Abstract
The blood-brain barrier (BBB) represents the interface between the brain and other body tissues. Its ability to protect the brain from harmful compounds has attracted the attention of both clinicians and investigators. However, far from being a simple physical barrier, the BBB is a complex, heterogeneous and dynamic tissue. The integrated function of the cerebral microvasculature, tight junction proteins, brain microvascular endothelial cells (BMEC), cellular transport pathways and enzymatic machinery jointly contribute to normal BBB integrity. Aging, systemic diseases and ischemic injury can disrupt these processes, resulting in a decline in overall BBB function and integrity. Based on the published literature, we propose that age- and disease-related BBB alterations play a key role in diminishing the ability of older patients to recover from acute ischemic stroke (AIS). Moreover, we also review evidence linking deficits in the cerebral microvasculature and BBB integrity to dementia, medication-related cognitive decline, white matter disease (leukoaraiosis), as well as related geriatric syndromes including delirium, gait disorders and urinary incontinence. Priority areas for a future research agenda include strategies to improve clinicians' ability to diagnose, prevent and manage BBB abnormalities. In future years, in vivo measures such as functional and contrast-enhanced neuroimaging will be used to evaluate BBB integrity in older adults while also assessing the effectiveness of interventions, some targeting inflammatory pathways known to disrupt the BBB, for their ability to prevent or slow the progression of these complex multifactorial geriatric syndromes.
Keywords: Blood-brain barrier, Stroke, Dementia, Delirium, Pharmacology
INTRODUCTION
A major role of the cerebral microvasculature is to support the integrity of the blood-brain barrier (BBB), regulating the passage of substances between the central nervous system and the systemic circulation across the life span. BBB functional integrity requires the coordination of diverse cellular activities, e.g. tight junction barrier properties, transport functions and enzyme processes (1). Compromise of the cerebral microvasculature may predispose older adults to a variety of common clinical conditions (2). In contrast to conventional large vessel strokes which present with acute onset of focal neurologic deficits, cerebral microvascular disease may evolve insidiously, with subtle deficits in gait and cognition. Increasing evidence from both experimental and clinical studies suggests that BBB impairment is an important contributor to progressive age-associated neurological decline (2).
This review will summarize the impact of aging on BBB integrity and will also discuss the potential consequences of these changes on the risk of development of age-related functional decline. More specifically, the contribution of decreasing BBB function and integrity to the increased risk of ischemia-related neuronal injury, white matter disease (leukoaraiosis), delirium and medication-related cognitive decline in the elderly will be discussed. These diseases play an important role in numerous geriatric syndromes that involve loss of mobility, impaired executive function and incontinence. Since the goal of this review is to primarily discuss the BBB in the context of clinically relevant geriatric issues, the interested reader is referred to excellent reviews pertaining to BBB biology (3-6).
BBB STRUCTURE AND FUNCTION
The Neurovascular Unit
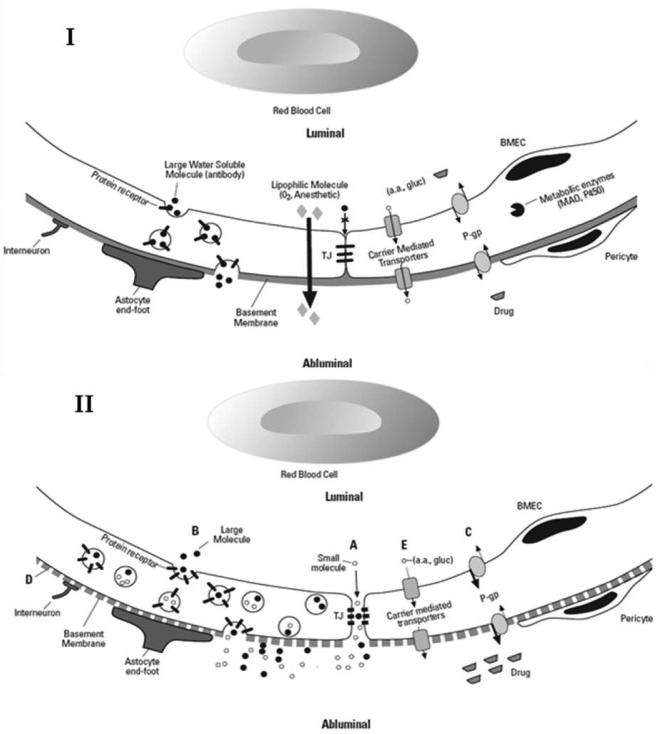
The BBB prevents circulating substances from gaining unlimited access to brain parenchyma. The neurovascular unit (NVU) consists of vascular endothelial cells and pericytes, as well as closely juxtaposed neurons and astrocytes. The NVU is the principal functional unit within the BBB (Fig. 1) (1, 6). Tissue culture studies provide insights into the nature of interactions between constituent cells and their contribution to BBB integrity (1, 5, 6). Cells forming the NVU regulate brain microvascular endothelial cell (BMEC) proliferation, migration and vascular branching in response to injuries e.g. trauma or hypoxia (6). Additional structural support is provided by the BMEC basement membrane where layers of type IV collagen, fibronectin, heparin sulfate and laminin create a negatively-charged barrier (5). Finally, neural activity may also modulate BBB function through noradrenergic, serotonergic and cholinergic receptors expressed by BMECs (6). Inability to fully recapitulate NVU interactions limits the value of in vitro models.
Figure 1. The Neurovascular Unit with normal and disrupted BBB.
(I) Functional barrier composed of transport systems and metabolic enzymes. The structural barrier includes tight junctions and basement membrane. (II) Disrupted BBB with (A) Increased paracellular permeability due to tight junction disruption (B) increased transcellular permeability via upregulated transcytosis (C) drug and toxin accumulation due to decreased efflux via P-gp (D) basement membrane disruption (E) decreased nutrient transport TJ = tight junctions, BMEC = brain microvascular endothelial cell, P-gp = permeability glycoprotein, a.a. = amino acids, gluc = glucose, MAO = monoamine oxidase inhibitor, P450 = drug metabolizing enzyme system
Brain Microvascular Endothelial Cell Properties
BMECs, located at the interface between the brain and its blood supply, are the primary site for production and function of BBB-related structural proteins, enzymes and membrane transporters (Fig. 1) (6). The close physical apposition of BMECs to each other, achieved by tight junctions (TJ's) (Fig. 1) (1), severely restricts solute flux through the paracellular space (1). TJ's are composed of an elaborate assembly of structural proteins that provide strong physical linkages between BMECs by tying together the cytoskeleton of adjacent cells (6).
Transport proteins are responsible for the selective transcellular movement of endogenous and exogenous substances across the luminal and abluminal plasma membranes of the BMEC (Fig. 1). For example glucose transporters (GLUT) passively move glucose across the BBB via carrier-mediated transport/facilitative diffusion, providing a constant energy supply for neuronal activity (1). Other carriers similarly facilitate the passive entry of additional hexoses, nitrogenous bases, nucleosides, fatty acids, amines and amino acids. Included among these amino acids is L-Dopa (3,4-dihydroxy-L-phenylalanine), prescribed for the treatment of Parkinson's disease (1). There are also active transporters present at the BBB. An example is P-glycoprotein (P-gp or permeability glycoprotein), an efflux transporter that plays a role in the development of multidrug resistance to antibiotics, antifungals and antineoplastic agents (1, 5, 7). Metabolizing enzymes within BMECs neutralize drugs and other potentially toxic compounds (1, 5).
Cerebral Microvascular Heterogeneity
A concept emerging from BBB research is the heterogeneity of the cellular composition and regional specialization of the brain microvasculature (4). BMECs obtained from capillaries, venules and arterioles reveal significant differences in properties which are relevant to BBB function (4, 8). For example, immunohistochemical staining reveals clear differences in BMEC surface marker expression and enzymatic activity for each segment of the brain microvasculature (4). Moreover, BBB properties may display regional heterogeneity between different brain lobes, between cortical and subcortical areas as well as between gray and white matter (4, 7, 9). As a result, broad generalizations should be avoided when discussing the BBB.
Determinants of BBB Permeability
Physical properties of a given solute and relevant aspects of BBB behavior contribute to the ability of a substance to cross the BBB. Smaller size and lipid solubility favor passage across the BBB: specifically lipophilic molecules <500 daltons (Fig. 1) (7). For example, anesthetic gases cross the BBB readily, whereas large proteins such as antibodies are excluded from the brain. During episodes of inflammation or trauma, producing BBB damage, fluid and proteins leak into the brain (Fig. 2), leading to vasogenic edema. Due to the rigid cranial vault even small amounts of brain swelling can raise intracranial pressure, causing life-threatening brain herniation (5). However even much more subtle increases in BBB permeability allow entry of macromolecules such as proteases, immunoglobulins and cytokines into the parenchyma which could impair cognition and also contribute to neurodegenerative conditions like Alzheimer's dementia (2, 10).
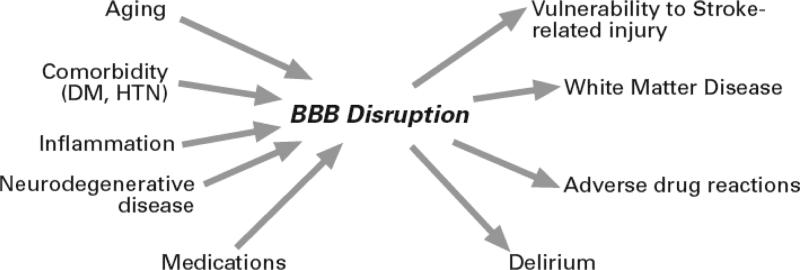
Figure 2. Multifactorial Contribution to Blood-Brain Barrier Pathogenesis.
Comorbidities and medications compound BBB changes accompanying aging. Compromise of the BBB is also associated with several age-related disorders.
AGE-RELATED CHANGES IN THE BBB
Age-related changes in BBB properties are apparent at anatomic and physiologic levels (11). These include decreased cortical and white matter microvascular density (12, 13), decreased capillary lumen size with increased tortuosity (11), as well as a reduced number of mitochondria per endothelial cell suggesting that energy dependent processes within the BMEC may be impaired (11).
Functional in vitro studies demonstrate age-related defects in the transport of glucose, amino acids and hormones across the BBB (14). Resulting deficits in glucose and choline bioavailability in the aged brain could contribute to older adults’ vulnerability to hypoglycemia and cognitive decline (15). More recently, the inclusion of novel ligands in positron emission tomography (PET) studies has permitted the in vivo assessment of specific BBB transport systems. For example, studies using radiolabeled verapamil, a ligand for the P-gp BBB transport system, have revealed lower activity in healthy elderly subjects as compared to younger controls (16). Age-related defects in cellular transport suggest functional mechanisms contribute to BBB impairment, although declines in BBB integrity may be multi-factorial (Table 1).
Table I. Major Determinants of BBB Integrity.
The main components of the BBB as well as specific protein products from each category with a possible role in age-related BBB changes and clinical conditions are listed.
| BBB Component | Defining Gene Product | Impact of Aging | Clinical Geriatric Relevance |
|---|---|---|---|
| Tight Junctions | Occludin | ? | AIS, AD, DM |
| Zonula Occludins | ? | AIS, PD | |
| Claudin-5 | ? | AIS | |
| Claudin-2 | ? | Sepsis | |
| Gap Junctions | Connexin-43 | ? | Sex differences in AIS outcomes |
| Non-specific tanscytosis | Aquaporin | ? | Hypoxia, AIS Hypoglycemia |
| Efflux Pumps | P-gp | ↓ activity | Adverse drug reactions |
| Delirium, AD, PD | |||
| WMD, Epilepsy | |||
| Brain tumors | |||
| Carrier-mediated transporter | GLUT-1 | ↓ activity | Hypoglycemia, MCI |
| Epilepsy, AIS, AD | |||
| Amino acid transporter | ↓ activity | PD, Epilepsy | |
| Methionine-enkephalin transporter | ↓ activity | Delirium | |
| Choline transporter | ↓ activity | Dementia | |
| Enzymes | Glutathione transferase | ? | PD |
| Basal Lamina | Matrix Metalloproteinase | ? | AIS, PD, AD |
| c-Fn | ? | AIS | |
| Laminin | ? | AIS | |
| Surface Receptors | ICAM-1 | ? | Neuroinflammation |
| CD31 | ? | AIS, WMD | |
| RAGE | ↑ activity | AD | |
| LRP | ↓ activity | AD | |
AIS = acute ischemic stroke, DM = Diabetes mellitus, PD = Parkinson's disease, P-gp = permeability glycoprotein, AD = Alzheimer's disease, WMD = White Matter Disease, GLUT-1 = glucose transporter 1, MCI = Mild Cognitive Impairment, c-Fn = c-fibronectin, ICAM-1 = intercellular adhesion molecule-1, CD31 = platelet endothelial cell adhesion molecule-1, RAGE = receptor for advanced glycation end products, LRP = low-density lipoprotein receptor-related protein
Since regional heterogeneity also pertains to BBB aging, it has been suggested that such changes contribute to the well-recognized selective vulnerability of specific cortical and hippocampal brain circuits in old age (9, 17, 18). For example, increased vascular permeability appears particularly prevalent from vessels in the aged mouse hippocampus compared to other regions (9). This is more pronounced in the senescence accelerated mouse (SAM) which exhibits premature memory impairment. Neurologically intact human subjects undergoing spinal anesthesia show a gradual age-related increase in cerebral spinal fluid (CSF)/serum protein concentrations (11). Since serum proteins do not normally cross the BBB, their presence in CSF is believed to reflect BBB disruption. This suggests that initial BBB alterations may be subtle and not become evident clinically until late in life, especially when disease or ischemic injury cause additional damage. Following seizures, old rats exhibit greater BBB disruption (19), providing additional support of an age-related increase in this vulnerability.
Hypertension, hyperlipidemia, diabetes mellitus and certain medications contribute to microvascular injury (2, 20), representing risk factors for BBB disruption which may confound changes seen with aging (Fig. 3). A limitation of CSF and neuroimaging studies is that they do not provide information concerning the mechanisms underlying enhanced BBB vulnerability in old age. It is also undetermined if BBB changes are compensatory responses to events involving an aged/diseased brain, or if a compromised BBB contributes to age/disease-related decrements of brain performance.
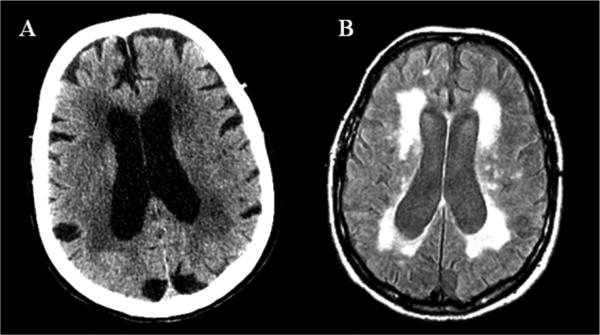
Figure 3.
Neuroimaging of an 84 year old woman with a history of gait impairment and urinary incontinence. (A) hypodense lesions on CT and (B) increased signal intensity on FLAIR MRI within cerebral white matter.
BBB, AGING AND STROKE
Effects of Stroke on the BBB
In animal models BBB disruption can be detected minutes after middle cerebral artery occlusion (MCAO) and precedes neuronal injury (21). Therefore, BBB disruption may represent an initial and decisive step in acute ischemic stroke (AIS) pathophysiology (21-23). A second opening of the BBB occurs several hours later resulting in a biphasic temporal pattern to BBB breakdown following AIS (22). In animal studies, Evan's blue extravasation, an index of BBB permeability, correlates with infarct size (21). BBB disruption, assessed in humans in vivo through magnetic resonance imaging (MRI) (23) and single photon emission computed tomography (SPECT) studies (24), has also been linked to worse Rankin scores (23), hemorrhagic transformation (23) and poorer neurologic recovery at hospital discharge (24). Moreover, following MCAO injury aged female rats demonstrate evidence of greater BBB permeability compared to young animals (21). Thus, BBB impairment may contribute to the increased prevalence and severity of cerebrovascular disease in the elderly by predisposing to ischemic insult and exacerbating stroke-related injury and diminishing recovery.
Mechanisms of BBB Injury during Stroke
Markers of BBB dysfunction such as basal lamina constituents matrix metalloproteinase (MMP-9) and c- fibronectin (c-Fn) predict malignant MCA infarction offering support to the concept that the BMEC's basal lamina plays a role in ischemic injury (25). Other BBB components have also been implicated. Stroke in spontaneously hypertensive rats causes disruption of the TJ protein claudin-5 in immunolabeled brain microvessels (3). Electron microscopy of BMEC following transient focal ischemia in rats reveals increased pinocytosis suggesting that the transcellular route, rather than or in addition to the paracellular route, may mediate BBB disruption in AIS (26). Diminished astrocytic response following stroke in old rats indicates that different NVU elements are affected (27). Interference with one or more BBB components at different times may occur during the evolution of stroke (22). Mediators of BBB disruption in conditions of ischemia that have been investigated include CCL2 (macrophage chemoattractant protein-1 or MCP-1), vascular endothelial growth factor (VEGF), tissue plasminogen activator (tPA) and nitric oxide. Thus, inflammation and proinflammatory molecules could contribute to worsened stroke-related outcomes via BBB disruption (28). It is unknown if peripheral increases in inflammatory mediators shown to be linked to aging and frailty (29), are associated with similar hyperinflammatory responses within the central nervous system (CNS). Other mechanisms possibly contributing to worsened AIS outcomes include age/disease-related declines in CNS angiogenesis, neurotransmission, neural plasticity and neurogenesis.
Functional recovery from an AIS event may also be influenced by medications (e.g. antipsychotics or sedatives) and systemic illnesses (e.g. sepsis or renal failure) (28). These factors can compromise CNS function and also may disrupt the BBB (Fig. 3) (5, 6) thus exacerbating stroke-related injury and recovery. Many seemingly peripheral conditions such as arthritis and sarcopenia may also play a role in the ability of an older adult to recover from AIS.
BBB and Lacunar Stroke
Lacunar infarcts which represent 25% of ischemic strokes are often clinically silent. Involving small penetrating cerebral arteries, lacunes are attributed to cerebral atherosclerosis and lipohyalinosis although absence of pathologic evidence of occluded vessels has raised doubts about this theory (30). Alternatively, it is proposed that BBB leakage might account for vascular changes, edema and neuronal damage seen in lacunar infarcts. BBB breakdown could initiate the process leading to lipohyalinosis, lumen narrowing, blood flow reduction and ischemia. Animal studies indicate that plasma leakage into the vessel wall and surrounding brain parenchyma is an initial step in lacunar strokes (30). Magnetic resonance imaging of humans with lacunar stroke reveals diffuse BBB dysfunction (30).
Sex Differences in Stroke and BBB Integrity
The impact of sex on stroke susceptibility appears age-dependent since AIS and stroke-related deaths are more common in older postmenopausal women (31). Furthermore, while young female mice have smaller stroke volumes and improved functional outcome than their male counterparts following MCAO, this advantage is no longer present after the menopause and appears to be estrogen-dependent (32). Interestingly, endothelial expression of connexin-43, a key gap-junction BBB component that may serve as a conduit by which astrocytes and endothelial cells interact, is regulated by sex hormones (33). It is also observed that declines in circulating estrogen result in loss of BBB integrity in animal models (33). In spite of these intriguing animal studies, current human trials do not support use of estrogen replacement to address this sex-related vulnerability since hormone replacement has been associated with a greater incidence of stroke in post-menopausal women (34).
BBB AND WHITE MATTER DISEASE
White Matter Disease Characteristics
Clinicians are often confronted with the presence of white matter signal abnormalities (WMSA) on brain imaging studies of older adults. These abnormalities appear as hypodense lesions on CT and as increased signal intensity on FLAIR or T2-weighted MRI sequences. They are distributed bilaterally in a patchy or diffuse pattern involving selected periventricular regions (Fig. 4) (12). MRI is sensitive for detecting WMSA (also referred to as leukoaraiosis or white matter hyperintensities), which are seen in over 85% of subjects 75 or older (35). New neuroimaging methodology using diffusion tensor imaging can better localize areas affected by WMSA and identify the specific neural tracts involved. Though WMSA are frequently asymptomatic, several studies have established a relationship with common geriatric syndromes such as cognitive impairment in the form executive dysfunction, gait abnormalities, urinary incontinence and depression (36, 37). It is not merely the total volume of white matter involvement that determines the type and degree of deficits, but also the specific regions affected by WMSA (36). In a study of gait impairment in community dwelling elderly, posterior and frontal WMSA were affected preferentially and likely represent interruption to long tracts involved with motor-sensory integration necessary for complex functions such as gait (36). Follow-up studies revealed that this is a slowly progressive process, with subjects having more extensive WMSA burden and greater declines in gait also demonstrating an accelerated rate of WMSA accrual (36).
Evidence of BBB Disruption in White Matter Disease
Accumulating evidence supports the hypothesis that injury to the cerebral microvasculature in general, and the BBB in particular, plays a role in the pathogenesis of WMSA (12). The symmetry of these lesions is inconsistent with focal cerebral infarction, making a more generalized process involving the cerebral microvasculature more likely. Arterial blood supply to deeper hemispheric white matter is particularly vulnerable since it relies on long penetrating arteries originating from the surface of the brain that travel a considerable distance before reaching the periventricular white matter (13). It has been proposed that this unique vascular anatomy and the paucity of relevant collateral blood flow predispose individuals to the development of microvascular changes which then lead to BBB leakage and the accrual of WMSAs. Vascular risk factors such as hypertension, diabetes mellitus, smoking and hypercholesterolemia all of which have been linked to WMSA (35, 38), have also been shown to result in relevant changes involving BMEC and the BBB in animal models (Fig. 3) (6). Moreover, treatment of hypertension may reduce the risk of developing WMSA (38).
Clinical evidence for BBB dysfunction in WMSA is provided by findings of elevated levels of serum markers of endothelial dysfunction such as intercellular adhesion molecule I (ICAMI) and an increased CSF/serum albumin ratio in individuals with WMSA (39). Contrast-enhanced MRIs in patients with WMSA have detected leakage of contrast agent in affected areas indicating modest increases in BBB permeability (40). While several neuropathologic studies have reported on atherosclerotic changes consisting of vessel wall thickening and narrowed vascular lumen in these individuals, another study of older subjects without vascular risk factors did not reveal histopathologic changes of atherosclerosis in regions effected by WMSA (12). However, the presence of decreased capillary density involving both white matter affected by WMSA and histologically normal white matter in the same individual suggests that such microvascular pathology may precede parenchymal involvement (13). Evidence that alterations in the BBB underlie, in part or in whole, manifestations of WMSA, comes from studies of spontaneously hypertensive rats (SHR). These investigations using contrast MRI have revealed that increased vascular permeability preceded the formation of WMSA (20). In a recent study comparing in vivo MRI scans to brain pathology in elderly subjects, areas of WMSA showed evidence of BBB compromise as indicated by a reduction in the expression of both the endothelial marker CD31 and the efflux transporter P-gp (12). These findings are corroborated by decreased P-gp activity on PET scan in areas of white matter abnormalities (17).
Formation of White Matter Lesions as a Result of BBB Impairment
BBB impairment can cause damage to perivascular white matter through several different mechanisms. Perivascular edema and altered electrolyte concentrations in interstitial fluid can lead to myelin loss, gliosis and neuronal injury. Deficiencies of BBB transport can also result in accumulation of toxins harmful to neuronal and glial cells (12, 17). Infiltration of serum proteins such as fibrinogen and plasmin protease into the brain tissue via a leaky BBB can adversely impact white matter directly or indirectly via enhancement of microglial phagocytosis, the latter possibly yielding considerable “bystander damage”. Alternative explanations of WMSA pathology include leakage of CSF or impaired venous drainage. More than one mechanism may be responsible for WMSA based on the underlying etiology and the involved brain region, as suggested by findings that deeper subcortical white matter lesions exhibit different rates of progression and clinical significance as compared to periventricular white matter lesions (41).
Animal Models of White Matter Disease
Poor consensus regarding WMSA pathogenesis may partly derive from a lack of animal models that faithfully replicate relevant radiographic, vascular and parenchymal changes. The MCAO animal model of acute ischemia is widely used and produces white matter abnormalities within moments of occlusion, yet does not truly reflect WMSAs which result from chronic ischemia (20). Bilateral common carotid occlusion in rats and bilateral common carotid stenosis in gerbils chronically reduce cerebral blood flow, inducing a prolonged hypoperfusion that produces diffuse white matter disease with histopathology characteristic of WMSA (20). Nevertheless, the fact that the rodent brain contains proportionately little white matter (20), limits the translational relevance of this model and highlights the need to develop better animal models for the conduct of pathophysiological and preclinical studies.
In summary, BBB disturbances play a role in development of WMD and provide a unifying theory to explain this condition. BMEC dysfunction explains the diffuse distribution of WMD as well as its progressive course of cumulative neuronal damage from ongoing microvascular compromise. Though BBB impairment could be secondary to existing disease, studies to date indicate that BBB changes likely initiate the process of white matter abnormalities (12, 13, 20). Tools available to measure BBB function in the clinical setting include contrast-enhanced MRI and CSF analysis. Functional neuroimaging such as PET scan with radiolabeled verapamil as well as circulating markers of endothelial dysfunction such as ICAMI, vWF (Von Willebrand factor) and thrombomodulin represent important research tools which require validation in the clinical setting. Based on these techniques it may be possible to detect damage to the brain microvasculature signaling the need for vascular risk factor management, especially blood pressure control, to reduce progression of WMD.
BBB AND DELIRIUM
Delirium is characterized by an acute worsening of global cognitive functioning with decreased attention and altered consciousness that is reversible. Although BBB dysfunction has not been yet demonstrated in typical geriatric patients experiencing delirium, elevated levels of calcium binding protein S100B expressed by astrocytes in patients with delirium may reflect BBB leakiness (42). Moreover, many of the predisposing (e.g. aging, dementia, WMSA) and precipitating (e.g. stroke, sepsis, hypoxemia) factors associated with delirium are also known to contribute to BBB disruption (Fig. 3). Since delirium is predominantly seen in elderly patients with dementia, WMSA (43) and other comorbid conditions with the potential to compromise BBB integrity, it is likely that many such risk factors may be shared (Fig. 3) (44, 45). Efforts to elucidate these factors will break new ground in providing potential insights into the pathophysiology of these multifactorial geriatric syndromes (45).
Mechanisms of BBB Impairment in Delirium
Mechanisms leading to delirium remain poorly understood although intriguing insights have begun to emerge. A recent prospective study evaluated 42 elderly subjects undergoing cardiac surgery (46) with one-third developing delirium. From among different categories of inflammatory mediators measured in the serum, only elevations in the proinflammatory chemokine CCL2 were associated with the development of post-operative delirium. Since parenchymal neural cells are major producers of CCL2 (47), it is possible that elevated peripheral levels of this chemokine may, at least in part, be reflective of brain and/or BBB injury. At the same time, evidence also exists to indicate that elevations in CCL-2 levels, irrespective of source, may play a primary role in both BBB disruption and inflammatory changes within the brain (48). A role for CCL-2 as a potential contributor to delirium is supported by studies indicating that CCL2 disrupts the integrity of BMEC TJ's, while also promoting macrophage migration through the BBB (47). Moreover, evidence that both aging and opioid medications are associated with elevated CCL-2 levels, indicates that some distinct risk factors for delirium may potentially mediate their effects through common shared pathways (45). One candidate pathway is the BBB efflux transporter P-gp, which is impaired with aging, leading to accumulation of medications, inflammatory molecules and toxins within the brain parenchyma that may contribute to delirium (16). Since the efflux of centrally-derived opiate met-enkephalins is decreased in old age, accumulation of endogenous and exogenous opiates within the aged brain could also raise the risk of delirium.
BBB AND POLYPHARMACY
Adverse CNS Effects of Medications in Elderly
Older patients are more likely to experience adverse CNS effects to pharmaceuticals commonly prescribed for psychiatric conditions, sleep problems, pain and urinary incontinence (49). When such events do occur, they tend to be more severe for a given dose (49). Altered BBB permeability may contribute to such vulnerability (7).
Anticholinergic medications prescribed for overactive bladder (OAB) have been associated with adverse effects on cognition, behavior, sleep and gait when taken by healthy older adults (50). It has been proposed that antimuscarinic agents decrease urge symptoms and incontinence through peripheral effects, while centrally-mediated cognitive side effects could result from a more highly permeable BBB (50). Trospium has been promoted as a safer anticholinergic alternative since this quaternary ammonium compound is less lipophilic and thus should be less likely to cross an intact BBB (51). Healthy individuals over age 50 had less REM sleep disturbances following a single dose of trospium than following a single dose of oxybutynin and tolterodine (51). More research in age-related changes in the BBB is needed to determine whether alterations in BBB integrity or other factors contribute to older adults’ enhanced sensitivity to many other medications including benzodiazepines , psychotropics , anesthetics , anti-emetics , or narcotics (49).
Evidence for BBB Involvement in Adverse Drug Reactions
The increased magnitude of cognitive impairment seen in elderly receiving benzodiazepines was not associated with altered plasma concentrations, indicating that altered pharmacokinetics were not primarily responsible for the heightened CNS sensitivity to benzodiazepines in elderly patients (49). Thus, alterations in BBB permeability leading to higher brain concentrations is a plausible explanation for these findings. Additional evidence comes from glycopryrronium, another quaternary ammonium structure anticholinergic agent with reduced penetration of the BBB, which is associated with faster recovery from anesthesia then atropine (52), suggesting that the degree of BBB permeability may be responsible for sedating side-effects. These finding imply that altered transport and permeability of CNS acting drugs by a compromised BBB may result in poor tolerability by aged persons. Interestingly older rats show an apparent increase in BBB permeability following intraperitoneal injection of haloperidol when compared to younger rats, suggesting that the elderly may also be at greater risk of medication-induced BBB injury (53).
POTENTIAL IMPACT AND FUTURE DIRECTIONS OF BBB RESEARCH IN GERIATRIC CARE
Identification of the key contributors to BBB impairment during aging would highlight prospective targets capable of improving outcomes for older patients. Efforts to delineate contributions from different elements of the neurovascular unit (e.g. distinguishing BMEC from astrocytes) while also differentiating components of the microvasculature (e.g. capillaries versus arterioles and venules) in a regionally-specific manner (e.g. gray versus white matter) should allow definition of the effects of aging on the BBB. The role of astrocytes, pericytes and neurons in fostering age-related changes in BMEC has not been investigated and the extent altered performance of these cells underlies diminished BBB capacity during the course of normal aging is unclear.
The temporal pattern of BBB alterations can clarify whether BBB changes are the cause of neurologic disorders or a secondary manifestation of other neurologic insults. The timing of BBB opening could be transient (as may be the case in delirium and AIS) or chronic and insidious (as may occur with WMSA). Chronic changes in the BBB may predispose to common neurodegenerative diseases such as Alzheimer's and Parkinson's disease (3). Longitudinal studies can be aimed at following developments in the BBB during aging and make correlations with clinical finding and modifiable disease risk factors (Fig. 3).
Defining BBB components impaired with aging may facilitate development of diagnostic tools such as neuroimaging and serum markers that reflect BBB function. Advantages of PET and MRI studies are that they can be done noninvasively in vivo and allow localization of the disease process. Techniques such as PET scans measuring P-gp activity and gadolinium MRI may eventually become more routine in clinical practice. Though the serum and CSF markers most indicative of BBB breakdown and endothelial damage are yet to be established, ICAM-1, S100, vWF, laminin, CCL2 and MMP-9 are a few which have been investigated and may be helpful in monitoring the progression of BBB impairment. BBB assessment tools may aid bedside evaluations by providing diagnostic and prognostic data. Accurate measure of BBB function may permit dose adjustments of psychoactive drugs appropriate for the elderly similar to renal dosing of medications according to GFR in order to avoid adverse effects. Markers of BBB breakdown such as CCL2 and c-Fn could be valuable in assessing the risk of delirium in hospitalized patients by indicating the need for preventive measures to reduce complications of aspiration pneumonia and falls thus shortening lengths of hospital stay. In the case of stroke, determinants of BBB function could better define the therapeutic window for revascularization therapies and minimize complications from thrombolytics.
Clarification of factors involved in BBB opening during aging can be exploited to optimize CNS delivery of centrally acting medications such as chemotherapy for brain tumors and neuroprotective agents for stroke (7). Definition of the transport processes affected by aging might be used in designing drugs that take advantage of age-related changes in BBB function. If structural BBB changes predominate, drugs can be packaged in nanoparticles that navigate small openings in tight junctions, while if receptor-mediated transcytosis is up-regulated these processes can be exploited to direct therapeutic agents across the BBB. Knowledge of enzymatic changes at the BBB will guide the design for a prodrug which is converted to its active form by BBB enzymes. Effective delivery of drugs used in stroke as well as other aspects of geriatric care should depend on the changes to the BBB.
Strategies to bolster the BBB have the potential to reduce cerebral edema and hemorrhagic transformation following AIS. Preserving the BBB may limit neurologic injury from WMD, neurodegenerative diseases and delirium. The efficacy of proposed BBB treatments such as statin drugs, angiotensin converting enzyme inhibitors, thiazolidinediones, resveratrol, nutritional supplements and exercise should be studied using comprehensive geriatric assessments and in vivo measures of BBB function. Interventions to improve brain imaging measures of BBB permeability as well as validated measures of physical performance, gait and cognitive function in older adults will be critical.
Extending the study of BBB properties to include sex differences will help elucidate the hormonal influences on BBB properties. Changing levels of sex hormones may underlie the greater degree of stroke-related BBB disruption that occurs with aging and female sex; an important area of research in light of the very high rate of stroke deaths in postmenopausal women (31).
A multidisciplinary approach involving geriatricians, neurologists and vascular biologists offers the best means of shedding light on these aspects of the aging BBB. Collaborations between scientists and clinicians will ultimately determine what changes in the BBB underlie healthy aging and disease.
CONCLUSION
Impairment of the BBB occurs as we age and may account for the increased susceptibility to stroke, WMD, delirium as well as other age-associated neurologic conditions. This age-related susceptibility to neurologic disease may be related to an altered BBB. Alterations in BBB tight junctions, cellular transporters and enzymatic processes have been implicated in age-related impairment of the BBB. Insights into the balance of BBB properties altered in aged subjects and in response to age-related medical conditions are likely to mold the approach to geriatric patients. Translational research in aged subjects will provide targets for more effective diagnostic tools and treatments to limit effects of vascular disease on the aging brain. Cerebrovascular disease in the elderly can be viewed as a spectrum with large vessel disease causing acute, focal neurologic deficits on one end and microvascular disease causing insidious and diffuse cerebral changes on the other. Multidisciplinary collaboration is needed to extend the expanding knowledge of the BBB into improved care for our geriatric population.
ACKNOWLEDGMENTS
This work was supported by grants R01NS061525 (JP), R21NS057241 (JP), R01NS050505 (LM), R01NS055215 (LM) and R01AG022092 (LW, GAK) from the National Institutes of Health, as well as the Travelers Research Institute on Health Promotion and Aging Endowment (NZ) and the Citicorp Chair in Geriatrics and Gerontology (GAK).
Footnotes
Author Contributions: All authors contributed to the manuscript.
Sponsor's role: The sponsors played no role in the preparation of the manuscript.
REFERENCES
- 1.Wolburg H, Noell S, Mack A, et al. Brain endothelial cells and the glio-vascular complex. Cell Tissue Res. 2009;335:75–96. doi: 10.1007/s00441-008-0658-9. [DOI] [PubMed] [Google Scholar]
- 2.Farrall AJ, Wardlaw JM. Blood-brain barrier: Ageing and microvascular disease - systematic review and meta-analysis. Neurobiol Aging. 2009;30:337–352. doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 3.Neuwelt E, Abbott NJ, Abrey L, et al. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008;7:84–96. doi: 10.1016/S1474-4422(07)70326-5. [DOI] [PubMed] [Google Scholar]
- 4.Ge S, Song L, Pachter JS. Where is the blood-brain barrier ... really? J Neurosci Res. 2005;79:421–427. doi: 10.1002/jnr.20313. [DOI] [PubMed] [Google Scholar]
- 5.Persidsky Y, Ramirez SH, Haorah J, et al. Blood-brain barrier: Structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1:223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 6.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 7.Cecchelli R, Berezowski V, Lundquist S, et al. Modelling of the blood-brain barrier in drug discovery and development. Nat Rev Drug Discov. 2007;6:650–661. doi: 10.1038/nrd2368. [DOI] [PubMed] [Google Scholar]
- 8.Macdonald JA, Murugesan N, Pachter JS. Endothelial cell heterogeneity of blood-brain barrier gene expression along the cerebral microvasculature. J Neurosci Res. 2009;88:1457–1474. doi: 10.1002/jnr.22316. [DOI] [PubMed] [Google Scholar]
- 9.Pelegri C, Canudas AM, del Valle J, et al. Increased permeability of blood-brain barrier on the hippocampus of a murine model of senescence. Mech Ageing Dev. 2007;128:522–528. doi: 10.1016/j.mad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Bowman GL, Kaye JA, Moore M, et al. Blood-brain barrier impairment in Alzheimer disease: Stability and functional significance. Neurology. 2007;68:1809–1814. doi: 10.1212/01.wnl.0000262031.18018.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah GN, Mooradian AD. Age-related changes in the blood-brain barrier. Exp Gerontol. 1997;32:501–519. doi: 10.1016/s0531-5565(96)00158-1. [DOI] [PubMed] [Google Scholar]
- 12.Young VG, Halliday GM, Kril JJ. Neuropathologic correlates of white matter hyperintensities. Neurology. 2008;71:804–811. doi: 10.1212/01.wnl.0000319691.50117.54. [DOI] [PubMed] [Google Scholar]
- 13.Brown WR, Moody DM, Thore CR, et al. Vascular dementia in leukoaraiosis may be a consequence of capillary loss not only in the lesions, but in normal-appearing white matter and cortex as well. J Neurol Sci. 2007;257:62–66. doi: 10.1016/j.jns.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mooradian AD. Potential mechanisms of the age-related changes in the blood-brain barrier. Neurobiol Aging. 1994;15:751–755. doi: 10.1016/0197-4580(94)90058-2. discussion 761-752, 767. [DOI] [PubMed] [Google Scholar]
- 15.Choi JY, Morris JC, Hsu CY. Aging and cerebrovascular disease. Neurol Clin. 1998;16:687–711. doi: 10.1016/s0733-8619(05)70089-x. [DOI] [PubMed] [Google Scholar]
- 16.Toornvliet R, van Berckel BN, Luurtsema G, et al. Effect of age on functional P-glycoprotein in the blood-brain barrier measured by use of (R)-[(11)C]verapamil and positron emission tomography. Clin Pharmacol Ther. 2006;79:540–548. doi: 10.1016/j.clpt.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Bartels AL, Kortekaas R, Bart J, et al. Blood-brain barrier P-glycoprotein function decreases in specific brain regions with aging: A possible role in progressive neurodegeneration. Neurobiol Aging. 2008;30:1818–1824. doi: 10.1016/j.neurobiolaging.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Hof PR, Morrison JH. The aging brain: morphomolecular senescence of cortical circuits. Trends Neurosci. 2004;27:607–613. doi: 10.1016/j.tins.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Oztas B, Kaya M, Camurcu S. Age related changes in the effect of electroconvulsive shock on the blood brain barrier permeability in rats. Mech Ageing Dev. 1990;51:149–155. doi: 10.1016/0047-6374(90)90097-y. [DOI] [PubMed] [Google Scholar]
- 20.Hainsworth AH, Markus HS. Do in vivo experimental models reflect human cerebral small vessel disease? A systematic review. J Cereb Blood Flow Metab. 2008;28:1877–1891. doi: 10.1038/jcbfm.2008.91. [DOI] [PubMed] [Google Scholar]
- 21.DiNapoli VA, Huber JD, Houser K, et al. Early disruptions of the blood-brain barrier may contribute to exacerbated neuronal damage and prolonged functional recovery following stroke in aged rats. Neurobiol Aging. 2008;29:753–764. doi: 10.1016/j.neurobiolaging.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strbian D, Durukan A, Pitkonen M, et al. The blood-brain barrier is continuously open for several weeks following transient focal cerebral ischemia. Neuroscience. 2008;153:175–181. doi: 10.1016/j.neuroscience.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Latour LL, Kang DW, Ezzeddine MA, et al. Early blood-brain barrier disruption in human focal brain ischemia. Ann Neurol. 2004;56:468–477. doi: 10.1002/ana.20199. [DOI] [PubMed] [Google Scholar]
- 24.Lorberboym M, Lampl Y, Sadeh M. Correlation of 99mTc-DTPA SPECT of the blood-brain barrier with neurologic outcome after acute stroke. J Nucl Med. 2003;44:1898–1904. [PubMed] [Google Scholar]
- 25.Serena J, Blanco M, Castellanos M, et al. The prediction of malignant cerebral infarction by molecular brain barrier disruption markers. Stroke. 2005;36:1921–1926. doi: 10.1161/01.STR.0000177870.14967.94. [DOI] [PubMed] [Google Scholar]
- 26.Cipolla MJ, Crete R, Vitullo L, et al. Transcellular transport as a mechanism of blood-brain barrier disruption during stroke. Front Biosci. 2004;9:777–785. doi: 10.2741/1282. [DOI] [PubMed] [Google Scholar]
- 27.Kharlamov A, Kharlamov E, Armstrong DM. Age-dependent increase in infarct volume following photochemically induced cerebral infarction: Putative role of astroglia. J Gerontol A Biol Sci Med Sci. 2000;55:B135–141. doi: 10.1093/gerona/55.3.b135. discussion B142-133. [DOI] [PubMed] [Google Scholar]
- 28.McColl BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci. 2008;28:9451–9462. doi: 10.1523/JNEUROSCI.2674-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: Toward a better understanding of physiology and etiology: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 30.Wardlaw JM, Doubal F, Armitage P, et al. Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann Neurol. 2009;65:194–202. doi: 10.1002/ana.21549. [DOI] [PubMed] [Google Scholar]
- 31.Turtzo LC, McCullough LD. Sex Differences in Stroke. Cerebrovasc Dis. 2008;26:462–474. doi: 10.1159/000155983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu F, Yuan R, Benashski SE, et al. Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab. 2009;29:792–802. doi: 10.1038/jcbfm.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson AC, Clemente L, Liu T, et al. Reproductive hormones regulate the selective permeability of the blood-brain barrier. Biochim Biophys Acta. 2008;1782:401–407. doi: 10.1016/j.bbadis.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 35.Lindgren A, Roijer A, Rudling O, et al. Cerebral lesions on magnetic resonance imaging, heart disease, and vascular risk factors in subjects without stroke. A population-based study. Stroke. 1994;25:929–934. doi: 10.1161/01.str.25.5.929. [DOI] [PubMed] [Google Scholar]
- 36.Wolfson L, Wei X, Hall CB, et al. Accrual of MRI white matter abnormalities in elderly with normal and impaired mobility. J Neurol Sci. 2005;232:23–27. doi: 10.1016/j.jns.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 37.Kuchel GA, Moscufo N, Guttmann CR, et al. Localization of brain white matter hyperintensities and urinary incontinence in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2009;64:902–9. doi: 10.1093/gerona/glp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dufouil C, de Kersaint-Gilly A, Besancon V, et al. Longitudinal study of blood pressure and white matter hyperintensities: The EVA MRI Cohort. Neurology. 2001;56:921–926. doi: 10.1212/wnl.56.7.921. [DOI] [PubMed] [Google Scholar]
- 39.Hassan A, Hunt BJ, Sullivan M, et al. Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain. 2003;126:424–432. doi: 10.1093/brain/awg040. [DOI] [PubMed] [Google Scholar]
- 40.Hanyu H, Asano T, Tanaka Y, et al. Increased blood-brain barrier permeability in white matter lesions of Binswanger's disease evaluated by contrast-enhanced MRI. Dement Geriatr Cogn Disord. 2002;14:1–6. doi: 10.1159/000058326. [DOI] [PubMed] [Google Scholar]
- 41.Simpson JE, Fernando MS, Clark L, et al. White matter lesions in an unselected cohort of the elderly: Astrocytic, microglial and oligodendrocyte precursor cell responses. Neuropathol Appl Neurobiol. 2007;33:410–419. doi: 10.1111/j.1365-2990.2007.00828.x. [DOI] [PubMed] [Google Scholar]
- 42.van Munster BC, Korevaar JC, Korse CM, et al. Serum S100B in elderly patients with and without delirium. Int J Geriatr Psychiatry. 2010;25:234–239. doi: 10.1002/gps.2326. [DOI] [PubMed] [Google Scholar]
- 43.Alsop DC, Fearing MA, Johnson K, et al. The role of neuroimaging in elucidating delirium pathophysiology. J Gerontol A Biol Sci Med Sci. 2006;61:1287–1293. doi: 10.1093/gerona/61.12.1287. [DOI] [PubMed] [Google Scholar]
- 44.Tinetti ME, Inouye SK, Gill TM, et al. Shared risk factors for falls, incontinence, and functional dependence. Unifying the approach to geriatric syndromes. JAMA. 1995;273:1348–1353. [PubMed] [Google Scholar]
- 45.Inouye SK, Studenski S, Tinetti ME, et al. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780–791. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rudolph JL, Ramlawi B, Kuchel GA, et al. Chemokines are associated with delirium after cardiac surgery. J Gerontol A Biol Sci Med Sci. 2008;63:184–189. doi: 10.1093/gerona/63.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dzenko KA, Song L, Ge S, et al. CCR2 expression by brain microvascular endothelial cells is critical for macrophage transendothelial migration in response to CCL2. Microvasc Res. 2005;70:53–64. doi: 10.1016/j.mvr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Ge S, Song L, Serwanski DR, et al. Transcellular transport of CCL2 across brain microvascular endothelial cells. J Neurochem. 2008;104:1219–1232. doi: 10.1111/j.1471-4159.2007.05056.x. [DOI] [PubMed] [Google Scholar]
- 49.ElDesoky ES. Pharmacokinetic-pharmacodynamic crisis in the elderly. Am J Ther. 2007;14:488–498. doi: 10.1097/01.mjt.0000183719.84390.4d. [DOI] [PubMed] [Google Scholar]
- 50.Kay GG, Abou-Donia MB, Messer WS, Jr, et al. Antimuscarinic drugs for overactive bladder and their potential effects on cognitive function in older patients. J Am Geriatr Soc. 2005;53:2195–2201. doi: 10.1111/j.1532-5415.2005.00537.x. [DOI] [PubMed] [Google Scholar]
- 51.Diefenbach K, Arold G, Wollny A, et al. Effects on sleep of anticholinergics used for overactive bladder treatment in healthy volunteers aged > or = 50 years. BJU Int. 2005;95:346–349. doi: 10.1111/j.1464-410X.2005.05296.x. [DOI] [PubMed] [Google Scholar]
- 52.Sheref SE. Pattern of CNS recovery following reversal of neuromuscular blockade. Comparison of atropine and glycopyrrolate. Br J Anaesth. 1985;57:188–191. doi: 10.1093/bja/57.2.188. [DOI] [PubMed] [Google Scholar]
- 53.Saija A, Princi P, Imperatore C, et al. Ageing influences haloperidol-induced changes in the permeability of the blood-brain barrier in the rat. J Pharm Pharmacol. 1992;44:450–452. doi: 10.1111/j.2042-7158.1992.tb03644.x. [DOI] [PubMed] [Google Scholar]