Abstract
Purpose
Valproic acid (VA) is an antiepileptic drug (AED) and histone deacetylase (HDAC) inhibitor taken by patients with glioblastoma (GB) to manage seizures, and it can modulate the biologic effects of radiation therapy (RT). We investigated whether VA use during RT for GB was associated with overall survival (OS).
Methods and Materials
Medical records of 544 adults with GB were retrospectively reviewed. Analyses were performed to determine the association of Radiation Therapy Oncology Group recursive partitioning analysis (RTOG RPA) class, seizure history, and concurrent temozolomide (TMZ) and AED use during RT with OS.
Results
Seizures before the end of RT were noted in 217 (40%) patients, and 403 (74%) were taking an AED during RT; 29 (7%) were taking VA. Median OS in patients taking VA was 16.9 months (vs 13.6 months taking another AED, P=.16). Among patients taking an AED during RT, OS was associated with VA (P=.047; hazard ratio [HR], 0.67; 95% confidence interval [CI], 0.27–1.07), and RTOG RPA class (P<.0001; HR, 1.49; 95% CI, 1.37–1.61). Of the 5 most common AEDs, only VA was associated with OS. Median OS of patients receiving VA and TMZ during RT was 23.9 months (vs 15.2 months for patients taking another AED, P=.26). When the analysis was restricted to patients who received concurrent TMZ, VA use was marginally associated with OS (P=.057; HR, 0.54; 95% CI, −0.09 to 1.17), independently of RTOG RPA class and seizure history.
Conclusions
VA use during RT for GB was associated with improved OS, independently of RTOG RPA, seizure history, and concurrent TMZ use. Further studies of treatment that combines HDAC inhibitors and RT are warranted.
Introduction
Glioblastoma (GB) is the most common primary malignant brain tumor in adults and is associated with a very poor prognosis (1). Standard treatment of GB consists of maximum safe tumor resection followed by external beam radiation therapy (RT) with concomitant and adjuvant temozolomide (TMZ) chemotherapy. This strategy yields a median survival of 14.6 months, with 2- and 5-year survival rates of 27.2% and 9.8%, respectively (2). More effective therapies are needed to improve GB outcomes.
Malignant gliomas have been shown to be driven by epigenetic aberrations (3). Epigenetic modifiers, such as histone deacetylase (HDAC) inhibitors, have been shown to sensitize cancer cells to ionizing radiation, while protecting normal cells and tissues from deleterious effects of RT (4). Valproic acid (VA) is an antiepileptic drug (AED) and HDAC inhibitor used to control seizures in patients with brain tumors (5, 6). Investigators have noted that exposing glioma cell lines U251 and SF539 to VA led to histone hyperacetylation, increased cellular radiosensitivity, and synergistic tumor-growth delay in animals treated with a combination of VA and RT (7). Similarly, it was recently reported that VA sensitized the glioma cell lines D384 and T98 to ionizing radiation, with or without TMZ (8). Importantly, VA can sensitize glioma cells for as long as 24 hours after radiation exposure (9).
The purpose of this study was to investigate the association of VA use during RT for GB and survival. Our hypothesis was that the use of VA during RT for GB may affect outcome.
Methods and Materials
Patients
This retrospective study was conducted with permission from the institutional review board. Eligible patients were identified using electronic institutional databases. Medical records of patients 18 to 70 years old at the time of histologic diagnosis (confirmed by a neuropathologist at our institution) of primary GB between 1998 and 2008 who underwent external beam RT were studied. Patients were excluded if more than 1 patient or treatment characteristic described herein were unavailable in medical records.
Patient characteristics analyzed included age at diagnosis, Karnofsky performance status (KPS), mental status, neurologic functional status, duration of symptoms before diagnosis, and clear documentation of a seizure at any time before the last fraction of RT. Treatment characteristics analyzed included extent of surgery (biopsy, subtotal resection, or gross total resection), RT dose and fractionation, and use of chemotherapy during RT. AEDs used for more than half the duration of RT were recorded for analysis.
Patient and treatment characteristics were used to assign patients to a Radiation Therapy Oncology Group recursive partitioning analysis (RTOG RPA) class (Table 1). Because there is no staging system for GB, the validated RTOG RPA classification system is used clinically for prognosis (10, 11).
Table 1.
Radiation Therapy Oncology Group recursive partitioning analysis classification system and criteria
| RTOG RPA Class | Criteria |
|---|---|
| III | Age < 50 y, KPS 90–100 |
| IV | Age < 50 y, KPS < 90 |
| Age ≥ 50 y, KPS ≥ 70, tumor resected, patient working | |
| V | Age ≥ 50 y, KPS ≥ 70, tumor resected, patient not able to work |
| Age ≥ 50 y, KPS ≥ 70, tumor not resected, RT dose > 54.4 Gy | |
| Age ≥ 50 y, KPS < 70, mental status normal | |
| VI | Age ≥ 50 y, KPS ≥ 70, tumor not resected, RT |
| dose ≤ 54.4 Gy | |
| Age ≥ 50 y, KPS < 70, mental status abnormal |
Abbreviations: KPS = Karnofsky performance status; RT = radiation therapy; RTOG RPA = Radiation Therapy Oncology Group recursive partitioning analysis.
Statistical analysis
Differences in the distribution of patient and treatment characteristics were assessed using χ2 tests and unpaired t tests. Overall survival (OS) was defined as time from start of RT to death or last follow-up (censored February 2, 2012). Kaplan-Meier analysis with log–rank tests were used to evaluate differences in OS. Cox regression models were built to evaluate the association of RTOG RPA class, concurrent TMZ use during RT, seizure before the end of RT, and AED use during RT with OS. Hazard ratios (HR) with 95% confidence intervals (CI) were reported. Analyses were carried out using WinSTAT for Microsoft Excel (Version 2009.1).
Results
Patient and treatment characteristics
Five hundred forty-four patients met criteria for study. Median age was 56 years (range, 18–70 years), and 69.7% of patients were ≥50 years. Most patients were in RTOG RPA class IV or V: III = 99 (18%), IV = 181 (33%), V = 212 (39%), VI = 38 (7%), unknown = 14 (3%). Seizure was noted before the end of RT in 217 (40%) patients. However, 403 (74%) patients were taking an AED during RT, suggesting that many took AEDs to prevent seizures.
Table 2 presents patient and treatment characteristics, grouped by use or nonuse of an AED during RT. There was no significant difference in age group, KPS, duration of symptoms, neurologic function, RT dose, and concurrent use of TMZ during RT between the groups. AED use was significantly more common in men, patients with abnormal mental status, patients who underwent surgery, and, as expected, patients with a history of seizures. Of the 403 patients taking an AED during RT, VA was used by 29 (7%). Table 2 also presents patient and treatment characteristics, grouped by use of VA or another AED during RT. There was a greater prevalence of a seizure history among patients using VA compared with other AEDs, suggesting that VA was used less frequently as a prophylactic AED. There was no statistically significant difference in any other variables, including RTOG RPA class.
Table 2.
Patient and treatment characteristics by use of antiepileptic drug
| Characteristic | No AED (n=128) | AED (n=403) | P value* | VA (n=29) | Non-VA AED (n=374) | P value* |
|---|---|---|---|---|---|---|
| Sex, n (%) | ||||||
| M | 72 (56.3) | 270 (67.0) | .027 | 16 (55.2) | 254 (67.9) | .16 |
| F | 56 (43.8) | 133 (33.0) | 13 (44.8) | 120 (32.1) | ||
| Age, y | ||||||
| Median | 57 | 56 | 53 | 56 | ||
| Age, n (%) | ||||||
| <50 | 37 (28.9) | 124 (30.8) | .993† | 10 (34.5) | 114 (30.5) | .54† |
| ≥50 | 91 (71.1) | 279 (69.2) | 19 (65.5) | 260 (69.5) | ||
| KPS, n (%) | ||||||
| ≥90 | 62 (48.4) | 190 (47.1) | .127 | 11 (37.9) | 179 (47.9) | .517 |
| ≥70 <90 | 56 (43.8) | 158 (39.2) | 13 (44.8) | 145 (38.8) | ||
| <70 | 8 (6.3) | 51 (12.7) | 5 (17.2) | 46 (12.3) | ||
| Mental status, n (%) | ||||||
| Normal | 108 (84.4) | 300 (74.4) | .025 | 19 (65.5) | 281 (75.1) | .154 |
| Abnormal | 18 (14.1) | 93 (23.1) | 10 (34.5) | 83 (22.2) | ||
| Data missing | 2 (1.6) | 10 (2.5) | 0 (0.0) | 10 (2.7) | ||
| Symptom length, n (%) | ||||||
| <12 wk | 98 (76.6) | 312 (77.4) | .306† | 22 (75.9) | 290 (77.5) | .621† |
| ≥12 wk | 30 (23.4) | 90 (22.3) | 7 (24.1) | 83 (22.2) | ||
| Data missing | 0 (0.0) | 1 (0.2) | 0 (0.0) | 1 (0.3) | ||
| Neurologic functional status, n (%) | ||||||
| Working | 54 (42.2) | 156 (38.7) | .503 | 11 (37.9) | 145 (38.8) | .858 |
| Not working | 72 (56.3) | 239 (59.3) | 18 (62.1) | 221 (59.1) | ||
| Data missing | 2 (1.6) | 8 (2.0) | 0 (0.0) | 8 (2.1) | ||
| RTOG RPA, n (%) | ||||||
| Class VI | 7 (5.5) | 31 (7.7) | .25 | 2 (6.9) | 29 (7.8) | .99 |
| Class V | 59 (46.1) | 149 (37.0) | 11 (37.9) | 138 (36.9) | ||
| Class IV | 36 (28.1) | 141 (35.0) | 11 (37.9) | 130 (34.8) | ||
| Class III | 24 (18.8) | 74 (18.4) | 5 (17.2) | 69 (18.4) | ||
| Unknown | 2 (1.6) | 8 (2.0) | 0 (0.0) | 8 (2.1) | ||
| Surgical extent, n (%) | ||||||
| Biopsy | 28 (21.9) | 49 (12.2) | .017 | 6 (20.7) | 43 (11.5) | .341 |
| STR | 49 (38.3) | 192 (47.6) | 12 (41.4) | 180 (48.1) | ||
| GTR | 51 (39.8) | 161 (40.0) | 11 (37.9) | 150 (40.1) | ||
| Radiation dose, n (%) | ||||||
| >54.4 Gy | 110 (85.9) | 330 (81.9) | .969† | 25 (86.2) | 305 (81.6) | .532† |
| ≤54.4 Gy | 15 (11.7) | 54 (13.4) | 4 (13.8) | 50 (13.4) | ||
| Data missing | 3 (2.3) | 19 (4.7) | 0 (0.0) | 19 (5.1) | ||
| Concurrent chemotherapy, n (%) | ||||||
| TMZ | 51 (39.8) | 134 (33.3) | .208 | 12 (41.4) | 122 (32.6) | .692 |
| Other chemotherapy‡ | 10 (7.8) | 50 (12.4) | 3 (10.3) | 47 (12.6) | ||
| None | 67 (52.3) | 221 (54.8) | 15 (51.7) | 206 (55.1) | ||
| Seizure before end of RT, n (%) | ||||||
| Yes | 4 (3.1) | 221 (54.8) | <.001 | 22 (75.9) | 189 (50.5) | .009 |
| No | 124 (96.9) | 191 (47.4) | 7 (24.1) | 184 (49.2) | ||
| Data missing | 0 (0.0) | 1 (0.2) | 0 (0.0) | 1 (0.3) | ||
Abbreviations: AED = antiepileptic drug; GTR = gross total resection; RTOG RPA = Radiation Therapy Oncology Group recursive partitioning analysis; STR = subtotal resection; TMZ = temozolomide.
χ2
t test.
Other chemotherapy included carmustine, lomustine, procarbazine, vincristine, and cisplatin.
Survival
The median OS of the entire cohort was 14 months (range, 0–197 months). Median OS was 17.6, 16.4, 11.4, and 8 months in RTOG RPA classes III, IV, V, and VI, respectively (P<.0001); 16.2 and 12.8 months in patients taking and not taking TMZ during RT, respectively (P=.027); 13.8 and 13.5 months in patients taking and not taking an AED during RT, respectively (P=.98); and 13.2 and 14.7 months in patients with and without a history of seizures, respectively (P=.13). Cox regression analysis revealed that OS was associated with RTOG RPA class (P<.0001; HR, 1.47; 95% CI, 1.36–1.58) and TMZ use during RT (P=.025; HR, 0.80; 95% CI, 0.61–0.99) but not with AED use during RT (P=.25; HR, 1.13; 95% CI, 0.92–1.33) or seizure history (P=.67; HR, 0.95; 95% CI, 0.72–1.18) on multivariable analysis.
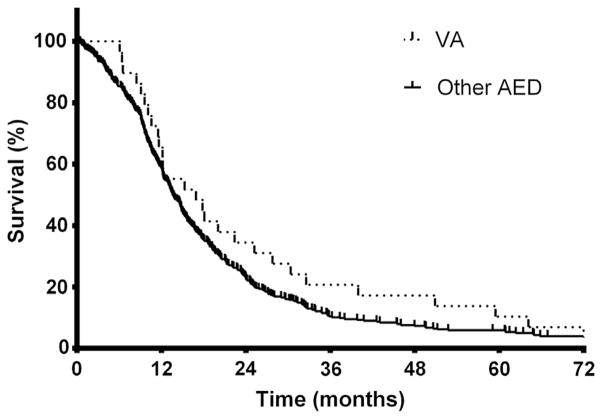
Because of the observed differences in patients taking or not taking an AED during RT, additional analyses were carried out in the patients taking AEDs during RT. Among these patients, median OS of patients taking VA was 16.9 months, compared with 13.6 months in patients using another AED (Fig. 1, P= .16). As noted in Table 3, multivariable Cox regression analysis revealed that OS was associated with VA use during RT and with RTOG RPA class but not with TMZ use during RT or a history of seizures. Cox regression analyses showed no association of OS with any of the other most commonly used AEDs (phenytoin, levetiracetam, carbamazepine, phenobarbital) (Table 3).
Fig. 1.
Overall survival in glioblastoma patients by valproic acid (VA, n=29) or other antiepileptic drug (AED, n=374) use. Median survival was 16.9 or 13.6 months in patients receiving VA or another AED during radiation therapy, respectively (P=.16 by log–rank test). Estimated 2-year and 5-year survival was 34% and 10% or 23% and 5%, respectively.
Table 3.
Multivariable Cox regression models
| Variables | P value | HR | 95% CI |
|---|---|---|---|
| Valproic acid model | |||
| RTOG RPA class | <.001 | 1.49 | 1.37–1.61 |
| Concurrent TMZ | .373 | 0.9 | 0.68–1.12 |
| Seizure history | .136 | 1.17 | 0.96–1.38 |
| Concurrent valproic acid (n=29) | .047 | 0.67 | 0.27–1.07 |
| Phenytoin model | |||
| RTOG RPA class | <.001 | 1.46 | 1.35–1.57 |
| Concurrent TMZ | .04 | 0.81 | 0.63–1.01 |
| Seizure history | .244 | 1.11 | 0.93–1.29 |
| Concurrent phenytoin (n=234) | .267 | 1.11 | 0.93–1.29 |
| Levetiracetam model | |||
| RTOG RPA class | <.001 | 1.53 | 1.42–1.65 |
| Concurrent TMZ | .22 | 0.86 | 0.63–1.10 |
| Seizure history | .543 | 1.06 | 0.87–1.25 |
| Concurrent levetiracetam (n=92) | .549 | 0.92 | 0.65–1.19 |
| Carbamazepine model | |||
| RTOG RPA class | <.001 | 1.46 | 1.35–1.56 |
| Concurrent TMZ | .021 | 0.8 | 0.61–0.99 |
| Seizure history | .225 | 1.12 | 0.94–1.30 |
| Concurrent carbamazepine (n=28) | .757 | 1.07 | 0.66–1.47 |
| Phenobarbital model | |||
| RTOG RPA class | <.001 | 1.45 | 1.34–1.56 |
| Concurrent TMZ | .011 | 0.78 | 0.59–0.97 |
| Seizure history | .192 | 1.13 | 0.95–1.31 |
| Concurrent phenobarbital (n=27) | .26 | 0.79 | 0.37–1.20 |
Abbreviations: CI = confidence interval; HR = hazard ratio; RTOG RPA = Radiation Therapy Oncology Group; TMZ = temozolomide.
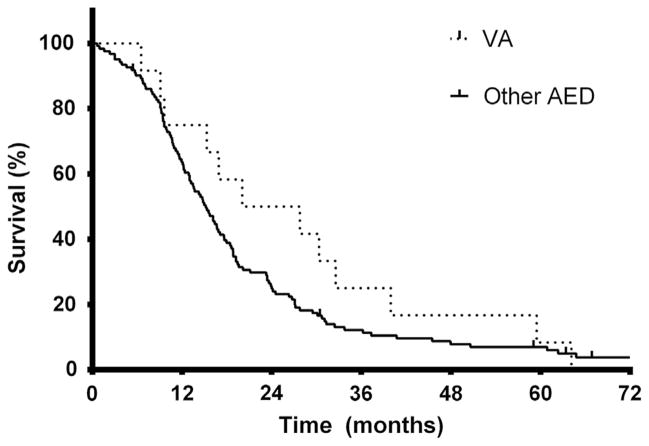
Because concurrent TMZ during RT is the current standard treatment for GB, further analysis was limited to patients who received concurrent TMZ during RT. Patients receiving VA and TMZ during RT had a median OS of 23.9 months, compared with 15.1 months in patients not receiving VA (Fig. 2) (P=.25). Cox regression analysis of patients receiving TMZ during RT revealed that VA use during RT was associated with longer OS with borderline significance (P=.06; HR, 0.54; 95% CI, −0.09–1.17), independently of RTOG RPA class (P=.002; HR, 1.39; 95% CI, 1.18–1.6) and seizure history (P=.007; HR, 1.69; 95% CI, 1.31–2.07). Similar findings were noted among patients not taking TMZ during RT, with the use of VA during RT marginally associated with OS (P=.14; HR, 0.68; 95% CI, 0.16–1.20), with RTOG RPA class but not seizure history associated with OS (P<.001; HR, 1.58; 95% CI, 1.42–1.74 and P=.93; HR, 0.99; 95% CI, 0.73–1.24).
Fig. 2.
Overall survival in glioblastoma patients taking temo-zolomide during radiation therapy by valproic acid (VA, n=12) or other antiepileptic drug (AED, n=122) use. Median survival was 23.9 or 15.2 months in patients receiving VA or another AED during chemoradiation therapy, respectively (P=.25 by log–rank test). Estimated 2-year and 5-year survival was 50% and 8% or 25% and 6%, respectively.
Discussion
This retrospective analysis of GB patients was designed to evaluate the association of VA use during RT with survival. We found that use of the HDAC inhibitor VA during RT was associated with improved OS. Importantly, we controlled for confounding variables by performing multivariable analyses of other factors associated with OS in GB patients, such as RTOG RPA classification (which consists of age, performance status, extent of tumor resection, RT dose, neurologic functional status, and mental status), seizure history, and concurrent TMZ use. Interestingly, among the AEDs used by the patients studied, VA was the only drug associated with prolonged survival. These results suggest that VA may be an effective radiation-effect modifier and a valuable part of the armamentarium for GB.
Our patients represented a typical cohort of GB patients, according to demographic statistics in the United States (1). RTOG RPA class IV or V criteria were met in 74.2% of patients, similar to the original RTOG RPA study (66.0%) (10), and the subsequent RTOG validation study (74.3%) (11). Seizures were noted in 54.0% of GB patients before the end of RT, which is consistent with that reported in GB patients (30%–50%) (6).
The use of AEDs in GB patients varies considerably. Guidelines do not recommend the use of prophylactic AEDs in patients with brain tumors (12). Nevertheless, 47.4% of patients in the present series were taking AEDs in the absence of seizures. Reports from Europe suggest that VA is the AED of choice for GB patients (13). We found few patients (7%) taking VA during RT. Another study of GB patients in the United States during the same period as the present study noted that 6% of patients received VA during RT. We speculate that VA was used infrequently because it is associated with more side effects than more recently developed AEDs, although the long record of safety in neurologic and psychiatric disorders is noteworthy.
The OS of patients in the present cohort is similar to previous estimations (median, 14 months) (2). The most powerful predictor of OS in the present study was RTOG RPA class. A recent study conducted using an adaptation of the RTOG RPA classification system found 95% CIs of median survival of 15–21 months, 13–16 months, and 9–12 months in classes III, IV, and V, respectively (14), compared with 17.6, 16.4, and 11.4 months in the present study, suggesting that our patient classification was consistent with the outcomes of a cotemporaneous cohort. Moreover, TMZ use was associated with longer survival, which is consistent with data from a randomized controlled trial (2).
Previous and more limited analyses have also suggested an association between VA and OS in GB patients. A study of 168 GB patients who underwent surgery, RT, and chemotherapy reported significantly improved survival among patients receiving a non-hepatic enzyme–inducing AED (86.4% were taking VA), although it was not specified whether AEDs were given during RT. The authors suggested that patients taking hepatic enzyme-inducing AEDs metabolize chemotherapy more efficiently, thus abrogating therapeutic effects, or alternatively that VA inhibited chemotherapy metabolism and increased bioavailability, to enhance therapeutic effects (15). Although our findings support the latter hypothesis, inasmuch as patients taking TMZ during RT were found to have longer survival if they were also taking VA, we did not find significantly worse survival in patients taking hepatic enzyme-inducing AEDs (such as phenytoin or carbamazepine). Moreover, a recent study by the RTOG (0525) did not find an association of dose-intensified TMZ and longer survival, suggesting that hepatic enzyme inhibition by VA (estimated to be modest, at approximately 5%) and increased bioavailability would probably not account for differences in survival.
A study of glioma patients with epilepsy found that GB patients receiving VA had a median OS of 20 months (vs 15 months if using another AED). This finding is consistent with the present study, with a median OS of 16.9 months in patients taking VA (vs 13.7 months if taking alternative AEDs). Another report of 101 patients with newly diagnosed GB who underwent chemo-radiation therapy with TMZ indicated that 38 patients took VA at some time during therapy with TMZ (≥25 of these received VA during RT). Median OS of patients taking VA was 26.39 months, compared with 14.03 or 14.69 months, respectively, for patients not taking an AED or taking a hepatic enzyme-inducing AED. Despite the trend of longer survival among patients taking VA and prolonged survival similar to the present study (median OS of 23.9 months), multivariable analysis did not suggest a significant association of VA use and survival, after controlling for RTOG RPA classification.
A recent retrospective analysis of the EORTC/NCIC 26981-22981/CE.3 trial assessed the association of AED use and survival of GB (16). Of the 573 patients participating in the trial between 2000 and 2002, 68% were taking an AED at time of enrollment, which is a proportion similar to the present study (74%). VA was used by 17.4% of patients. Reason for, or duration of, AED use was not recorded. Median OS among patients taking VA during RT was 12.78 months. Among patients taking VA during RT, median OS was 10.09 and 17.35 months when not taking and taking TMZ, respectively. respectively. In a multivariable Cox regression analysis, among patients taking TMZ during RT, use of VA was significantly associated with longer survival, compared with patients not taking an AED, or taking a hepatic enzyme-inducing AED. Multivariable analysis was carried out using some prognostic variables (age, extent of surgery, corticosteroid administration, mental status) but not performance status, neurologic functional status, or RT dose. Moreover, seizure history, which was not analyzed, in previous studies has been associated with outcome in patients with high-grade gliomas and therefore could be a major confounding factor. The present analysis has overcome some of the limitations of the EORTC/NCIC analysis and, like that study, supports the hypothesis that the outcome in GB patients may be improved by taking VA during RT.
Other AEDs have been reported to have HDAC inhibitory properties, although VA is the most potent HDAC inhibitor of clinically available AEDs (17). Many have speculated that VA is not a potent enough HDAC inhibitor to be used in oncologic settings. However, VA has long been used as an AED and mood stabilizer, suggesting adequate central nervous system penetration and biologic effect. Moreover, Tsai and colleagues performed a retrospective analysis of 102 patients with GB; 7 patients received VA after first tumor resection but before second tumor resection. Histone 3 lysine 9 hyperacetylation was found in 4 of 7 patients on immunohistochemical staining of formalin-fixed, paraffin-embedded tumor tissue only after patients started taking VA. This study demonstrates that VA can modify the biologic target in some, but not all, patients receiving a dose titrated to target serum concentrations of 50 to 100 μg/mL (roughly equivalent to 0.5 mM) (18).
Relevant to the present study are previous studies suggesting that HDAC inhibitors, specifically VA, may protect neural tissues from therapeutic radiation effects. In a rat model of radiation spinal cord injury, investigators showed that animals receiving VA before and during RT were significantly less likely to manifest late radiation-related injury (paralysis) (19). In another study, hippocampal neurons were protected from radiation damage in vitro and in vivo by VA in colony formation and apoptosis assays (20). A clinical study from Australia demonstrated that cerebral radio-necrosis develops significantly later in patients taking VA than in patients not taking VA (21). Taken together, these findings suggest that VA is a unique radiation-effect modifier worthy of further study, with radiosensitizing properties in cancers and radioprotective properties in normal tissues.
Our study has several potential limitations. First, we retrospectively reviewed the outcomes in a heterogenous group treated over a 10-year period. Thus, this study may be affected by the biases inherent in any retrospective analysis. Nevertheless, our outcomes are consistent with the results of other studies. Second, the reason for and exact duration of AED use was not specified. However, by including seizure history in our analyses, we attempted to minimize bias introduced by patients receiving AEDs for seizure prevention (vs treatment of seizures). Third, the number of patients taking VA in our study was relatively small. We are optimistic that appropriately designed prospective trials (such as NCT00302159 and NCT00879437) will help assess the effect of VA during RT and TMZ in a larger cohort of GB patients.
In conclusion, our study offers an important perspective on the effect of VA on the outcome of GB patients treated with RT. We accounted for confounding factors by incorporating the RTOG RPA classification, which was not used in all the previous studies. Furthermore, seizure history and use of TMZ during RT are important factors included and adjusted for on multivariable analysis. Despite clear strengths of this study, we suggest that properly designed prospective studies be carried out to determine whether VA, or other HDAC inhibitors, can improve GB outcomes. Importantly, incorporating analyses of the potential protective effects of VA would be critical to fully understanding the therapeutic benefit in combination with RT.
Summary.
Valproic acid (VA) is an antiepileptic drug and histone deacetylase (HDAC) inhibitor taken by patients with glioblastoma to manage seizures; preclinical studies suggest that it can modulate the biologic effects of radiation therapy (RT). We investigated whether VA use during RT for glioblastoma was associated with overall survival by retrospective multivariable analysis in a large cohort. The results suggest that HDAC inhibitors, like VA, may improve the results of RT and should be subjected to clinical trials.
Acknowledgments
M.C. was supported by the Medical Student Summer Fellowship Research Program by the Brain Tumor Center at Memorial Sloan-Kettering Cancer Center.
Footnotes
Presented in part at the 54th Annual Meeting of the American Society for Radiation Oncology in (ASTRO) Boston, MA, October 28-31, 2012.
Conflict of interest: none.
References
- 1.CBTRUS. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumours Diagnosed in the United States in 2004–2008 (March 23, 2012 Revision) Hinsdale, IL: Central Brain Tumor Registry of the United States; 2012. [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munshi A, Kurland JF, Nishikawa T, et al. Histone deacetylase inhibitors radiosensitize human melanoma cells by suppressing DNA repair activity. Clin Cancer Res. 2005;11:4912–4922. doi: 10.1158/1078-0432.CCR-04-2088. [DOI] [PubMed] [Google Scholar]
- 5.Phiel CJ, Zhang F, Huang EY, et al. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- 6.van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007;6:421–430. doi: 10.1016/S1474-4422(07)70103-5. [DOI] [PubMed] [Google Scholar]
- 7.Camphausen K, Cerna D, Scott T, et al. Enhancement of in vitro and in vivo tumor cell radiosensitivity by valproic acid. Int J Cancer. 2005;114:380–386. doi: 10.1002/ijc.20774. [DOI] [PubMed] [Google Scholar]
- 8.Van Nifterik KA, Van den Berg J, Slotman BJ, et al. Valproic acid sensitizes human glioma cells for temozolomide and gamma-radiation. J Neurooncol. 2012;107:61–67. doi: 10.1007/s11060-011-0725-z. [DOI] [PubMed] [Google Scholar]
- 9.Chinnaiyan P, Cerna D, Burgan WE, et al. Postradiation sensitization of the histone deacetylase inhibitor valproic acid. Clin Cancer Res. 2008;14:5410–5415. doi: 10.1158/1078-0432.CCR-08-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curran WJ, Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 11.Scott CB, Scarantino C, Urtasun R, et al. Validation and predictive power of Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis classes for malignant glioma patients: A report using RTOG 90-06. Int J Radiat Oncol Biol Phys. 1998;40:51–55. doi: 10.1016/s0360-3016(97)00485-9. [DOI] [PubMed] [Google Scholar]
- 12.Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: Anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54:1886–1893. doi: 10.1212/wnl.54.10.1886. [DOI] [PubMed] [Google Scholar]
- 13.van Breemen MS, Rijsman RM, Taphoorn MJ, et al. Efficacy of anti-epileptic drugs in patients with gliomas and seizures. J Neurol. 2009;256:1519–1526. doi: 10.1007/s00415-009-5156-9. [DOI] [PubMed] [Google Scholar]
- 14.Mirimanoff RO, Gorlia T, Mason W, et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma: Recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J Clin Oncol. 2006;24:2563–2569. doi: 10.1200/JCO.2005.04.5963. [DOI] [PubMed] [Google Scholar]
- 15.Oberndorfer S, Piribauer M, Marosi C, et al. P450 enzyme inducing and non-enzyme inducing antiepileptics in glioblastoma patients treated with standard chemotherapy. J Neurooncol. 2005;72:255–260. doi: 10.1007/s11060-004-2338-2. [DOI] [PubMed] [Google Scholar]
- 16.Weller M, Gorlia T, Cairncross JG, et al. Prolonged survival with valproic acid use in the EORTC/NCIC temozolomide trial for glioblastoma. Neurology. 2011;77:1156–1164. doi: 10.1212/WNL.0b013e31822f02e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eyal S, Yagen B, Sobol E, et al. The activity of antiepileptic drugs as histone deacetylase inhibitors. Epilepsia. 2004;45:737–744. doi: 10.1111/j.0013-9580.2004.00104.x. [DOI] [PubMed] [Google Scholar]
- 18.Tsai H-C, Wei K-C, Tsai C-N, et al. Effect of valproic acid on the outcome of glioblastoma multiforme. B J Neurosurg. 2012;26:347–354. doi: 10.3109/02688697.2011.638996. [DOI] [PubMed] [Google Scholar]
- 19.Kornguth D, Su J, Li X, et al. Valproic acid shows normal tissue protection in a rat spinal cord model. Int J Radiat Oncol Biol Phys. 2008;72:S697–S698. [Google Scholar]
- 20.Thotala D, Sweeney K, Leahy K, et al. Valproic Acid enhances radiation therapy by protecting normal hippocampal neurons and sensitizing malignant glioblastoma cells in vivo and in vitro. Int J Radiat Oncol Biol Phys. 2011;81:S669. [Google Scholar]
- 21.Ruben JD, Dally M, Bailey M, et al. Cerebral radiation necrosis: Incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys. 2006;65:499–508. doi: 10.1016/j.ijrobp.2005.12.002. [DOI] [PubMed] [Google Scholar]