Abstract
We undertook a community-level aggregate analysis in South Carolina, USA, to examine associations between mother-child conditions from a Medicaid cohort of pregnant women and their children using spatially interpolated arsenic (As) and lead (Pb) concentrations in three geographic case areas and a control area. Weeks of gestation at birth was significantly negatively correlated with higher estimated As (rs=−0.28, p=0.01) and Pb (rs=−0.26, p=0.02) concentrations in one case area. Higher estimated Pb concentrations were consistently positively associated with frequency of black mothers (all p<0.02) and negatively associated with frequency of white mothers (all p<0.01), suggesting a racial disparity with respect to Pb.
Keywords: arsenic, lead, geographic information systems, racial disparity, soil
Introduction
Children are highly susceptible to environmental metals and can be exposed both prenatally, in infancy, and during childhood. Lead (Pb), an anthropogenic environmental contaminant, is found throughout the world; sources include industrial processes, cigarette smoke, historical use of leaded gasoline, and Pb-based paint in older homes (Patrick, 2006). In a meta-analysis by Wigle et al. (2007), maternal exposure to environmental Pb was associated with a variety of neurological outcomes in their children, including lower IQ scores, both fine and visual motor function deficits, and other negative health outcomes, including reduced height, anemia, immune system dysfunction, preterm birth and even early fetal death.
Environmental arsenic (As), unlike Pb, is both naturally occurring and from anthropogenic sources (Mandal and Suzuki, 2002). High As concentrations in soil, however, are usually a result of point source industrial emissions or pesticide application (Gerr et al., 2000; Hinwood et al., 2004). Arsenic can also leach into soils from chromated copper arsenate (CCA) treated wood, which has been used for commercial applications, and, until recently, for decks and children's playground equipment (Ljung et al., 2007). Like Pb, exposure to environmental As can negatively impact children's IQ scores (Calderón et al., 2001). Children may also metabolize ingested As differently than adults (Chowdhury et al., 2003; Concha et al., 1998; Kalman et al., 1990), which may indicate a differential exposure risk for children as compared to adults. However, study findings have been inconsistent.
Racial disparities have been well documented for potential environmental exposures to Pb, and, to a lesser extent, As. Diawara et al. (2006) and Campanella and Mielke (2008) both found associations of higher Pb soil concentrations with higher populations of minorities in Pueblo, CO, and New Orleans, LA, respectively. When characterizing children with elevated blood Pb levels in the United States (US), minority and low-income children consistently are at greater risk. In a CDC study of blood Pb levels in children in the United States aged 1-19 from 1999-2002, 1.4% of black children had elevated blood Pb levels (≥10 μg dL−1) compared to only 0.5% of white children (CDC, 2005). For As, Caldwell et al. (2009), using data collected as part of the 2003-2004 National Health and Nutrition Examination Survey (NHANES), reported that the mean total urinary As concentration for non-Hispanic whites of all ages was 7.12 μg L−1, compared to 11.6 μg L−1 for non-Hispanic blacks of all ages.
In an ongoing study in South Carolina (SC), data from a cohort of Medicaid mother-child pairs were analyzed to assess intellectual disability (ID; previously classified as mental retardation) and developmental delay (DD) outcomes in children (Cai et al., 2011; Zhen et al., 2009; Zhen et al., 2008) and potential associations with soil metal concentrations (McDermott et al., 2011; Liu et al., 2010). Building upon those studies, we undertook an aggregate analysis of mother-child variables at the United States (US) Census 2000 block group level, and spatially interpolated soil As and Pb concentrations in case areas with significantly higher ID and/or DD prevalence rates, and in a control area with ID and DD prevalence rates similar to the state background rate. We hypothesize that mother-child variables, including race/ethnicity of the mothers, child's gestational age, and birth weight, will be significantly associated with estimated soil As and Pb concentrations. We also identified community-level environmental sources of As and Pb including median year home built by block group, emissions of As and Pb from industrial areas, and road coverage, and hypothesize that older homes, quantities of both on-site land and air releases from industrial sources of As or Pb, and densities of roads will be associated with either As (industry only) or Pb (roads, median year home built, and industry) in soils as well as mother-child variables.
Methods
Study Population
Using a Medicaid data set, a cohort of women giving birth from 1996-2001 was identified (n ≈ 150,000; Zhen et al., 2009). For the selected case and control geographic areas, aggregate data for block groups were supplied by the state's Office of Research and Statistics (ORS). The aggregate data were based on the geocoded maternal residential location during the sixth month of pregnancy (which identified where mothers lived and whether their child received a diagnosis of ID or DD during the first five years of life), and included the following variables: weeks of gestation at birth, mother's age (in years) at the time of the birth, baby birth weight (in grams), and mother's self-identified race (black, white, or other). For continuous variables, the mean was obtained and, for categorical variables, a frequency was obtained. If there were no aggregate measures available for a block group, there were either 1) no mothers living in that particular block group who were members of the Medicaid cohort, or 2) the total number of mothers in the Medicaid cohort was < 5, as Medicaid restricts access to aggregate data unless n ≥ 5.
Site Identification and Sampling
Using mother-child pairs in the Medicaid cohort, three case areas (Areas 4, 5, and 22) and a control area (Area 1) were identified using Bayesian local likelihood modeling (Kim et al., 2010; Kim et al. 2009; Zhen et al., 2008). Each case area contained a cluster of ID and/or ID/DD with a prevalence of ID and/or ID/DD that was significantly higher than the state background prevalence, as well as a gradient of risk around the cluster. The control area (Area 1) had an ID and ID/DD prevalence rate that was not significantly different from the state background prevalence. All areas were selected based solely on data associated with the ID/DD health measures, and did not take into account environmental conditions; in other words, there was no selection based on potential anthropogenic sources of soil metals. All block groups within sampling locations were categorized as either urbanized areas (UA; population >50,000) or urbanized clusters (UC; population 2,500-50,000; US Census, 2009). Other sampling area characteristics are listed in Table 1.
Table 1.
Sampling area characteristics for the control Area 1 and case Areas 4, 5, and 22, and kriging results for estimated arsenic (As) and lead (Pb) concentrations.
| Area | Area Size (km2) | Block Groups (metals)a | Block Groups (health)b | Average Mean Asc | Average Mean Pbc | RMSESd As | RMSES Pb | Average Median Year Home Builte |
|---|---|---|---|---|---|---|---|---|
| Area 1 | 160 | 28 | 25 | 1.1 | 15.0 | 0.8424 | 1.017 | 1975 |
| Area 4 | 140 | 78 | 70 | 4.7 | 62.5 | 1.151 | 1.001 | 1943 |
| Area 5 | 64 | 64 | 55 | 4.8 | 87.9 | 0.9991 | 1.135 | 1963 |
| Area 22 | 114 | 99 | 82 | 4.8 | 97.6 | 1.142 | 1.023 | 1958 |
Number of block groups for which estimated mean As and Pb concentrations calculated
Number of block groups for which aggregate mother-child measures were available
in mg kg−1; average of all block group mean metal concentrations for each sampling area
Root mean square error standardized
Median year home built by block group obtained from Census 2000 data; average of all block group median year home built for each sampling area
A 120-node grid was overlaid on each area and surface soil samples were collected at approximately each grid node as described previously (Aelion et al., 2007; Aelion et al., 2008; Aelion et al., 2009a; Aelion et al., 2009b; Davis et al., 2009). A sterile spatula was used to collect ~100 g of surface soil (top 5 cm) and samples were placed in sterile Whirl-Pak® bags (Nasco, Fort Collins, CO) and refrigerated until analysis for As and Pb concentrations (in mg kg−1), as well as barium (Ba), beryllium (Be), chromium (Cr), copper (Cu), manganese (Mn), nickel (Ni),and mercury (Hg), by an independent analytical laboratory using inductively-coupled plasma optical emission spectroscopy (ICP-OES). Duplicate samples were collected at 10% of the sample locations for quality assurance/quality (QA/QC) control purposes. Minimum detection limit (MDL) concentrations were ~0.5 mg kg−1 for As, Ba, Cr, Cu, Pb, Mn, and Ni, ~0.1 mg kg−1 for Be, and ~0.005 mg kg−1 for Hg. Samples measured below the MDL were recorded as 0 mg kg−1 for all subsequent analyses.
A hand-held GPS device (Garmin eTrex; Garmin, Olathe, KS, USA) was used to identify and store the latitude and longitude of each sample location. The range of error was 1-15 m and each latitude/longitude coordinate was verified using Google Earth. The resolution of each grid was not identical because some areas were larger than others. Distance between nodes varied from 0.5 km to 2.0 km for sampling areas. Using the measured soil As and Pb concentrations and the corresponding sample location latitude/longitude coordinate, the As and Pb concentrations were mapped using ArcMap Version 10 (ESRI, Redlands, CA) for each of the four sampling areas. Using the Geostatistical Wizard application in ArcMap, As and Pb concentrations were spatially interpolated across each sampling area using the ordinary kriging method. A Box-Cox transformation was used to normalize Pb concentrations, and the number of neighbors was optimized in each analysis to minimize the root mean squared error (RMSE) for both As and Pb. It is expected that estimated concentrations at the boundaries of the grid may have more error associated with them because those nodes have fewer neighbors. However, because sampling was carried out along the boundaries, this error is minimized.
The kriged As and Pb soil concentration layer files for each sampling area were saved as raster files (with cell size set to 30 m by 30 m), and these raster layers were used to subset block groups that were either wholly or partially within each sampling area. The zonal statistics function in ArcMap was implemented to calculate mean estimated As and Pb concentration for each block group located within each sampling area.
Community Level Exposures-Roads, Median Year Home Built and Facilities
To assess roads, a Topologically Integrated Geographic Encoding and Referencing (TIGER) road shapefile for SC was downloaded from the US Census website. The As and Pb kriged raster layers were used to subset roads that were located within the sampling areas, and the summed area of roads within each block group was calculated. Then, using the area (either whole or partial) of each block group located within the sampling areas, a percent road coverage value was calculated by dividing the summed road area of each block group by the block group area. Median year home built by block group was obtained using the US Census 2000 Data Engine software for SC for all block groups in sampling areas (US Census, 2001).
The Environmental Protection Agency (EPA) Toxic Release Inventory (TRI) was used to separately identify Pb and As emitters in SC from 2000-2009 (US EPA, 2011). Block groups within each sampling area were identified that contained, or were adjacent to, a block group containing at least one As and/or Pb TRI facility. Block groups were also identified that were within 5 km distance of at least one As and/or Pb TRI facility. A five kilometers distance was chosen because this is often used as a buffer in exposure studies (Rava et al., 2012; Tanyanont and Vichit-Vadakan, 2012; Zubero et al., 2009). To provide information on potential sources of metals as might occur from long-range air transport, facilities within 50 km or less of each sampling area were identified. The total on-site air (both fugitive and stack air releases) and land (disposal at an on-site landfill) As and Pb releases (in thousand pounds) were obtained for each year for all facilities, and average per-year air and surface releases were calculated by dividing the total air or surface release by the number of years the facility reported releases from 2000-2009.
Statistical Analysis
Analysis with Spearman's rank correlation was used to evaluate associations between mother-child variables and mean estimated As and Pb concentrations, percent road coverage, and median year home built by block group. Associations were assessed for each sampling area separately. Correlations were also assessed separately (by sampling area) for both block groups within each sampling area that contained, or were adjacent to, a block group containing at least one As and/or Pb TRI facility, and block groups that were within a 5-km distance of at least one As and/or Pb TRI facility. An analysis of variance, using a generalized linear model with a random statement (strip was the random variable) was used to compare mother-child variables, mean estimated As and Pb concentrations, percent road coverage, and both average per-year on site air and surface releases separately between sampling areas. A Tukey adjustment was made for multiple comparisons, and the alpha level was set to 0.05 for all analyses. We also completed a cluster analysis of principal component analysis (PCA) factors for Area 22, using measured soil metal concentrations. In PCA, metals that cluster together are predicted to derive from similar sources, and those that do not cluster together are predicted to derive from different sources. PCA was carried out previously for the control Area 1, as well as case Areas 4 and 5 (Davis et al., 2009). SAS Version 9.2 (SAS Institute, Cary, NC) was used for all statistical analyses.
Results
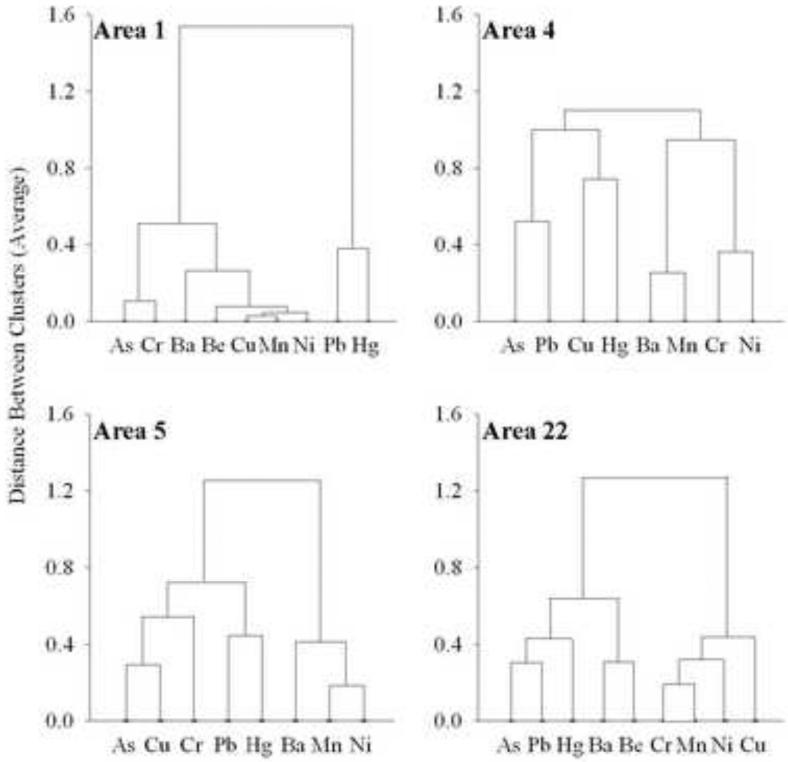
For kriged As concentrations, the RMSE standardized value (RMSES; standardized for the range of data) was closest to 1 for Area 5 (0.9991; Table 1). For kriged Pb concentrations, the RMSE standardized values for kriging models of all sampling areas were close to 1 (Table 1). Overall, RMSES were closer to 1 for interpolation of Pb as compared to As in sampling locations. The mean estimated soil As and Pb concentrations by block group for the case areas (4, 5, and 22; Table 1) were significantly higher than for the control Area 1 (p <0.0001). Estimated mean As and Pb concentrations were significantly correlated in all case areas: Area 4 (rs=0.31, p=0.005), Area 5 (rs=0.65, p<0.0001), and Area 22 (rs=0.49, p<0.0001), as well as in the control Area 1 (rs=0.65, p=0.002). Based on prior cluster analysis using PCA (Davis et al., 2009), the control Area 1 (Figure 1) had no clustering of As and Pb. For case sampling areas, As and Pb were loosely clustered in Area 5, and were more closely clustered in both Areas 4 and 22 (Figure 1).
Figure 1.
Cluster analysis of principal component analysis (PCA) factors for arsenic (As), barium (Ba), beryllium (Be), chromium (Cr), copper (Cu), lead (Pb), manganese (Mn), mercury (Hg), and nickel (Ni) soil concentrations for the Control area 1 and Case areas 4, 5, and 22.
The ranges, means and standard deviations of mother-child measures by block group for each sampling area are shown in Table 2. Mean infant weight at birth by block group was significantly higher for both the control Area 1 (p=0.0064) and case Area 5 (p=0.0001), as compared to Area 22. The mean weeks of gestation at birth for all four sampling areas ranged from 35.5 to 42 weeks, and the mean mother's age ranged from 15 to 35 years. Mean weeks of gestation for both the control Area 1 and case Area 5 was significantly higher than for case Area 22 (p=0.003 and p=0.007, respectively), and mean age of the mother at birth was significantly higher for case Area 22 as compared to case Area 4 (p=0.01).
Table 2.
Range and mean (standard deviation) of mother-child measures by block group for the control Area 1 and case Areas 4, 5, and 22.
| Measures | Area 1 | Area 4 | Area 5 | Area 22 |
|---|---|---|---|---|
| Infant birth weight (g) | 3060-3790 | 2296-3799 | 2334-4040 | 2646-3771 |
| 3262* (169) | 3156 (265) | 3263* (272) | 3099* (167) | |
| Weeks gestation at birth | 37.8-42 | 35.5-41 | 36-42 | 36-41 |
| 39.1* (0.87) | 38.5 (0.99) | 38.9* (0.74) | 38.5* (0.68) | |
| Mother age at birth (years) | 21-26.7 | 17-30.2 | 15-33 | 18.9-35 |
| 23 (1.3) | 22.3* (2.2) | 23 (3) | 23.6* (2.1) | |
| Proportion of black mothers (%) | 8.3-97 | 3.4-100 | 5.9-100 | 44.2-100 |
| 52.1* (27.9) | 58.2* (30.2) | 41.2* (29.3) | 85.1* (14.9) | |
| Proportion of white mothers (%) | 3.2-100 | 2.4-100 | 6.9-100 | 1.9-100 |
| 51.4* (10.3) | 48.5* (30.7) | 67.4* (25.3) | 20.5* (23.8) | |
Denotes significant difference (α was adjusted for multiple comparisons)
The range in ethnic/racial diversity was large for all sampling area (Table 2). In case Area 22, the mean proportion of black mothers was significantly higher (all p<0.0001), and the mean proportion of white mothers was significantly lower (all p<0.0001), as compared to the other sampling locations. The mean proportion of black mothers for case Area 4 was also significantly higher than for case Area 5 (p=0.0001).
Mean estimated As and Pb (Table 3) concentrations by block group were significantly correlated with mother-child measures in all sampling areas. In case Area 22, both mean estimated soil As and Pb concentrations by block group were significantly negatively correlated with mean weeks of gestation. Mean estimated As concentrations by block group were significantly positively associated with the proportion of black mothers in case Areas 4 and 22 (Table 3), and mean estimated Pb concentrations and black mothers were significantly positively associated in the control Area 1, and in case Areas 4 and 22. The proportion of white mothers was significantly negatively associated with mean estimated Pb concentrations by block group in all sampling areas (Table 3).
Table 3.
Significant Spearman correlations and p-values for comparisons between mean estimated arsenic (As) and lead (Pb) concentrations and mother-child measures, median year home built, and percent road coverage by block group for the control Area 1 and case Areas 4, 5, and 22.
| Arsenic (As) | Lead (Pb) | |||||||
|---|---|---|---|---|---|---|---|---|
| Measures | Area 1 | Area 4 | Area 5 | Area 22 | Area 1 | Area 4 | Area 5 | Area 22 |
| Weeks gestation | NAa | NA | NA | −0.28 0.01 |
NA | NA | NA | −0.26 0.017 |
| Proportion of black mothers (%) | NA | 0.28 0.02 |
NA | 0.31 0.007 |
0.50 0.02 |
0.44 0.0004 |
NA | 0.46 <0.0001 |
| Proportion of white mothers (%) | NA | NA | NA | NA | −0.52 0.007 |
−0.53 <0.0001 |
−0.35 0.011 |
−0.41 0.0004 |
| Median year home built | NA | −0.29 0.01 |
−0.45 0.001 |
NA | −0.39 0.04 |
−0.34 0.002 |
−0.39 0.001 |
−0.67 <0.0001 |
| Percent road coverage | NA | 0.35 0.002 |
0.32 0.01 |
NA | 0.45 0.02 |
0.60 <0.0001 |
0.43 0.0004 |
0.47 <0.0001 |
Not applicable
The mean median year home built by block group was oldest for case Area 4 and newest for the control Area 1 (Table 1); however, differences in median home age between sampling areas were not significant (data not shown). The median year home built by block group was significantly negatively associated with Pb concentrations in all case areas, and with As concentrations in case Areas 4 and 5 (Table 3). Percent road coverage by block group was significantly associated with Pb in all sampling locations, and with As only in case Areas 4 and 5. Mean percent road coverage by block group was significantly higher for case Areas 5 and 22 than for the control Area 1 (p-values < 0.02).
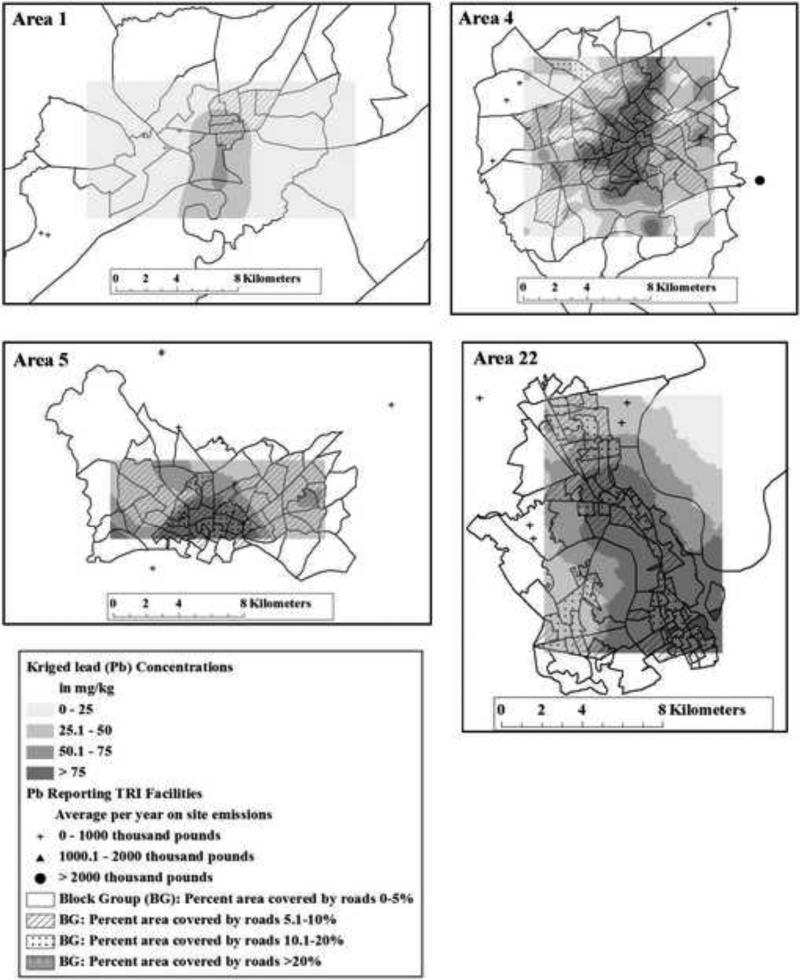
Twelve As-emitting TRI facilities were located within 50 km of any sampling location (data not shown). Kriged soil Pb concentrations, nearby Pb-emitting TRI facilities and percent road coverage by block group are shown in Figure 2 for the control Area 1 and case Areas 4, 5, and 22. For all sampling areas, most nearby Pb-emitting facilities released on average 1000 pounds per year or less of Pb on-site to both air and surface combined. Only case Area 4 (Figure 2) had a nearby facility releasing an average of >2000 thousand pounds per year of Pb. For all TRI facilities within 50 km, the control Area 1 had the highest average on-site air release of Pb (128.9 thousand pounds per year) and the case Area 22 had the highest mean average on-site surface release of Pb (279.1 thousand pounds per year).
Figure 2.
Kriged soil lead (Pb) concentrations (shaded grey areas), Pb-emitting (to either air and/or surface land) Toxic Release Inventory (TRI) facilities (dots), and percent area road coverage (crosshatch) by block group for the Control area 1 and Case areas 4, 5, and 22.
In case Areas 4, 5 and 22 (Figure 2), higher mean percent road coverage in block groups (indicated by crosshatched block groups) was associated with higher estimated soil Pb concentrations. Mean percent road coverage for case Areas 4, 5, 22 and the control Area 1 were 5.6%, 6.2%, 9.4%, and 3.3%, respectively. The control Area 1 generally had much lower estimated soil Pb concentrations (Figure 2), and lower percent area road coverage by block group, with a maximum of only 9.2%.
Discussion
It appears that in this Medicaid cohort, there is a racial disparity with regards to potential exposure to high soil As and Pb concentrations. For As in case Areas 4 and 22, and for Pb in case Areas 4 and 22, as well as the control Area 1, higher estimated mean soil concentrations were associated with higher frequencies of black mothers. Associations were stronger for Pb, where all Spearman correlation coefficients were >0.4. The frequency of white mothers was significantly negatively correlated with mean estimated soil Pb concentrations in all sampling areas. Thus, lower numbers of white mothers and higher numbers of black mothers were living in block groups with higher mean estimated soil Pb concentrations.
Studies have observed racial disparities with respect to environmental contaminants in other locations in the US; however, most of these studies have also found associations with income or some other measure of socioeconomic status (SES). For example, Campanella and Mielke (2008) found in New Orleans, LA, that as the percentage of black individuals in a block group increased, the mean median income decreased and soil Pb concentrations increased. In a previous study, we found significant positive associations between higher estimated soil Pb concentrations and both larger populations of black, non-Hispanic Latino, individuals and individuals of lower SES using Census 2000 data (Aelion et al., in revision).
It is known that contextual or neighborhood characteristics play an important role in health disparities, in addition to individual characteristics such as those examined in this study. In our Medicaid cohort study population, all individuals are low SES. However, regardless of SES, racial disparities exist in these residential areas with respect to environmental contamination, and thus to potential exposure. This is especially important for children, who may be at higher risk not only for exposure to environmental Pb, but also for detrimental health effects associated with exposure (Khan et al., 2010; Wigle et al., 2007). Oyana and Margai (2010) found that higher proportions of minority children (especially black children) in Chicago, IL, USA, had elevated blood lead levels (BLL; ≥ 10 μg dL−1) from 1997-2003, and that neighborhoods with clusters of children with high BLL often had many older (pre-1950) homes, as well as residents with lower income (based on median household income). We also found older homes in our case areas, and median year home built was significantly negatively correlated with mean estimated Pb concentrations.
With respect to health data, there were few significant correlations observed between mother-child measures and either mean estimated soil As or Pb concentrations. In case Area 22, weeks of gestation at birth was significantly negatively correlated with both mean estimated soil As and Pb concentrations. This is of note because Area 22 also had significantly lower average weeks of gestation as compared to both the control Area 1 and case Area 5. Studies examining the relationship between As or Pb exposure and weeks of gestation have been inconsistent. Torres-Sanchez et al. (1999) found a significant association between preterm birth and umbilical cord blood Pb levels that were ≥5.1 μg dL−1 in a case-cohort study of women categorized by birth (preterm or term) in Mexico City, Mexico. However, Berkowitz et al. (2006) did not find a significant association between preterm birth and exposure to Pb from a Pb smelter in Idaho, USA. For As, Myers et al. (2010) did not find a significant association between preterm birth and drinking water with As concentrations >50 μg L−1 for women in inner Mongolia, China. However, in a cross-sectional study of women in Bangladesh, Ahmad et al. (2001) found that exposed women (characterized as those drinking water with As concentrations >0.05 mg L−1) had significantly more preterm births (p = 0.018) than unexposed women. While these studies looked at biological measures to assess exposure, we used soil metal concentrations as a surrogate for exposure and also found a significant association.
In the three case areas, both As and Pb were clustered with other metals known to come from anthropogenic sources, and clustered closely with each other (particularly in Areas 4 and 22). In Davis et al. (2009), we concluded that both Pb and Hg were from anthropogenic sources in the control Area 1, and in case Areas 4 and 5, and that As was from both natural and anthropogenic sources, varying by sampling area. Lead has also been concluded to be from anthropogenic sources in other studies (Rodríguez Martín et al., 2006; Diawara et al., 2006; Möller et al., 2005; Li et al., 2004). In the control Area 1, As and Pb were not clustered closely, suggesting As is most likely to be from sources different than Pb. This is also supported by the generally low As concentrations observed in the Area 1 as compared to the case areas. In case Area 22, not previously analyzed using PCA, As was closely clustered with both Pb and Hg, suggesting similar and anthropogenic sources for As in this sampling area.
While sources of As and Pb may be anthropogenic in our case areas, it appears that emissions from TRI facilities were not associated with higher estimated soil As and Pb concentrations. There were few As-emitting TRI facilities in SC and only 12 were located within 50 km of any sampling area. This indicates that As may be from sources at further distances if airborne, or from sources, such as agriculture, which is a predominant land use in the state.
Percent road coverage was significantly associated with estimated soil Pb concentrations by block group in all sampling areas. Historical release of Pb from car exhaust may have contributed to current soil Pb concentrations to a greater extent than recent Pb emissions from local industrial facilities, assuming there was dispersal of car exhaust particles to nearby soils. Since most nearby facilities emitted, on average, less than 1000 thousand pounds of Pb per year, these sources may not emit enough Pb to impact soils, or the emitted Pb may travel farther. Wei et al. (2009) found that higher Pb concentrations were associated spatially with both main roads and higher traffic density in Urumqi, China, which has a population of approximately 2.5 million. Traffic patterns may also be important, as Ewen et al. (2009) found that heavy metals from car exhaust were higher in areas with more congestion and stop/start traffic patterns in Thessaloniki, Greece, the country's second largest city. In our urban areas, block groups with higher percent coverage of roads would have similar traffic patterns to those observed by Ewen et al. (2009).
This study has limitations due to the use of aggregate measures of mother-child variables and estimated As and Pb concentrations at the block group level; therefore, we cannot necessarily generalize results to individuals. Also, since this population is comprised of mother-child pairs in a Medicaid cohort, our results may not be transferable to other populations. We are also not able to quantify all other sources of As and Pb that may be important, such as application of pesticides containing As, leaching of As from CCA-treated wood, and both As and Pb emitters not subject to TRI reporting, nor other metals that may potentially confound the relationship between As and Pb, and both mother-child and neighborhood variables. Finally, temporality may be an issue because information on mothers and children was collected from 1996-2001, and soil samples and facility releases were collected more recently (2000-2011). However, given the chemical stability of Pb in the environment, one would not expect concentrations to change greatly in a short period of time (Rodriguez-Salazar et al., 2011; Wu et al., 2010).
Regardless of the potential limitations of the study, several associations were significant and warrant further investigation. Higher proportions of black mothers were associated with higher estimated As and Pb soil concentrations in both ID/DD case and control areas, suggesting a racial disparity in this Medicaid cohort within these areas for potential exposure to both As and Pb. Associations of estimated As and Pb soil concentrations with mother-child health outcome measures were observed for weeks of gestation at birth in one case area, which also had significantly lower average weeks of gestation at birth than other sampling areas. Finally, some, but not all, environmental factors were significant. The year a home was built may play a role in these areas with respect to potential exposure to Pb. Surface land and air emissions from TRI facilities were not important predictors of higher estimated soil Pb concentrations as compared to percent road coverage in the block groups, potentially due to the ubiquitous nature of vehicular traffic and the relatively small number of industrial facilities.
Research Highlights.
We observed a racial disparity for women for potential exposure to As and Pb in soil
Lower weeks gestation was associated with higher As and Pb concentrations in one area
Year home built and roads were predictors of high Pb soil concentrations
Acknowledgements
Funding for this research was provided by a National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH) grant (grant number 2R01ES012895-04A2). We thank J. Davis, M. Engle, S. Jayasinghe, and F. Nemeth for help with sampling.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aelion CM, Davis HT. Use of a general toxicity test to predict heavy metal concentrations in residential soils. Chemosphere. 2007;67:1043–1049. doi: 10.1016/j.chemosphere.2006.10.042. [DOI] [PubMed] [Google Scholar]
- Aelion CM, Davis HT, McDermott S, Lawson AB. Metal concentrations in rural topsoil in South Carolina: potential for human health impact. Science of the Total Environment. 2008;402:149–156. doi: 10.1016/j.scitotenv.2008.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aelion CM, Davis HT, Liu Y, Lawson AB, McDermott S. Validation of Bayesian kriging of arsenic, chromium, lead and mercury in surface soils based on internode sampling. Environmental Science and Technology. 2009a;43:4432–4438. doi: 10.1021/es803322w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aelion CM, Davis HT, McDermott S, Lawson AB. Soil metal concentrations and toxicity: associations with distances to industrial facilities and implications for human health. Science of the Total Environment. 2009b;407:2216–2223. doi: 10.1016/j.scitotenv.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad SA, Salim Ullah Sayed MH, Barua S, Khan MH, Faruquee MH, Jalil A, Hadi SA, Talukder HK. Arsenic in drinking water and pregnancy outcomes. Environmental Health Perspectives. 2001;109:629–631. doi: 10.1289/ehp.01109629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz Z, Price-Green P, Bove FJ, Kaye WE. Lead exposure and birth outcomes in five communities in Shoshone county, Idaho. International Journal of Hygiene and Environmental Health. 2006;209:123–132. doi: 10.1016/j.ijheh.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Cai B, Lawson AB, McDermott S, Aelion CM. Variable selection for spatial latent predictors under Bayesian spatial model. Statistical Modelling. 2011;11:535–555. [Google Scholar]
- Calderón J, Navarro ME, Jimenez-Capdeville ME, Santos-Diaz MA, Golden A, Rodriguez-Leyva I, Borja-Aburto V, Díaz-Barriga F. Exposure to arsenic and lead and neuropsychological development in Mexican children. Environmental Research Section A. 2001;85:69–76. doi: 10.1006/enrs.2000.4106. [DOI] [PubMed] [Google Scholar]
- Caldwell KL, Jones RL, Verdon CP, Jarrett JM, Caudill SP, Osterloh JD. Levels of urinary total and speciated arsenic in the US population: National Health and Nutrition Examination Survey 2003-2004. Journal of Exposure Science and Environmental Epidemiology. 2009;19:59–68. doi: 10.1038/jes.2008.32. [DOI] [PubMed] [Google Scholar]
- Campanella R, Mielke HW. Human geography of New Orleans’ high-lead geochemical setting. Environmental Geochemistry and Health. 2008;30:531–540. doi: 10.1007/s10653-008-9190-9. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control (CDC) Blood lead levels—United States 1999-2002. Morbidity and Mortality Weekly. 2005;54:513–516. [PubMed] [Google Scholar]
- Chowdhury UK, Rahman MM, Sengupta MK, Lodh D, Chanda CR, Roy S, Quamruzzaman Q, Tokunaga H, Ando M, Chakraborti D. Pattern of excretion of arsenic compounds [arsenite, arsenate, MMA(V), DMA(V)] in urine of children compared to adults from an arsenic exposed area in Bangladesh. Journal of Environmental Science and Health, Part A. 2003;38:87–113. doi: 10.1081/ese-120016883. [DOI] [PubMed] [Google Scholar]
- Concha G, Nermell B, Vahter M. Metabolism of inorganic arsenic in children with chronic high arsenic exposure in Northern Argentina. Environmental Health Perspectives. 1998;106:355–359. doi: 10.1289/ehp.98106355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis HT, Aelion CM, McDermott S, Lawson AB. Identifying natural and anthropogenic sources of metals in urban and rural soils using GIS-based data, PCA, and spatial interpolation. Environmental Pollution. 2009;157:2378–2385. doi: 10.1016/j.envpol.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diawara MM, Litt JS, Unis D, Alfonso N, Martinez L, Crock JG, Smith DB, Carsella J. Arsenic, cadmium, lead, and mercury in surface soils, Pueblo, Colorado: implications for population health risk. Environmental Geochemistry and Health. 2006;28:297–315. doi: 10.1007/s10653-005-9000-6. [DOI] [PubMed] [Google Scholar]
- Ewen C, Anagnostopoulou MA, Ward NI. Monitoring of heavy metal levels in roadside dusts of Thessaloniki, Greece in relation to motor vehicle traffic density and flow. Environmental Monitoring and Assessment. 2009;157:483–498. doi: 10.1007/s10661-008-0550-9. [DOI] [PubMed] [Google Scholar]
- Gerr F, Letz R, Ryan PB, Green RC. Neurological effects of environmental exposure to arsenic in dust and soil among humans. NeuroToxicology. 2000;21:475–488. [PubMed] [Google Scholar]
- Hinwood AL, Sim MR, Jolley D, de Klerk N, Bastone EB, Gerostamoulos J, Drummer OH. Exposure to inorganic arsenic in soil increases urinary inorganic arsenic concentrations of residents living in old mining areas. Environmental Geochemistry and Health. 2004;26:27–36. doi: 10.1023/b:egah.0000020897.15564.93. [DOI] [PubMed] [Google Scholar]
- Kalman DA, Hughes J, van Belle G, Burbacher T, Bolgiano D, Coble K, Mottet NK, Polissar L. The effect of variable environmental arsenic contamination on urinary concentrations of arsenic species. Environmental Health Perspectives. 1990;89:145–151. doi: 10.1289/ehp.9089145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan DA, Qayyum S, Saleem S, Ansari WM, Khan FA. Lead exposure and its adverse health effects among occupational worker's children. Toxicology and Industrial Health. 2010;26:497–504. doi: 10.1177/0748233710373085. [DOI] [PubMed] [Google Scholar]
- Kim J-I, Lawson AB, McDermott S, Aelion CM. Variable selection for spatial random field predictors under a Bayesian mixed hierarchical spatial model. Spatial and Spatiotemporal Epidemiology. 2009;1:95–102. doi: 10.1016/j.sste.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-I, Lawson A, McDermott S, Aelion CM. Bayesian spatial modeling of disease risk in relation to multivariate environmental risk fields. Statistics in Medicine. 2010;29:142–157. doi: 10.1002/sim.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Lee S, Wong S, Shi W, Thornton I. The study of metal contamination in urban soils of Hong Kong using a GIS-based approach. Environmental Pollution. 2004;129:113–124. doi: 10.1016/j.envpol.2003.09.030. [DOI] [PubMed] [Google Scholar]
- Liu Y, McDermott S, Lawson AB, Aelion CM. The relationship between mental retardation and developmental delays in children and the levels of arsenic, mercury, and lead in soil samples taken near their mother's residence during pregnancy. International Journal of Hygiene and Environmental Health. 2010;213:116–123. doi: 10.1016/j.ijheh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung K, Oomen A, Duits M, Selinus O, Berglund M. Bioaccessibility of metals in urban playground soils. Journal of Environmental Science and Health Part A. 2007;42:1241–1250. doi: 10.1080/10934520701435684. [DOI] [PubMed] [Google Scholar]
- Mandal BK, Suzuki KT. Arsenic round the world: a review. Talanta. 2002;58:201–235. [PubMed] [Google Scholar]
- McDermott S, Wu J, Cai B, Lawson AB, Aelion CM. Probability of intellectual disability is associated with soil concentrations of arsenic and lead. Chemosphere. 2011;84:31–38. doi: 10.1016/j.chemosphere.2011.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller A, Müller HW, Abdullah A, Abdelgawad G, Utermann J. Urban soil pollution in Damascus, Syria: concentrations and patterns of heavy metals in the soils of the Damascus Ghouta. Geoderma. 2005;124:63–71. [Google Scholar]
- Myers SL, Lobdell DT, Liu Z, Xia Y, Ren H, Li Y, Kwok RK, Mumford JL, Mendola P. Maternal drinking water arsenic exposure and perinatal outcomes in Inner Mongolia, China. Journal of Epidemiology and Community Health. 2010;64:325–329. doi: 10.1136/jech.2008.084392. [DOI] [PubMed] [Google Scholar]
- Oyana TJ, Margai FM. Spatial patterns and health disparities in pediatric lead exposure in Chicago: characteristics and profiles of high-risk neighborhoods. The Professional Geographer. 2010;62:46–65. [Google Scholar]
- Patrick L. Lead toxicity: a review of the literature. Part 1: exposure, evaluation, and treatment. Alternative Medicine Review. 2006;11:2–22. [PubMed] [Google Scholar]
- Rava M, Crainicianu C, Marcon A, Cazzoletti L, Pironi V, Silocchi C, Ricci P, de Marco R. Proximity to wood industries and respiratory symptoms in children: a sensitivity analysis. Environment International. 2012;38:37–44. doi: 10.1016/j.envint.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Rodríguez Martín JA, López Arias M, Grau Corbí JM. Heavy metal contents in agricultural topsoils in the Ebro basin (Spain). Application of multivariate geostatistical methods to study spatial variations. Environmental Pollution. 2006;144:1001–1012. doi: 10.1016/j.envpol.2006.01.045. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Salazar MT, Morton-Bermea O, Hernandez-Alvarez E, Lozano R, Tapia-Cruz V. The study of metal contamination in urban topsoils of Mexico City using GIS. Environmental Earth Sciences. 2011;62:899–905. [Google Scholar]
- Tanyanont W, Vichit-Vadakan N. Exposure to volatile organic compounds and health risks among residents in an area affected by a petrochemical complex in Rayong, Thailand. Southeast Asian Journal of Tropical Medicine and Public Health. 2012;43:201–211. [PubMed] [Google Scholar]
- Torres-Sanchez LE, Berkowitz G, Lopez-Carrillo L, Torres-Arreola L, Rios C, Lopez-Cervantes R. Intrauterine lead exposure and preterm birth. Environmental Research. 1999;81:297–301. doi: 10.1006/enrs.1999.3984. [DOI] [PubMed] [Google Scholar]
- United States (US) Census Bureau [3 March 2011];Census 2000 Urban and Rural Classification. 2009 Available: http://www.census.gov/geo/www/ua/ua_2k.html.
- US Census 2000 Summary File 1 [South Carolina]/prepared by the U.S. Census Bureau, 2001.
- US Environmental Protection Agency (US EPA) [11 November 2011];Toxic Release Inventory (TRI) Explorer. Available: http://iaspub.epa.gov/triexplorer/tri_release.chemical.
- Wei B, Jiang F, Li X, Mu S. Spatial distribution and contamination assessment of heavy metals in urban road dusts from Urumqui, NW China. Microchemical Journal. 2009;93:147–152. [Google Scholar]
- Wigle DT, Arbuckle TE, Walker M, Wade MG, Liu S, Krewski D. Environmental hazards: evidence for effects on child health. Journal of Toxicology and Environmental Health Part B. 2007;10:3–39. doi: 10.1080/10937400601034563. [DOI] [PubMed] [Google Scholar]
- Wu SA, Xia XH, Lin CY, Chen X, Zhou CH. Levels of arsenic and heavy metals in the rural soils of Beijing and their changes over the last two decades (1985-2008). Journal of Hazardous Materials. 2010;1-3:860–868. doi: 10.1016/j.jhazmat.2010.03.084. [DOI] [PubMed] [Google Scholar]
- Zhen H, Lawson AB, McDermott S, Pande Lamichhane A, Aelion CM. A spatial analysis of mental retardation of unknown cause and maternal residence during pregnancy. Geospatial Health. 2008;2:173–182. doi: 10.4081/gh.2008.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen H, McDermott S, Lawson AB, Aelion CM. Are clusters of mental retardation correlated with clusters of developmental delay? Geospatial Health. 2009;4:17–26. doi: 10.4081/gh.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubero MB, Ibarluzea JM, Aurrekoetxea JJ, Rivera J, Parera J, Abad E, Goni F, Lopez R, Etxeandia A, Rodriguez C, Saenz JR. Serum levels of polychlorinated dibenzodioxins and dibenzofurans and PCBs in the general population living near an urban waste treatment plant in Biscay, Basque Country. Chemosphere. 2009;76:784–791. doi: 10.1016/j.chemosphere.2009.04.061. [DOI] [PubMed] [Google Scholar]