Abstract
The peroxisome proliferator‐activated receptors, PPARα, PPARβ/δ and PPARγ, are ligand‐activated transcriptional factors belonging to the nuclear receptors superfamily and they are known to play important roles in glucose and lipid metabolism. Experimental studies in animal models of metabolic diseases have also revealed that activation of PPARs protects against the vascular complications of diabetes, hypertension, atherosclerosis, myocardial infarction and stroke, through exerting their anti‐inflammatory, anti‐atherogenic and antioxidant effects. In clinical trials and post‐market surveillance, agonists of PPARs have been shown to effectively prevent cardiovascular events. However, adverse effects, particularly for PPARγ agonists, are also observed with the use of investigational PPAR agonists and even some approved drugs. Further exploration of underlying mechanisms is needed to develop novel ways of PPAR activation without causing serious side effects. This article reviews the cardiovascular effects of PPARs, with emphasis on the therapeutic potential of PPAR agonists in combating metabolic vascular diseases.
Linked Articles
This article is part of a themed section on Chinese Innovation in Cardiovascular Drug Discovery. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2015.172.issue-23
Abbreviations
- Ang II
angiotensin II
- EC
endothelial cell
- RXR
retinoid X receptor
- TCM
traditional Chinese medicine
- VSMC
vascular smooth muscle cell
Tables of Links
| TARGETS |
|---|
| Nuclear hormone receptors a |
| PPARα, NR1C1 |
| PPARβ/δ, NR1C2 |
| PPARγ, NR1C3 |
| RXR, retinoid X receptor |
| Enzymes b |
| Akt |
| Casein kinase 2 |
| Cdk5, cyclin‐dependent kinase 5 |
| Endothelial NOS |
| Glycogen synthase kinase‐3 |
| GPCR c |
| 5‐HT2B receptor |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,cAlexander et al., 2013a, 2013b, 2013c).
Introduction
The physiological and pathophysiological roles of the nuclear receptors, PPARα, PPARβ/δ and PPARγ, have been much less studied in terms of their effects on the vascular system, compared with their functions in glucose and lipid metabolism. PPARs are expressed in endothelial cells (ECs), vascular smooth muscle cells (VSMCs) and macrophages. Activation of PPARs in the vasculature is mostly anti‐inflammatory and antioxidant. It is therefore protective against endothelial dysfunction, atherosclerosis and vascular remodelling in vivo. The ongoing clinical use or trials of synthetic PPAR agonists support the predicted vascular benefits of PPAR activation although some adverse effects have been reported. This review will focus on how PPARα, PPARβ/δ and PPARγ regulate vascular function through different targets in ECs, VSMCs and macrophages. In particular, we wish to provide an overview of the clinically relevant aspects and controversies of the use of PPAR agonists for the treatment of cardiovascular complications associated with metabolic disorders. This information increases the potential therapeutic value of PPAR agonists against vascular dysfunction and justifies further development of novel PPAR agonists.
Transcriptional regulation and physiological functions of PPARs
PPARs heterodimerize with retinoid X receptors (RXR) and bind to specific DNA regions of target genes (AGGTCAXAGGTCA, with X being a random nucleotide) termed as peroxisome proliferator hormone response elements (Gearing et al., 1993). PPAR‐RXR heterodimers remain bound with co‐repressor protein complex in the absence of ligand but ligand activation triggers conformational changes of PPAR‐RXR which releases the co‐repressor complex and, subsequently, the heterodimer assembles with co‐activators to activate the transcription of target genes (DiRenzo et al., 1997). Furthermore, activated PPARs can interact with other transcription factors, such as AP‐1 and NF‐κB, resulting in transcriptional repression (Delerive et al., 1999). These transcriptional regulation and key functions of PPARs are summarized in Figure 1.
Figure 1.
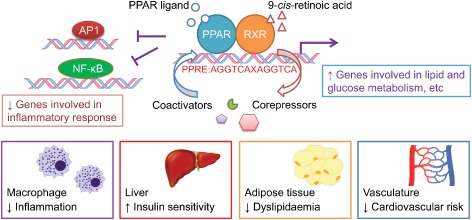
Transcriptional regulation and key functions of PPAR family. Ligand binding to PPAR recruits coactivators in replacement of corepressors to induce transcription and inhibits other transcriptional factors such as AP‐1 and NF‐κB, regulating different functions.
The endogenous ligands for PPARs are fatty acids and eicosanoids. Both PPARα and PPARδ are activated by a wide variety of saturated and unsaturated fatty acids while PPARγ interacts only with polyunsaturated fatty acids (Xu et al., 1999). Differences also exist between eicosanoids as activators of PPARs. For instance, LTB4 and 8(S)‐hydroxyeicosa‐5,8,11,13‐tetraenoic acid (8(S)‐HETE) are PPARα ligands; 9‐ and 13‐hydroxyoctadecadienoic acids (9‐ and 13‐HODEs), and 15‐deoxy‐Δ12,14‐PGJ2 are agonists for PPARγ whereas prostacyclin, PGA1, and PGD2 are agonists for PPARδ (Forman et al., 1997; Kliewer et al., 1997).
PPARs have different levels of expression in different tissues. PPARα is predominantly expressed in cells with high rates of fatty acid catabolism such as liver, heart, kidney and skeletal muscle (Braissant et al., 1996) to regulate fatty acid oxidation systems. PPARγ is mainly associated with adipose tissue to regulate adipocyte differentiation and thus, lipid and energy storage (Rosen and Spiegelman, 2001). PPARδ is ubiquitously expressed at much higher levels than PPARγ and PPARα, markedly in brain, adipose tissue and skin (Kliewer et al., 1992). It is implicated in fatty acid oxidation and energy dissipation, improving lipid profiles and reducing adiposity (Wang et al., 2003).
Cardiovascular effects of PPARα activation and deletion
PPARα activation limits inflammatory responses in ECs and VSMCs (Plutzky, 2011) as well as macrophages (Neve et al., 2001) by inhibiting NF‐κB and AP‐1 and improves lipid profile, thus contributing to the anti‐atherogenic action of PPARα agonists. PPARα inhibits the activities of NF‐κB and AP‐1 through direct interactions with p65 and c‐Jun subunits, respectively (Delerive et al., 1999), or through induction of IκBα which sequesters NF‐κB in the cytoplasm (Delerive et al., 2000). PPARα agonists were developed to treat dyslipidaemia. For example, fibric acid derivatives (fibrates), including clofibrate, ciprofibrate, fenofibrate and gemfibrozil, reduce triglyceride and raise high‐density lipoprotein (HDL) with modest effect to lower low‐density lipoprotein (Remick et al., 2008). Fenofibrate retards the development of atherosclerosis in ApoE−/− and LDLR−/− mice (Duez et al., 2002; Srivastava et al., 2006). However, atherosclerosis develops more slowly in PPARα−/− mice (Tordjman et al., 2007), while another study shows that PPARα deficiency in macrophages increases atherosclerosis (Babaev et al., 2007). Such conflicting data may raise concerns over the relevance of the choice of mouse models used to reveal the roles of molecular targets in the development of atherosclerosis. Using tissue‐specific gene deletion, mice are probably better than global knockout mice as more selective activity can be investigated with less effects on the whole body. Deletion of PPARα further increased the plasma level of triglyceride in ApoE−/− mice which might cause increased atherosclerosis (Babaev et al., 2007). Interestingly, fenofibrate exerts some PPARα‐independent actions in human microvascular ECs, reducing endothelin‐1 expression via inhibition of glycogen synthase kinase‐3 (Glineur et al., 2013).
Clinical perspective of PPARα agonists
Several clinical studies evaluated the vascular effects of fibrates. In these trials, gemfibrozil alone reduces cardiovascular events such as coronary heart disease, myocardial infarction and stroke in type 2 diabetic patients without any statin use (Asztalos et al., 2008). By contrast, the more potent PPARα agonist, fenofibrate, shows no overall benefit in reducing macrovascular events in diabetic patients (Group et al., 2010). On the other hand, fenofibrate prevents microvascular complications such as diabetic retinopathy (Chen et al., 2013). This agonist also ameliorates coronary flow velocity reverse and arterial stiffness in patients with hypertriglyceridaemia (Wang et al., 2013). It appears that fenofibrate may preferentially regulate those cellular targets which control for microvascular dysfunction. In particular, many clinical trials have found that fibrates are more effective in patients with high triglyceride and low‐HDL cholesterol (Fruchart et al., 1998; Tenkanen et al., 2006), both of which are risk factors for cardiovascular diseases, implying a higher effectiveness to protect vascular function in such patients. Patients with high triglyceride and low‐HDL cholesterol have not been the basis of an entire clinical trial, so the vasoprotective effect of fibrates may have been underestimated in the trials so far (Tenkanen et al., 2006; Scott et al., 2009).
Four fibrates are available for clinical application to treat hyperlipidaemia. The US Food and Drug Administration (FDA) approved fenofibrate and gemfibrozil but not ciprofibrate and bezafibrate while the latter two are available elsewhere except in the United States. Treating mice and rats with fibrates increases incidence of hepatocellular carcinogenesis but no significant toxic or carcinogenic effects have been reported in patients (Gonzalez and Shah, 2008). The underlying mechanism attributed to this differential effect among species is currently unclear but the different levels of PPARα expression might play a role. Notably, mouse liver expresses about 10‐fold more PPARα than human liver, implying that PPARα signalling might be much stronger in mice and thus recruit more molecular targets to cause more severe toxicity. Furthermore, the hepatocarcinogenicity of fibrates in rodents may be independent of their binding to PPAR. Fibrates are known to affect NADH cytochrome c reductase activity, disrupting mitochondrial electron respiratory chain (Scatena et al., 2003). Because peroxisomal β‐oxidation is more active in rodents than in humans (Perrone et al., 1998), this process may be a factor in the higher level of hepatocarcinogenicity in rodents. Identifying the mechanisms behind adverse drug effects is inherently difficult but new synthetic agonists should be modified to be more targeted, with minimal side effects. Correct dosing is also a critical factor but it is important to achieve the beneficial effects without severe side effects. More potent and specific PPARα agonists including AVE8134, DRF10945, GW590735 and LY518674 have been developed and currently under investigation in phase II trials.
PPARγ linking to cardiovascular complications in diabetes and obesity
Extensive studies support that PPARγ activation by thiazolidinediones (TZDs), such as troglitazone, rosiglitazone and pioglitazone, confers vascular benefits in diabetic animals. Pioglitazone (Huang et al., 2008) and rosiglitazone (Wong et al., 2011) improve endothelial function in diabetic and obese mice by stimulating NO production and lowering oxidative stress. Existing in vivo data indicate that PPARγ activation by both pharmacological ligands (Chen et al., 2001) and genetic overexpression (Hu et al., 2010) attenuates the occurrence of atherosclerosis in ApoE−/− mice. PPARγ activation in monocytes/macrophages is anti‐inflammatory by reducing inflammatory molecules and by inducing the generation of IL‐1 receptor antagonist (Jiang et al., 1998; Meier et al., 2002). VSMC‐specific PPARγ knockout aggravates angiotensin II (Ang II)‐induced vascular remodelling and impairment of endothelium‐dependent relaxations (Marchesi et al., 2012), atherosclerosis (Chang et al., 2012) and vascular calcification (Woldt et al., 2012) in mice, thus revealing a vasoprotective property of PPARγ agonists.
TZDs suppress the vasoconstrictor effects of endothelin‐1 (Tian et al., 2010), Ang II (Yuen et al., 2011) and 5‐HT2B receptor agonists (Liu et al., 2012), suggesting a promising strategy to delay the development of hypertension and vascular dysfunction. However, contradictory findings on BP regulation are reported in animals with VSMC‐selective PPARγ deletion. Hypertension [systolic BP: ∼130 vs. 120 mmHg (day) and ∼145 vs. 135 mmHg (night) for transgenic vs. control mice] (Halabi et al., 2008) was accompanied by increased RhoA and Rho kinase activity (Pelham et al., 2012). However, in another study, hypotension (systolic: 96 ± 27 vs. 123 ± 14 mmHg and diastolic: 84 ± 15 vs. 102 ± 14 mmHg for knockout vs. control mice in daytime) was described, accompanied with reduced vasoconstriction and increased vasodilatation in aortas (Chang et al., 2009). These contradictory results between nuclear receptor deficiency and agonists might be due to compensation mechanisms and/or the absence of active repression of gene transcription by unliganded nuclear receptors. Apart from these considerations, the discrepancies between findings of genetic deletion and pharmacological activation may be due to the PPAR‐independent activities of PPAR ligands, summarized in Table 1. Pioglitazone restores blood flow recovery in ischaemic muscle of diabetic mice via the Akt‐VEGF pathway (Biscetti et al., 2009). Troglitazone causes dephosphorylation of endothelial NOS at Ser116 to increase endothelial NO (Cho et al., 2004), inhibits ECs proliferation by suppressing casein kinase 2 (Lee et al., 2006b) and triggers apoptosis of VSMCs by activating the Gadd45 and p53 pathway (Okura et al., 2000).
Table 1.
PPAR‐independent effects of clinically used PPAR ligands in the vascular system
| Agonist | Model | Pathway | Effect | Reference |
|---|---|---|---|---|
| PPARα | ||||
| Fenofibrate | Human microvascular ECs (HMEC‐1) | Inhibit glycogen synthase kinase‐3 | Reduce endothelin‐1 expression | Glineur et al., 2013 |
| PPARγ | ||||
| Pioglitazone | Streptozotocin‐diabetic mice with hind limb ischaemia | Stimulate Akt‐VEGF pathway | Improve blood flow recovery in ischaemic muscle | Biscetti et al., 2009 |
| Troglitazone | Bovine aortic endothelial cells | Induce endothelial NOS dephosphorylation at Ser116 | Increase NO production | Cho et al., 2004 |
| HUVECs | Suppress casein kinase 2 activity | Inhibit proliferation of ECs | Lee et al., 2006b | |
| Rat aortic VSMCs | Activate Gadd45 and p53 pathway | Induce apoptosis of VSMCs | Okura et al., 2000 |
Rosiglitazone increases angiogenesis and functional recovery after focal cerebral ischaemia (Chu et al., 2006) and pioglitazone enhances angiogenesis and blood flow recovery in hind limb ischaemia in diabetic mice through activating endothelial NO synthesis (Huang et al., 2008). Besides, PPARγ activation favours angiogenesis through increasing endothelial progenitor cell (EPC) formation (Tousoulis et al., 2008) and inhibiting EPC apoptosis (Zhang et al., 2013). Of interest, EC‐specific PPARγ−/− mice show dyslipidaemia and rosiglitazone therapy is unable to lower serum levels of free fatty acid and triglyceride (Kanda et al., 2009), implying that the PPARγ in ECs plays a significant role in lipid metabolism which may in turn affect vascular functions; and thus endothelial PPARγ could be a therapeutic target.
The promising data on vasoprotective effects of TZDs in animal studies led to several clinical trials in patients. A meta‐analysis confirmed the prevention of diabetes‐associated vascular complications by pioglitazone (Lincoff et al., 2007) and another clinical study also shows that pioglitazone promoted endothelial function in non‐diabetic patients at high cardiovascular risk (Campia et al., 2006). In the PROactive clinical trial, pioglitazone reduced non‐fatal myocardial infarction and stroke, but it did not affect the risk of coronary revascularization while increasing leg revascularization in type 2 diabetic patients (Wilcox et al., 2008). Pioglitazone also improved aortic elasticity and decreased inflammation and disease progression, with minimal safety concerns, in patients with rheumatoid arthritis (Marder et al., 2013). A number of clinical studies now show that TZDs reduce BP independent of their insulin‐sensitizing properties, suggesting a direct vascular benefit (Giles and Sander, 2007). In addition, PPARγ agonists inhibit atherosclerosis in diabetic patients (Nissen et al., 2008).
Deciphering the adverse effects of PPARγ agonists
TZDs were launched in the late 1990s, as an effective means of restoring insulin sensitivity in type 2 diabetes. However, troglitazone was removed from the market in 2000 because of liver failure in diabetic patients (Kohlroser et al., 2000). Rosiglitazone and pioglitazone currently remain marketed but their application is seriously questioned as adverse effects have been reported. Meta‐analyses from randomized trials and Medicare databases show that rosiglitazone was associated with increased risk of stroke, myocardial infarction, heart failure and death (Nissen and Wolski, 2007; Graham et al., 2010). As a result, rosiglitazone has also been withdrawn from Europe by the European Medicines Agency. However, the FDA removed restrictions in November 2013, based on all available data including the re‐adjudicated RECORD trial showing no increase of cardiovascular incidence with rosiglitazone therapy (Mahaffey et al., 2013). Likewise, pioglitazone has been associated with bone fracture, heart failure and bladder cancer (Vallarino et al., 2013). Meanwhile, new selective PPARγ modulators are being synthesized and tested for potential therapeutic efficacy and safety. For example, a PPARγ ligand SR1664 improved insulin sensitivity without causing significant adverse effects on fluid retention or bone formation (Choi et al., 2011). SR1664, unlike classical PPARγ agonists, has a unique mode of binding to PPARγ with high affinity, blocking the Cdk5‐mediated phosphorylation of PPARγ but not acting as a transcriptional agonist. This difference in action might explain the fewer side effects of this drug. It may therefore be possible to develop a new class of antidiabetic drug with fewer side effects by specifically targeting the Cdk‐mediated phosphorylation of PPARγ. Additionally, the development of tissue‐specific agonists may enhance the therapeutic benefits and reduce deleterious effects.
PPARδ in vascular dysfunction
Several PPARδ agonists consistently correct dyslipidaemia and improve insulin and glucose tolerance in animal models of obesity and diabetes (Wang et al., 2003; Lee et al., 2006a). Dyslipidaemia and inflammation in macrophage are common contributors to the development of atherosclerosis. PPARδ agonists favourably modulate lipid metabolism and attenuate inflammation, thus reducing the susceptibility to atherosclerosis in both ApoE−/− and LDLR−/− mice (Barish et al., 2008; Bojic et al., 2014). Conversely, PPARδ−/− bone marrow transplants reduce atherosclerotic lesion area in LDLR−/− mice because of the elevated availability of inflammatory suppressor Bcl‐6 (Lee et al., 2003). This discrepancy is probably related to the different actions of PPARδ activation in different cell types.
PPARδ agonists directly induce endothelium‐dependent relaxation when used at higher concentrations (Jimenez et al., 2010) and can restore endothelial function in animal models of both type 1 (Quintela et al., 2012) and type 2 (Tian et al., 2012) diabetes through increasing NO bioavailability and suppressing generation of NADPH oxidase‐derived superoxide anions. In hypertensive rats, GW0742 prevented the development of endothelial dysfunction and hypertension by enhancing endothelial NOS activity, and expression of antioxidant genes and regulators of G protein‐coupled signalling proteins (RGS) 5, limiting NADPH oxidase activity and reducing expressions of proinflammatory and pro‐atherogenic genes (Zarzuelo et al., 2011; 2013). A recent study also suggested that PPARδ plays a crucial rule for vasoprotective effect of the commonly used antidiabetic drug metformin in obese mice (Cheang et al., 2014).
Clinical translation of PPARδ agonists
Clinical trials on selected PPARδ agonists have assessed both metabolic and vascular outcomes. No severe side effects have been so far reported, except for GW1516 which induced cancer in several organs in rodents (Gupta et al., 2004; Pollock et al., 2010) and its development has been stopped. MBX‐8025, another PPARδ agonist, normalized lipid profiles and C‐reactive protein levels in patients with the metabolic syndrome and has completed a phase II clinical trial (Choi et al., 2012). More long‐term clinical studies are needed to further evaluate the safety profile before any of these PPARδ ligands can be marketed for clinical treatment of metabolic and vascular complications. To conclude, protective effects of PPARδ agonists against metabolic and vascular diseases are strongly indicated by resulst from animal models but, unlike the other isoforms, none of the PPARδ agonists has been approved for clinical use. Furthermore, PPARδ is ubiquitously expressed and any differential mechanism of PPARδ action in different tissues should be explored for developing new PPARδ agonists with improved efficacy and safety. Developing such agonists with high tissue specificity may reduce adverse effects.
Dual PPAR agonism
Because both fibrates and TZDs exerted favourable effects on lipid and glucose metabolism as well as inhibitory effects on vascular inflammation, interest has grown to develop and explore the clinical outcomes of dual PPARα/γ agonists. Indeed, dual PPARα/γ agonists such as ragaglitazar (Chakrabarti et al., 2003) and muraglitazar (Mittra et al., 2007) provide better glycaemic control than conventional TZDs. Unfortunately, the development of diverse dual PPARα/γ agonists has not met with the anticipated success. Their development was halted in late‐phase trials because of reported side effects such as increased cardiovascular risk (muraglitazar), carcinogenicity (ragaglitazar and MK‐767), liver toxicity (imiglitazar) and renal injury (tesaglitazar) (Fievet et al., 2006). The effect of dual PPARα/γ agonists on atherogenesis is now also being debated. For instance, GW2331 (Claudel et al., 2001), LY465608 (Zuckerman et al., 2002) and tesaglitazar (Zadelaar et al., 2006) attenuated atherosclerosis in ApoE−/− mice however, treatment with compound 3q ((S)‐3‐(4‐(2‐carbazol(phenoxazin)‐9‐yl‐ethoxy)phenyl)‐2‐ethoxy‐propionic acid) increases atherosclerotic plaque formation associated with an increased oxidative stress in these mice (Calkin et al., 2007). The reasons for these disparate findings are unclear, possibly related to the different potency of these drugs to activate PPARα and PPARγ together with differential transcriptional outcomes. For instance, muraglitazar and tesaglitazar preferentially act on PPARγ, possibly accounting for some of the adverse effects. Therefore, a balanced PPARα/γ agonist, aleglitazar, was developed, but disappointingly again, its development is terminated at phase III AleCardio trial because of safety concerns with increased risk for bone fractures, heart failure and gastrointestinal bleeding (Lincoff et al., 2013). This outcome has strongly discouraged the further development of safer dual PPARα/γ ligands. Dual PPARα/γ agonists primarily acting on PPARα may provide another option in the reduction of side effects. On the other hand, dual PPARα/δ agonists are less investigated with GFT505 being shown to favourably modulate lipid and glucose metabolism in patients with metabolic syndrome (Cariou et al., 2011). However, it has only completed a phase II clinical trial, so its safety profile remains to be evaluated.
Pan‐PPAR activator
Bezafibrate is a pan‐PPAR activator activating all three PPAR subtypes. It has been approved by the FDA for the treatment of hyperlipidaemia, despite the reports, in some countries, of renal and hepatobiliary disorders. With beneficial effects on glucose and insulin tolerance, bezafibrate decreased the incidence of and delayed the onset of type 2 diabetes in patients with impaired fasting glucose concentrations (Tenenbaum et al., 2004) and in obese patients (Tenenbaum et al., 2005a). Moreover, bezafibrate decreased the risk of myocardial infarction and cardiac mortality in patients with metabolic syndrome and with a history of recent myocardial infarction and/or stable angina (Tenenbaum et al., 2005b). The benefits of bezafibrate appear to justify the development of newer pharmacological agents targeting more than one PPAR isoform. Consequently, several pan‐PPAR agonists were synthesized. However, the majority of them (DRL 11605, GW‐625019, indeglitazar, netoglitazone, sipoglitazar and sodelglitazar) have been terminated because of serious safety concerns.
Traditional Chinese compounds acting as PPAR ligands
Traditional Chinese medicine (TCM) compounds have long been used against metabolic disease and the related cardiovascular complications. TCM is an attractive resource in the design of new PPAR agonists to reduce cardiovascular risks. Screening the TCM database containing more than 30 000 candidates, two TCM compounds, (S)‐tryptophan‐betaxanthin and berberrubine, have been identified as potential lead compounds targeting more than one PPAR (Chen et al., 2012). By in silico identification, two other TCM candidates, 5‐hydroxy‐L‐tryptophan and abrine, were found to bind to PPARγ (Chen and Chen, 2014). In addition, honokiol from TCM Magnolia bark was also in silico predicted to bind to the ligand‐binding domain of PPARγ and prevent ed hyperglycaemia in diabetic mice (Atanasov et al., 2013). Formononetin, the predominant component of San‐ao decoction, which is one of the most popular TCM formulae for asthma, was demonstrated to activate PPARγ (Zhou et al., 2009). Through their action on PPARs, such TCM compounds may prevent inflammatory diseases, hyperglycaemia and cardiovascular diseases, apart from their originally known therapeutic functions. They warrant further investigation as novel therapeutic agents. More importantly, TCM is deployed as many different combinations of compounds to treat human diseases and the investigation of the active components together with mechanisms of action is crucial for drug development. The efficacy and safety of identified compounds can be ensured and improved using modern analytical and chemical techniques.
Conclusion
The results of many animal studies taken together have demonstrated that activation of PPARα, PPARγ and PPARδ with their corresponding agonists produced clear vasoprotective effects, including inhibition of vascular inflammation, oxidative stress and atherogenic development. These findings stimulated intense interest in using PPAR agonists as another therapeutic approach to vascular dysfunction. Disappointingly, a number of drugs targeting PPARs have failed clinical trials because of safety concerns. Only drugs that activate PPARα (ciprofibrate, fenofibrate and gemfibrozil) and PPARγ (pioglitazone and rosiglitazone) and one pan‐PPAR agonist (bezafibrate) have reached the market, so far. PPARα agonists and bezafibrate are available for the treatment of hyperlipidaemia associated with metabolic syndrome while PPARγ agonists are used to correct hyperglycaemia in type 2 diabetic patients. Clinical studies reveal that several PPAR agonists have not achieved the expected levels of cardiovascular benefits or that the benefits are significantly offset by unwanted outcomes such as malignancy, liver and renal toxicity and increased cardiovascular risk. The cardiovascular benefits and side effects are summarized in Figure 2. Despite these disadvantages, PPARs are important players in the control of lipid and glucose homeostasis. Therefore, new PPAR ligands are being developed, in the hope that these new drugs will produce fewer side effects. With deeper understanding of the regulatory mechanisms of PPAR activation, for example, co‐repressor/co‐activators of PPARs, epigenetic modifiers and intermediate metabolites that may activate certain enzymes or induce post‐translational modification, new insights could be useful in developing novel or tissue‐targeted PPAR agonists with more tolerable side effects for better prevention and management of cardiovascular events associated with metabolic diseases.
Figure 2.
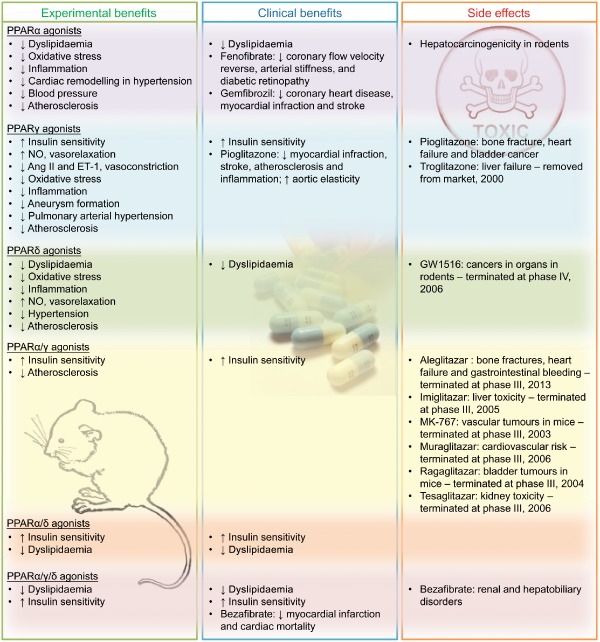
Cardiovascular effects of PPAR agonists in animal and clinical studies. The list shows the cardiovascular benefits shown in rodents (experimental benefits) and in patients with metabolic syndrome (clinical benefits) and the adverse effects of agonists for PPARs.
Conflict of interest
None declared.
Acknowledgements
This study is supported by Hong Kong Research Grants Council (CUHK2/CRF/12G and T12/402/13N) and Natural Science Foundation of China (91339117 and 2012CB517805).
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M et al (2013a). The Concise Guide to PHARMACOLOGY 2013/14: Nuclear Hormone Receptors. Br J Pharmacol 170: 1652–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M et al (2013b). The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol 170: 1797–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M et al (2013c). The Concise Guide to PHARMACOLOGY 2013/14: G Protein‐Coupled Receptors. Br J Pharmacol 170: 1459–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asztalos BF, Collins D, Horvath KV, Bloomfield HE, Robins SJ, Schaefer EJ (2008). Relation of gemfibrozil treatment and high‐density lipoprotein subpopulation profile with cardiovascular events in the Veterans Affairs High‐Density Lipoprotein Intervention Trial. Metabolism 57: 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanasov AG, Wang JN, Gu SP, Bu J, Kramer MP, Baumgartner L et al (2013). Honokiol: a non‐adipogenic PPARγ agonist from nature. Biochim Biophys Acta 1830: 4813–4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaev VR, Ishiguro H, Ding L, Yancey PG, Dove DE, Kovacs WJ et al (2007). Macrophage expression of peroxisome proliferator‐activated receptor‐alpha reduces atherosclerosis in low‐density lipoprotein receptor‐deficient mice. Circulation 116: 1404–1412. [DOI] [PubMed] [Google Scholar]
- Barish GD, Atkins AR, Downes M, Olson P, Chong LW, Nelson M et al (2008). PPARdelta regulates multiple proinflammatory pathways to suppress atherosclerosis. Proc Natl Acad Sci U S A 105: 4271–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscetti F, Straface G, Arena V, Stigliano E, Pecorini G, Rizzo P et al (2009). Pioglitazone enhances collateral blood flow in ischemic hindlimb of diabetic mice through an Akt‐dependent VEGF‐mediated mechanism, regardless of PPARgamma stimulation. Cardiovasc Diabetol 8: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojic LA, Burke AC, Chhoker SS, Telford DE, Sutherland BG, Edwards JY et al (2014). Peroxisome proliferator‐activated receptor delta agonist GW1516 attenuates diet‐induced aortic inflammation, insulin resistance, and atherosclerosis in low‐density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol 34: 52–60. [DOI] [PubMed] [Google Scholar]
- Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W (1996). Differential expression of peroxisome proliferator‐activated receptors (PPARs): tissue distribution of PPAR‐alpha, ‐beta, and ‐gamma in the adult rat. Endocrinology 137: 354–366. [DOI] [PubMed] [Google Scholar]
- Calkin AC, Allen TJ, Lassila M, Tikellis C, Jandeleit‐Dahm KA, Thomas MC (2007). Increased atherosclerosis following treatment with a dual PPAR agonist in the ApoE knockout mouse. Atherosclerosis 195: 17–22. [DOI] [PubMed] [Google Scholar]
- Campia U, Matuskey LA, Panza JA (2006). Peroxisome proliferator‐activated receptor‐gamma activation with pioglitazone improves endothelium‐dependent dilation in nondiabetic patients with major cardiovascular risk factors. Circulation 113: 867–875. [DOI] [PubMed] [Google Scholar]
- Cariou B, Zaïr Y, Staels B, Bruckert E (2011). Effects of the new dual PPAR alpha/delta agonist GFT505 on lipid and glucose homeostasis in abdominally obese patients with combined dyslipidemia or impaired glucose metabolism. Diabetes Care 34: 2008–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti R, Vikramadithyan RK, Misra P, Hiriyan J, Raichur S, Damarla RK et al (2003). Ragaglitazar: a novel PPAR alpha PPAR gamma agonist with potent lipid‐lowering and insulin‐sensitizing efficacy in animal models. Br J Pharmacol 140: 527–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Villacorta L, Zhang J, Garcia‐Barrio MT, Yang K, Hamblin M et al (2009). Vascular smooth muscle cell‐selective peroxisome proliferator‐activated receptor‐deletion leads to hypotension. Circulation 119: 2161–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C et al (2012). Loss of perivascular adipose tissue on peroxisome proliferator‐activated receptor‐γ deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation 126: 1067–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheang WS, Tian XY, Wong WT, Lau CW, Lee SS, Chen ZY et al (2014). Metformin protects endothelial function in diet‐induced obese mice by inhibition of endoplasmic reticulum stress through 5′ adenosine monophosphate‐activated protein kinase‐peroxisome proliferator‐activated receptor δ pathway. Arterioscler Thromb Vasc Biol 34: 830–836. [DOI] [PubMed] [Google Scholar]
- Chen KC, Chen CY (2014). In silico identification of potent PPAR‐γ agonists from traditional Chinese medicine: a bioactivity prediction, virtual screening, and molecular dynamics study. Evid Based Complement Alternat Med 2014: 192452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KC, Chang SS, Huang HJ, Lin TL, Wu YJ, Chen CY (2012). Three‐in‐one agonists for PPAR‐α, PPAR‐γ, and PPAR‐δ from traditional Chinese medicine. J Biomol Struct Dyn 30: 662–683. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hu Y, Lin M, Jenkins AJ, Keech AC, Mott R et al (2013). Therapeutic effects of PPARα agonists on diabetic retinopathy in type 1 diabetes models. Diabetes 62: 261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Ishibashi S, Perrey S, Osuga J, Gotoda T, Kitamine T et al (2001). Troglitazone inhibits atherosclerosis in apolipoprotein E‐knockout mice: pleiotropic effects on CD36 expression and HDL. Arterioscler Thromb Vasc Biol 21: 372–377. [DOI] [PubMed] [Google Scholar]
- Cho DH, Choi YJ, Jo SA, Jo I (2004). Nitric oxide production and regulation of endothelial nitric‐oxide synthase phosphorylation by prolonged treatment with troglitazone: evidence for involvement of peroxisome proliferator‐activated receptor (PPAR) gamma‐dependent and PPARgamma‐independent signaling pathways. J Biol Chem 279: 2499–2506. [DOI] [PubMed] [Google Scholar]
- Choi JH, Banks AS, Kamenecka TM, Busby SA, Chalmers MJ, Kumar N et al (2011). Antidiabetic actions of a non‐agonist PPARγ ligand blocking Cdk5‐mediated phosphorylation. Nature 477: 477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YJ, Roberts BK, Wang X, Geaney JC, Naim S, Wojnoonski K et al (2012). Effects of the PPAR‐δ agonist MBX‐8025 on atherogenic dyslipidemia. Atherosclerosis 220: 470–476. [DOI] [PubMed] [Google Scholar]
- Chu K, Lee ST, Koo JS, Jung KH, Kim EH, Sinn DI et al (2006). Peroxisome proliferator‐activated receptor‐gamma‐agonist, rosiglitazone, promotes angiogenesis after focal cerebral ischemia. Brain Res 1093: 208–218. [DOI] [PubMed] [Google Scholar]
- Claudel T, Leibowitz MD, Fievet C, Tailleux A, Wagner B, Repa JJ et al (2001). Reduction of atherosclerosis in apolipoprotein E knockout mice by activation of the retinoid X receptor. Proc Natl Acad Sci U S A 98: 2610–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delerive P, De Bosscher K, Besnard S, Vanden Berghe W, Peters JM, Gonzalez FJ et al (1999). Peroxisome proliferator‐activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross‐talk with transcription factors NF‐kappaB and AP‐1. J Biol Chem 274: 32048–32054. [DOI] [PubMed] [Google Scholar]
- Delerive P, Gervois P, Fruchart JC, Staels B (2000). Induction of IkappaBalpha expression as a mechanism contributing to the anti‐inflammatory activities of peroxisome proliferator‐activated receptor‐alpha activators. J Biol Chem 275: 36703–36707. [DOI] [PubMed] [Google Scholar]
- DiRenzo J, Soderstrom M, Kurokawa R, Ogliastro MH, Ricote M, Ingrey S et al (1997). Peroxisome proliferator‐activated receptors and retinoic acid receptors differentially control the interactions of retinoid X receptor heterodimers with ligands, coactivators, and corepressors. Mol Cell Biol 17: 2166–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duez H, Chao YS, Hernandez M, Torpier G, Poulain P, Mundt S et al (2002). Reduction of atherosclerosis by the peroxisome proliferator‐activated receptor alpha agonist fenofibrate in mice. J Biol Chem 277: 48051–48057. [DOI] [PubMed] [Google Scholar]
- Fievet C, Fruchart JC, Staels B (2006). PPARalpha and PPARgamma dual agonists for the treatment of type 2 diabetes and the metabolic syndrome. Curr Opin Pharmacol 6: 606–614. [DOI] [PubMed] [Google Scholar]
- Forman BM, Chen J, Evans RM (1997). Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator‐activated receptors alpha and delta. Proc Natl Acad Sci U S A 94: 4312–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruchart JC, Brewer B, Leitersdorf E, Brochier M, Brown G, Calvo C et al (1998). Consensus for the use of fibrates in the treatment of dyslipoproteinemia and coronary heart disease. Am J Cardiol 81: 912–917. [DOI] [PubMed] [Google Scholar]
- Gearing KL, Gottlicher M, Teboul M, Widmark E, Gustafsson JA (1993). Interaction of the peroxisome‐proliferator‐activated receptor and retinoid X receptor. Proc Natl Acad Sci U S A 90: 1440–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles TD, Sander GE (2007). Effects of thiazolidinediones on blood pressure. Curr Hypertens Rep 9: 332–337. [DOI] [PubMed] [Google Scholar]
- Glineur C, Gross B, Neve B, Rommens C, Chew GT, Martin‐Nizard F et al (2013). Fenofibrate inhibits endothelin‐1 expression by peroxisome proliferator‐activated receptor alpha‐dependent and independent mechanisms in human endothelial cells. Arterioscler Thromb Vasc Biol 33: 621–628. [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ, Shah YM (2008). PPARalpha: mechanism of species differences and hepatocarcinogenesis of peroxisome proliferators. Toxicology 246: 2–8. [DOI] [PubMed] [Google Scholar]
- Graham DJ, Ouellet‐Hellstrom R, MaCurdy TE, Ali F, Sholley C, Worrall C et al (2010). Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA 304: 411–418. [DOI] [PubMed] [Google Scholar]
- Group AS, Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA et al (2010). Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 362: 1563–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RA, Wang D, Katkuri S, Wang H, Dey SK, DuBois RN (2004). Activation of nuclear hormone receptor peroxisome proliferator‐activated receptor‐delta accelerates intestinal adenoma growth. Nat Med 10: 245–247. [DOI] [PubMed] [Google Scholar]
- Halabi CM, Beyer AM, De Lange WJ, Keen HL, Baumbach GL, Faraci FM et al (2008). Interference with PPAR gamma function in smooth muscle causes vascular dysfunction and hypertension. Cell Metab 7: 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Zhang XJ, Liu CX, Wang XP, Zhang Y (2010). PPARgamma1‐induced caveolin‐1 enhances cholesterol efflux and attenuates atherosclerosis in apolipoprotein E‐deficient mice. J Vasc Res 47: 69–79. [DOI] [PubMed] [Google Scholar]
- Huang PH, Sata M, Nishimatsu H, Sumi M, Hirata Y, Nagai R (2008). Pioglitazone ameliorates endothelial dysfunction and restores ischemia‐induced angiogenesis in diabetic mice. Biomed Pharmacother 62: 46–52. [DOI] [PubMed] [Google Scholar]
- Jiang C, Ting AT, Seed B (1998). PPAR‐gamma agonists inhibit production of monocyte inflammatory cytokines. Nature 391: 82–86. [DOI] [PubMed] [Google Scholar]
- Jimenez R, Sanchez M, Zarzuelo MJ, Romero M, Quintela AM, Lopez‐Sepulveda R et al (2010). Endothelium‐dependent vasodilator effects of peroxisome proliferator‐activated receptor beta agonists via the phosphatidyl‐inositol‐3 kinase‐Akt pathway. J Pharmacol Exp Ther 332: 554–561. [DOI] [PubMed] [Google Scholar]
- Kanda T, Brown JD, Orasanu G, Vogel S, Gonzalez FJ, Sartoretto J et al (2009). PPARgamma in the endothelium regulates metabolic responses to high‐fat diet in mice. J Clin Invest 119: 110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Umesono K, Mangelsdorf DJ, Evans RM (1992). Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature 355: 446–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS et al (1997). Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator‐activated receptors alpha and gamma. Proc Natl Acad Sci U S A 94: 4318–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlroser J, Mathai J, Reichheld J, Banner BF, Bonkovsky HL (2000). Hepatotoxicity due to troglitazone: report of two cases and review of adverse events reported to the United States Food and Drug Administration. Am J Gastroenterol 95: 272–276. [DOI] [PubMed] [Google Scholar]
- Lee CH, Chawla A, Urbiztondo N, Liao D, Boisvert WA, Evans RM (2003). Transcriptional repression of atherogenic inflammation: modulation by PPAR delta. Science 302: 453–457. [DOI] [PubMed] [Google Scholar]
- Lee CH, Olson P, Hevener A, Mehl I, Chong LW, Olefsky JM et al (2006a). PPARdelta regulates glucose metabolism and insulin sensitivity. Proc Natl Acad Sci U S A 103: 3444–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Park JH, Lee S, Lim HJ, Jang Y, Park HY (2006b). Troglitazone inhibits endothelial cell proliferation through suppression of casein kinase 2 activity. Biochem Biophys Res Commun 346: 83–88. [DOI] [PubMed] [Google Scholar]
- Lincoff AM, Wolski K, Nicholls SJ, Nissen SE (2007). Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta‐analysis of randomized trials. JAMA 298: 1180–1188. [DOI] [PubMed] [Google Scholar]
- Lincoff AM, Tardif JC, Neal B, Nicholls SJ, Ryden L, Schwartz GG et al (2013). Evaluation of the dual peroxisome proliferator‐activated receptor α/γ agonist aleglitazar to reduce cardiovascular events in patients with acute coronary syndrome and type 2 diabetes mellitus: rationale and design of the AleCardio trial. Am Heart J 166: 429–434. [DOI] [PubMed] [Google Scholar]
- Liu Y, Tian XY, Mao G, Fang X, Fung ML, Shyy JY et al (2012). Peroxisome proliferator‐activated receptor‐γ ameliorates pulmonary arterial hypertension by inhibiting 5‐hydroxytryptamine 2B receptor. Hypertension 60: 1471–1478. [DOI] [PubMed] [Google Scholar]
- Mahaffey KW, Hafley G, Dickerson S, Burns S, Tourt‐Uhlig S, White J et al (2013). Results of a reevaluation of cardiovascular outcomes in the RECORD trial. Am Heart J 166: 240–249.e1. [DOI] [PubMed] [Google Scholar]
- Marchesi C, Rehman A, Rautureau Y, Kasal DA, Briet M, Leibowitz A et al (2012). Protective role of vascular smooth muscle cell PPAR in angiotensin II‐induced vascular disease. Cardiovasc Res 97: 562–570. [DOI] [PubMed] [Google Scholar]
- Marder W, Khalatbari S, Myles JD, Hench R, Lustig S, Yalavarthi S et al (2013). The peroxisome proliferator activated receptor‐γ pioglitazone improves vascular function and decreases disease activity in patients with rheumatoid arthritis. J Am Heart Assoc 2: e000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier CA, Chicheportiche R, Juge‐Aubry CE, Dreyer MG, Dayer JM (2002). Regulation of the interleukin‐1 receptor antagonist in THP‐1 cells by ligands of the peroxisome proliferator‐activated receptor gamma. Cytokine 18: 320–328. [DOI] [PubMed] [Google Scholar]
- Mittra S, Sangle G, Tandon R, Sharma S, Roy S, Khanna V et al (2007). Increase in weight induced by muraglitazar, a dual PPARalpha/gamma agonist, in db/db mice: adipogenesis/or oedema? Br J Pharmacol 150: 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve BP, Corseaux D, Chinetti G, Zawadzki C, Fruchart JC, Duriez P et al (2001). PPARalpha agonists inhibit tissue factor expression in human monocytes and macrophages. Circulation 103: 207–212. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Wolski K (2007). Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356: 2457–2471. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Nicholls SJ, Wolski K, Nesto R, Kupfer S, Perez A et al (2008). Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA 299: 1561–1573. [DOI] [PubMed] [Google Scholar]
- Okura T, Nakamura M, Takata Y, Watanabe S, Kitami Y, Hiwada K (2000). Troglitazone induces apoptosis via the p53 and Gadd45 pathway in vascular smooth muscle cells. Eur J Pharmacol 407: 227–235. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP et al; NC‐IUPHAR (2014). The IUPHAR/BPS Guide to PHARMACOLOGY: an expert‐driven knowledge base of drug targets and their ligands. Nucl. Acids Res. 42 (Database Issue): D1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham CJ, Ketsawatsomkron P, Groh S, Grobe JL, de Lange WJ, Ibeawuchi SR et al (2012). Cullin‐3 regulates vascular smooth muscle function and arterial blood pressure via PPARγ and RhoA/Rho‐kinase. Cell Metab 16: 462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone CE, Shao L, Williams GM (1998). Effect of rodent hepatocarcinogenic peroxisome proliferators on fatty acyl‐CoA oxidase, DNA synthesis, and apoptosis in cultured human and rat hepatocytes. Toxicol Appl Pharmacol 150: 277–286. [DOI] [PubMed] [Google Scholar]
- Plutzky J (2011). The PPAR‐RXR transcriptional complex in the vasculature: energy in the balance. Circ Res 108: 1002–1016. [DOI] [PubMed] [Google Scholar]
- Pollock CB, Rodriguez O, Martin PL, Albanese C, Li X, Kopelovich L et al (2010). Induction of metastatic gastric cancer by peroxisome proliferator‐activated receptorδ activation. PPAR Res 2010: 571783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintela AM, Jimenez R, Gomez‐Guzman M, Zarzuelo MJ, Galindo P, Sanchez M et al (2012). Activation of peroxisome proliferator‐activated receptor‐β/‐δ (PPARβ/δ) prevents endothelial dysfunction in type 1 diabetic rats. Free Radic Biol Med 53: 730–741. [DOI] [PubMed] [Google Scholar]
- Remick J, Weintraub H, Setton R, Offenbacher J, Fisher E, Schwartzbard A (2008). Fibrate therapy: an update. Cardiol Rev 16: 129–141. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM (2001). PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem 276: 37731–37734. [DOI] [PubMed] [Google Scholar]
- Scatena R, Bottoni P, Vincenzoni F, Messana I, Martorana GE, Nocca G et al (2003). Bezafibrate induces a mitochondrial derangement in human cell lines: a PPAR‐independent mechanism for a peroxisome proliferator. Chem Res Toxicol 16: 1440–1447. [DOI] [PubMed] [Google Scholar]
- Scott R, O'Brien R, Fulcher G, Pardy C, d'Emden M, Tse D et al (2009). Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care 32: 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava RA, Jahagirdar R, Azhar S, Sharma S, Bisgaier CL (2006). Peroxisome proliferator‐activated receptor‐alpha selective ligand reduces adiposity, improves insulin sensitivity and inhibits atherosclerosis in LDL receptor‐deficient mice. Mol Cell Biochem 285: 35–50. [DOI] [PubMed] [Google Scholar]
- Tenenbaum A, Motro M, Fisman EZ, Schwammenthal E, Adler Y, Goldenberg I et al (2004). Peroxisome proliferator‐activated receptor ligand bezafibrate for prevention of type 2 diabetes mellitus in patients with coronary artery disease. Circulation 109: 2197–2202. [DOI] [PubMed] [Google Scholar]
- Tenenbaum A, Motro M, Fisman EZ, Adler Y, Shemesh J, Tanne D et al (2005a). Effect of bezafibrate on incidence of type 2 diabetes mellitus in obese patients. Eur Heart J 26: 2032–2038. [DOI] [PubMed] [Google Scholar]
- Tenenbaum A, Motro M, Fisman EZ, Tanne D, Boyko V, Behar S (2005b). Bezafibrate for the secondary prevention of myocardial infarction in patients with metabolic syndrome. Arch Intern Med 165: 1154–1160. [DOI] [PubMed] [Google Scholar]
- Tenkanen L, Manttari M, Kovanen PT, Virkkunen H, Manninen V (2006). Gemfibrozil in the treatment of dyslipidemia: an 18‐year mortality follow‐up of the Helsinki Heart Study. Arch Intern Med 166: 743–748. [DOI] [PubMed] [Google Scholar]
- Tian J, Wong WT, Tian XY, Zhang P, Huang Y, Wang N (2010). Rosiglitazone attenuates endothelin‐1‐induced vasoconstriction by upregulating endothelial expression of endothelin B receptor. Hypertension 56: 129–135. [DOI] [PubMed] [Google Scholar]
- Tian XY, Wong WT, Wang NP, Lu Y, Cheang WS, Liu J et al (2012). PPARδ activation protects endothelial function in diabetic mice. Diabetes 61: 3285–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordjman KM, Semenkovich CF, Coleman T, Yudovich R, Bak S, Osher E et al (2007). Absence of peroxisome proliferator‐activated receptor‐alpha abolishes hypertension and attenuates atherosclerosis in the Tsukuba hypertensive mouse. Hypertension 50: 945–951. [DOI] [PubMed] [Google Scholar]
- Tousoulis D, Andreou I, Antoniades C, Tentolouris C, Stefanadis C (2008). Role of inflammation and oxidative stress in endothelial progenitor cell function and mobilization: therapeutic implications for cardiovascular diseases. Atherosclerosis 201: 236–247. [DOI] [PubMed] [Google Scholar]
- Vallarino C, Perez A, Fusco G, Liang H, Bron M, Manne S et al (2013). Comparing pioglitazone to insulin with respect to cancer, cardiovascular and bone fracture endpoints, using propensity score weights. Clin Drug Investig 33: 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, He L, Liu J, Yu J, Feng X, Li F et al (2013). Coronary flow velocity reserve is improved by PPAR‐α agonist fenofibrate in patients with hypertriglyceridemia. Cardiovasc Ther 31: 161–167. [DOI] [PubMed] [Google Scholar]
- Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H et al (2003). Peroxisome‐proliferator‐activated receptor delta activates fat metabolism to prevent obesity. Cell 113: 159–170. [DOI] [PubMed] [Google Scholar]
- Wilcox R, Kupfer S, Erdmann E (2008). Effects of pioglitazone on major adverse cardiovascular events in high‐risk patients with type 2 diabetes: results from PROspective pioglitAzone Clinical Trial In macro Vascular Events (PROactive 10). Am Heart J 155: 712–717. [DOI] [PubMed] [Google Scholar]
- Woldt E, Terrand J, Mlih M, Matz RL, Bruban V, Coudane F et al (2012). The nuclear hormone receptor PPARγ counteracts vascular calcification by inhibiting Wnt5a signalling in vascular smooth muscle cells. Nat Commun 3: 1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WT, Tian XY, Xu AM, Yu J, Lau CW, Hoo RLC et al (2011). Adiponectin is required for PPAR gamma‐mediated improvement of endothelial function in diabetic mice. Cell Metab 14: 104–115. [DOI] [PubMed] [Google Scholar]
- Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ et al (1999). Molecular recognition of fatty acids by peroxisome proliferator‐activated receptors. Mol Cell 3: 397–403. [DOI] [PubMed] [Google Scholar]
- Yuen CY, Wong WT, Tian XY, Wong SL, Lau CW, Yu J et al (2011). Telmisartan inhibits vasoconstriction via PPARγ‐dependent expression and activation of endothelial nitric oxide synthase. Cardiovasc Res 90: 122–129. [DOI] [PubMed] [Google Scholar]
- Zadelaar AS, Boesten LS, Jukema JW, van Vlijmen BJ, Kooistra T, Emeis JJ et al (2006). Dual PPARalpha/gamma agonist tesaglitazar reduces atherosclerosis in insulin‐resistant and hypercholesterolemic ApoE*3Leiden mice. Arterioscler Thromb Vasc Biol 26: 2560–2566. [DOI] [PubMed] [Google Scholar]
- Zarzuelo MJ, Jimenez R, Galindo P, Sanchez M, Nieto A, Romero M et al (2011). Antihypertensive effects of peroxisome proliferator‐activated receptor‐β activation in spontaneously hypertensive rats. Hypertension 58: 733–743. [DOI] [PubMed] [Google Scholar]
- Zarzuelo MJ, Gomez‐Guzman M, Jimenez R, Quintela AM, Romero M, Sanchez M et al (2013). Effects of peroxisome proliferator‐activated receptor‐β activation in endothelin‐dependent hypertension. Cardiovasc Res 99: 622–631. [DOI] [PubMed] [Google Scholar]
- Zhang HF, Wang L, Yuan HJ, Ma YH, Wang YF, Hu ZY et al (2013). PPAR‐γ agonist pioglitazone prevents apoptosis of endothelial progenitor cells from rat bone marrow. Cell Biol Int 37: 430–435. [DOI] [PubMed] [Google Scholar]
- Zhou L, Tang YP, Gao L, Fan XS, Liu CM, Wu DK (2009). Separation, characterization and dose‐effect relationship of the PPARgamma‐activating bio‐active constituents in the Chinese herb formulation ‘San‐Ao decoction. Molecules 14: 3942–3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman SH, Kauffman RF, Evans GF (2002). Peroxisome proliferator‐activated receptor alpha, gamma coagonist LY465608 inhibits macrophage activation and atherosclerosis in apolipoprotein E knockout mice. Lipids 37: 487–494. [DOI] [PubMed] [Google Scholar]


