The degradation of cartilage is characterized by a loss of extracellular matrix (ECM) and the appearance of clusters of hypertrophic chondrocytes (1). The transmembrane proteoglycan syndecan is involved in the activation of aggrecanase-2 (ADAMTS5), which, together with other metalloproteinases, promotes matrix degradation (2). Syndecan-4 is a receptor for a number of ECM constituents and is a key regulator of cartilage degradation in osteoarthritis (OA) (2). Syndecans bind to laminins containing the alpha 4 subunit (3). Laminin alpha 4 chain (LAMA4) was first described as a constituent of basal membranes in blood vessels, the heart, and the kidney. Mutations in the murine gene encoding LAMA4 are associated with endothelial defects, cardiomyopathy, and chronic kidney disease (4, 5). We demonstrate that LAMA4 can be detected by immunohistochemistry in human osteoarthritic cartilage and that it colocalizes with syndecan-4 around hypertrophic chondrocytes. Moreover, treatment of human chondrocyte cultures with a function-inhibiting antibody to LAMA4 leads to a marked decrease in the transcription of the matrix metalloproteinase 3 (MMP3) gene, pointing to a possible regulatory role of laminin in human osteoarthritic cartilage.
Human cartilage specimens were obtained after total knee replacement surgery in 20 patients who had given written consent as approved by the local ethics committee. Samples from apparently healthy areas between the femoral condyles and macroscopically damaged specimens were stained with safranin O and classified according to the Osteoarthritis Research Society International (OARSI) criteria (6). Two-µm-thick sections were incubated with the primary antibodies to LAMA4 (Abcam, Cambridge, UK) and syndecan-4 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After application of a secondary antibody (biotinylated multi-link swine anti-goat, mouse, rabbit; Dako, Glostrup, Denmark), visualization was performed with streptavidin peroxidase (StreptAB HRP; Dako) and AEC solution (Dako). The intensity of the signal was assessed semi-quantitatively (+, ++,+++) by two independent investigators and samples with discordant adjudications were excluded. In vitro we blocked LAMA4 with the LAMA4 antibody 2A3 in human chondrocyte monolayer cultures from grade 0-I OA (7). Chondrocytes were pretreated with 2A3 blocking antibody (1:10) for 72 h, and then incubated with 10 ng/mL interleukin (IL)-1β (Sigma Aldrich, Vienna, Austria) or vehicle for 3 h. The control group was treated with an irrelevant immunoglobulin to rule out a non-specific effect of the 2A3 antibody, obtained from JJ Jones (Department of Cell and Molecular Biology, Northwestern University, Chicago, II, USA) (7).
RNA isolation transcription into cDNA and real-time polymerase chain reaction (PCR) were performed as described elsewhere (8). We used primers for ADAMTS5 (fw: gcggagtatgtggaggagac rev: ggaatcctcaccacgtcagt), LAMA4 (fw: gcggccgagaaatgca rev: agtcgcagggcacacattc), MMP13 (fw: tgagctggactcattgtcgg rev: gattcccgcgagatttgtagg), syndecan-4 (fw: cagggtctgggagccaagt rev: gcacagtgctggacattgaca), and MMP3 (fw: tggcattcagtccctctatgg rev: aggacaaagcaggatcacagtt) as well as glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (fw: ccacatcgctcagacaccat rev: accaggcgcccaatacg) as a control gene.
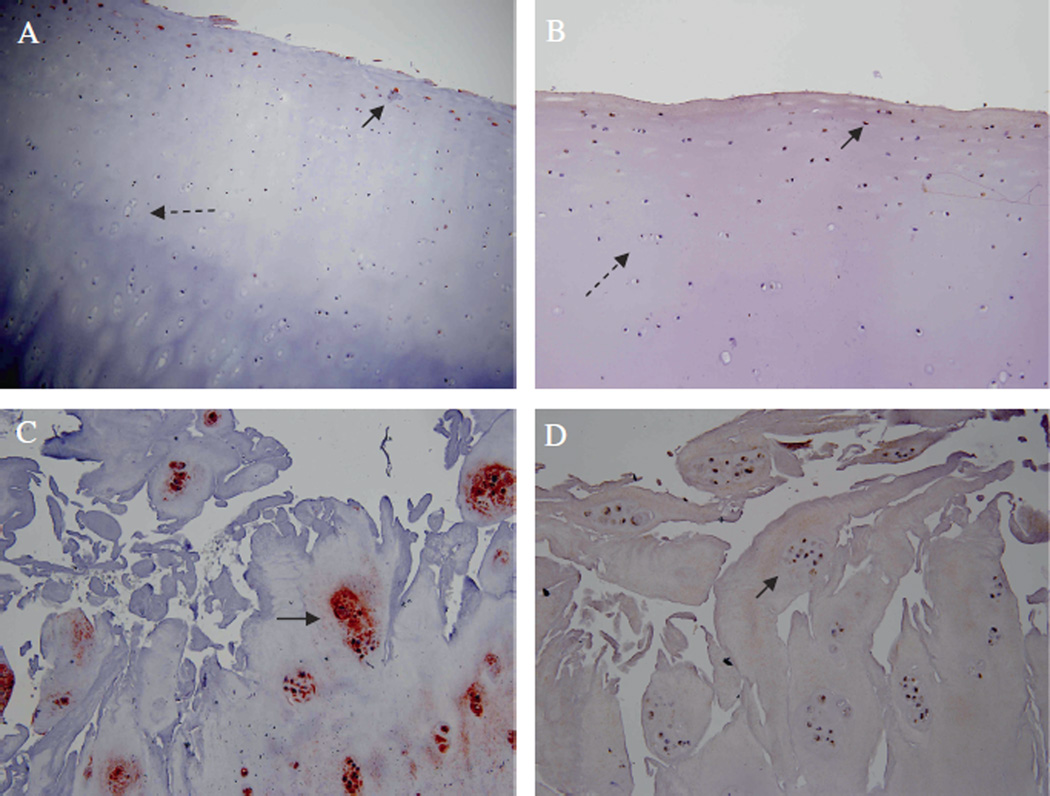
By immunohistochemistry, LAMA4 expression was significantly increased in the grade IV area of cartilage compared with grade 0-I areas (p < 0.04). In areas of samples with grade 0-I histological damage, LAMA4 was only occasionally detected in surface layer cells. Grade IV areas of the cartilage co-expressed LAMA4 and syndecan-4 (Figure 1).
Figure 1.
Negative immunohistochemical staining for (A) LAMA4 and (B) syndecan-4 in arthritic cartilage (dashed arrows) grade 0 according to the OARSI criteria for osteoarthritis. Only occasional cells in the surface layer show weak staining (+) (black arrows). Positive immunohistochemical staining (+++) for (C) LAMA4 and (D) syndecan-4 in arthritic cartilage lesions grade IV hypertrophic chondrocyte clusters according to the OARSI criteria for osteoarthritis (black arrows).
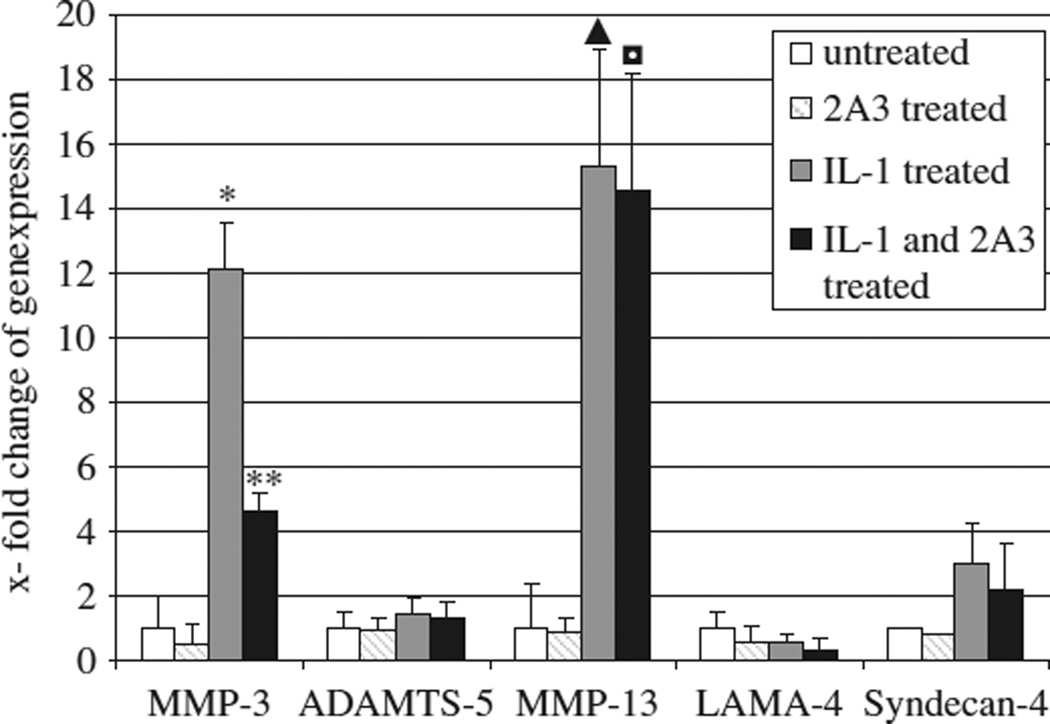
As expected, IL-1 stimulation led to a significant increase of MMP3 transcription (p < 0.03). Neutralizing LAMA4 in cultured human chondrocytes led to a significant downregulation of MMP3 after IL-1 pre-stimulation (p < 0.02). Moreover, syndecan-4 and MMP13 transcription was markedly enhanced after IL-1 exposition (p < 0.03); however, the LAMA4 antibody did not significantly affect the expression of ADAMTS5, MMP13, or syndecan-4 (Figure 2).
Figure 2.
Gene expression of MMP3, MMP13, ADAMTS5, LAMA4, and syndecan-4 in primary human chondrocytes of grade 0-I human osteoarthritic specimens in vitro when exposed to IL-1 (10 ng/mL) and to 2A3 antibody (monoclonal antibody blocking LAMA4). RT-PCR was performed after 3 h of treatment. MMP3 expression increased after exposure to IL-1 (*p < 0.03) and this was significantly inhibited by 2A3 (**p < 0.02). MMP13 expression was significantly elevated after IL-1 treatment (▲p < 0.03) as well as IL-1 and 2A3 treatment (■p < 0.04). Syndecan-4 expression was enhanced by IL-1, but not affected by blocking LAMA4. The results are presented as mean ± standard deviation (SD). Real-time RT-PCR results are expressed as mean ± SD percentage over control. Comparison between two groups was performed using Student’s t test. Differences between two groups were considered significant at the p < 0.05 level.
In syndecan-4 knock-out mice, less cartilage damage occurs compared to that in wild-type mice (2). In cartilage with high-grade lesions, the syndecan ligand LAMA4 colocalizes with syndecan-4, especially in hypertrophic areas. This observation concurs with a functional interaction of syndecan-4 with proteinases (2). Moreover, a functional context between laminins, syndecan, and MMP3 has been elucidated (3). During proteolytic cleavage of the ECM, cryptic fragments of LAMA4 bind to syndecans and thus modulate growth factor effects (9). Activation of syndecans and LAMA4 may be a consequence of an attempt to repair damaged tissue or to initiate and/or support tissue remodelling. Our in vitro data also support a deleterious role for LAMA4 in the perpetuation of cartilage degeneration.
In conclusion, we have demonstrated that LAMA4 protein is manifestly expressed by chondrocytes in human osteoarthritic cartilage showing high-grade damage. With regard to the search for innovative therapeutic targets, our data indicate that LAMA4 may have a role in perpetuating cartilage damage in OA.
References
- 1.Aigner T, Stove J. Collagens – major component of the physiological cartilage matrix, major target of cartilage degeneration, major tool in cartilage repair. Adv Drug Deliv Rev. 2003;55:1569–1593. doi: 10.1016/j.addr.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Echtermeyer F, Bertrand J, Dreier R, Meinecke I, Neugebauer K, Fuerst M, et al. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med. 2009;15:1072–1076. doi: 10.1038/nm.1998. [DOI] [PubMed] [Google Scholar]
- 3.Yamashita H, Goto A, Kadowaki T, Kitagawa Y. Mammalian and Drosophila cells adhere to the laminin alpha4 LG4 domain through syndecans, but not glypicans. Biochem J. 2004;382:933–943. doi: 10.1042/BJ20040558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Hoshijima M, Lam J, Zhou Z, Jokiel A, Dalton ND, et al. Cardiomyopathy associated with microcirculation dysfunction in laminin alpha4 chain-deficient mice. J Biol Chem. 2006;281:213–220. doi: 10.1074/jbc.M505061200. [DOI] [PubMed] [Google Scholar]
- 5.Abrass CK, Hansen KM, Patton BL. Laminin alpha4-null mutant mice develop chronic kidney disease with persistent overexpression of platelet-derived growth factor. Am J Pathol. 2010;176:839–849. doi: 10.2353/ajpath.2010.090570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rutgers M, van Pelt MJ, Dhert WJ, Creemers LB, Saris DB. Evaluation of histological scoring systems for tissue-engineered, repaired and osteoarthritic cartilage. Osteoarthritis Cartilage. 2010;18:12–23. doi: 10.1016/j.joca.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 7.DeHahn KC, Gonzales M, Gonzalez AM, Hopkinson SB, Chandel NS, Brunelle JK, et al. The alpha4 laminin subunit regulates endothelial cell survival. Exp Cell Res. 2004;294:281–289. doi: 10.1016/j.yexcr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Stradner MH, Hermann J, Angerer H, Setznagl D, Sunk IG, Windhager R, et al. Spingosine-1-phosphate stimulates proliferation and counteracts interleukin-1 induced nitric oxide formation in articular chondrocytes. Osteoarthritis Cartilage. 2008;16:305–311. doi: 10.1016/j.joca.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Yamashita H, Goto C, Tajima R, Koparal AT, Kobori M, Ohki Y, et al. Cryptic fragment alpha4 LG4-5 derived from laminin alpha4 chain inhibits de novo adipogenesis by modulating the effect of fibroblast growth factor-2. Dev Growth Differ. 2008;50:97–107. doi: 10.1111/j.1440-169X.2007.00979.x. [DOI] [PubMed] [Google Scholar]