Abstract
Rationale
Varenicline is an α4β2 nicotinic acetylcholine receptor partial agonist that has been found to be effective for treating tobacco dependence. However, the subjective and behavioral mediators of its efficacy are not known.
Objectives
Using multiple sessions of laboratory-based assessment, this double-blind, placebo-controlled experiment was designed to test if varenicline reduced both tonic and cue-provoked tobacco cravings, and if it attenuated perceived reward from smoking.
Methods
Participants in the present analysis include 100 smokers who were scheduled for three assessment sessions: at baseline, before receiving medication; at mid-run-in, 5– 7 days after beginning medication; and after full dosage was reached, 12–15 days. Following overnight abstinence, each session included assessment of tonic craving, reactivity (including craving) to smoking cues, expected value of a cigarette, smoking behavior, and self-reported reward following smoking.
Results
Varenicline, compared to placebo, reduced tonic craving, cue-provoked craving by the final assessment, the expected value of cigarettes, number of puffs and time spent smoking, and self-reported reward (i.e., satisfaction) from smoking.
Conclusions
Results showing that varenicline reduced tonic craving levels and perceived reward from smoking are consistent with reports from clinical trials, strengthening the evidence in support of these subjective mechanisms of action. This is the first placebo-controlled study to demonstrate that varenicline reduced cue-provoked cravings, thereby offering another potential mediator of its therapeutic effects. Findings may aid in the development of more targeted interventions for tobacco dependence.
Keywords: Tobacco, Nicotine, Smoking, Reward, Reinforcement, Craving, Cues, Conditioned stimuli
Varenicline is an α4β2 nicotinic acetylcholine receptor partial agonist that received FDA approval for the treatment of nicotine dependence in 2006. In theory, the agonist effects of varenicline should reduce nicotine withdrawal symptoms and cravings to smoke, while the antagonist effects should block satisfaction and reinforcement from smoking. The standard dosing schedule includes a 7-day run-up period prior to the target smoking quit date, and up to 12 weeks of use, although extended use may improve maintenance of abstinence (Tonstad et al. 2006).
The clinical efficacy of varenicline has been established via multiple randomized controlled trials (RCTs; Gonzales et al. 2006; Jorenby et al. 2006; Nides et al. 2006; Oncken et al. 2006). Less clear are the bio-behavioral mechanisms under-lying its clinical efficacy. In particular, there is little information about the subjectively experienced and behavioral effects of varenicline during discrete conditions known to motivate tobacco smoking. The current experiment examined two phenomena with particular relevance to smoking cessation: (1) cravings to smoke—both tonic and cue-provoked—and (2) the potency of reward from smoking.
Cravings to smoke
Associative processes appear to play a major role in maintaining nicotine dependence (Baker et al. 2004) and precipitating post-cessation relapse (Brandon et al. 2007). Cue-provoked cravings are thought to develop via classical conditioning (Pavlovian) mechanisms. Smokers, as well as other substance abusers, reliably demonstrate reactivity to cues that have previously been associated with their drug use—in this case, cigarette smoking (Carter and Tiffany 1999). The most robust index of cue-reactivity is self-reported ratings of cigarette cravings, but physiological reactivity is also observed (e.g., responses in heart rate, skin conductance, and neural activation; Brody et al. 2002; Carter and Tiffany 1999). Moreover, the magnitude of cue-provoked craving has been found in at least one study to predict short- and long-term smoking cessation outcomes (Waters et al. 2004). In general, findings support that cue-reactivity contributes to the maintenance of smoking and the failure to achieve and maintain tobacco abstinence (Ferguson and Shiffman 2009; Shiffman et al. 2004; Niaura et al. 1989), although see Perkins (2009) for an alternative perspective. This conclusion is further supported by naturalistic research demonstrating that the vast majority of smoking relapse episodes appear to occur in response to cues that had been previously paired with smoking (Shiffman 1982; Shiffman et al. 1996). In theory, the most effective pharmacotherapies would be those that function by reducing both tonic (or abstinence-induced) craving as well as phasic, cue-provoked craving that is superimposed upon tonic levels (Tiffany et al. 2000; Shiffman et al. 2003; Waters et al. 2004). Indeed, this is the rationale for interventions that combine medications that primarily affect tonic craving (e.g., nicotine transdermal patch) with those that may be used in response to cue-provoked craving (e.g., nicotine gum). In a paper presented at the 2007 meeting of the Society for Research on Nicotine and Tobacco, Niaura et al. reported that varenicline reduced tonic craving levels, but not cue-provoked craving. However, that study tested participants after only a single dose of varenicline. Because of the antagonistic properties of varenicline, any smoking while on the medication (i.e., during the 1-week run-up period) could conceivably constitute extinction trials of any conditioned cues present during smoking. Therefore, in the current study, we assessed both tonic and cue-provoked cravings in participants who reached therapeutic dosage of the drug by following the prescribed dosing schedule.
Reward potency
A unique characteristic of varenicline compared to other pharmacotherapies for treating tobacco dependence is that its nicotine antagonist properties should reduce the reward potency of nicotine. Thus, smoking while on varenicline should be less reinforcing, thereby less likely to lead to additional smoking. This has at least two clinical implications: (1) during the run-up period, cigarettes should become progressively less rewarding and reinforcing to smokers (which, in turn, should also contribute to the extinction of cue-provoked cravings), setting the stage for a more successful cessation attempt; and (2) any post-cessation smoking (i.e., a “lapse”) should be less likely to produce additional smoking, and the eventual return to regular smoking (a full relapse). The latter is particularly important, given the high rate of smoking relapse, and the fact that approximately 90% of initial lapses eventually lead to full relapse (Brandon et al. 1990). Any reduction in lapse–relapse conversion has very high clinical significance. Retrospective self-report data from the varenicline RCTs suggest that varenicline reduced self-reported smoking satisfaction compared to placebo (Gonzales et al. 2006; Jorenby et al. 2006; West et al. 2008), and two short-term experimental studies reported similar findings when self-reported smoking reward was assessed immediately after smoking (Patterson et al. 2009; Perkins et al. 2010b).
Current study
The specific purpose of this study was to understand the subjective and behavioral mechanisms that underlie the established clinical efficacy of varenicline for treating tobacco dependence. Varenicline was the first non-nicotine smoking cessation medication developed specifically for this purpose based on theoretical mechanisms of action. As a nicotinic partial agonist, its use should lead to the extinction of conditioned responses to smoking cues and to attenuated reward from smoking itself. Our study was designed to test these two predictions via multimodal assessment. Positive findings would support the theoretical mechanisms of action of varenicline. Such results would also provide patients and providers additional information about the subjective effects of varenicline that could be useful in their decisions about whether to use the medication. Negative findings would support future investigation into alternative mechanisms of action. Both findings could contribute to continuing efforts to develop more highly targeted medications for treating tobacco dependence.
We proposed the following primary hypotheses:
Tonic (i.e., non-cue-provoked) craving levels would be lower in participants receiving varenicline versus placebo.
Cue-provoked cravings would be lower in participants receiving varenicline versus placebo. (Heart rate and skin conductance were collected as secondary physiological indices of cue reactivity, see Carter and Tiffany 1999.)
Two primary indices of nicotine reward (direct self-report ratings and a cigarette choice index) would be lower in participants receiving varenicline versus placebo. (Secondary, indirect indices of nicotine reward include smoking topography variables.)
Method
Participants
To achieve a final target sample size of 100 following attrition, we screened 573 smokers from the community and randomized 163 non-treatment seeking daily smokers to the two conditions. Inclusion criteria were (a) 18– 60 years of age, (b) smoked at least 15 cigarettes daily, (c) expired-air carbon monoxide (CO)≥8 ppm, and (d) medically eligible to receive varenicline. Smokers who were using other smoking cessation medications, or who had current mood or psychotic disorders (as determined by the Structured Clinical Interview for DSM Disorders [SCID; First et al. 1996]) were excluded. The first assessment session was attended by 114 participants: 58 in the placebo condition and 56 in the varenicline condition. Session 2 was completed by 49 and 39 in the two conditions, respectively, and session 3 by 54 and 46 participants. The final sample comprised the 100 smokers who completed both critical assessment sessions (sessions 1 and 3). Their key demographic and smoking-related variables are provided in Table 1. This study was approved by the university's institutional review board, and therefore complied with the standards of the 1964 Declaration of Helsinki. All participants provided informed consent after receiving written and verbal descriptions of the medications (with known risks), the dosing schedule, and the content of the assessment sessions, including that they would be smoking during these assessments.
Table 1. Baseline characteristics, with means (and SDs) or percentages.
| Variable | Condition | |
|---|---|---|
|
| ||
| Varenicline (n=46) | Placebo (n=54) | |
| Demographic variables | ||
| Gender (% female) | 39.1% | 38.9% |
| Age | 45.8 (9.4) | 41.2 (11.5)* |
| Education (% ≤HS degree) | 34.8% | 46.3% |
| Median household income | $20,000 | $15,000 |
| Race (% African American) | 32.6% | 20.4% |
| Ethnicity (% Hispanic) | 13.3% | 14.8% |
| Smoking-related variables | ||
| Cigarettes/day | 22.9 (9.2) | 26.1 (11.5) |
| Years of daily smoking | 26.6 (11.6) | 23.9 (11.6) |
| FTND | 5.4 (2.2) | 6.2 (1.9) |
| CO | 30.63 (15.7) | 31.19 (15.1) |
| Contemplation ladder | 6.65 (2.30) | 5.46 (3.01)* |
p<.05
Baseline screening and dosing regimen
Following a telephone screening, potential participants were scheduled for a baseline evaluation and screening. The baseline evaluation comprised the informed consent procedure, screening for inclusion and exclusion criteria, and a medical evaluation, including a basic metabolic panel and pregnancy test for women of child-bearing age. Participants also completed the Fagerström Test of Nicotine Dependence (FTND; Heatherton et al. 1991), a validated six-item measure of nicotine dependence, and the Contemplation Ladder, an 11-point single item scale of cessation motivation (Biener and Abrams 1991). Smokers who passed the baseline screening were randomly assigned to receive varenicline or placebo in a double-blind, dose-escalating design. To track the change in dependent measures over time and/or dosage, participants were scheduled for three laboratory assessments over the course of 15 days. Varenicline dosing used the following schedule: 0.5 mg on days 1–3, 0.5 mg BID dosing on days 4–7, and 1 mg BID dosing on days 8–15 to achieve the therapeutic dose of 2 mg/day. Placebo treatment followed the same dispensing schedule. Both varenicline and placebo tablets were provided by Pfizer, Inc. Random assignment was conducted by the Research Pharmacy of Moffitt Cancer Center, so that both experimenters and participants were blind to experimental condition (i.e., double-blind).
Medication was dispensed at the first laboratory assessment with instructions to start the first dose the day after the first assessment. The second assessment occurred at midtitration, (i.e., days 5–7) of the medication regimen. The third and final assessment occurred once the therapeutic dose was reached (i.e., days 12–15). All three laboratory assessments were conducted following overnight (12 h) abstinence and consisted of completion of questionnaires, a cue-reactivity test, and a smoking reward test described in detail below. Participants were instructed to smoke at their normal rate between sessions.
Assessment sessions
Medication-related measures
A 12-item self-report measure assessed the severity of common varenicline side effects (e.g., nausea, flatulence, and dry mouth), at the beginning of each assessment. Participants used a four-point rating scale to indicate to what degree they had experienced each of the potential symptoms. These ratings were averaged to create side effect severity scores. To assess medication adherence, participants were asked to bring in their remaining medication to assessments 2 and 3. An experimenter counted the remaining tablets and calculated the percentage of tablets consumed. To assess the fidelity of the blind, at both the second and third assessments, participants were asked to guess which medication they had received (i.e., varenicline or placebo).
Initial smoking-related measures
Upon arrival to each assessment session, participants' breath carbon monoxide (CO) levels were measured to verify overnight abstinence (i.e., CO value<8 ppm or below 50% of initial baseline value). (Those who had not abstained were rescheduled, if possible, within the 3–4-day assessment window.) Participants then completed three smoking-related questionnaires. The Questionnaire of Smoking Urges (QSU), our primary measure of tonic craving, is a 32-item instrument, including two separate factor scales that roughly correspond to the desire to smoke for its pleasurable effects (positive reinforcement) or to remove unpleasant feelings of negative affect or withdrawal (negative reinforcement; Tiffany and Drobes 1991). The Wisconsin Smoking Withdrawal Scale (WSWS) is a 28-item self-report scale that loads on seven factors with good subscale reliability (Welsch et al. 1999). It includes a highly reliable (α=0.89) four-item craving subscale. The QSU and the WSWS permitted examination of whether medication assignment produced differences in tonic craving and other withdrawal symptoms. The cigarette choice procedure (CCP; Griffiths et al. 1993; Kidorf et al. 1995) is a measure of the subjective expected value of a cigarette. Participants were asked to choose between hypothetically smoking a cigarette now or receiving a small amount of money (from 10 cents up to $6 in increments of 10 cents). A crossover value, at and above which participants prefer money, was obtained (Reid et al. 2007). This first administration of the CCP was designed to assess the general effect of drug condition upon smokers' expected value of a cigarette.
Cue reactivity
An established picture-viewing paradigm was used to assess cue reactivity (Drobes 2002). Smoking-related and neutral control cues were randomly presented to each participant, while subjective (craving) and psychophysiological (heart rate and skin conductance) measures were obtained. The smoking cues included pictures that have elicited substantial craving reports in previous studies, as well as physiological arousal in our prior research (Carter et al. 2006; Gilbert and Rabinovich 2003). The neutral cues comprised pictures selected from the International Affective Picture System (IAPS; Center for the Study of Emotion and Attention 1995), and included objects, people, and situations that have been rated as neither pleasant, unpleasant, nor arousing. A total of 24 pictures with 12 pictures from each category (smoking and neutral) were presented randomly during each session. Different sets of pictures were used across the three assessment sessions to minimize habituation. Pictures were displayed for 6 s each on a 20″ computer monitor located 2.5 ft. in front of participants, controlled by software that synchronized cue presentations with physiological data collection.
Psychophysiological measures were collected throughout each picture-viewing interval, as well as during a 2-s baseline prior to each picture onset. Heart rate (HR) was detected using Ag-AgCl electrodes (with electrolyte gel) placed on each forearm, with signals passed through a Coulbourn (V75-01) bioamplifier with bandpass settings of 8 and 40 Hz. Inter-beat (R-R) intervals were detected to the nearest millisecond using a Schmitt trigger, with values edited offline to correct for artifact, then converted to beats per minute for each half-second period. For skin conductance (SC), two Ag-AgCl electrodes (with unibase conductance medium) were placed on the hypothenar eminence on the non-dominant palm, and an isolated skin conductance coupler (Coulbourn V71-23) created a constant voltage (0.5 V) circuit. The resulting signal was sampled at a rate of 20 Hz and calibrated to detect a range of 0–40 μS. HR and SC were both expressed as the deviation of each 0.5-s value during picture viewing from the 2-s baseline, with signals averaged over trials within each picture category. The average HR waveform was scored for the initial deceleration (D1) following picture onset, as well as subsequent acceleratory (A1) and deceleratory (D2) components. For SC, we scored the average and maximum change during seconds 2 through 6 following picture onset.
Following the offset of each picture, subjective smoking craving ratings were collected via computer. To minimize session length and participant burden, cue-induced craving was assessed via a single item: How strong was your craving to smoke a cigarette? Responses were obtained using a 0 to 20 scale, with anchor labels of “None” and Very Strong” placed at each end of the scale. Coefficient alpha reliabilities for this instrument within session and cue type ranged from 0.95 to 0.98. A variable (12–20 s) inter-trial interval occurred prior to the next picture presentation. The cue reactivity assessment took approximately 20 min to complete.
Smoking reward and behavior
Following the cue reactivity assessment, we assessed the effect of varenicline on reward from smoking, as determined by self-report measures obtained immediately after smoking and behavioral observation of smoking topography. As an analog of an initial lapse, participants were first given half of one of their own cigarettes and instructed to smoke it ad libitum. They then rated their response to that cigarette using the Modified Cigarette Evaluation Questionnaire (mCEQ) and completed the CCP to assess their expected value of an additional cigarette. The mCEQ (Cappelleri et al. 2007; Rose et al. 1998, 2000, 2001; Westman et al. 1992) is a self-rated scale that assesses the participant's response to a smoked cigarette. Twelve items are scored in five domains: satisfaction, psychological reward, aversion, sensory feelings (one item, labeled respiratory sensations), and reduction in craving. Similar to the procedure used by Perkins et al. (2006), participants were then given another (full) cigarette and told that they may smoke as much or as little as desired over the subsequent 20 min. Smoking topography was obtained by videotaping participants during the smoking period and scoring smoking behaviors, including latency to cigarette, number of puffs, and total time spent smoking. Immediately after smoking, participants completed the mCEQ again.
Participation duration and payment
Each assessment session lasted approximately 1–1.5 h. Participants were paid $25 for completing the baseline evaluation, $40 for completing the first assessment, $60 for completing the second assessment, and $75 for completing the third assessment. Participation in the study concluded, and medication was discontinued after the third assessment, resulting in a total participation duration of approximately 15 days.
Results
Overview of primary analyses
To test our hypotheses, we assessed condition effects (varenicline vs. placebo) across the assessment time points via mixed-model repeated measures analyses. Specifically, the models included effects for condition (varenicline vs. placebo), assessment time (assessments 1, 2, and 3), and the interaction of these two factors as fixed effects, with time as a random effect. Primary dependent variables examined with these analyses included: tonic craving (QSU and WSWS craving scale), smoking reward, and expected value (mCEQ and CCP crossover value). These analyses were also conducted on the secondary dependent variables of nicotine withdrawal (remaining WSWS scales) and smoking topography variables (latency to first puff, total number of puffs, and total time spent smoking). Smoking cue-provoked craving ratings, and the secondary dependent variables of heart rate and skin conductance responses to cues were analyzed similar to the models above, with the addition of neutral cue-provoked responses included as a third-level parameter, with repeated effects. This provided an estimate of the influence of smoking-related cues, while controlling for response magnitude.
Preliminary analyses
Baseline characteristics
As seen in Table 1, significant baseline differences were found on age and motivation to quit smoking (Contemplation Ladder). However, repeating the primary analyses reported below with these two variables as covariates did not alter the results.
Attrition and missed Sessions
After breaking the blind, it was revealed that greater attrition from assessments 1 to 3 occurred for the varenicline (17.9%) condition compared to the placebo condition (7.4%), although this difference did not reach statistical significance, χ2 (1, N= 114)=3.18, p=.08. Moreover, 30.4% of varenicline participants missed assessment 2, compared to 15.5% of placebo participants, which also approached significance, χ2 (1, N=114)=3.56, p=.06. Among those who completed the study (i.e., attended assessments 1 and 3), attendance at assessment 2 did not differ by condition (varenicline, 82.6%; placebo, 92.6%), χ2 (1, N=100)=2.35, p=.13. Although more participants in the varenicline condition failed their CO screen to verify overnight smoking abstinence (25% vs. 13%), this difference was not statistically significant, χ2 (1, N=121)=2.78, p=.10. To examine other potential determinants of attrition, we compared those who completed both assessments 1 and 3 against those who did not complete assessment 3, using variables collected during the baseline screening and assessment 1. At assessment 1, those who did not complete the study reported higher QSU (tonic craving) scores [(M=6.27, SD=0.88 vs. M=5.15, SD=1.21), t(112)= 3.34, p<.01]. Also, for the second cigarette smoked ad lib (the full cigarette), study noncompleters reported lower smoking satisfaction [(M=3.51, SD=1.34 vs. M=4.91, SD=1.76), t(111)=−2.75, p<.01], psychological reward [(M=2.53, SD=1.56 vs. M=3.69, SD=1.60), t(111)=−2.47, p<.05], and respiratory sensations [(M=2.62, SD=1.26 vs. M=3.99, SD=2.08), t(111 or 21.68)=−3.38, p<.01], as measured by the mCEQ. We found no significant differences between study completers and non-completers on other variables (all ps>.08).
Medication adherence
Unfortunately, not all participants complied with instructions to return their remaining medication. At assessments 2 and 3, compliance rates were 76.32% and 89.13% for the varenicline condition and 79.59% and 83.33% within the placebo condition. Among those who complied, adherence for taking the 0.5-mg dose was 98.89% for those randomized to take varenicline and 99.73% for their placebo counterparts. Adherence for taking the 1-mg dose was 96.98% and 98.33%, respectively. Because of the high adherence rates and the equivalent compliance and adherence rates between conditions (all ps>.40), we included all participants in subsequent analyses.
Side effects
At assessment 1 (prior to medication), there was no difference between varenicline and placebo conditions on average side effects severity scores [(M=0.06, SD=0.27 vs. M=0.02, SD=0.05), F(1, 97)=1.30, p=.26]. To examine the influence of treatment condition on side effects severity scores, separate ANCOVAs were conducted for assessment 2 and 3 (controlling for assessment 1 scores). Participants randomized to the varenicline condition reported higher levels of side effects at assessment 2 [(M=0.10, SD=0.15 vs. M=0.04, SD=0.06), F(1, 83)= 7.28, p<.01] and assessment 3 [(M=0.09, SD=0.11 vs. M=0.04, SD=0.10), F(1, 96)=6.16, p≤.05], relative to their placebo counterparts. Overall, participants in both conditions reported very few side effects that they rated as either moderate or severe. The highest frequency of these occurred at assessment 2 for nausea (varenicline= 5.2%, placebo=0%) and dry mouth (varenicline=5.2%, placebo=2.0%).
Cue-reactivity manipulation check
We examined potential differences, at assessment 1 (i.e., prior to medication dosing), in self-reported craving and psychophysiological indices from the different cue stimuli (smoking vs. neutral). As expected, the smoking cues evoked higher levels of self-reported craving, relative to neutral cues [(M=15.31, SD=4.90 vs. M=6.38, SD=5.69), t(192)= 11.70, p<.001]. However, there were no differences between cues for any of the psychophysiological variables (all ps>.13).
Hypothesis tests
Tables 2 and 3 list the marginal means of the dependent measures. The mixed-models analyses resulted in several time×condition interactions, indicating differential change over time as a function of condition assignment (see Tables 4 and 5).
Table 2. Marginal means (and standard errors) for craving and withdrawal as a function of assessment time and condition.
| Assessment session | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Assessment 1 | Assessment 2 | Assessment 3 | |||||
|
|
|
|
|||||
| Placebo | Varenicline | Placebo | Varenicline | Placebo | Varenicline | ||
| Craving scales | Cue-provoked craving (0–20) | 10.96 (0.84) | 10.58 (1.03) | 10.43 (0.84) | 9.03 (1.03) | 9.90 (0.84) | 7.48 (1.03)* |
| Smoke cue craving (0–20) | 15.72 (0.70) | 14.73 (0.75) | 15.29 (0.72) | 12.85 (0.80)* | 14.61 (0.69) | 11.18 (0.76)* | |
| Neutral cue craving (0–20) | 6.29 (0.67) | 6.42 (0.72) | 5.31 (0.68) | 4.83 (0.75) | 5.28 (0.67) | 3.73 (0.73) | |
| QSU total: tonic craving (1–7) | 5.10 (0.19) | 5.21 (0.21) | 4.87 (0.20) | 4.39 (0.22)* | 4.93 (0.19) | 3.53 (0.21)*** | |
| QSU F1: tonic craving (1–7) | 6.14 (0.21) | 6.30 (0.23) | 5.70 (0.22) | 5.28 (0.24)* | 5.84 (0.21) | 4.30 (0.23)*** | |
| QSU F2: tonic craving (1–7) | 4.06 (0.20) | 4.11 (0.22) | 4.03 (0.21) | 3.49 (0.24)* | 4.02 (0.20) | 2.76 (0.22)*** | |
| WSWS scales (0–8) | Tonic craving | 5.46 (0.24) | 5.61 (0.25) | 5.27 (0.24) | 4.59 (0.27)* | 5.21 (0.24) | 3.75 (0.25)*** |
| Anger | 2.71 (0.27) | 2.82 (0.29) | 2.77 (0.27) | 2.79 (0.30) | 2.63 (0.27) | 2.41 (0.29) | |
| Anxiety | 3.81 (0.23) | 4.09 (0.24) | 3.61 (0.23) | 3.69 (0.26) | 3.61 (0.23) | 3.16 (0.24) | |
| Sleep | 3.14 (0.27) | 3.63 (0.29) | 2.85 (0.28) | 3.37 (0.30) | 2.83 (0.27) | 3.47 (0.29) | |
| Hunger | 3.56 (0.23) | 3.96 (0.25) | 3.49 (0.23) | 4.16 (0.26) | 3.47 (0.23) | 3.60 (0.25) | |
| Concentration | 2.46 (0.21) | 2.68 (0.22) | 2.53 (0.21) | 2.35 (0.24) | 2.48 (0.21) | 2.26 (0.22) | |
| Sadness | 2.19 (0.17) | 2.34 (0.18) | 2.11 (0.17) | 2.07 (0.19) | 2.41 (0.17) | 2.07 (0.18) | |
Condition comparisons within assessment session were conducted only when full model revealed condition × time interaction. Cue-provoked craving values reflect responses to smoking cues while controlling for responses to neutral cues. Total, factor 1 and factor 2
QSU Questionnaire of Smoking Urges, WSWS Wisconsin Smoking Withdrawal Scale
p<.05;
p<.01;
p<.001
Table 3. Marginal means (and standard errors) for smoking reward/reinforcement for the one half cigarette and full cigarette as a function of assessment time and condition.
| Assessment session | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Assessment 1 | Assessment 2 | Assessment 3 | |||||
|
|
|
|
|||||
| Placebo | Varenicline | Placebo | Varenicline | Placebo | Varenicline | ||
| One half cigarette | |||||||
| Cigarette choice cross-over value ($) | 4.33 (0.27) | 4.04 (0.29) | 4.10 (0.27) | 3.19 (0.30)* | 4.17 (0.26) | 2.65 (0.29)*** | |
| mCEQ scales (1–7) | Smoking satisfaction | 5.44 (0.24) | 4.70 (0.26)* | 5.05 (0.24) | 4.08 (0.27) | 4.77 (0.24) | 3.72 (0.26) |
| Psychological reward | 4.00 (0.20) | 3.60 (0.21) | 3.59 (0.20) | 3.11 (0.23) | 3.38 (0.20) | 2.65 (0.21) | |
| Aversion | 2.59 (0.20) | 2.65 (0.21) | 2.70 (0.20) | 2.64 (0.23) | 2.55 (0.20) | 2.57 (0.21) | |
| Respiratory sensations | 4.39 (0.28) | 3.78 (0.30) | 4.14 (0.28) | 3.31 (0.31) | 2.98 (0.28) | 3.15 (0.30) | |
| Craving reduction | 5.17 (0.24) | 5.09 (0.26) | 4.84 (0.25) | 4.67 (0.27) | 4.85 (0.24) | 4.48 (0.26) | |
| Smoking topography | Latency | 3.76 (0.62) | 4.60 (0.63) | 4.15 (0.68) | 4.97 (0.70) | 5.08 (0.66) | 4.60 (0.67) |
| # Puffs | 8.44 (0.38) | 8.04 (0.28) | 8.22 (0.39) | 7.52 (0.41) | 7.88 (0.39) | 7.48 (0.40) | |
| Time smoking | 132.63 (6.68) | 143.73 (6.65) | 127.42 (7.11) | 132.21 (7.32) | 123.75 (6.97) | 134.70 (7.18) | |
| Full cigarette | |||||||
| Cigarette choice cross-over value ($) | 3.77 (0.28) | 3.40 (0.30) | 3.71 (0.28) | 2.85 (0.31)* | 3.72 (0.28) | 2.46 (0.30)** | |
| mCEQ scales (1–7) | Smoking satisfaction | 4.99 (0.26) | 4.8 (0.28) | 4.72 (0.26) | 3.34 (0.29)** | 4.70 (0.26) | 3.13 (0.28)*** |
| Psychological reward | 3.78 (0.22) | 3.59 (0.23) | 3.49 (0.22) | 2.80 (0.25)* | 3.50 (0.22) | 2.46 (0.23)** | |
| Aversion | 1.97 (0.20) | 2.32 (0.22) | 2.42 (0.21) | 2.52 (0.23) | 2.19 (0.20) | 2.26 (0.22) | |
| Respiratory sensations | 4.17 (0.28) | 3.78 (0.30) | 3.89 (0.28) | 2.75 (0.31)* | 3.69 (0.28) | 2.59 (0.30)** | |
| Craving reduction | 5.69 (0.25) | 5.46 (0.27) | 5.53 (0.26) | 4.91 (0.29)* | 5.21 (0.25) | 4.13 (0.27)** | |
| Smoking topography | Latency | 3.67 (0.75) | 5.33 (0.77) | 5.42 (0.77) | 6.40 (0.83) | 4.52 (0.79) | 6.85 (0.80) |
| # Puffs | 9.58 (0.72) | 9.07 (0.72) | 10.07 (0.74) | 7.67 (0.79)* | 9.86 (0.76) | 6.05 (0.77)** | |
| Time smoking | 192.62 (17.76) | 200.40 (17.83) | 203.39 (18.36) | 177.94 (19.70) | 196.73 (19.07) | 131.86 (19.13)* | |
Condition comparisons within assessment session were conducted only when full model revealed condition × time interaction. Latency is the duration of time (in seconds) from when participants were given the opportunity to smoke until they lit their cigarette. # Puffs is the total number of puffs taken. Time smoking is the total time spent smoking (in seconds)
mCEQ Modified Cigarette Evaluation Questionnaire
p<.05;
p<.01;
p<.001
Table 4. Parameter estimates (and standard errors) of random effects models for craving and withdrawal with assessment time and condition effects.
| Cue-provoked craving |
Smoke cue craving |
Neutral cue craving |
QSU total: tonic craving |
QSUF1: tonic craving |
QSU F2: tonic craving |
WSWS scales | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| WSWS: tonic craving |
WSWS: anger | WSWS: anxiety | WSWS: sleep | WSWS: hunger | WSWS: concentration |
WSWS: sadness | |||||||
| Intercept | 10.97 (0.66)*** | 15.79 (0.64)*** | 6.16 (0.74)*** | 5.05 (0.16)*** | 6.05 (0.17)*** | 4.06 (0.19)*** | 5.44 (0.21)*** | 2.74 (0.28)*** | 3.78 (0.21)*** | 3.10 (0.27)*** | 3.55 (0.22)*** | 2.48 (0.22)*** | 2.13 (0.17)*** |
| Time | −0.53 (0.23)* | −0.57 (0.33) | −0.50 (0.29) | −0.09 (0.10) | −0.15 (0.12) | −0.02 (0.11) | −0.13 (0.12) | −0.04 (0.12) | −0.10 (0.12) | −0.15 (0.11) | −0.04(0.11) | 0.01 (0.12) | 0.11 (0.09) |
| Condition | −0.40 (0.97) | −1.03 (0.94) | 0.15 (1.09) | 0.16 (0.24) | 0.25 (0.25) | 0.07 (0.28) | 0.15 (0.31) | 0.13 (0.41) | 0.32 (0.31) | 0.48 (0.39) | 0.51 (0.32) | 0.17 (0.32) | 0.17 (0.25) |
| Time × condition | −1.02 (0.33)** | −1.23 (0.50)* | −0.78 (0.43) | −0.75 (0.15)*** | −0.85 (0.17)*** | −0.65 (0.16)*** | −0.81 (0.18)*** | −0.17 (0.18) | −0.36 (0.17) | 0.08 (0.16) | −0.14 (0.16) | −0.22 (0.17) | −0.25 (0.14) |
QSU Questionnaire of Smoking Urges, WSWS Wisconsin Smoking Withdrawal Scale
p<.05;
p<.01;
p<.001
Table 5. Parameter estimates (and standard errors) of random effects models for smoking reward variables, with assessment time and condition effects.
| Cigarette choice cross-over value ($) |
mCEQ scales | Smoking topogrciphy variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Smoking satisfaction |
Psychological reward |
Aversion | Respiratory sensations |
Craving reduction | Latency | # Puffs | Time smoking | |||
| One half cigarette | Intercept | 4.28 (0.25)*** | 5.42 (0.22)*** | 3.97 (0.19)*** | 2.63 (0.17)*** | 4.37 (0.26)*** | 5.12 (0.23)*** | 3.65 (0.63)*** | 8.46 (0.36)*** | 132.43 (6.37)*** |
| Time | −0.08 (0.11) | −0.34 (0.10)** | −0.31 (0.09)** | −0.02 (0.10) | −0.21 (0.11) | −0.16 (0.12) | 0.65 (0.42) | −0.28 (0.17) | −4.46 (3.48) | |
| Condition | −0.29 (0.37) | −0.77 (0.32)* | −0.38 (0.28) | 0.03 (0.26) | −0.64 (0.38) | −0.06 (0.34) | 1.03 (0.90) | −0.47 (0.51) | 9.57 (9.07) | |
| Time × condition | −0.61 (0.16)*** | −0.15 (0.15) | −0.16 (0.13) | −0.02 (0.15) | −0.11 (0.17) | −0.14 (0.18) | −0.61 (59) | −0.02 (0.24) | −0.33 (4.93) | |
| Full cigarette | Intercept | 3.76 (0.27)*** | 4.95 (0.24)*** | 3.73 (0.21)*** | 2.07 (0.18)*** | 4.15 (0.27)*** | 5.71 (0.21)*** | 4.06 (0.72)*** | 9.70 (0.62)*** | 195.03 (15.95)*** |
| Time | −0.03 (0.10) | −0.14 (0.11) | −0.14 (0.10) | 0.11 (0.10) | −0.23 (0.11)* | −0.24 (0.14) | 0.46 (0.41) | 0.14 (0.39) | 3.45 (10.38) | |
| Condition | −0.38 (0.40) | −0.33 (0.35) | −0.20 (0.31) | 0.30 (0.26) | −0.49 (0.40) | −0.22 (0.32) | 1.35 (1.04) | −0.60 (0.87) | 8.00 (22.69) | |
| Time × condition | −0.45 (0.15)** | −0.69 (0.16)*** | −0.42 (0.15)** | −0.14 (0.15) | −0.36 (0.16)* | −0.43 (0.21)* | 0.30 (0.57) | −1.67 (0.55)** | −36.51 (14.57)* | |
Latency is the duration of time (in seconds) from when participants were given the opportunity to smoke until they lit their cigarette. # Puffs is the total number of puffs taken. Time smoking is the total time spent smoking (in seconds)
mCEQ Modified Cigarette Evaluation Questionnaire
p<.05;
p<.01;
p<.001
Tonic craving
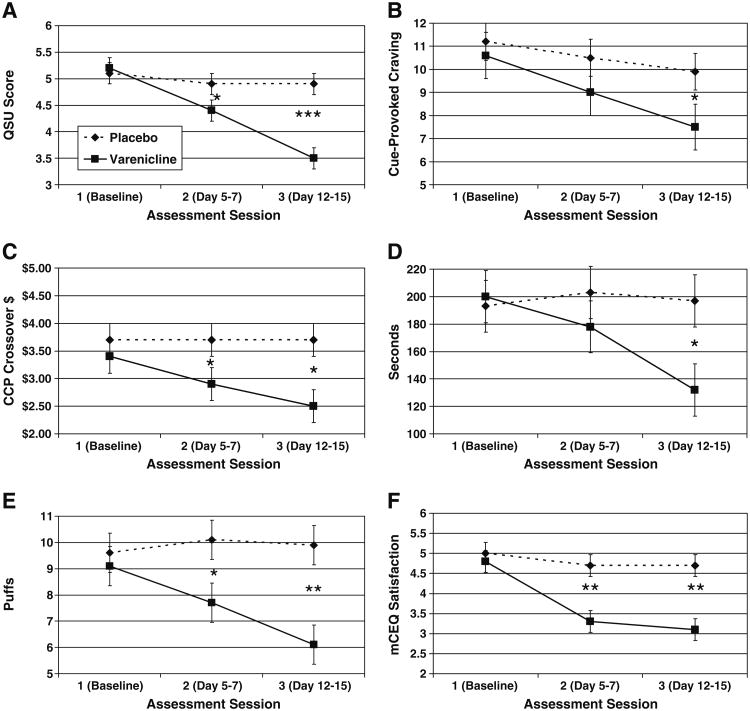
As shown in Table 2 and Fig. 1a, participants receiving varenicline rated their cravings lower than those receiving placebo at both post-dosing assessments (assessments 2 and 3). This pattern was consistently observed across QSU total and the positive and negative reinforcement factors, as well as the Craving scale of the WSWS. Note that none of the other scales of the WSWS showed these effects.
Fig. 1.
Comparison of varenicline (solid line) versus placebo (dotted line) across sessions on a tonic craving (Questionnaire of Smoking Urges, total score), b cue-provoked craving (ratings in response to smoking images, controlling for neutral images), c expected reinforcement value (cigarette choice procedure cross-over value) before the full cigarette, d total time smoking (seconds), e total number of puffs smoked, and f perceived reward (mCEQ Satisfaction Scale) following the full cigarette. Error bars indicate standard error. *p<.05; **p<.01; ***p<.001
Cue-provoked craving
A similar pattern emerged for cravings in response to smoking cues and cue-provoked cravings (in which neutral cues were controlled), although significant differences for the latter emerged only at assessment 3, in that those who received varenicline showed less cue-provoked craving, compared to their placebo counterparts (see Tables 2 and 4, and Fig. 1b). These analyses were conducted with a reduced sample size due to equipment problems (assessment 1, 97%; assessment 2, 97%; and assessment 3, 95%).
Physiological reactivity
We found no significant time × condition interactions for the initial HR deceleration during cue presentation, but we did find interactions for the subsequent acceleratory and deceleratory HR components. Differences between conditions emerged only at assessment 3, as those in the varenicline condition had diminished heart rate reactivity to cue presentation [increase: (M=4.53, SD=2.70 vs. 6.05, SD=3.77), p<.05; decrease: (M=−3.66, SD=2.01 vs. −5.32, SD=3.69), p<.01]. Because there were no response differences between cue types, the current findings indicated that those taking varenicline had dampened responses to stimuli in general (i.e., not specific to smoking cues). There were no significant effects on SC reactivity.
Cigarette reward
Significant time × condition interactions for the mCEQ were found after participants smoked the full cigarette in the laboratory, but not after smoking the one half cigarette. As hypothesized, smokers who received varenicline reported less satisfaction, psychological reward, craving reduction, and respiratory sensations than placebo-receiving smokers, at both assessments 2 and 3 (see Tables 3 and 5; and Fig. 1f for satisfaction scale following full cigarette). Moreover, during both of these sessions, the varenicline condition indicated on the cigarette choice procedure that a hypothetical cigarette was less valuable to them (i.e., they had a lower cross-over point) compared to the placebo condition. On this measure of expected value of smoking, significant time × condition interactions were found prior to smoking both the one half cigarette and full cigarette (see Fig. 1c for full cigarette).
Smoking topography
Actual smoking behavior was assessed as a secondary index of smoking reward. No effects of time × condition were found for the one half cigarette. However, as hypothesized, smokers on varenicline took fewer puffs and spent less time smoking the full cigarette compared to those on placebo, and the time × condition interaction was significant (see Tables 3 and 5, and Fig. 1d, e). These condition effects emerged for number of puffs taken at assessment 2 and 3, and for total time spent smoking at assessment 3. Note, however, that due to equipment problems and participant behavior (e.g., facing away from the camera while smoking), these analyses were conducted with a reduced sample size that varied by index (assessment 1, 86–90%; assessment 2, 80–85%; assessment 3, 73–76%).
Fidelity of the blind
At the second assessment, 44.7% of those in the varenicline condition and 34.7% of those in the placebo condition guessed that they had received varenicline, which was not statistically significant, χ2 (1, N=87)=0.91, p=.34. Differences did emerge at assessment 3, when 60.9% of participants in the varenicline condition guessed that they had received varenicline, compared to 31.5% of those in the placebo condition, χ2 (1, N=100)=8.67, p<.01. Thus, condition assignment only appeared to have an impact on participants' beliefs that they were taking varenicline once full dosage was reached. Correlational analyses revealed that participants with higher side effect reports at assessment 2 were more likely to guess that they were in the varenicline condition at both assessments 2 and 3 [r(85)=.38, p<.001; r(85)=.24, p<.05]. When accuracy of guessing was included in the mixed model analyses and tested as a potential moderator of observed condition × time effects, it acted as a significant moderator of the following dependent measures: both tonic craving scales (QSU and WSWS), cue-provoked craving, and mCEQ smoking satisfaction. That is, for these variables, group differences that emerged over assessment sessions were strongest amongst those participants who correctly guessed their conditions. Guessing the correct condition did not moderate group differences on the other mCEQ scales, the CCP crossover values, or the smoking topography measures. Of course, it is not possible to determine whether the medication effects reported earlier for these craving and smoking satisfaction measures were driven by participants' guesses about their condition, or if their guesses were driven by the differential therapeutic and/or side effects of the medication.
Discussion
The goal of this study was to test, in a controlled, double-blind design, the hypothesized subjective and behavioral effects of varenicline that may underlie its clinical efficacy. Specifically, we assessed the effect of varenicline over the recommended run-up period upon tonic craving, cue-provoked craving, perceived reward from smoking, and smoking behavior.
With respect to craving, we found, as expected, that varenicline reduced tonic (or abstinence-induced) craving compared to placebo, replicating the 2007 conference report by Niaura et al., as well as the general finding that steady-state medications (including bupropion and nicotine transdermal patch) tend to reduce tonic, or background, craving levels (Ferguson and Shiffman 2009). However, we also found that varenicline reduced cue-provoked cravings. The difference between varenicline and placebo did not reach significance until the end of the medication run-up period, which may explain why Niaura et al. did not find such an effect following a single dose. It may be that full therapeutic blood levels are necessary before this effect is revealed, or that smokers must have the opportunity to extinguish conditioned responses to smoking cues by smoking while on varenicline. To test these alternatives would require studies that systematically control, during the run-in period, medication dosing, cigarette consumption, and exposure to smoking-related cues. To our knowledge, this is the first placebo-controlled study that has shown a reduction in cue-provoked craving by varenicline or any other steady-state medication, although in an fMRI study, Franklin et al. (2011) reported that varenicline diminished cue-provoked ventral striatum and medial orbitofrontal cortex responses. Our results are also consistent with findings that varenicline reduced cue-induced reinstatement of nicotine-seeking in the rat—although only when combined with a nicotine prime (O'Connor et al. 2010); however, findings have been inconsistent in this domain (Wouda et al. 2011).
We also found that varenicline reduced perceived reward from smoking, as indicated by the scales of the mCEQ, replicating Patterson et al. (2009) and Perkins et al. (2010), and consistent with the retrospective reports of patients in the clinical trials (e.g., West et al. 2008). We found this effect only after smoking the full cigarette, so it appears that the half cigarette was not sufficient for producing differential medication effects upon reward. Additionally, the expected value of smoking was lower for patients on varenicline compared to placebo; that is, they showed relatively less preference for cigarettes over money. Smokers in the varenicline condition also took fewer puffs and spent less time smoking than smokers on placebo when they were provided with a full cigarette. As noted above, the reduced reward from smoking during the run-up period may facilitate extinction of classically conditioned responses to smoking-related stimuli, accounting for the reduction of cue-provoked cravings by the end of the run-up.
Aside from craving, no other withdrawal symptoms were reduced by varenicline. However, participants had only abstained for 12 h prior to the assessment sessions. Although some withdrawal symptoms can appear within this period (Hendricks et al. 2006), they are relatively mild and may not be significantly affected by medication. We also failed to find cue-specific reactivity on the psychophysiological indices of heart rate and skin conductance, which precluded the appearance of medication effects. These indices are less robust measures of cue-reactivity than is self-reported craving (Carter and Tiffany 1999), which is why they were relegated to secondary status in our design.
The primary limitation of the study was the relatively high attrition and no-show rate. This study attracted a sample of smokers of generally low socio-economic status, including a median household income of only $15,000–20,000. Although there are multiple challenges to conducting experimental research with this population (e.g., difficulty contacting, transportation barriers, and compliance issues), they are also increasingly representative of today's population of smokers (Litvin and Brandon 2010). Upon breaking the blind, we were particularly surprised to see the trend of lower compliance in the varenicline condition, including failure to pass the CO screen to verify overnight abstinence. We would have predicted, if anything, better abstinence compliance among those using varenicline, as is found in the treatment literature. However, the smokers in this study were nontreatment-seeking, with mean Contemplation Ladder scores indicating that they were not ready to quit. Among such smokers, the reduction in smoking satisfaction may be frustrating, and it perhaps therefore leads to attempts to compensate by increasing smoking over the short term (akin to the “extinction burst” phenomenon found in animal studies of drug self-administration; e.g., Harris et al. 2007). This explanation is consistent with our finding that study noncompleters reported higher cravings, but lower levels of reward following smoking, compared to study completers.
The previous point illustrates the second limitation of this research, which concerns external validity. Because of attrition, which may have been due to adverse responses to the medication in the varenicline condition, or disappointment about minimal effects in the placebo condition, a conservative conclusion might be that the results may generalize to only those smokers who respond to and/or can tolerate varenicline. Also relevant to external validity, participants in this study were not attempting to quit smoking, and the laboratory environment and tasks were artificial compared to natural smoking situations. As is often the case, some degree of external validity was sacrificed to maximize internal validity, which allows for stronger causal conclusions. In this case, however, the results—particularly those indicating attenuated reward from smoking while using varenicline—complement the retrospective reports of patients enrolled in clinical trials (West et al. 2008). Thus, studies that maximized either internal or external validity have now produced converging results.
A third limitation concerns the fidelity of the blind. By the time full clinical dosage had been reached, participants were able to guess their condition significantly better than chance, and the accuracy of their guesses moderated the group differences found on the craving and smoking satisfaction measures. Few placebo-controlled studies of varenicline have reported smokers' guesses about medication, but our results are similar to those reported elsewhere (Perkins et al. 2010a). It is interesting that we found that accuracy of guessing condition moderated the group differences on the most direct subjective measures (craving and smoking satisfaction), but not on the less direct or behavioral measures (cigarette choice crossover value, smoking topography, as well as the other mCEQ scales). We must be concerned that inclinications about their condition assignment—based perhaps upon experience of side effects—may have influenced participants' subjective ratings through mechanisms such as demand or expectancy effects. There is evidence that expectancies about smoking cessation medications can influence smokers' perceived response to the medication (e.g., Darredeau and Barrett 2010; Fucito and Juliano 2007). However, any causality between accurately guessing the condition and perceived benefits of the medication could plausibly flow in the opposite direction as well; that is, true therapeutic responses (or lack thereof) to medication may cue participants as to their condition assignment. Direction of causality cannot be determined from basic placebo-controlled studies such as ours. However, there is a legitimate concern about the fidelity of blinds in both clinical trials and laboratory studies, and it suggests a need for assessing participants' perceptions of condition assignment, as well as other methodological enhancements, including the use of active, rather than inert, placebos in pharmacotherapy research (Fisher and Greenberg 1993).
Additionally, it would have been ideal to have data on smoking behavior between assessment sessions to test for differential effects by condition. Not only would this provide another index of smoking behavior in response to condition but also amount of smoking between assessments could theoretically influence each of the dependent measures, and it would be ideal to control for variability in smoking. Although we instructed participants to continue their usual rate of smoking, we also asked them to record their smoking. Unfortunately, however, compliance was so poor on this task that we had to exclude it from analyses. A final limitation worth noting was the loss of some topography data from logistical problems. Asking participants to smoke through a computerized topography device might have reduced data loss, but at the possible cost of external validity (Blank et al. 2009).
In conclusion, the current results supported, via a controlled laboratory study, both of the hypothesized subjective and behavioral mechanisms thought to mediate the clinical efficacy of varenicline: reduced craving (both tonic and cue-provoked) and diminished reward from smoking. Future longitudinal studies with treatment-seeking smokers are needed to test the actual mediation of these variables upon smoking cessation outcomes. The eventual calibration of such mediation effects could guide the further development of pharmacotherapies (or even behavioral interventions) that target these key variables.
Acknowledgments
This study was funded by Pfizer, Inc. via Investigator Initiated Research Grant #GA3051LP. The authors thank Kristen M. Sismilich, Monica S. Carrington, and Brittany Weisenthal for their work on the project.
Footnotes
Conflicts of interest Dr. Brandon has served on the Varenicline Advisory Board for Pfizer and consulted on the development of the online behavioral adjuvant for varenicline users.
Contributor Information
Thomas H. Brandon, Email: thomas.brandon@moffitt.org, Department of Psychology, University of South Florida, Tampa, FL, USA; University of South Florida, Tampa, FL, USA; Tobacco Research and Intervention Program, H. Lee Moffitt Cancer Center and Research Institute, 4115 E. Fowler Ave., Tampa, FL 33617, USA.
David J. Drobes, University of South Florida, Tampa, FL, USA; Moffitt Cancer Center, Tampa, FL, USA
Marina Unrod, University of South Florida, Tampa, FL, USA; Moffitt Cancer Center, Tampa, FL, USA.
Bryan W. Heckman, University of South Florida, Tampa, FL, USA; Moffitt Cancer Center, Tampa, FL, USA
Jason A. Oliver, University of South Florida, Tampa, FL, USA; Moffitt Cancer Center, Tampa, FL, USA
Richard C. Roetzheim, University of South Florida, Tampa, FL, USA; Moffitt Cancer Center, Tampa, FL, USA
Sloan Beth Karver, University of South Florida, Tampa, FL, USA; Moffitt Cancer Center, Tampa, FL, USA.
Brent J. Small, University of South Florida, Tampa, FL, USA; Moffitt Cancer Center, Tampa, FL, USA
References
- Baker TB, Brandon TH, Chassin L. Motivational influences on cigarette smoking. Ann Rev Psychol. 2004;55:463–491. doi: 10.1146/annurev.psych.55.090902.142054. [DOI] [PubMed] [Google Scholar]
- Biener L, Abrams DB. The Contemplation Ladder: validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991;10:360–365. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- Blank MD, Disharoon S, Eissenberg T. Comparison of methods for measurement of smoking behavior: mouthpiece-based computerized devices versus direct observation. Nicotine Tob Res. 2009;11:896–903. doi: 10.1093/ntr/ntp083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH, Tiffany ST, Obremski KM, Baker TB. Post-cessation cigarette use: the process of relapse. Addict Behav. 1990;15:105–114. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Vidrine JI, Litvin EB. Relapse and relapse prevention. Ann Rev Clin Psychol. 2007;3:257–284. doi: 10.1146/annurev.clinpsy.3.022806.091455. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, et al. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analysis and reliability of the modified cigarette evaluation questionnaire. Addict Behav. 2007;32:912–923. doi: 10.1016/j.addbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Carter BL, Robinson JD, Lam CY, et al. A psychometric evaluation of cigarette stimuli used in a cue reactivity study. Nicotine Tob Res. 2006;8:361–369. doi: 10.1080/14622200600670215. [DOI] [PubMed] [Google Scholar]
- Center for the Study of Emotion and Attention. The International Affective Picture System [photographic slides] The Center for Research in Psychophysiology, University of Florida; Gainesville: 1995. [Google Scholar]
- Darredeau C, Barrett SP. The role of nicotine content information in smokers' subjective responses to nicotine and placebo inhalers. Hum Psychopharmacol. 2010;25:577–581. doi: 10.1002/hup.1159. [DOI] [PubMed] [Google Scholar]
- Drobes DJ. Cue reactivity in alcohol and tobacco dependence. Alcohol Clin Exp Res. 2002;26:1928–1929. doi: 10.1097/01.ALC.0000040983.23182.3A. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat. 2009;36:235–243. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) American Psychiatric Press, Inc.; Washington: 1996. [Google Scholar]
- Fisher S, Greenberg RP. How sound is the double-blind design for evaluating psychotropic drugs? J Nerv Ment Dis. 1993;181:345–350. doi: 10.1097/00005053-199306000-00002. [DOI] [PubMed] [Google Scholar]
- Franklin T, Wang Z, Suh JJ, et al. Effects of varenicline on smoking cue-triggered neural and craving responses. Arch Gen Psychiatry. 2011;68:516–526. doi: 10.1001/archgenpsychiatry.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucito LM, Juliano LM. Effects of instructions on responses to the nicotine patch: a laboratory study. Psychopharmacol. 2007;194:475–483. doi: 10.1007/s00213-007-0851-7. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Rabinovich NE. International smoking images series (with neutral counterparts, version 1.2) Integrative Neuroscience Laboratory, Department of Psychology, Southern Illinois University; Carbondale: 2003. [Google Scholar]
- Gonzales D, Rennard SI, Nides M, et al. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Troisi JR, Silverman K, Mumford GK. Multiple-choice procedure: an efficient approach for investigating drug reinforcement in humans. Behav Pharmacol. 1993;4:3–13. [PubMed] [Google Scholar]
- Harris AC, Pentel PR, Lesage MG. Prevalence, magnitude, and correlates of an extinction burst in drug-seeking behavior in rats trained to self-administer nicotine during unlimited access (23 h/day) sessions. Psychopharmacology. 2007;194:395–402. doi: 10.1007/s00213-007-0848-2. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, Brandon TH. The early time course of smoking withdrawal effects. Psychopharmacology. 2006;187:385–395. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kidorf M, Stitzer ML, Griffiths RR. Evaluating the reinforcement value of clinic-based privileges through a multiple choice procedure. Drug Alcohol Depend. 1995;39:167–172. doi: 10.1016/0376-8716(95)01136-7. [DOI] [PubMed] [Google Scholar]
- Litvin EB, Brandon TH. Testing the influence of external and internal cues on smoking motivation using a community sample. Exp Clin Psychopharmacol. 2010;18:61–70. doi: 10.1037/a0017414. [DOI] [PubMed] [Google Scholar]
- Niaura R, Abrams D, Demuth B, Pinto R. Responses to smoking-related stimuli and early relapse to smoking. Addict Behav. 1989;14:419–428. doi: 10.1016/0306-4603(89)90029-4. [DOI] [PubMed] [Google Scholar]
- Nides M, Oncken C, Gonzales D, et al. Smoking cessation with varenicline, a selective α4β2 nicotinic receptor partial agonist. Arch Intern Med. 2006;166:1561–1568. doi: 10.1001/archinte.166.15.1561. [DOI] [PubMed] [Google Scholar]
- O'Connor EC, Parker D, Rollema H, Mead AN. The alpha4beta2 nicotinic acetylcholine-receptor partial agonist varenicline inhibits both nicotine self-administration following repeated dosing and reinstatement of nicotine seeking in rats. Psychopharmacol. 2010;208:365–376. doi: 10.1007/s00213-009-1739-5. [DOI] [PubMed] [Google Scholar]
- Oncken C, Gonzales D, Nides M, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med. 2006;166:1571–1577. doi: 10.1001/archinte.166.15.1571. [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser AA, et al. Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry. 2009;65:144–149. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA. Does smoking cue-induced craving tell us anything important about nicotine dependence? Addiction. 2009;104:1610–1616. doi: 10.1111/j.1360-0443.2009.02550.x. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Doyle T, Ciccocioppo M, Conklin C, Sayette M, Caggiula A. Sex differences in the influence of nicotine dose instructions on the reinforcing and self-reported rewarding effects of smoking. Psychopharmacology. 2006;184:600–607. doi: 10.1007/s00213-005-0103-7. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Fonte CA, et al. Cross-validation of a new procedure for early screening of smoking cessation medications in humans. Clin Pharmacol Ther. 2010a;88:109–114. doi: 10.1038/clpt.2010.65. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Mercincavage M, Fonte CA, Lerman C. Varenicline's effects on acute smoking behavior and reward and their association with subsequent abstinence. Psychopharmacology. 2010b;210:45–51. doi: 10.1007/s00213-010-1816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Palamar J, Raghavan S, Flammino F. Effects of topiramate on cue-induced cigarette craving and the response to a smoked cigarette in briefly abstinent smokers. Psychopharmacology. 2007;192:147–158. doi: 10.1007/s00213-007-0755-6. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC. Nicotine-mecamylamine treatment for smoking cessation: the role of pre-cessation therapy. Exp Clin Psychopharmacol. 1998;6:331–343. doi: 10.1037//1064-1297.6.3.331. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Johnson M. Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacol Biochem Behav. 2000;67:71–81. doi: 10.1016/s0091-3057(00)00301-4. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC. Acute effects of nicotine and mecamylamine on tobacco withdrawal symptoms, cigarette reward and ad lib smoking. Pharmacol Biochem Behav. 2001;68:187–197. doi: 10.1016/s0091-3057(00)00465-2. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Relapse following smoking cessation: a situational analysis. J Consult Clin Psychol. 1982;50:71–86. doi: 10.1037//0022-006x.50.1.71. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Shadel WG, Niaura R, et al. Efficacy of acute administration of nicotine gum in relief of cue-provoked cigarette craving. Psychopharmacology. 2003;166:343–350. doi: 10.1007/s00213-002-1338-1. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis MH, et al. Immediate antecedents of cigarette smoking: an analysis from ecological momentary assessment. J Abnorm Psychol. 2004;111:531–545. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Cox LS, Elash CA. Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. J Consult Clin Psychol. 2000;68:233–240. doi: 10.1037//0022-006x.68.2.233. [DOI] [PubMed] [Google Scholar]
- Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. Effect of maintenance therapy with varenicline on smoking cessation. JAMA. 2006;296:64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Cue-provoked craving and nicotine replacement therapy in smoking cessation. J Consult Clin Psychol. 2004;72:1136–1143. doi: 10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharmacol. 1999;7:354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology. 2008;197:371–377. doi: 10.1007/s00213-007-1041-3. [DOI] [PubMed] [Google Scholar]
- Westman EC, Levin ED, Rose JE. Smoking while wearing the nicotine patch: is smoking satisfying or harmful? (Abstract) Clin Res. 1992;40:871A. [Google Scholar]
- Wouda JA, Riga D, De Vries W, et al. Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology. 2011 doi: 10.1007/s00213-011-2213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]