Abstract
Background and Purpose
Cardiac fibrosis is a common feature of advanced coronary heart disease and is characteristic of heart disease. However, currently available drugs against cardiac fibrosis are still very limited. Here, we have assessed the role of isopropyl 3‐(3,4‐dihydroxyphenyl)‐2‐hydroxylpropanoate (IDHP), a new metabolite of Danshen Dripping Pills, in cardiac fibrosis mediated by the β‐adrenoceptor agonist, isoprenaline, and its underlying mechanisms.
Experimental Approach
Identification of IDHP was identified by mass spectrometry, and proton and carbon nuclear magnetic resonance spectra. Myocardial collagen was quantitatively assessed with Picrosirius Red staining. Expression of mRNA for collagen was evaluated with real‐time PCR. Phosphorylated and total p38 MAPK, NADPH oxidase (NOX) and superoxide dismutase (SOD) were analysed by Western blot. Generation of reactive oxygen species (ROS) generation was evaluated by dihydroethidium (DHE) fluorescent staining. NOX2 was knocked down using specific siRNA.
Key Results
IDHP attenuated β‐adrenoceptor mediated cardiac fibrosis in vivo and inhibited isoprenaline‐induced proliferation of neonatal rat cardiac fibroblasts (NRCFs) and collagen I synthesis in vitro. Phosphorylation of p38 MAPK, which is an important mediator in the pathogenesis of isoprenaline‐induced cardiac fibrosis, was inhibited by IDHP. This inhibition of phospho‐p38 by IDHP was dependent on decreased generation of ROS. These effects of IDHP were abolished in NRCFs treated with siRNA for NOX2.
Conclusions and Implications
IDHP attenuated the cardiac fibrosis induced by isoprenaline through a NOX2/ROS/p38 pathway. These novel findings suggest that IDHP is a potential pharmacological candidate for the treatment of cardiac fibrosis, induced by β‐adrenoceptor agonists.
Linked Articles
This article is part of a themed section on Chinese Innovation in Cardiovascular Drug Discovery. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2015.172.issue-23
Abbreviations
- CCK‐8
Cell Counting Kit‐8
- DHE
dihydroethidium
- ECM
extracellular matrix
- IDHP
isopropyl 3‐(3,4‐dihydroxyphenyl)‐2‐hydroxylpropanoate
- NAC
N‐acetylcysteine
- NOX
NADPH oxidase
- NRCFs
neonatal rat cardiac fibroblasts
- ROS
reactive oxygen species
- SOD
superoxide dismutase
Tables of Links
| TARGETS |
|---|
| GPCRs a |
| β2‐adrenoceptors |
| Enzymes b |
| p38 |
| LIGANDS |
|---|
| Collagen |
| Isoprenaline |
| SB202190 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guideto PHARMACOLOGY 2013/14 (a,bAlexander et al., 2013a, [Link]).
Introduction
Cardiac fibrosis is the common feature of advanced coronary heart disease, hypertension and cardiomyopathy (Zeisberg et al., 2007) and results in serious complications including heart failure, ventricular arrhythmia and sudden death (St John Sutton et al., 2003). At present, cardiac fibrosis is the key therapeutic target to prevent these serious complications (Du et al., 2009).
The excessive activation of the sympathetic nervous system with increased plasma catecholamine level is involved in the process of cardiac fibrosis caused by various factors, such as pressure overload, ischaemia and cardiomyopathy (Cohn, 1990). Sustained activation of β‐adrenoceptors leads to pathological cardiac fibrosis and the synthetic β‐adrenoceptor agonist, isoprenaline, has been widely used to induce models of cardiac fibrosis (Teerlink et al., 1994; Kiriazis et al., 2008). Cardiac fibrosis induced by β‐adrenoceptor stimulation involves many mechanisms including inflammatory activation (Feng et al., 2009), oxidative stress (Zhang et al., 2005a) and MMP activation (Hori et al., 2011). Oxidative stress caused by the accumulation of reactive oxygen species (ROS) (Krifka et al., 2013) is the main mechanism of cardiac fibrosis (Engberding et al., 2004) through activating intracellular signal pathways. A critical signalling pathway induced by isoprenaline is that involving ROS and p38 MAPK, which contributes to the development of cardiac fibrosis (Zhang et al., 2005b; Xu et al., 2011).
Salvia miltiorrhiza (Danshen) is a valued Chinese traditional herb for the treatment of cardiovascular diseases (Ge et al., 2014; Lu et al., 2014) by increasing blood flow (Gao et al., 2005). Compound Danshen Formulae consisting of Danshen and other traditional Chinese medicines have been used in myocardial infarction (Jun et al., 2014), myocardial ischaemia/reperfusion injury (Wang et al., 2014) and heart failure (H Li et al., 2011a). We have also found that Danshensu can inhibit proliferation of cardiac fibroblasts (Lu et al., 2014). However, the mechanisms underlying the action of compound Danshen formulae in the regulation of fibrosis during pathological remodelling is still unclear.
In a previous study, we identified a new metabolite of Danshen Dripping Pills, isopropyl 3‐(3,4‐dihydroxyphenyl)‐2‐hydroxylpropanoate (IDHP), using metabolomic techniques (Xiaohui et al., 2007). Here, we have found that IDHP attenuated cardiac fibrosis induced by a β‐adrenoceptor agonist. The anti‐fibrosis effect of IDHP was dependent on NADPH oxidase (NOX)2 and the ROS/p38 pathway.
Methods
Animals and treatment
All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of Peking University Health Science Center. Rats were housed in a temperature‐ and humidity‐controlled room on a 12 h light/dark cycle and given free access to water and food. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 32 animals were used in the experiments described here.
Male Sprague‐Dawley rats, 6–8 weeks old and weighing 280–320 g, were obtained from the Animal Department of Peking University Health Science Center (Beijing, China). The cardiac fibrosis model was established by s.c. injection of isoprenaline (0.25 mg·kg−1·d−1, dissolved in saline, Sigma–Aldrich, St. Louis, MO, USA) once daily for 7 consecutive days. Rats were randomly divided into four groups: (i) control group, daily s.c. injection with physiological saline as negative control; (ii) ISO group, after three days pretreatment with physiological saline, isoprenaline (0.25 mg·kg−1·d−1) was injected s.c. for 7 consecutive days; (iii) IDHP + ISO group, after 3 days of pretreatment with IDHP (50 mg·kg−1·d−1), isoprenaline (0.25 mg·kg−1·d−1) and IDHP (50 mg·kg−1·d−1) were injected s.c. for seven consecutive days; (iv) IDHP (50 mg·kg−1·d−1) was injected s.c. for ten consecutive days.
Culture of neonatal rat cardiac fibroblasts (NRCFs)
Primary NRCFs were harvested from ventricles of 1–3‐day‐old rats as previously described (Jiang et al., 2013). Ventricles were minced and digested with trypsin (0.1%) and collagenase II (50 U·mL−1) (Life Technologies, Grand Island, NY, USA). Cells were collected and plated for 2 h at 37°C. Cardiomyocytes, the unattached cells, were removed and the remaining cardiac fibroblasts were cultured in DMEM (Life Technologies) with 10% FBS (HyClone, Logan, UT, USA), 100 U·mL−1 penicillin, 100 μg·mL−1 streptomycin at 37°C with 5% CO2. Subconfluent (>90% confluency) NRCFs at the second to third passage were used in the experiments.
Histological analysis
Heart tissues were fixed with 4% paraformaldehyde solution (pH 7.4), embedded in paraffin, sectioned into 5 μm slices, and stained with Picrosirius Red. The ratio of stained fibrotic area to total ventricular area were calculated and used as the collagen volume fraction. Ten randomly selected fields from each section were collected to evaluate left ventricular fibrosis.
Real‐time PCR
Total RNA was extracted from left ventricular tissues of rat hearts, using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and quantified by measuring the absorbance at 260 nm. One microgram of total RNA was used for the preparation of cDNA by reverse transcription. The expression of rat type I and III collagen mRNA was determined with real‐time PCR (Eppendorf Mastercycler ep realplex, Eppendorf, Hamburg, Germany). The reaction conditions included denaturation at 95°C for 15 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s. The primer sequences used in this study were as follows: type I collagen, forward 5′‐ATCAGCCCAAACCCCAAGGAGA‐3′, reverse 5′‐CG CAGGAAGGTCAGCTGGATAG‐3′; type III collagen, forward 5′‐CGAGGA CCTA AAGATAACCGGC‐3′, reverse 5′‐GTTGCAGCTGAGATCGAGCCAC‐3′; GADPH, forward 5′‐TCCCTCAAGGTTGTCAGCAA‐3′; reverse 5′‐AGATCCACAACGGAT ACAT T‐3′. The gene expression level was calculated by 2−(ΔCT).
elisa for type I collagen
The amount of collagen type I in NRCF cytoplasm was evaluated by elisa (EIAAB, Wuhan, China) according to the manufacturer's instructions. Absorbances were read at 450 nm (Microplate Reader Model 550, Bio‐Rad, Hercules, CA, USA). All samples were assayed in triplicate.
Cell Counting Kit‐8 (CCK)‐8 for proliferation of NRCFs
The proliferation of NRCFs was evaluated using the CCK‐8 (Dojindo, Kumamoto, Japan). NRCFs were cultured in 96‐well plates. After incubation in serum‐free medium for 24 h, NRCFs were treated for 24 h. The supernatant was removed, 10 μL CCK‐8 was added to each well containing 100 μL of serum‐free medium, and the plate was incubated at 37°C for 3–4 h. The optical density was read at 450 nm (Microplate Reader Model 550, Bio‐Rad).
BrdU elisa for NRCFs proliferation
NRCFs were seeded in 96‐well plates at a density of 1.5 × 104 cells·mL−1. When they had reached 50–60% confluence, cells were incubated with serum‐free medium for 24 h, and then with 10 μL BrdU per well (10 μmol·L−1, final concentration) for 8–10 h before sample collection. Samples were collected and measured with Proliferation elisa BrdU kit (Roche, Basel, Switzerland).
Western blot
The expressions of phospho‐p38, p38, NOX2, NOX4, SOD1 and SOD2 (antibodies from Santa Cruz Biotechnology, Dallas, TX, USA) were examined by Western blot. All cell samples were lysed in lysis buffer. The protein concentration was assessed by BCA protein assay kit (Life Technologies). Proteins (40 mg) were separated by electrophoresis on 10% SDS polyacrylamide gel and transferred to PVDF membranes. The membranes were analysed with antibodies according to the supplier's protocol, and immunolabelled bands were visualized by use of the Pierce ECL Western blotting substrate (Thermo Fisher Scientific, San Jose, CA, USA).
Detection of intracellular ROS
Intracellular ROS generation was estimated using dihydroethidium (DHE) (Molecular Probes, Eugene, OR, USA) fluorescent staining. Cells were loaded with 10 μM DHE at 37°C for 30 min and washed with HBSS. After treatment, cells were washed twice with HBSS. The intensity of DHE fluorescence was measured using an inverted fluorescence microscope (Leica, Solms, Germany), to assay intracellular ROS generation.
Detection of NOX activity
The activity of NOX was determined by chemiluminescence assay as described previously (Hsieh et al., 2012). After incubation, the cells were scraped and centrifuged at 300× g for 5 min at 4°C. The cell pellet was resuspended in a volume (100 μL per well) of ice‐cold lysis buffer and the cell suspension was kept on ice for 30 min, and then centrifuged at 16000× g for 5 min at 4°C. The suspension was used to detect NOX activity using a chemiluminescence assay (Qiagen, Hilden, Germany).
Assessment of NOX2 knockdown
The specific siRNAs targeting rat NOX2 were synthesized with the sequence 5′‐GCC UGA AUU UCA ACU GCA UTT‐3′. The scrambled sequences were synthesized with the sequence 5′‐UUC UCC GAA CGU GUC ACG UTT‐3′. The day before transfection, cells were seeded in 6 well plate in 10% FBS/DMEM without antibiotics and grown overnight. On the day of transfection, ScreenFect™ A (Incella, Eggenstein‐Leopoldshafen, Germany) – siRNA complexes were prepared in dilution buffer according to the manufacturer's protocol. Cells were infected with either 60 pmol NOX2 siRNAs or scrambled siRNA complexes for 24 h in opti‐MEM I (Life Technologies). NOX2 expression was assessed by Western blot.
Data analysis
All data are expressed as mean ± SEM and were analysed with Graph Pad Prism 5 (GraphPad Software, La Jolla, CA, USA). The data were from eight rats per group in the in vivo experiments and from at least five independent experiments in vitro. Statistical significance was tested using one‐way anova with Tukey's post hoc test, Kruskal–Wallis test with Dunn's post hoc test and two way anova. P < 0.05 was considered as statistically significant.
Materials
IDHP was synthesized by us, using the method described by Bai et al., 2014. SB202190 was supplied by Sigma‐Aldrich.
Results
Identification of IDHP
The chemical structure of IDHP is shown in Figure 1A. We applied MS, 1H NMR and 13C NMR methods to ensure the identity and purity of the IDHP synthesized. The result of MS showed that the peak of molecular ion of IDHP was in m/z 239.0965 (Figure 1B) and data from 1H NMR and 13C NMR showed the synthetic compound was IDHP (Figure 1C and 1D). We also evaluated the purity of IDHP. The chemical purity of the sample of IDHP used in these experiments was 96.4%, estimated by the area normalization method.and the main impurity was methanol. The optical purity of IDHP was 99.5%.
Figure 1.
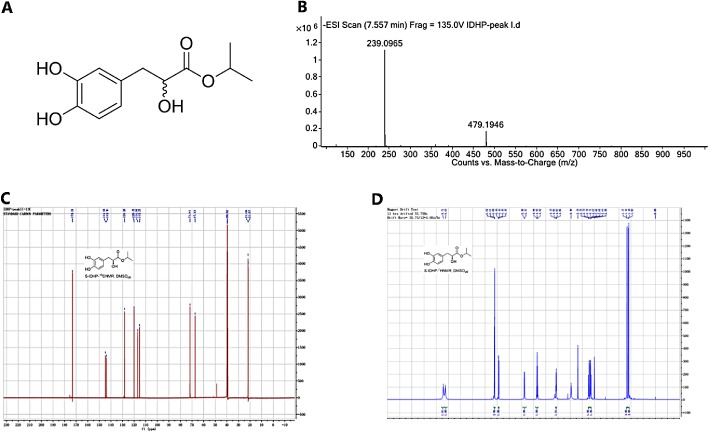
Identification of IDHP. (A) Structural formulas of IDHP. (B) The MS of IDHP. (C) 1H NMR spectra of IDHP. (D) 13C‐NMR spectra of IDHP.
IDHP inhibited cardiac fibrosis induced by ISO
Cardiac fibrosis in rats was quantitatively determined with two different methods, which offered robust morphological and biochemical data. The distribution of collagen fibres in hearts was visualized by staining with Picrosirius Red. IDHP clearly inhibited the extent of isoprenaline‐induced cardiac fibrosis (Figure 2A and 2B).
Figure 2.
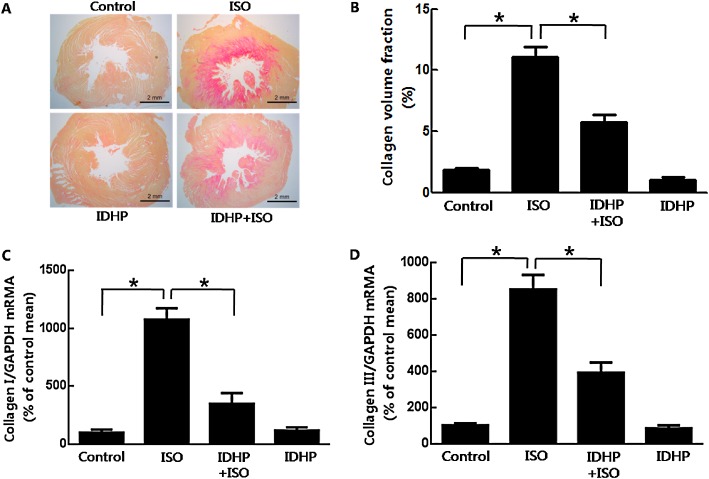
IDHP inhibited isoprenaline (ISO)‐induced cardiac fibrosis. (A) Images show Picrosirius Red‐stained collagen in cardiac interstitium (bar = 2 mm). (B) Collagen volume fraction (%) was quantified from stained sections. (C, D) Expression of mRNA for collagen I and III were detected by real‐time PCR in cardiac tissue. Data are representative of eight samples per group. *P < 0.05, significantly different as indicated.
Cardiac fibrosis resulted from the increased synthesis and decreased degradation of extracellular matrix (ECM), which is mainly composed of type I collagen (85%) and type III collagen (11%) (Van der Heiden et al., 2010; Chen et al., 2012). The mRNA levels of types I and III collagen were measured by real‐time PCR. IDHP pretreatment significantly reduced the expression of mRNA for types I and III collagen, compared with that after isoprenaline administration (Figure 2C and 2D).
IDHP attenuated isoprenaline‐induced NRCFs proliferation and collagen synthesis
Cardiac fibroblasts are critical in the accumulation of ECM ,as these cells proliferate, are transformed into myofibroblasts and secrete collagen, following ischaemia and injury (Porter and Turner, 2009). Pretreatment with IDHP (0.1–10 μM) dose‐dependently inhibited isoprenaline‐induced proliferation of NRCFs, as measuered by either the CCK8 kit (Figure 3A) or BrdU assays (Figure 3B). Collagen I elisa assay showed that pretreatment with IDHP (0.1–10 μM) reduced isoprenaline‐induced collagen I generation in a dose‐dependent manner (Figure 3C). In addition, we assessed the effect of IDHP treatment given after isoprenaline on cardiac fibrosis and found it to inhibit isoprenaline‐induced proliferation of NRCFs (Supporting Information Fig. S1A and S1B).
Figure 3.
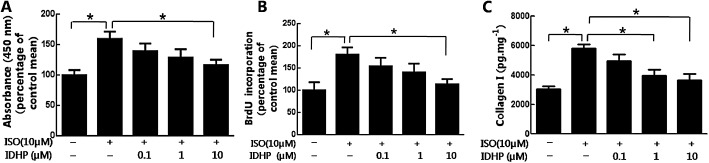
IDHP inhibited isoprenaline (ISO)‐induced proliferation of NRCFs and collagen synthesis. (A) NRCF proliferation was measured with CCK8 assay or (B) by BrdU elisa Kit. (C) The content of collagen type I in the cytoplasm of NRCFs was measured by elisa. After incubation in serum‐free medium for 24 h, NRCFs were pre‐treated with IDHP (0.1–10 μM) for 30 min, then stimulated by isoprenaline (10 μM) for 24 h. Data are representative of five independent experiments.*P < 0.05, significantly different as indicated.
IDHP decreased isoprenaline‐induced phosphorylation of p38 MAPK
p38 MAPK plays an important role in the proliferation of NRCFs (Akiyama‐Uchida et al., 2002; Kim et al., 2007). In our study, NRCF proliferation induced by isoprenaline was abolished by SB202190, an inhibitor of p38 MAPK (either CCK8; Figure 4A or BrdU assay; Figure 4B). In the presence of SB202190, isoprenaline‐induced cell proliferation was not further inhibited by IDHP pretreatment (Figure 4A and 4B), which suggested that the effects of IDHP on the proliferation of NRCFs were exerted mainly by decreasing p38 activation induced by isoprenaline. In addition, we showed, by Western blotting, that IDHP pretreatment decreased the levels of phospho‐p38 MAPK, induced by isoprenaline (Figure 4C and 4D), with no effect of IDHP alone, on phospho‐p38 MAPK (Supporting Information Fig. S2A).
Figure 4.
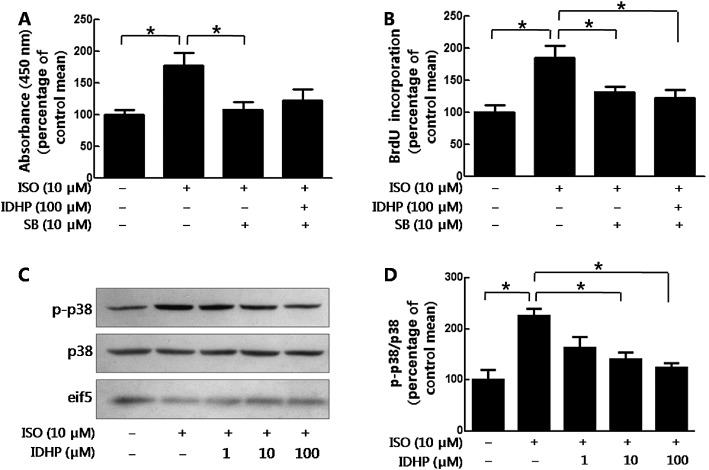
IDHP inhibited NRCFs proliferation by decreasing phosphorylation of p38. (A) NRCFs proliferation was measured with CCK8 assay or (B) by BrdU elisa Kit. Following pretreatment with SB202190 (SB; 10 μM) and/or IDHP (100 μM), NRCFs were stimulated by isoprenaline (ISO; 10 μM) for 24 h. Data summarise six independent experiments. (C) Following pretreatment with IDHP (1–100 μM) for 30 min, NRCFs were stimulated by isoprenaline (10 μM) for 15 min. Western blot analyses were performed using antibodies against phospho‐p38 MAPK, p38 MAPK, eif‐5 respectively. Panel (D) shows summary data from five independent experiments. *P < 0.05, significantly different as indicated.
IDHP inhibited isoprenaline‐induced ROS generation
ROS is an important endogenous regulator of fibroblast proliferation in the heart (Kim et al., 2001; Wedgwood et al., 2001; Lijnen et al., 2006). In the present study, we confirmed the mediation by ROS of the isoprenaline‐induced proliferation of NRCFs, using N‐acetylcysteine (NAC) to scavenge ROS, with CCK8 (Figure 5A) and BrdU incorporation assays (Figure 5B). Pretreatment with IDHP did not further inhibit isoprenaline‐induced cell proliferation in the presence of NAC (Figure 5A and 5B), suggesting that the effects of IDHP were mainly exerted through blocking ROS production. Direct assays of ROS generation showed that IDHP did inhibit isoprenaline‐induced ROS production (Figure 5C and 5D) but did not affect ROS levels in the absence of isoprenaline (Supporting Information Fig. S2B). In accordance with the results from the in vitro study, pretreatment with IDHP inhibited ROS generation induced by isoprenaline in vivo (Figure 5E and 5F).
Figure 5.
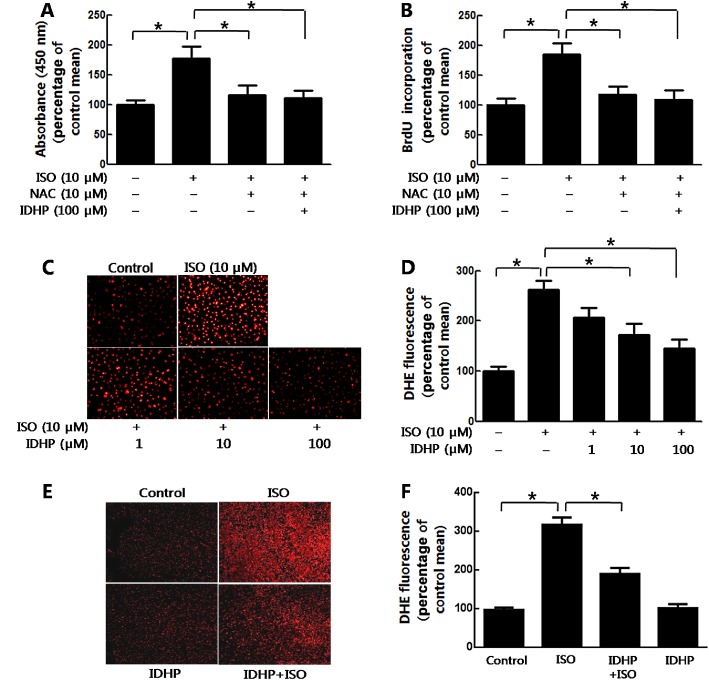
IDHP inhibited NRCFs proliferation through decreasing ROS levels. (A) NRCFs proliferation was measured with CCK8 assay or (B) by BrdU elisa Kit. Following pretreatment with NAC (10 μM) and/or IDHP (100 μM), NRCFs were stimulated by isoprenaline (ISO; 10 μM) for 24 h. Data summarise five independent experiments. (C) Staining of NRCFs for oxidative status with the fluorescent marker DHE . NRCFs were loaded with DHE for 30 min. Following pretreatment with IDHP (1–100 μM) for 30 min, NRCFs were stimulated by isoprenaline (ISO; 10 μM) for 30 min. (D) Densitometric analysis of DHE fluorescence. Data summarise five independent experiments. (E) Representative DHE staining of LV sections. (F) Densitometric analysis of DHE fluorescence from eight independent samples per group. *P < 0.05, significantly different as indicated.
ROS‐mediated isoprenaline‐induced p38 MAPK activation
The results shown earlier suggested that IDHP inhibited phospho‐p38 MAPK and ROS production to attenuate isoprenaline‐induced proliferation of NRCFs. However, the relationship between ROS generation and p38 MAPK in response to isoprenaline stimulation was still unclear. Our results showed that the scavenging of ROS by NAC inhibited isoprenaline‐induced phosphorylation of p38 MAPK (Figure 6A and 6B). However, inhibition of this phosphorylation with SB202190 did not abolish ROS production induced by isoprenaline (Figure 6C and 6D). These data indicated that IDHP inhibited proliferation of NRCFs via a signalling pathway involving ROS and then p38 phosphorylation.
Figure 6.
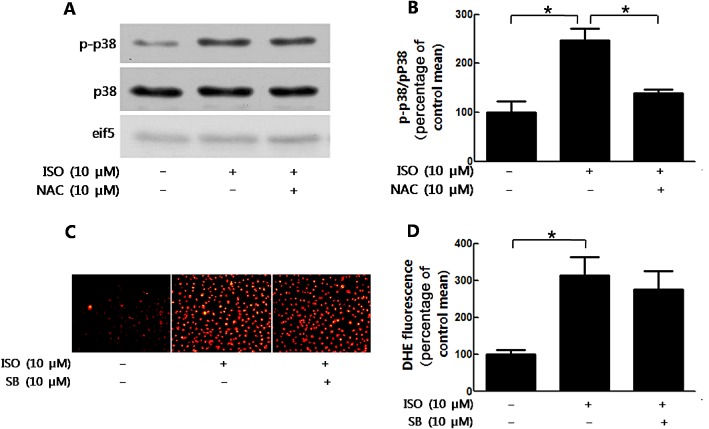
ROS generation mediated the phosphorylation of p38 induced by isoprenaline. (A) NRCFs were stimulated by isoprenaline (ISO; 10 μM) after NAC (10 μM) pretreatment. Cell lysates were immunoblotted with antibody against phospho‐p38 MAPK, p38 MAPK, eif‐5 respectively. (B) Quantification of phospho‐p38/p38 is shown. (C) DHE staining of NRCFs. After treatment with SB202190 (SB; 10 μM) for 30 min, NRCFs were stimulated with isoprenaline (10 μM) for 30 min. (D) The data shows analysis of DHE fluorescence. Data shown summarise five independent experiments. *P < 0.05, significantly different as indicated.
IDHP attenuated isoprenaline‐induced NRCF proliferation by blocking ROS generation via NOX2
NOX is the major source of ROS production (Lambeth, 2004) and in our experiemtns, IDHP clearly inhibited isoprenaline‐induced NOX activity (Figure 7A). NOX2 and NOX4 are the two main isoforms of NOX expressed in cardiac tissue and, here, Western blot analyses of NRCFs showed IDHP attenuated isoprenaline‐induced expression of NOX2 (Figure 7B), but not that of NOX4 (Figure 7C).
Figure 7.
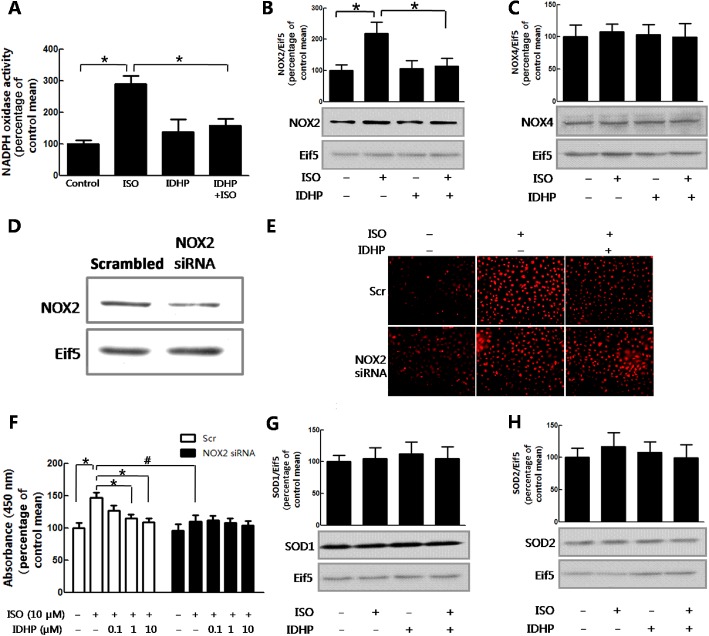
IDHP attenuation of isoprenaline‐induced proliferation of NRCFs was dependent on a NOX2/ROS pathway. (A) NOX activity was detected by chemiluminescence assay. (B) NOX2 or (C) NOX4 expression was detected by Western blot. NRCFs were stimulated by isoprenaline (ISO; 10 μM) after treatment with IDHP (10 μM). (D) Effect of NOX2 siRNA on NOX2 protein levels. NRCFs were transfected with NOX2 and scrambled siRNA for 48 h. Cell lysates were immunoblotted with NOX2, eif5 antibody. (E) Representative DHE staining of NRCFs. At 48 h post transfection, NRCFs were loaded with DHE for 30 min. Following pretreatment with IDHP (100 μM), NRCFs were stimulated by isoprenaline (10 μM) for 30 min. (F) NRCF proliferation was measured with the CCK8 assay. At 24 h post transfection in 6‐well plates, NRCFs were subcultured in 96‐well plate. At 48 h post transfection, NRCFs were incubated with isoprenaline (10 μM) for 24 h with IDHP (0.1–10 μM) pretreatment. The expression of (G) SOD1 and (H) SOD2 were investigated by Western blot. NRCFs were stimulated by isoprenaline (10 μM) after pretreatment with IDHP (10 μM). Data shown summarise five independent experiments. *P < 0.05, #P < 0.05, significantly different as indicated.
To confirm whether IDHP attenuated isoprenaline‐induced proliferation of NRCFs was dependent on ROS generated by NOX2, we used NOX2‐specific siRNA to silence expression of this enzyme (Figure 7D). Isoprenaline‐induced ROS generation was inhibited significantly in the NOX2 knockdown cells and pretreatment with IDHP did not further decrease ROS generation in cells with knockdown of NOX2 (Figure 7E). Similar results were obtained in terms of proliferation, with IDHP pretreatment of NOX2‐deficient cells showing no further attenuation of isoprenaline‐induced proliferation (Figure 7F). Taken together, our results showed that the attenuation of isoprenaline‐induced NRCF proliferation by IDHP was dependent on a NOX2/ROS pathway.
In addition, IDHP may also inhibit ROS accumulation through increasing ROS elimination. Superoxide dismutase (SOD) is a critical enzyme in the scavenging of ROS and SOD1 and SOD2 are intracellular components in cardiac tissue (Borgstahl et al., 1996; Chen et al., 2006; Cao et al., 2008). Our results indicated that expression of neither SOD1 nor SOD2 was regulated by IDHP (Figure 7G and 7H).
Discussion and conclusions
Compound Danshen Formulae have been shonw to inhibit fibrosis in several organs. Danshen attenuated hepatic fibrosis by inhibiting proliferation of vascular smooth muscle cells and collagen synthesis (Liu et al., 2009; Zhang et al., 2013). Danshen Compound Dripping Pill (Dantonic, Tasly Pharmaceuticals, Tianjin, China) reversed cardiac fibrosis through reducing expressions of TGF‐β1, p‐Smad3, Smad4, MMP‐9 and α‐SMA (Wei et al., 2013). However, Compound Danshen Formulae comprise many components, so that their pharmacological mechanisms are still poorly understood. In recent years, more and more research in traditional Chinese medicine has been focused on the effects of active ingredients from formulae. IDHP, a novel bioactive metabolite of Compound Danshen Formulae, was discovered by metabonomic techniques in a previous study. Here, we have demonstrated IDHP to block the cardiac fibrosis induced by a β‐adrenoceptor agonist, through the inhibition of a NOX2/ROS/p38 pathway (Figure 8).
Figure 8.
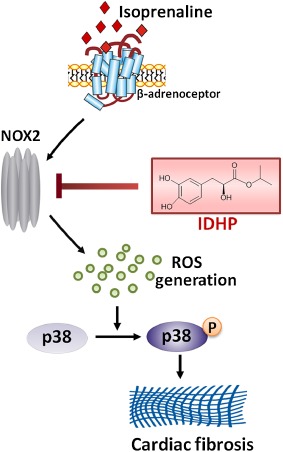
Summary scheme of the mechanisms underlying the inhibition by IDHP of isoprenaline‐induced cardiac fibrosis. IDHP inhibits the expression of NOX2, thus blocking the signalling pathway of NOX2/ROS/p38, by which isoprenaline induced fibrosis in rat heart.
In the mammalian heart, cardiomyocytes and cardiac fibroblasts account for 90% of the cells in the myocardium. Cardiac fibroblasts, accounting for 60–70% of the total population, are key cells not only in the maintenance of cardiac structure, but also in the synthesis of a range of bioactive molecules and of the ECM. Isoprenaline stimulated IL‐6 secretion by cardiac fibroblasts through p38 MAPK with activation of β‐adrenoceptors (Chen et al., 2012). Activation of these receptors by isoprenaline increased the synthesis and secretion of fibrillar collagen types I and III (Brooks and Conrad, 2009). Proliferation of NRCFs and fibrillar collagen synthesis are important features of cardiac fibrosis. Here, we found that IDHP attenuated cardiac fibrosis mediated by a β‐adrenoceptor agonist in vivo and inhibited isoprenaline‐induced proliferation of NRCFs and collagen I synthesis in vitro.
p38 MAPK regulates cellular response to growth, apoptosis and stress signals (Griendling et al., 2000) and is involved in fibrosis in several organs. Recent results with Ask1‐deficient (Ask1−/−) mice, suggested that ASK1/p38 signalling mediated renal fibrosis (Ma et al., 2014). In the mechanisms of hepatic fibrosis, theioacetamide‐induced activation of p38 increased proliferation and migration of hepatic stellate cells (Tsukada et al., 2005). In addition, p38 MAPK not only increased the expression of α1(I) collagen, but also increased stability of α1(I) collagen mRNA induced by TGF‐β treatment (Tsukada et al., 2005). IN the present context of cardiac fibrosis, p38 MAPK mediated the cardiac fibrosis induced by angiotensin II, through regulating cAMP‐response element binding protein (CREB) (L Li et al., 2011b).We have also found that p38 MAPK was crucially involved in isoprenaline‐induced cardiac fibrosis (Lu et al., 2014). In the present study, IDHP attenuated isoprenaline‐induced cardiac fibrosis through inhibiting the activation of p38 MAPK.
Oxidative stress plays a critical role in cardiac fibrosis with the burst of ROS production (Purnomo et al., 2013). Transgenic β2‐adrenoceptor activation enhanced ROS production in the processes of interstitial fibrosis (Xu et al., 2011). Isoprenaline‐induced ROS generation can activate p38 MAPK (Zhang et al., 2005b). Here, our study also showed that isoprenaline‐induced p38 activation was regulated by ROS production. ROS accumulation is due to an imbalance between ROS production and elimination (Grieve et al., 2004). ROS is generated from mitochondria, xanthine oxidase and NOX during the progression of cardiac diseases. NOX is the critical determinant of the redox state of the myocardium (Griendling et al., 2000). Among five subtypes of NOX, only NOX2 and NOX4 are expressed in cardiac myocytes and fibroblasts. NOX2 is known to be involved in angiotensin II induced cardiac fibrosis, as knockdown of NOX2 suppressed ROS generation and expression of procollagen I and III (Johar et al., 2006). NOX was the major source of ROS in a transgenic model with excessive activation of β2–adrenoceptors (Xu et al., 2011). In the present study, we demonstrated that NOX2 was critical in the isoprenaline‐induced proliferation of NRCFs, using siRNA to silence NOX2. Furthermore, IDHP inhibited isoprenaline‐induced NRCFs proliferation, an effect similarly dependent on a NOX2/ROS pathway.
Compound Danshen Tablet, a herbal preparation, protected the myocardium against ischaemia‐reperfusion injury through increasing Bcl‐2 expression, and decreasing Bax and caspase‐3 expression, by activating the Akt signalling pathway (Ren‐an et al., 2014). Danshen decreased serum levels of IL‐8, IL‐10 and TNF‐α in cerebral ischaemia‐reperfusion injury in an ischaemic stroke model (Liang et al., 2013). Meanwhile, expression of other pro‐inflammatory cytokines such as IL‐6 and CCL2 in HUVECs was significantly reduced by Danshen (Stumpf et al., 2013). These pro‐inflammatory cytokines enhance migration, proliferation and collagen secretion by cardiac myofibroblasts, which accelerate the process of remodelling (Porter and Turner, 2009). p38 MAPK mediates the production of pro‐inflammatory cytokines, induced by β‐adrenoceptor activation (Yin et al., 2006), and was also involved in apoptosis of cardiomyocytes induced by chronic isoprenaline stimulation (Jiao et al., 2007). IDHP exerted anti‐inflammatory activity by abolishing TNF‐α and IL‐1β secretion from BV‐2 mouse microglial cells stimulated by LPS (Wang et al., 2012). Therefore, the cardioprotective effects of IDHP would involve mechanisms other than the anti‐oxidative pathway (NOX2/ROS/p38) demonstrated here. Such other mechanisms of IDHP in attenuating cardiac remodelling need to be assessed in further studies.
In conclusion, our study indicated that IDHP prevented isoprenaline‐induced cardiac fibrosis through inhibiting a NOX2/ROS/p38 pathway. These findings suggest IDHP could be a potential candidate drug against cardiac fibrosis and have identified potential drug targets for the treatment of heart diseases.
Author contributions
XP. Z. and Z. L. designed the project and wrote the manuscript; Q. Yin and H. L designed the experiments and conducted the animal model; Y. B. performed identification of IDHP; A. T. and Q. Yang performed the cell culture and Western blot; C. Y. performed real‐time PCR and elisa experiments; J. W. participated in statistical analysis; T.‐P. F, Y. Z. and X. Z. reviewed and edited the paper. All authors agreed on the final version.
Conflict of interest
None.
Supporting information
Figure S1 Post‐treatment with IDHP inhibited isoprenaline‐induced NRCF proliferation. (A) NRCF proliferation was measured with CCK8 assay. NRCFs were stimulated by isoprenaline (10 μM) for 6 h, then were treated with IDHP (0.1–10 μM) for 18 h. Data shown are from five independent experiments. (B) NRCF proliferation was assayed with CCK8. NRCFs were stimulated by isoprenaline (10 μM) for 12 h, then were incubated with IDHP (0.1–10 μM) for 12 h. Data shown are from three independent experiments.*P < 0.05.
Figure S2 No significant effect of IDHP alone on phospho‐p38 and ROS production. (A) NRCFs were treated with IDHP (0.1–10 μM) and cell lysates were immunoblotted with antibody against phospho‐p38 MAPK, p38 MAPK, eif‐5 respectively. (B)Representative DHE staining of NRCFs. NRCFs were treated with IDHP (0.1–10 μM), or with isoprenaline (10 μM) as the positive control.
Acknowledgements
This work was supported by grants from the National Basic Research Program of China (No. 2011CB503903), the National Natural Science Foundation of China (Nos. 81270157, 81471893 and 81070078), Beijing Municipal Natural Science Foundation (No. 7102158), Changjiang Scholars and Innovative Research Team in University (IRT1174), the Project for Innovative Research Team of Research and Technology of Shaanxi Province (2013KCT‐24) and the National Scientific Instrument and Equipment Development Project of China (2013YQ170525).
References
- Akiyama‐Uchida Y, Ashizawa N, Ohtsuru A, Seto S, Tsukazaki T, Kikuchi H et al (2002). Norepinephrine enhances fibrosis mediated by TGF‐β in cardiac fibroblasts. Hypertension 40: 148–154. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL Spedding M et al (2013a). The Concise Guide to PHARMACOLOGY 2013/14: G Protein‐Coupled Receptors. Br J Pharmacol 170: 1459–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL Spedding M et al (2013b). The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol 170: 1797–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Zhang Q, Jia P, Yang L, Sun Y, Nan Y et al (2014). Improved process for pilot‐scale synthesis of Danshensu ((±)‐DSS) and its enantiomer derivatives. Organic Process Research & Development 18: 1667−1673. [Google Scholar]
- Borgstahl GE, Parge HE, Hickey MJ, Johnson MJ, Boissinot M, Hallewell RA et al (1996). Human mitochondrial manganese superoxide dismutase polymorphic variant Ile58Thr reduces activity by destabilizing the tetrameric interface. Biochemistry 35: 4287–4297. [DOI] [PubMed] [Google Scholar]
- Brooks WW, Conrad CH (2009). Isoproterenol‐induced myocardial injury and diastolic dysfunction in mice: structural and functional correlates. Comp Med 59: 339–343. [PMC free article] [PubMed] [Google Scholar]
- Cao X, Antonyuk SV, Seetharaman SV, Whitson LJ, Taylor AB, Holloway SP et al (2008). Structures of the G85R variant of SOD1 in familial amyotrophic lateral sclerosis. J Biol Chem 283: 16169–16177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Du J, Feng W, Song Y, Lu Z, Xu M et al (2012). beta‐Adrenergic receptors stimulate interleukin‐6 production through Epac‐dependent activation of PKCdelta/p38 MAPK signalling in neonatal mouse cardiac fibroblasts. Br J Pharmacol 166: 676–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Niroomand F, Liu Z, Zankl A, Katus H, Jahn L et al (2006). Expression of nitric oxide related enzymes in coronary heart disease. Basic Res Cardiol 101: 346–353. [DOI] [PubMed] [Google Scholar]
- Cohn J (1990). Abnormalities of peripheral sympathetic nervous system control in congestive heart failure. Circulation 82 (2 Suppl.): I59–I67. [PubMed] [Google Scholar]
- Du XJ, Xu Q, Lekgabe E, Gao XM, Kiriazis H, Moore XL et al (2009). Reversal of cardiac fibrosis and related dysfunction by relaxin. Ann N Y Acad Sci 1160: 278–284. [DOI] [PubMed] [Google Scholar]
- Engberding N, Spiekermann S, Schaefer A, Heineke A, Wiencke A, Muller M et al (2004). Allopurinol attenuates left ventricular remodeling and dysfunction after experimental myocardial infarction: a new action for an old drug? Circulation 110: 2175–2179. [DOI] [PubMed] [Google Scholar]
- Feng W, Li W, Liu W, Wang F, Li Y, Yan W (2009). IL‐17 induces myocardial fibrosis and enhances RANKL/OPG and MMP/TIMP signaling in isoproterenol‐induced heart failure. Exp Mol Pathol 87: 212–218. [DOI] [PubMed] [Google Scholar]
- Gao D, Song J, Hu J, Lin J, Zheng L, Cai J et al (2005). [Angiogenesis promoting effects of Chinese herbal medicine for activating blood circulation to remove stasis on chick embryo chorio‐allantoic membrane]. Zhongguo Zhong Xi Yi Jie He Za Zhi 25: 912–915. [PubMed] [Google Scholar]
- Ge G, Zhang Q, Ma J, Qiao Z, Huang J, Cheng W et al (2014). Protective effect of Salvia miltiorrhiza aqueous extract on myocardium oxidative injury in ischemic‐reperfusion rats. Gene 546: 97–103. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Ushio‐Fukai M (2000). NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86: 494–501. [DOI] [PubMed] [Google Scholar]
- Grieve DJ, Byrne JA, Cave AC, Shah AM (2004). Role of oxidative stress in cardiac remodelling after myocardial infarction. Heart Lung Circ 13: 132–138. [DOI] [PubMed] [Google Scholar]
- Hori Y, Yoshioka K, Kanai K, Hoshi F, Itoh N, Higuchi S‐I (2011). Spironolactone decreases isoproterenol‐induced ventricular fibrosis and matrix metalloproteinase‐2 in rats. Biol Pharm Bull 34: 61–65. [DOI] [PubMed] [Google Scholar]
- Hsieh HL, Lin CC, Shih RH, Hsiao LD, Yang CM (2012). NADPH oxidase‐mediated redox signal contributes to lipoteichoic acid‐induced MMP‐9 upregulation in brain astrocytes. J Neuroinflammation 9: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Li D, Deng Y, Teng F, Chen J, Xue S et al (2013). Salvianolic acid A, a novel matrix metalloproteinase‐9 inhibitor, prevents cardiac remodeling in spontaneously hypertensive rats. PLoS ONE 8: e59621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao X, Hu A, Tao L, Lopez B, Christopher T, Sun J et al (2007). Chronic isoproterenol stimulation induces cardiomyocyte apoptosis through p38‐cytokine‐iNOS pathway. FASEB J 21: 958.7. [Google Scholar]
- Johar S, Cave AC, Narayanapanicker A, Grieve DJ, Shah AM (2006). Aldosterone mediates angiotensin II‐induced interstitial cardiac fibrosis via a Nox2‐containing NADPH oxidase. FASEB J 20: 1546–1548. [DOI] [PubMed] [Google Scholar]
- St John Sutton M, Lee D, Rouleau JL, Goldman S, Plappert T, Braunwald E et al (2003). Left ventricular remodeling and ventricular arrhythmias after myocardial infarction. Circulation 107: 2577–2582. [DOI] [PubMed] [Google Scholar]
- Jun Y, Chunju Y, Qi A, Liuxia D, Guolong Y (2014). The effects of compound Danshen dripping pills and human umbilical cord blood mononuclear cell transplant after acute myocardial infarction. Exp Clin Transplant 12: 123–128. [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B‐Y, Han M‐J, Chung A‐S (2001). Effects of reactive oxygen species on proliferation of Chinese hamster lung fibroblast (V79) cells. Free Radic Biol Med 30: 686–698. [DOI] [PubMed] [Google Scholar]
- Kim CS, Kim J‐M, Nam SY, Yang KH, Jeong M, Kim HS et al (2007). Low‐dose of ionizing radiation enhances cell proliferation via transient ERK1/2 and p38 activation in normal human lung fibroblasts. J Radiat Res (Tokyo) 48: 407–415. [DOI] [PubMed] [Google Scholar]
- Kiriazis H, Wang K, Xu Q, Gao XM, Ming Z, Su Y et al (2008). Knockout of β1‐and β2‐adrenoceptors attenuates pressure overload‐induced cardiac hypertrophy and fibrosis. Br J Pharmacol 153: 684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krifka S, Spagnuolo G, Schmalz G, Schweikl H (2013). A review of adaptive mechanisms in cell responses towards oxidative stress caused by dental resin monomers. Biomaterials 34: 4555–4563. [DOI] [PubMed] [Google Scholar]
- Lambeth JD (2004). NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4: 181–189. [DOI] [PubMed] [Google Scholar]
- Li H, Zhang Y, Ma J (2011a). [Effects of yiqi huoxue compound combined with exercise therapy on MMP‐1 and collagen type III expressions of cardiac muscle in chronic heart failure rats]. Zhongguo Zhong Xi Yi Jie He Za Zhi 31: 955–960. [PubMed] [Google Scholar]
- Li L, Fan D, Wang C, Wang J‐Y, Cui X‐B, Wu D et al (2011b). Angiotensin II increases periostin expression via Ras/p38 MAPK/CREB and ERK1/2/TGF‐β1 pathways in cardiac fibroblasts. Cardiovasc Res 91: 80–89. [DOI] [PubMed] [Google Scholar]
- Liang X‐Y, Li H‐N, Yang X‐Y, Zhou W‐Y, Niu J‐G, Chen B‐D (2013). Effect of Danshen aqueous extract on serum hs‐CRP, IL‐8, IL‐10, TNF‐α levels, and IL‐10 mRNA, TNF‐α mRNA expression levels, cerebral TGF‐β1 positive expression level and its neuroprotective mechanisms in CIR rats. Mol Biol Rep 40: 3419–3427. [DOI] [PubMed] [Google Scholar]
- Lijnen P, Petrov V, Semplicini A, Fagard R (2006). Angiotensin II‐stimulated collagen production in cardiac fibroblasts is mediated by reactive oxygen species. J Hypertens 24: 757–766. [DOI] [PubMed] [Google Scholar]
- Liu C, Hu Y, Xu L, Liu C, Liu P (2009). Effect of Fuzheng Huayu formula and its actions against liver fibrosis. Chin Med 4: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Tian A, Wu J, Yang C, Xing R, Jia P et al (2014). Danshensu inhibits beta‐adrenergic receptors‐mediated cardiac fibrosis by ROS/p38 MAPK axis. Biol Pharm Bull 37: 961–967. [DOI] [PubMed] [Google Scholar]
- Ma FY, Tesch GH, Nikolic‐Paterson DJ (2014). ASK1/p38 signaling in renal tubular epithelial cells promotes renal fibrosis in the mouse obstructed kidney. Am J Physiol Renal Physiol 307: F1263–F1273. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C (2010). Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol 160: 1573–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP et al; NC‐IUPHAR (2014). The IUPHAR/BPS Guide to PHARMACOLOGY: an expert‐driven knowledge base of drug targets and their ligands. Nucl. Acids Res. 42 (Database Issue): D1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter KE, Turner NA (2009). Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther 123: 255–278. [DOI] [PubMed] [Google Scholar]
- Purnomo Y, Piccart Y, Coenen T, Prihadi JS, Lijnen PJ (2013). Oxidative stress and transforming growth factor‐beta1‐induced cardiac fibrosis. Cardiovasc Hematol Disord Drug Targets 13: 165–172. [DOI] [PubMed] [Google Scholar]
- Ren‐an Q, Juan L, Chuyuan L, Wenjuan F, Chunyan H, Xuemei Y et al (2014). Study of the protective mechanisms of Compound Danshen Tablet (Fufang Danshen Pian) against myocardial ischemia/reperfusion injury via the Akt‐eNOS signaling pathway in rats. J Ethnopharmacol 156: 190–198. [DOI] [PubMed] [Google Scholar]
- Stumpf C, Fan Q, Hintermann C, Raaz D, Kurfürst I, Losert S et al (2013). Anti‐inflammatory effects of Danshen on human vascular endothelial cells in culture. Am J Chin Med 41: 1065–1077. [DOI] [PubMed] [Google Scholar]
- Teerlink JR, Pfeffer JM, Pfeffer MA (1994). Progressive ventricular remodeling in response to diffuse isoproterenol‐induced myocardial necrosis in rats. Circ Res 75: 105–113. [DOI] [PubMed] [Google Scholar]
- Tsukada S, Westwick JK, Ikejima K, Sato N, Rippe RA (2005). SMAD and p38 MAPK signaling pathways independently regulate α1(I) collagen gene expression in unstimulated and transforming growth factor‐β‐stimulated hepatic stellate cells. J Biol Chem 280: 10055–10064. [DOI] [PubMed] [Google Scholar]
- Van der Heiden K, Cuhlmann S, Luong le A, Zakkar M, Evans PC (2010). Role of nuclear factor kappaB in cardiovascular health and disease. Clin Sci (Lond) 118: 593–605. [DOI] [PubMed] [Google Scholar]
- Wang S, Wang H, Jing H, Wang S, Kang L, Gao X et al (2012). Anti‐inflammatory effects of isopropyl 3‐(3, 4‐dihydroxyphenyl)‐2‐hydroxypropanoate, a novel metabolite from Danshen, on activated microglia. Chin J Physiol 55: 428–434. [DOI] [PubMed] [Google Scholar]
- Wang WD, Wang L, Cheng L, Yin XJ, Xu HY, Wang JL et al (2014). [Protection effect of Yindan Xinnaotong capsule and main compositions compatibility on myocardial ischemia/reperfusion injury]. Zhongguo Zhong Yao Za Zhi 39: 1690–1694. [PubMed] [Google Scholar]
- Wedgwood S, Dettman RW, Black SM (2001). ET‐1 stimulates pulmonary arterial smooth muscle cell proliferation via induction of reactive oxygen species. Am J Physiol Lung Cell Mol Physiol 281: L1058–L1067. [DOI] [PubMed] [Google Scholar]
- Wei XH, Liu YY, Li Q, Yan L, Hu BH, Pan CS et al (2013). Treatment with cardiotonic pills ((R)) after ischemia‐reperfusion ameliorates myocardial fibrosis in rats. Microcirculation 20: 17–29. [DOI] [PubMed] [Google Scholar]
- Xiaohui Z, Xinfeng Z, Xin Z, Shixiang W, Yinmao W, Jianbin Z (2007). Determination of the main bioactive metabolites of Radix Salvia miltiorrhizae in compound Danshen dripping pills and the tissue distribution of Danshensu in rabbit by SPE‐HPLC‐MSn. J Sep Sci 30: 851–857. [DOI] [PubMed] [Google Scholar]
- Xu Q, Dalic A, Fang L, Kiriazis H, Ritchie R, Sim K et al (2011). Myocardial oxidative stress contributes to transgenic β2‐adrenoceptor activation‐induced cardiomyopathy and heart failure. Br J Pharmacol 162: 1012–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Wang Y‐Y, Du J‐H, Li C, Lu Z‐Z, Han C et al (2006). Noncanonical cAMP pathway and p38 MAPK mediate β2‐adrenergic receptor‐induced IL‐6 production in neonatal mouse cardiac fibroblasts. J Mol Cell Cardiol 40: 384–393. [DOI] [PubMed] [Google Scholar]
- Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E et al (2007). Endothelial‐to‐mesenchymal transition contributes to cardiac fibrosis. Nat Med 13: 952–961. [DOI] [PubMed] [Google Scholar]
- Zhang G‐X, Kimura S, Nishiyama A, Shokoji T, Rahman M, Yao L et al (2005a). Cardiac oxidative stress in acute and chronic isoproterenol‐infused rats. Cardiovasc Res 65: 230–238. [DOI] [PubMed] [Google Scholar]
- Zhang G‐X, Kimura S, Nishiyama A, Shokoji T, Rahman M, Yao L et al (2005b). Cardiac oxidative stress in acute and chronic isoproterenol‐infused rats. Cardiovasc Res 65: 230–238. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang Y, Xie Y, Gao Y, Ma J, Yuan J et al (2013). Multitargeted inhibition of hepatic fibrosis in chronic iron‐overloaded mice by Salvia miltiorrhiza . J Ethnopharmacol 148: 671–681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Post‐treatment with IDHP inhibited isoprenaline‐induced NRCF proliferation. (A) NRCF proliferation was measured with CCK8 assay. NRCFs were stimulated by isoprenaline (10 μM) for 6 h, then were treated with IDHP (0.1–10 μM) for 18 h. Data shown are from five independent experiments. (B) NRCF proliferation was assayed with CCK8. NRCFs were stimulated by isoprenaline (10 μM) for 12 h, then were incubated with IDHP (0.1–10 μM) for 12 h. Data shown are from three independent experiments.*P < 0.05.
Figure S2 No significant effect of IDHP alone on phospho‐p38 and ROS production. (A) NRCFs were treated with IDHP (0.1–10 μM) and cell lysates were immunoblotted with antibody against phospho‐p38 MAPK, p38 MAPK, eif‐5 respectively. (B)Representative DHE staining of NRCFs. NRCFs were treated with IDHP (0.1–10 μM), or with isoprenaline (10 μM) as the positive control.


