regulated synthesis of versican, decorin, and biglycan: extracellular matrix proteoglycans that influence cellular phenotype. Crit Rev Eukaryot Gene Expr. 2004;14:203–234. doi: 10.1615/critreveukaryotgeneexpr.v14.i3.40. [DOI] [PubMed] [Google Scholar]
- 33.Merrilees MJ, Lemire JM, Fischer JW, et al. Retrovirally mediated overexpression of versican v3 by arterial smooth muscle cells induces tropoelastin synthesis and elastic fiber formation in vitro and in neointima after vascular injury. Circ Res. 2002;90:481–487. doi: 10.1161/hh0402.105791. [DOI] [PubMed] [Google Scholar]
- 34.Henderson DJ, Copp AJ. Versican expression is associated with chamber specification, septation, and valvulogenesis in the developing mouse heart. Circ Res. 1998;83:523–532. doi: 10.1161/01.res.83.5.523. [DOI] [PubMed] [Google Scholar]
- 35.Wagner M, Siddiqui MA. Signal transduction in early heart development (II): ventricular chamber specification, trabeculation, and heart valve formation. Exp Biol Med (Maywood) 2007;232:866–880. [PubMed] [Google Scholar]
- 36.Lara S, Evanko S, Wight T. Morphological evaluation of proteoglycans in cells and tissues. Methods Mol Biol. 2001;171:271–290. doi: 10.1385/1-59259-209-0:271. [DOI] [PubMed] [Google Scholar]
- 37.Harris EN, Weigel JA, Weigel PH. Endocytic function, glycosaminoglycan specificity, and antibody sensitivity of the recombinant human 190-kDa hyaluronan receptor for endocytosis (HARE) J Biol Chem. 2004;279:36201–36209. doi: 10.1074/jbc.M405322200. [DOI] [PubMed] [Google Scholar]
- 38.Allan LD, Chita SK, Al-Ghazali W, Crawford DC, Tynan M. Doppler echocardiographic evaluation of the normal human fetal heart. Br Heart J. 1987;57:528–533. doi: 10.1136/hrt.57.6.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta V, Tseng H, Lawrence BD, Jane Grande-Allen K. Effect of cyclic mechanical strain on glycosaminoglycan and proteoglycan synthesis by heart valve cells. Acta Biomater. 2009;5:531–540. doi: 10.1016/j.actbio.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta V, Werdenburg JA, Blevins TL, Grande-Allen KJ. Synthesis of glycosaminoglycans in differently loaded regions of collagen gels seeded with valvular interstitial cells. Tissue Eng. 2007;13:41–49. doi: 10.1089/ten.2006.0091. [DOI] [PubMed] [Google Scholar]
- 41.Balachandran K, Sucosky P, Jo H, Yoganathan AP. Elevated cyclic stretch alters matrix remodeling in aortic valve cusps: implications for degenerative aortic valve disease. Am J Physiol Heart Circ Physiol. 2009;296:H756–H764. doi: 10.1152/ajpheart.00900.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mol A, Bouten CV, Zünd G, et al. The relevance of large strains in functional tissue engineering of heart valves. Thorac Cardiovasc Surg. 2003;51:78–83. doi: 10.1055/s-2003-38993. [DOI] [PubMed] [Google Scholar]
- 43.Merryman WD, Lukoff HD, Long RA, Engelmayr GC, Jr, Hopkins RA, Sacks MS. Synergistic effects of cyclic tension and transforming growth factor-beta1 on the aortic valve myofibroblast. Cardiovasc Pathol. 2007;16:268–276. doi: 10.1016/j.carpath.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pauschinger M, Doerner A, Remppis A, Tannhauser R, Kuhl U, Schultheiss HP. Differential myocardial abundance of collagen type I and type III mRNA in dilated cardiomyopathy: effects of myocardial inflammation. Cardiovasc Res. 1998;37:123–129. doi: 10.1016/s0008-6363(97)00217-4. [DOI] [PubMed] [Google Scholar]
- 45.Marijianowski MM, Teeling P, Mann J, Becker AE. Dilated cardiomyopathy is associated with an increase in the type I/type III collagen ratio: a quantitative assessment. J Am Coll Cardiol. 1995;25:1263–1272. doi: 10.1016/0735-1097(94)00557-7. [DOI] [PubMed] [Google Scholar]
- 46.Sivakumar P, Gupta S, Sarkar S, Sen S. Upregulation of lysyl oxidase and MMPs during cardiac remodeling in human dilated cardiomyopathy. Mol Cell Biochem. 2008;307:159–167. doi: 10.1007/s11010-007-9595-2. [DOI] [PubMed] [Google Scholar]
- 47.Falkowski M, Schledzewski K, Hansen B, Goerdt S. Expression of stabilin-2, a novel fasciclin-like hyaluronan receptor protein, in murine sinusoidal endothelia, avascular tissues, and at solid/liquid interfaces. Histochem Cell Biol. 2003;120:361–369. doi: 10.1007/s00418-003-0585-5. [DOI] [PubMed] [Google Scholar]
- 48.Lindsley A, Li W, Wang J, Maeda N, Rogers R, Conway SJ. Comparison of the four mouse fasciclin-containing genes expression patterns during valvuloseptal morphogenesis. Gene Expr Patterns. 2005;5:593–600. doi: 10.1016/j.modgep.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 49.Gupta V, Barzilla JE, Mendez JS, et al. Abundance and location of proteoglycans and hyaluronan within normal and myxomatous mitral valves. Cardiovasc Pathol. 2009;18:191–197. doi: 10.1016/j.carpath.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hansen B, Longati P, Elvevold K, et al. Stabilin-1 and stabilin-2 are both directed into the early endocytic pathway in hepatic sinusoidal endothelium via interactions with clathrin/AP-2, independent of ligand binding. Exp Cell Res. 2005;303:160–173. doi: 10.1016/j.yexcr.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 51.Jung MY, Park SY, Kim IS. Stabilin-2 is involved in lymphocyte adhesion to the hepatic sinusoidal endothelium via the interaction with alphaMbeta2 integrin. J Leukoc Biol. 2007;82:1156–1165. doi: 10.1189/jlb.0107052. [DOI] [PubMed] [Google Scholar]
- 52.Park SY, Jung MY, Kim IS. Stabilin-2 mediates homophilic cell-cell interactions via its FAS1 domains. FEBS Lett. 2009;583:1375–1380. doi: 10.1016/j.febslet.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 53.Kyosseva SV, Harris EN, Weigel PH. The hyaluronan receptor for endocytosis mediates hyaluronan-dependent signal transduction via extracellular signal-regulated kinases. J Biol Chem. 2008;283:15047–15055. doi: 10.1074/jbc.M709921200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harris EN, Weigel PH. The ligand-binding profile of HARE: hyaluronan and chondroitin sulfates A, C, and D bind to overlapping sites distinct from the sites for heparin, acetylated low-density lipoprotein, dermatan sulfate, and CS-E. Glycobiology. 2008;18:638–648. doi: 10.1093/glycob/cwn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Norris RA, Moreno-Rodriguez RA, Sugi Y, et al. Periostin regulates atrioventricular valve maturation. Dev Biol. 2008;316:200–213. doi: 10.1016/j.ydbio.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snider P, Hinton RB, Moreno-Rodriguez RA, et al. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res. 2008;102:752–760. doi: 10.1161/CIRCRESAHA.107.159517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kern CB, Hoffman S, Moreno R, et al. Immunolocalization of chick periostin protein in the developing heart. Anat Rec A Discov Mol Cell Evol Biol. 2005;284:415–423. doi: 10.1002/ar.a.20193. [DOI] [PubMed] [Google Scholar]
- 58.Ushiki T. Collagen fibers, reticular fibers and elastic fibers: a comprehensive understanding from a morphological viewpoint. Arch Histol Cytol. 2002;65:109–126. doi: 10.1679/aohc.65.109. [DOI] [PubMed] [Google Scholar]
- 59.McDonald PC, Wilson JE, McNeill S, et al. The challenge of defining normality for human mitral and aortic valves: geometrical and compositional analysis. Cardiovasc Pathol. 2002;11:193–209. doi: 10.1016/s1054-8807(01)00102-8. [DOI] [PubMed] [Google Scholar]
- 60.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconjug J. 2002;19:249–255. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- 62.Derwin K, Soslowsky L, Kimura J, Plaas A. Proteoglycans and glycosaminoglycan fine structure in the mouse tail tendon fascicle. J Orthop Res. 2001;19:269–277. doi: 10.1016/S0736-0266(00)00032-2. [DOI] [PubMed] [Google Scholar]
- 63.Kunzelman KS, Cochran RP, Murphree SS, Ring WS, Verrier ED, Eberhart RC. Differential collagen distribution in the mitral valve and its influence on biomechanical behaviour. J Heart Valve Dis. 1993;2:236–244. [PubMed] [Google Scholar]
- 64.Vesely I. The role of elastin in aortic valve mechanics. J Biomech. 1998;31:115–123. doi: 10.1016/s0021-9290(97)00122-x. [DOI] [PubMed] [Google Scholar]
- 65.Balguid A, Rubbens M, Mol A, et al. The role of collagen crosslinks in biomechanical behavior of human aortic heart valve leaflets: relevance for tissue engineering. Tissue Eng. 2007;13:1501–1511. doi: 10.1089/ten.2006.0279. [DOI] [PubMed] [Google Scholar]
- 66.Stephens EH, de Jonge N, McNeill MP, Durst CA, Grande-Allen KJ. Age-related changes in material behavior of porcine mitral and aortic valves and correlation to matrix composition. Tissue Eng. 2009;16:867–878. doi: 10.1089/ten.tea.2009.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Toole BP. Hyaluronan in morphogenesis. Semin Cell Dev Biol. 2001;12:79–87. doi: 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]
- 68.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 69.Walker GA, Masters KS, Shah DN, Anseth KS, Leinwand LA. Valvular myofibroblast activation by transforming growth factor-beta: implications for pathological extracellular matrix remodeling in heart valve disease. Circ Res. 2004;95:253–260. doi: 10.1161/01.RES.0000136520.07995.aa. [DOI] [PubMed] [Google Scholar]
- 70.Disatian S, Orton EC. Autocrine serotonin and transforming growth factor beta 1 signaling mediates spontaneous myxomatous mitral valve disease. J Heart Valve Dis. 2009;18:44–51. [PubMed] [Google Scholar]
- 71.Sim EK, Muskawad S, Lim CS, et al. Comparison of human and porcine aortic valves. Clin Anat. 2003;16:193–196. doi: 10.1002/ca.10149. [DOI] [PubMed] [Google Scholar]
- 72.Vesely I. Reconstruction of loads in the fibrosa and ventricularis of porcine aortic valves. ASAIO J. 1996;42:M739–M746. doi: 10.1097/00002480-199609000-00087. [DOI] [PubMed] [Google Scholar]




