Abstract
Background and Purpose
Nuciferine, a constituent of lotus leaf, is an aromatic ring‐containing alkaloid, with antioxidative properties. We hypothesize nuciferine might affect vascular reactivity. This study aimed at determining the effects of nuciferine on vasomotor tone and the underlying mechanism
Experimental Approach
Nuciferine‐induced relaxations in rings of rat main mesenteric arteries were measured by wire myographs. Endothelial NOS (eNOS) was determined by immunoblotting. Intracellular NO production in HUVECs and Ca2+ level in both HUVECs and vascular smooth muscle cells (VSMCs) from rat mesenteric arteries were assessed by fluorescence imaging.
Key Results
Nuciferine induced relaxations in arterial segments pre‐contracted by KCl or phenylephrine. Nuciferine‐elicited arterial relaxations were reduced by removal of endothelium or by pretreatment with the eNOS inhibitor L‐NAME or the NO‐sensitive guanylyl cyclase inhibitor ODQ. In HUVECs, the phosphorylation of eNOS at Ser1177 and increase in cytosolic NO level induced by nuciferine were mediated by extracellular Ca2+ influx. Under endothelium‐free conditions, nuciferine attenuated CaCl2‐induced contraction in Ca2+‐free depolarizing medium. In the absence of extracellular calcium, nuciferine relieved the vasoconstriction induced by phenylephrine and the addition of CaCl2. Nuciferine also suppressed Ca2+ influx in Ca2+‐free K+‐containing solution in VSMCs.
Conclusions and Implications
Nuciferine has a vasorelaxant effect via both endothelium‐dependent and ‐independent mechanisms. These results suggest that nuciferine may have a therapeutic effect on vascular diseases associated with aberrant vasoconstriction.
Linked Articles
This article is part of a themed section on Chinese Innovation in Cardiovascular Drug Discovery. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2015.172.issue-23
Abbreviations
- [Ca2+]i
intracellular calcium
- [NO]i
intracellular NO
- DAF‐FM DA
4‐amino‐5‐methylamino‐2′,7′‐difluorofluorescein diacetate
- EDHF
endothelium‐derived hyperpolarizing factor
- eNOS
endothelial NOS
- Fluo‐4 AM
fluo‐4‐acetoxymethylester
- iNOS
inducible NOS
- L‐NAME
Nω‐nitro‐L‐arginine methyl ester
- ODQ
1H‐[1,2,4]oxadizolo[4,3‐a]quinoxalin‐1‐one
- VDCCs
voltage‐dependent Ca2+ channels
- VSMCs
vascular smooth muscle cells
Tables of Links
| TARGETS |
|---|
| Enzymes a |
| eNOS |
| iNOS |
| Ion channels b |
| KATP channels, Kir6.2 |
| VDCC, voltage‐dependent calcium channels |
| GPCRs c |
| α1‐adrenoceptors |
| β‐adrenoceptors |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,cAlexander et al., 2013a, 2013b, 2013c).
Introduction
Vascular dysfunction is a key factor in a variety of cardiovascular diseases such as hypertension, atherosclerosis, diabetes and stroke (Brunner et al., 2005). Increased vascular contractility and decreased dilatation are among the key features of endothelial dysfunction, which are associated with many other pathophysiogical processes such as pro‐inflammatory, proliferative and pro‐coagulatant states (Xiao et al., 2014). The endothelium plays a pivotal role in modulating vascular function by releasing vasoactive substances, such as NO, endothelin and PGs (Vanhoutte et al., 2009).
Nelumbo nucifera (commonly named lotus) leaf is a medicinal herb that has been used in traditional Chinese medicine for the treatment of fever, diarrhoea and bleeding. Several alkaloids extracted from lotus leaf have been shown to possess therapeutic potentials for obesity (Ono et al., 2006; Du et al., 2010), atherosclerosis (Ho et al., 2010) and infections (Kashiwada et al., 2005; Agnihotri et al., 2008). Nuciferine (PubChem CID: 10146), a constituent of lotus leaf, is an aromatic ring‐containing alkaloid (Figure 1A). Recently, nuciferine was shown to increase insulin secretion in isolated islets of pancreas via closure of KATP channels (Nguyen et al., 2012). In hamsters given a high‐fat diet, nuciferine treatment alleviated dyslipidaemia and liver steatosis by inhibition of the expression of hepatic genes linked to lipid metabolism (Avila et al., 2013).
Figure 1.
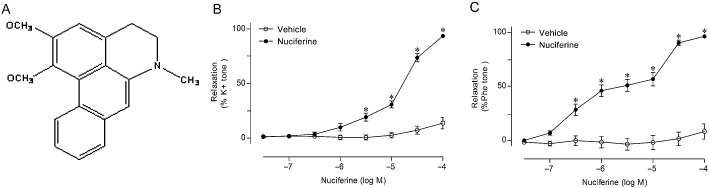
The structure of nuciferine and the effect of nuciferine on agonist‐induced contraction. In (A), the chemical structure of nuciferine. In (B) rings from rat main mesenteric arteries were pre‐contracted by 60 mM K+ or in (C) by 10 μM phenylephrine (Phe), followed by the cumulative addition of vehicle or nuciferine. Data shown are means ± SEM; n = 6–9. *P < 0.05, significantly different from vehicle (DMSO).
Although these effects of nuciferine on metabolism have now been described, little is known about its potential vascular activity. Earlier studies showed that the compounds extracted from lotus leaf, including nuciferine, exhibited antioxidative property (Wu et al., 2003; Huang et al., 2010). In view of the involvement of oxidative stress in the pathogenesis of vascular dysfunction, we hypothesized that nuciferine might affect vascular reactivity. Therefore, the present study aimed to investigate whether nuciferine affects vasomotor tone in isolated arteries and to elucidate the underlying mechanism(s).
Methods
Animals
All animal care and experimental protocols were in accordance with the Animal Management Rules of the Ministry of Health of China and the guidelines for the Care and Use of Laboratory Animals of Xi'an Jiaotong University (Approval No.: XJTULAC2013‐018). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 51 animals were used in the experiments described here. Female Sprague‐Dawley rats weighing 230–270 g were provided by the Experimental Animal Center of Xi'an Jiaotong University.
Preparation of arterial rings
Rats were killed by CO2 inhalation, and then the main mesenteric arteries were quickly isolated and immersed in cold oxygenated Krebs solution containing (mM): NaCl 119, NaHCO3 25, KCl 4.7, MgCl2 1, KH2PO4 1.2, CaCl2 2.5 and D‐glucose 11. The arteries were cleared of connective tissues, and then cut into ring segments, 2 mm long. For the endothelium‐denuded rings, the vessels were perfused with 0.1% Triton X‐100 for 10 s to remove the endothelium (Fountain et al., 2004).
Measurement of isometric force
Each experiment was performed on rings prepared from different rats. One of the rings from each rat was used to examine nuciferine‐induced relaxations for comparison with other groups, so the number of these experiments was larger than the others. The arterial rings were mounted in Multi Myograph System (Danish Myo Technology A/S, Aarhus, Denmark) to measure the isometric force. Briefly, two stainless steel wires (40 μm in diameter) were threaded through the ring's lumen and fixed to the jaws of the myograph. The mounted rings were immersed in temperature‐controlled (37°C) chamber baths containing 5 mL Krebs solution. During the course of the whole experiment, the solution was continuously oxygenated with a gas mixture of 95% O2 plus 5% CO2. The arterial rings were given a resting tension of 3 mN and then allowed to equilibrate for 60 min prior to experiment. The integrity of the functional endothelium was tested by obtaining a relaxation to ACh (1 μM) in rings precontracted with phenylephrine (10 μM). The endothelium was considered intact when such an ACh–induced relaxation was more than 85% of the pre‐contraction value to phenylephrine. In the experiments with endothelium‐denuded arterial rings, lack of relaxation in response to ACh was considered to show successful removal of the endothelium.
To determine the vasorelaxant effect of nuciferine, 60 mM KCl or 10 μM phenylephrine was used to pre‐constrict endothelium‐intact arterial rings. After a sustained contraction was obtained, the concentration‐dependent responses to nuciferine (0.03–100 μM) were examined. The time‐matched vehicle control (DMSO) group was also analysed. The final concentration of DMSO was 0.1% (v/v).
In order to evaluate the role of endothelium‐derived vasodilator factors, endothelium‐intact arteries were incubated for 30 min with each of the following inhibitors: 100 μM L‐NAME (NOS inhibitor), 1 μM indomethacin (nonselective COX inhibitor), 100 nM 1400W (inducible NOS inhibitor, iNOS inhibitor) and 3 μM ODQ (NO‐sensitive guanylyl cyclase inhibitor) before the addition of phenylephrine (10 μM) and nuciferine. L‐NAME plus indomethacin was used to assess the involvement of endothelium‐derived hyperpolarizing factor (EDHF) indirectly.
To examine the role of β‐adrenoceptors in the vasorelaxant effect of nuciferine, some endothelium‐denuded arterial rings were pretreated with 1 μM propranolol (β‐adrenoceptor antagonist) for 30 min before the addition of phenylephrine (10 μM) and nuciferine.
The effect of nuciferine on regulating Ca2+ influx via voltage‐dependent Ca2+ channels (VDCCs) was tested. After preincubation with nuciferine (1 or 10 μM) or 1 μM nifedipine (L‐type Ca2+ channel inhibitor) for 20 min, cumulative concentrations of CaCl2 (0.01–30.0 mM) were added in Ca2+‐free depolarizing medium containing KCl (60 mM) to trigger the contraction of endothelium‐denuded arteries.
Some endothelium‐denuded arterial segments were pretreated with nuciferine (1 or 10 μM) for 20 min, and then the concentration‐dependent responses to phenylephrine (0.003–1000.0 μM) were examined. To determine whether the vasorelaxation to nuciferine resulted from regulation of Ca2+ fluxes, the effects of nuciferine (1 or 10 μM) on the contractile response of arteries without endothelium to phenylephrine (10 μM) and the addition of CaCl2 (2 mM) in Ca2+ ‐free Krebs solution were studied.
Culture of HUVECs
HUVECs were obtained from umbilical cords, with the donors' permission and the approval of the hospital's Ethical Committee. The protocol for the isolation of HUVECs was approved by the Ethical Committee of Xi'an Jiaotong University and the cells cultured as previously described (Wang, 2002). Briefly, HUVECs were harvested by collagenase treatment of umbilical cord veins and cultured on plates coated with collagen. Cells were maintained in M199 supplemented with 16% FBS, 20 mM HEPES (pH 7.4), 1 ng·mL−1 of recombinant human fibroblast growth factor, 90 μg·mL−1 of heparin and antibiotics. HUVECs within six passages were used in the subsequent cell experiments.
Primary culture of vascular smooth muscle cells (VSMCs) from main mesenteric arteries of rats
VSMCs were cultured by the explant technique. Main mesenteric arteries from rats were isolated with removal of endothelium; and then cut into small pieces (∼1 mm2). The tissues were placed in culture dishes and cultured in 80% DMEM with 20% FBS, 100 μg·mL−1 streptomycin and 100 U·mL−1 penicillin at 37°C and 5% CO2 in air till 70% confluence. The identity of the primary cultured VSMCs was verified by positive staining for α‐smooth muscle actin (Abcam, Cambridge, UK) and negative staining for eNOS.
Measurement of intracellular NO ([NO]i) and calcium ([Ca2+]i) levels in HUVECs
[NO]i and [Ca2+]i were detected using fluorescent indicators DAF‐FM DA and Fluo‐4 AM respectively. After different treatment, cultured HUVECs seeded on glass coverslips were incubated in M199 medium containing 0.1 μM DAF‐FM DA or 5 μM Fluo‐4 AM for 20 min at 37°C, and then washed with PBS. Images were obtained using fluorescence microscopy (Olympus America, Inc., Melville, NY, USA) and evaluated using MOTIC software (Motic Company, Xiamen, China). For quantification purpose, three images were randomly taken per slide. The mean fluorescence intensity value of the three images was taken to be the slide value.
Measurement of [Ca2+]i in VSMCs by fluorescence microscopy
[Ca2+]i in VSMCs was measured as described by Cheang et al., (2013). Cultured VSMCs from rat main mesenteric arteries seeded on glass coverslips were incubated with 10 μM Fluo‐4 AM in normal physiological saline solution at 37°C for 1 h; and treated with DMSO (vehicle control), 10 μM nuciferine, or 1 μM nifedipine (positive control) for 20 min. The solution was then replaced with Ca2+‐free physiological saline solution, containing 60 mM K+. Fluorescence images were obtained using fluorescence microscopy continuously every 5 s. Ca2+ influx was stimulated by addition of 2 mM CaCl2. Changes in [Ca2+]i was expressed as a ratio (F1/F0) by comparing the fluorescence before (F0) and after (F1) adding CaCl2.
Western blotting analysis
Cultured HUVECs after different treatment were harvested to evaluate the level of endothelial NOS (eNOS) protein. The cell lysates were collected in ice‐cold RIPA buffer containing 1 mM EGTA, 1 mM EDTA, 1 mM sodium fluoride, 1 mM sodium orthovanadate, 100 μg·mL−1 phenylmethylsulfonyl fluoride, 2 mg·mL−1 β‐glycerolphosphate, 5 μg·mL−1 aprotinin and 1 μg·mL−1 leupeptin, and then centrifuged at 12 000 g for 20 min at 4°C. The protein concentration of supernatant was determined using the Bradford assay (Bio‐Rad, Hercules, CA). The samples after denaturation were separated on 8% SDS‐PAGE and then transferred to polyvinylidene difluoride membranes (Millipore, Billerica, USA). The membranes were blocked with 1% bovine serum albumin for 1 h and subsequently exposed to corresponding primary antibodies for total eNOS (9586S, Cell Signaling Technology) or phosphorylated eNOS at Ser1177 (9571, Cell Signaling Technology) (1:2000) at 4°C overnight. After washing with Tris‐buffered saline/Tween 20, the membranes were incubated with HRP‐conjugated secondary antibody, anti‐mouse (sc‐2005, Santa Cruz Biotechnology) or anti‐rabbit (sc‐2004, Santa Cruz Biotechnology) antibody (1:3000) for 1 h at room temperature. Then the membranes were developed using the enhanced chemiluminescence detection system (ECL, Amersham, Piscataway, NJ, USA). Phosphorylated eNOS and total eNOS correspond to the band at approximately 140 kDa. The level of β‐actin (sc‐47778, Santa Cruz Biotechnology) was used as an internal reference.
Data analysis
Data are expressed as mean ± SEM. For assays involving arterial rings, the number (n) refers to the number of rats, each providing 4–5 rings. Relaxation in each arterial segment is expressed as the percentage of the contraction induced by 60 mM KCl or 10 μM phenylephrine. pEC50 is the negative logarithm of the dilator concentration that produced half of the maximal relaxation (E max). pEC50 was calculated by nonlinear regression analysis using GraphPad Prism 5 (San Diego, CA, USA). Concentration–relaxation curves were analysed by two‐way anova followed by Bonferroni post hoc tests. Difference comparison among multiple groups was tested by one‐way anova. P < 0.05 was considered statistically significant.
Materials
Nuciferine (purity by HPLC > 98.0%) was from APP‐CHEM (YHI‐039, Xi'an, Shannxi, China). Phenylephrine, ACh, Nω‐nitro‐L‐arginine methyl ester (L‐NAME), 1H‐[1,2,4]oxadizolo[4,3‐a]quinoxalin‐1‐one (ODQ), indomethacin, 1400W, propranolol, nifedipine, BAPTA‐AM and DMSO were from Sigma Aldrich (St Louis, MO, USA). Fluo‐4‐acetoxymethylester (Fluo‐4 AM) and 4‐amino‐5‐methylamino‐2′,7′‐difluorofluorescein diacetate (DAF‐FM DA) were from Life Science, Ltd. (Eugene, OR, USA). Nuciferine, BAPTA‐AM, Fluo‐4 AM and DAF‐FM DA were dissolved in DMSO. Indomethacin was dissolved in ethanol. Other chemicals were dissolved in double distilled water. M199 medium, DMEM and FBS were from Invitrogen (Carlsbad, CA, USA). Antibodies for total eNOS and phosphorylated eNOS at Ser1177 were from Cell Signaling Technology (Danvers, MA, USA). Antibodies for β‐actin and HRP‐conjugated secondary antibody were from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Results
Nuciferine induced relaxation of isolated main mesenteric arteries in a concentration‐dependent manner
Compared with the time‐matched vehicle control (DMSO), nuciferine elicited concentration‐dependent relaxations in the arterial segments pre‐contracted by KCl or by phenylephrine under endothelium‐intact conditions (Figure 1 B and C). The derived pEC50 and Emax values are shown in Table 1. The contraction tone of each ring before the addition of vehicle or nuciferine was similar for both groups (Supporting Information Table S1). Nuciferine induced relaxation in mesenteric arteries from male rats in the same manner (Supporting Information Fig. S1A and B). Additionally, nuciferine caused concentration‐dependent relaxation in aortas and renal arteries in both male and female rats (Supporting Information Fig. S2).
Table 1.
pEC 50 and E max (%) values for nuciferine‐induced relaxations of rings from mesenteric arteries
| Treatment | pEC50 | E max (%) |
|---|---|---|
| Vehicle (60 mM KCl) | 13.6 ± 5.3 | |
| +nuciferine | 4.82 ± 0.08 | 93.5 ± 1.1* |
| Vehicle (Phe) | 8.2 ± 7.1 | |
| +nuciferine | 5.64 ± 0.23 | 96.2 ± 0.8* |
| Control (Phe) | 5.60 ± 0.25 | 97.0 ± 0.7 |
| +L‐NAME | 4.97 ± 0.10* | 97.7 ± 0.6 |
| +Indomethacin | 5.60 ± 0.21 | 95.9 ± 1.4 |
| +ODQ | 4.86 ± 0.05* | 98.5 ± 0.8 |
| +1400W | 5.97 ± 0.16 | 100.4 ± 0.3 |
| L‐NAME + Indomethacin (Phe) | 4.78 ± 0.05 | 97.6 ± 1 |
| ‐Endo | 4.49 ± 0.07* | 94.1 ± 1.2 |
Phe, phenylephrine; ‐Endo, endothelium‐denuded rings. Data shown are means ± SEM; n = 6–10. *P < 0.05, significant difference between control and treatment group
Nuciferine‐evoked relaxation in arteries was dependent on endothelium‐derived vasorelaxing factors and endothelium
Preincubation with a NOS inhibitor L‐NAME or the NO‐sensitive guanylyl cyclase inhibitor ODQ, partly attenuated the nuciferine‐induced concentration–response curves in endothelium‐intact arterial rings, even though the maximal relaxation did not change. By contrast, the non‐selective COX inhibitor indomethacin or the inducible NOS inhibitor 1400 W did not modulate nuciferine‐induced relaxation (Figure 2A and B and Table 1). The effect of L‐NAME plus indomethacin on the nuciferine‐induced relaxation of arteries with endothelium and the effect of nuciferine on arteries without endothelium were compared. The results showed that arteries without endothelium exhibited a decreased value of pEC50, but the maximal relaxation was unchanged (Figure 2C and Table 1). Incubation with L‐NAME or ODQ and removal of endothelium increased contraction tone as the production of relaxing factors from endothelium was inhibited (Supporting Information Table S1).
Figure 2.
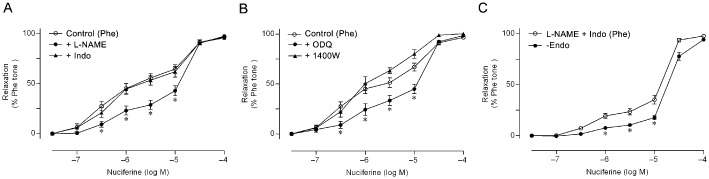
The roles of endothelium‐derived relaxing factors in nuciferine‐triggered vasorelaxation. Effects of L‐NAME, indomethacin (Indo), ODQ and 1400 W on nuciferine‐induced relaxation of arterial rings pre‐contracted by phenylephrine (Phe) (A and B). *P < 0.05, significantly different from control. In ( C ), the role of EDHF in the nuciferine‐induced relaxation of arteries with endothelium and without endothelium (‐endo). Data shown are means ± SEM; n = 7–10. *P < 0.05, significantly different from L‐NAME+Indo.
Nuciferine‐induced vasorelaxation in arteries was independent of β‐adrenoceptors on VSMCs
Nuciferine‐induced concentration‐dependent relaxation was unaffected by propranolol in the endothelium‐denuded arteries (data not shown), suggesting minimal contribution by β‐adrenoceptors on VSMCs to the nuciferine‐induced vasorelaxation.
Calcium regulation was involved in the nuciferine‐induced relaxation of arteries
Compared with control, 20 min treatment of endothelium‐denuded rings with nuciferine (1 or 10 μM) in Ca2+‐free 60 mM K+‐containing Krebs solution shifted the CaCl2‐induced contraction curves towards the right (Figure 3A). Incubation with nuciferine also attenuated phenylephrine‐induced contractions in arterial rings without endothelium (Figure 3B). The pEC50 value of CaCl2 or phenylephrine‐induced contraction was significantly decreased in endothelium‐denuded rings (Table 2).
Figure 3.
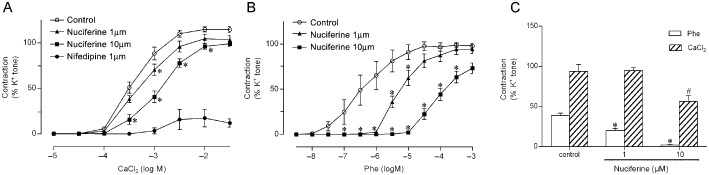
Involvement of calcium regulation in nuciferine‐induced relaxation. CaCl2‐induced dose‐dependent contractions in the absence and presence of nuciferine (1 or 10 μM) in arterial rings without endothelium in Ca2+‐free depolarizing solution, with nifedipine as a positive control (A). In (B), the effects of nuciferine on the contractile response of arterial rings to phenylephrine (Phe). *P < 0.05, significantly different from control (DMSO). In (C), The mechanism mentioned earlier is associated with the intracellular and/or extracellular Ca2+ regulation (C). Contractile response of each segment is expressed as a percentage of contraction by KCl (60 mM). Data shown are means ± SEM; n = 5. *P < 0.05, significantly different from control (phenylephrine), # P < 0.05 compared with control (CaCl2)
Table 2.
pEC 50 values of different agonists contracting the endothelium‐denuded mesenteric arteries after nuciferine incubation
| Agonist | Control | Nuciferine 1 μM | Nuciferine 10 μM |
|---|---|---|---|
| CaCl2 | 3.40 ± 0.08 | 3.26 ± 0.05 | 2.87 ± 0.09* , ** |
| Phenylephrine | 6.41 ± 0.32 | 5.19 ± 0.10* | 4.04 ± 0.09* , ** |
Data are means ± SEM; n = 5. *P < 0.05, significantly different from control (DMSO). **P < 0.05, significantly different from nuciferine (1 μM).
To further test the inhibitory role of extracellular Ca2+ influx and/or intracellular Ca2+ release in the relaxant effect of nuciferine on arteries, the rings without endothelium were incubated with Ca2+‐free Krebs solution containing DMSO (control) or nuciferine (1 or 10 μM) for 20 min, then 10 μM phenylephrine was added to the bath solution to induce contraction via intracellular Ca2+ release. When the maximal contraction was obtained, further contraction via extracellular Ca2+ influx was tested by the addition of 2 mM CaCl2 into the bath solution in the presence of phenylephrine (Figure 3C). The results showed nuciferine alleviated the contractions of arterial rings induced by phenylephrine and the addition of CaCl2 via inhibition of the intracellular Ca2+ release and extracellular Ca2+ influx respectively. Similar effects were also obtained in arteries from male rats (Supporting Information Fig. S1C and D).
Increased [Ca2+]i by nuciferine promoted the generation of NO in HUVECs mediated by eNOS phosphorylation
Cultured HUVECs were incubated with nuciferine (1 or 3 μM). Under these conditions, the MTT test showed no cytotoxicity from nuciferine in the HUVECs, and cell morphology was also not affected (data not shown). Application of nuciferine stimulated the rise in [NO]i and [Ca2+]i, as reflected by the increased fluorescence intensity (Figure 4A and B). The ratio of phosphorylated eNOS to total eNOS was determined to reflect the activation of eNOS. The phosphorylated eNOS at Ser1177 in HUVECs was elevated by a 30 min incubation with nuciferine (Figure 4C). In addition, the elevated eNOS phosphorylation and [NO]i levels induced by nuciferine were abolished by pretreatment with BAPTA‐AM, a Ca2+ chelator. These results indicated that the nuciferine‐induced NO production was mediated by eNOS phosphorylation, following raised [Ca2+]i in HUVECs.
Figure 4.
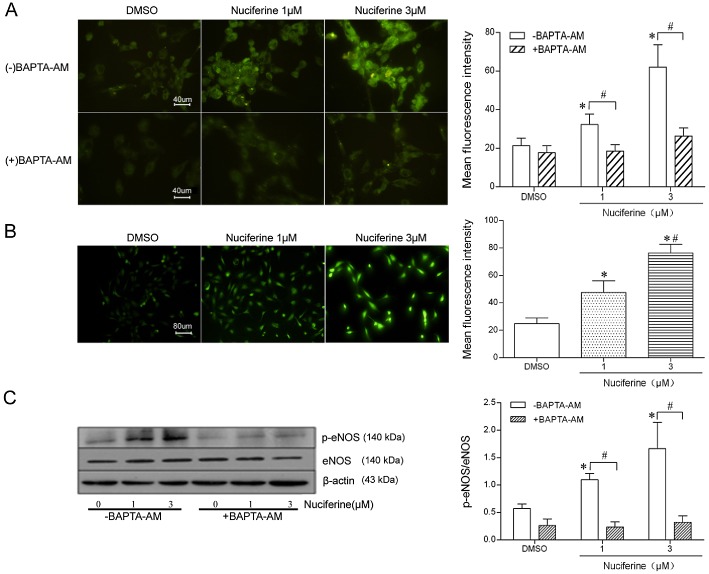
The production of NO induced by nuciferine is mediated by eNOS phosphorylation via increased [Ca2+]i in HUVECs. (A) HUVECs were pretreated with the Ca2+ chelator BAPTA‐AM (0 or 5 μM for 3 h) and then stimulated with nuciferine (1 or 3 μM for 30 min). HUVECs were labelled with the fluorescent NO indicator DAF‐FM DA. The mean fluorescent intensity was evaluated (40× objective). Data shown are means ± SEM; n = 5. *P < 0.05, significantly different from DMSO (‐BAPTA‐AM), # P < 0.05, significantly different as indicated. (B) HUVECs were labelled with the fluorescent Ca2+ indicator Fluo‐4 AM (20× objective). Data shown are means ± SEM; n = 5. *P < 0.05, significantly different from DMSO, # P < 0.05, significantly different from nuciferine (1 μM). (C) Protein was extracted and subjected to immunoblotting for phospho‐eNOS (Ser1177), total eNOS and β‐actin. Western blots are representative of five independent experiments. *P < 0.05, significantly different from DMSO (‐BAPTA‐AM), # P < 0.05, significantly different as indicated.
Nuciferine suppressed [Ca2+]i rise in VSMCs from rat main mesenteric arteries
In Ca2+‐free depolarizing solution, addition of 2 mM CaCl2 triggered Ca2+ influx in primary cultured VSMCs from main mesenteric arteries of rats (Figure 5). Pretreatment for 20 min with 10 μM nuciferine reduced the maximal increase of [Ca2+]i. As a positive control, nifedipine (1 μM) also abolished Ca2+ influx. Its effect was the same in VSMCs from male rats (Supporting Information Figure S3).
Figure 5.
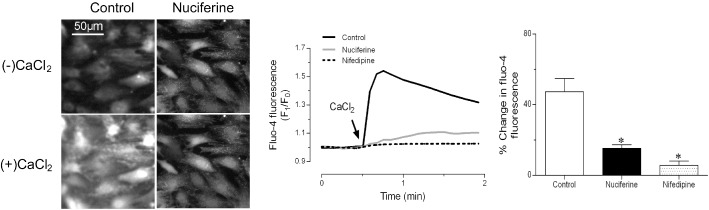
Nuciferine inhibited Ca2+ influx in VSMCs from rat mesenteric arteries. (A) Representative images. (B) Representative time–course of responses and (C) summarized graph of maximal change in Fluo‐4 AM fluorescence intensity, showing the addition of 2 mM CaCl2‐triggered Ca2+ influx in VSMCs was attenuated by preincubation with nuciferine (10 μM for 20 min) in Ca2+‐free 60 mM K +‐containing solution. Nifedipine (1 μM for 20 min) also abolished Ca2+ influx in VSMCs as positive control. Data shown are means ± SEM; n = 5. *P < 0.05, significantly different from control (DMSO).
Discussion
The present study shows novel findings regarding an acute vasorelaxant effect of nuciferine. Nuciferine induced dose‐dependent relaxation of arterial rings through both endothelium‐dependent and ‐independent mechanisms, which were supported by the following observations: (i) endothelium‐derived NO/guanylyl cyclase‐related mechanisms accounted for part of the nuciferine‐induced relaxation; (ii) eNOS activation was mediated by influx of extracellular Ca2+ in HUVECs; (iii) intracellular calcium ([Ca2+]i) regulation was involved in the nuciferine‐induced relaxation of arteries without endothelium; (iv) nuciferine inhibited Ca2+ influx in VSMCs from rat mesenteric arteries; (v) in addition to NO‐mediated vasorelaxation to nuciferine, other endothelium‐derived factors like EDHF might also associated with the relaxant effect of nuciferine; and (vi) K+ channels, β‐adrenoceptors or prostacyclin had little effect on the vasorelaxation to nuciferine. These results reveal that the NO/guanylyl cyclase pathway in endothelial cells and cytoplasmic calcium regulation in VSMCs were correlated with the nuciferine‐evoked relaxation. There was no difference between arterial samples from male and female rats in terms of the vasorelaxation to nuciferine.
Endothelial cells contribute importantly to the regulation of the vascular tension by synthesis and release of several vasodilators such as NO that causes vascular relaxation by increasing production of cGMP in VSMCs (Wong et al., 2010). The removal of endothelium attenuated the relaxation of arterial rings to nuciferine, suggesting that the relaxation was endothelium‐dependent. L‐NAME, a NOS inhibitor, partly suppressed the relaxation of nuciferine, indicating that the vasorelaxant effect of nuciferine was partly associated with NO release. The NO‐sensitive guanylyl cyclase inhibitor ODQ also reduced nuciferine‐induced relaxation. The results clearly demonstrate that nuciferine can elicit a NO/guanylyl cyclase‐dependent vasodilation. By contrast, iNOS played no role in the enhanced NO release, which was confirmed using the iNOS inhibitor 1400 W.
Calcium/calmodulin‐dependent PKII is an important regulator in the rapid activation of eNOS (Cai et al., 2007). The present results showed that the application of nuciferine to HUVECs increased the intracellular Ca2+ level, eNOS phosphorylation at Ser1177 and cytosolic NO production. Pretreatment with the Ca2+ chelator BAPTA‐AM abolished the nuciferine‐induced eNOS phosphorylation and increased cytosolic NO level. The nuciferine‐induced increase in eNOS activation was dependent on extracellular Ca2+ influx in endothelial cells. These results demonstrate that the stimulated rise in cytosolic Ca2+ was causally linked to nuciferine‐induced eNOS phosphorylation, which then augmented NO production in endothelial cells.
The endothelium‐derived vasodilators, prostacyclin and EDHF, also participate in the regulation of vasomotor tone. Prostacyclin induced‐vasodilation is dependent on activation of adenylyl cyclase in most arteries and, in some conditions on VSMC hyperpolarization (Feletou et al., 2011). Pretreatment with the non‐selective COX inhibitor indomethacin did not modulate the relaxant effect of nuciferine in this study, revealing a negligible role for prostacyclin in the response to nuciferine. EDHF‐mediated relaxation is associated with the opening of calcium‐activated potassium channels in endothelial cells, which results in endothelium‐dependent hyperpolarization in VSMCs (Feletou, 2006). The concentration–response curves of nuciferine revealed that the endothelium‐denuded arteries exhibited a decreased value of pEC50, compared with the endothelium‐intact arteries, after pretreatment with L‐NAME plus indomethacin, suggesting indirectly that other endothelium‐derived vasodilators, such as EDHF, could be involved in the relaxation induced by nuciferine.
In endothelium‐denuded arterial rings, nuciferine still caused relaxation. Thus, it is conceivable that nuciferine may directly act on VSMCs. K+ channels and β‐adrenoceptors both affect vascular tension. Activation of K+ channels causes hyperpolarization of the membrane potential and closure of VDCCs in VSMCs, resulting in vasorelaxation (Quilley and Qiu, 2005; Eichhorn and Dobrev, 2007). β‐adrenoceptor agonists elicit vasodilation through the activation of adenylyl cyclase and the consequently increased cAMP in VSMCs (Huang and Kwok, 1998). In the present study, based on the observation on KCl‐contracted arteries (Figure 1B), it is unlikely that opening K+ channels participated in the nuciferine‐induced vasorelaxation. Furthermore, the β‐adrenoceptor antagonist propranolol did not affect the endothelium‐independent relaxation to nuciferine in arteries, suggesting that β‐adrenoceptors were also not linked to the vasorelaxation.
In VSMCs, contractions induced by raised extracellular K+ are known to activate VDCCs, thus increasing influx of extracellular Ca2+ via membrane depolarization (Anfinogenova et al., 2004). Nuciferine markedly attenuated the contractions of arterial rings induced by 60 mM KCl, suggesting that nuciferine could inhibit the extracellular Ca2+ influx via VDCCs. Phenylephrine acting on α1‐adrenoceptors also raises [Ca2+]i and leads to contraction through the inositol phosphate cascade mechanism in the first phase; and subsequently, through Ca2+ release from sarcoplasmic reticulum, or through enhanced extracellular calcium influx via VDCCs mainly or receptor‐operated Ca2+ channels (ROCCs) in the second phase (Lee et al., 2001). We found that incubation with nuciferine caused a rightward shift of the phenylephrine‐induced contraction curves in Ca2+‐containing solutions, implying that nuciferine may restrict intracellular Ca2+ mobilization and/or extracellular Ca2+ influx. Subsequently, the effect of nuciferine (1 or 10 μM) on phenylephrine‐induced contractions was studied in Ca2+‐free medium. In the absence of extracellular Ca2+, nuciferine still inhibited these contractions, probably via inhibition of intracellular Ca2+ release. Subsequent addition of CaCl2 induced contraction, which was reduced by nuciferine as well, probably via inhibition of extracellular Ca2+ influx. CaCl2‐evoked contraction of endothelium‐denuded arteries in a Ca2+‐free depolarizing solution was repressed by nuciferine pretreatment, a finding providing further support for the notion that nuciferine inhibited Ca2+ influx. The inhibitory effect of nuciferine on Ca2+ influx was also confirmed by Fluo‐4 fluorescence imaging where nuciferine reduced the [Ca2+]i rise in VSMCs from rat main mesenteric arteries upon the application of CaCl2. The result indicated that nuciferine‐induced relaxation may be associated with the inhibition of Ca2+ influx through VDCCs and of Ca2+ release in VSMCs.
Nuciferine has been shown to exert beneficial therapeutic effects, promoting insulin secretion (Nguyen et al., 2012) and alleviating dyslipidaemia (Avila et al., 2013). The present study showed that nuciferine relaxed arteries and inhibited contraction. Therefore, nuciferine may have therapeutic potential to normalise vascular tension, which is impaired under cardiovascular pathologies such as hypertension, but further investigations on animals are needed. Notably, nuciferine relaxed arteries through enhancement of NO production and through blocking the rise in intracellular Ca2+ (by Ca2+ influx and by intracellular Ca2+ release). Nuciferine probably acts on several targets that are worth being identified in future studies for drug development.
In summary, this study demonstrated that the primary vascular effects of nuciferine were attributable to increased NO production by the activation of eNOS following a rise in [Ca2+]i levels in endothelial cells and, in VSMCs, to a suppression of [Ca2+]i levels. Our experimental findings suggest that nuciferine could provide beneficial effects in conditions related to abnormal vasoconstriction.
Author contributions
X. W. and W. S. C. performed experiments, analysed the data and wrote the paper. H. Y., M. Z., J. N., Z. L., Z. Z., L. X. and B. L. performed experiments. N. W. and Y. H. designed the research and wrote the paper.
Conflict of interest
The authors have declared no conflict of interest.
Supporting information
Figure S1 Nuciferine induced relaxation of rings from the main mesenteric arteries in both male and female rats. The rings of the main mesenteric arteries from male and female rats pre‐contracted by 60 mM K+ (A) or 10 μM phenylephrine (Phe) (B) were relaxed by nuciferine concentration‐dependently. The effects of nuciferine on the contractile response of endothelium‐free mesenteric arterial rings to CaCl2 (C) or phenylephrine (D) in male rats were shown. Data shown are means ± SEM. n = 5. *P < 0.05 compared with control (DMSO).
Figure S2 Nuciferine induced relaxation of rings from aortas and renal arteries from male and female rats. The aortic (A and B) and renal arterial (C and D) rings from male and female rats pre‐contracted by 60 mM K+ or 10 μM phenylephrine (Phe) were relaxed by nuciferine. Data shown are mean ± SEM. n = 5.
Figure S3 Nuciferine inhibited Ca2+ influx in VSMCs from main mesenteric arteries of male Sprague‐Dawley rats. Summarized graph of maximal change in Fluo‐4 AM fluorescence intensity showing the addition of 2 mM CaCl2‐triggered Ca2+ influx in VSMCs was attenuated by preincubation with nuciferine (10 μM for 20 min) in Ca2+‐free 60 mM K+‐containing solution. Nifedipine (1 μM for 20 min) also abolished Ca2+ influx in VSMCs as positive control. Data shown are means ± SEM. n = 5. *P < 0.05 compared with control (DMSO).
Table S1 The contraction tone and final tone values for nuciferine‐induced relaxations of mesenteric arteries. Data shown are means ± SEM. *P < 0.05, significant difference between control and treatment groups; n = 6–10.
Acknowledgements
We thank Mr. Chi Wai Lau and Jian Liu for their technical assistance in the arterial ring experiment. This work was supported by the grants from the National Natural Science Foundation of China (81220108005 and 81300242).
References
- Agnihotri VK, ElSohly HN, Khan SI, Jacob MR, Joshi VC, Smillie T et al (2008). Constituents of Nelumbo nucifera leaves and their antimalarial and antifungal activity. Phytochem Lett 1: 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M et al (2013a). The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol 170: 1797–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA et al (2013b). The Concise Guide to PHARMACOLOGY 2013/14: Ion Channels. Br J Pharmacol 170: 1607–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M et al (2013c). The Concise Guide to PHARMACOLOGY 2013/14: G Protein‐Coupled Receptors. Br J Pharmacol 170: 1459–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anfinogenova YJ, Baskakov MB, Kovalev IV, Kilin AA, Dulin NO, Orlov SN (2004). Cell‐volume‐dependent vascular smooth muscle contraction: role of Na+, K + , 2Cl‐ cotransport, intracellular Cl‐ and L‐type Ca2+ channels. Pflugers Arch 449: 42–55. [DOI] [PubMed] [Google Scholar]
- Avila MA, Guo F, Yang X, Li X, Feng R, Guan C et al (2013). Nuciferine prevents hepatic steatosis and injury induced by a high‐fat diet in hamsters. PLoS ONE 8: e63770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner H, Cockcroft JR, Deanfield J, Donald A, Ferrannini E, Halcox J et al (2005). Endothelial function and dysfunction. Part II: association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J Hypertens 23: 233–246. [DOI] [PubMed] [Google Scholar]
- Cai H, Liu D, Garcia JGN (2007). CaM kinase II‐dependent pathophysiological signalling in endothelial cells. Cardiovasc Res 77: 30–34. [DOI] [PubMed] [Google Scholar]
- Cheang WS, Lam MY, Wong WT, Tian XY, Lau CW, Zhu Z et al (2013). Menthol relaxes rat aortae, mesenteric and coronary arteries by inhibiting calcium influx. Eur J Pharmacol 702: 79–84. [DOI] [PubMed] [Google Scholar]
- Du H, You J‐S, Zhao X, Park J‐Y, Kim S‐H, Chang K‐J (2010). Antiobesity and hypolipidemic effects of lotus leaf hot water extract with taurine supplementation in rats fed a high fat diet. J Biomed Sci 17 (Suppl. 1): S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichhorn B, Dobrev D (2007). Vascular large conductance calcium‐activated potassium channels: functional role and therapeutic potential. Naunyn Schmiedebergs Arch Pharmacol 376: 145–155. [DOI] [PubMed] [Google Scholar]
- Feletou M (2006). Endothelium‐derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol 26: 1215–1225. [DOI] [PubMed] [Google Scholar]
- Feletou M, Huang Y, Vanhoutte PM (2011). Endothelium‐mediated control of vascular tone: COX‐1 and COX‐2 products. Br J Pharmacol 164: 894–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain SJ, Cheong A, Flemming R, Mair L, Sivaprasadarao A, Beech DJ (2004). Functional up‐regulation of KCNA gene family expression in murine mesenteric resistance artery smooth muscle. J Physiol 556: 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho H‐H, Hsu L‐S, Chan K‐C, Chen H‐M, Wu C‐H, Wang C‐J (2010). Extract from the leaf of nucifera reduced the development of atherosclerosis via inhibition of vascular smooth muscle cell proliferation and migration. Food Chem Toxicol 48: 159–168. [DOI] [PubMed] [Google Scholar]
- Huang B, Ban X, He J, Tong J, Tian J, Wang Y (2010). Hepatoprotective and antioxidant activity of ethanolic extracts of edible lotus (Nelumbo nucifera Gaertn.) leaves. Food Chem 120: 873–878. [Google Scholar]
- Huang Y, Kwok KH (1998). Beta‐adrenoceptor‐mediated relaxation inhibited by tetrapentylammonium ions in rat mesenteric artery. Life Sci 62: PL19–PL25. [DOI] [PubMed] [Google Scholar]
- Kashiwada Y, Aoshima A, Ikeshiro Y, Chen Y‐P, Furukawa H, Itoigawa M et al (2005). Anti‐HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera, and structure–activity correlations with related alkaloids. Bioorg Med Chem 13: 443–448. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Poburko D, Sahota P, Sandhu J, Ruehlmann DO, van Breemen C (2001). The mechanism of phenylephrine‐mediated [Ca(2+)](i) oscillations underlying tonic contraction in the rabbit inferior vena cava. J Physiol 534 (Pt 3): 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C (2010). Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol 160: 1573–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KH, Ta TN, Pham THM, Nguyen QT, Pham HD, Mishra S et al (2012). Nuciferine stimulates insulin secretion from beta cells – an in vitro comparison with glibenclamide. J Ethnopharmacol 142: 488–495. [DOI] [PubMed] [Google Scholar]
- Ono Y, Hattori E, Fukaya Y, Imai S, Ohizumi Y (2006). Anti‐obesity effect of Nelumbo nucifera leaves extract in mice and rats. J Ethnopharmacol 106: 238–244. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP et al; NC‐IUPHAR (2014). The IUPHAR/BPS Guide to PHARMACOLOGY: an expert‐driven knowledge base of drug targets and their ligands. Nucl. Acids Res. 42 (Database Issue): D1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilley J, Qiu Y (2005). K(+)‐induced vasodilation in the rat kidney is dependent on the endothelium and activation of K+ channels. Eur J Pharmacol 508: 193–199. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Shimokawa H, Tang EHC, Feletou M (2009). Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 196: 193–222. [DOI] [PubMed] [Google Scholar]
- Wang N (2002). Constitutive activation of peroxisome proliferator‐activated receptor‐gamma suppresses pro‐inflammatory adhesion molecules in human vascular endothelial cells. J Biol Chem 277: 34176–34181. [DOI] [PubMed] [Google Scholar]
- Wong WT, Wong SL, Tian XY, Huang Y (2010). Endothelial dysfunction: the common consequence in diabetes and hypertension. J Cardiovasc Pharmacol 55: 300–307. [DOI] [PubMed] [Google Scholar]
- Wu MJ, Wang L, Weng CY, Yen JH (2003). Antioxidant activity of methanol extract of the lotus leaf (Nelumbo nucifera Gertn. Am J Chin Med 31: 687–698. [DOI] [PubMed] [Google Scholar]
- Xiao L, Liu Y, Wang N (2014). New paradigms in inflammatory signaling in vascular endothelial cells. Am J Physiol Heart Circ Physiol 306: H317–H325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Nuciferine induced relaxation of rings from the main mesenteric arteries in both male and female rats. The rings of the main mesenteric arteries from male and female rats pre‐contracted by 60 mM K+ (A) or 10 μM phenylephrine (Phe) (B) were relaxed by nuciferine concentration‐dependently. The effects of nuciferine on the contractile response of endothelium‐free mesenteric arterial rings to CaCl2 (C) or phenylephrine (D) in male rats were shown. Data shown are means ± SEM. n = 5. *P < 0.05 compared with control (DMSO).
Figure S2 Nuciferine induced relaxation of rings from aortas and renal arteries from male and female rats. The aortic (A and B) and renal arterial (C and D) rings from male and female rats pre‐contracted by 60 mM K+ or 10 μM phenylephrine (Phe) were relaxed by nuciferine. Data shown are mean ± SEM. n = 5.
Figure S3 Nuciferine inhibited Ca2+ influx in VSMCs from main mesenteric arteries of male Sprague‐Dawley rats. Summarized graph of maximal change in Fluo‐4 AM fluorescence intensity showing the addition of 2 mM CaCl2‐triggered Ca2+ influx in VSMCs was attenuated by preincubation with nuciferine (10 μM for 20 min) in Ca2+‐free 60 mM K+‐containing solution. Nifedipine (1 μM for 20 min) also abolished Ca2+ influx in VSMCs as positive control. Data shown are means ± SEM. n = 5. *P < 0.05 compared with control (DMSO).
Table S1 The contraction tone and final tone values for nuciferine‐induced relaxations of mesenteric arteries. Data shown are means ± SEM. *P < 0.05, significant difference between control and treatment groups; n = 6–10.


