Abstract
Fibromyalgia (FM) is characterized by chronic non-inflammatory widespread pain (CWP) and changes in sympathetic function. In attempt to elucidate the pathophysiological mechanisms of FM we used a well-established CWP animal model. We aimed to evaluate changes in cardiac autonomic balance and baroreflex function in response to CWP induction in rats. CWP was induced by two injections of acidic saline (pH 4.0, n=8) five days apart into the left gastrocnemius muscle. Control animals were injected twice with normal saline (pH 7.2, n=6). One day after the second injection of acidic saline or normal saline, the animals had pulse interval (PI) and systolic arterial pressure (SAP) variability, and spontaneous baroreflex sensitivity (BRS) evaluated. After induction of CWP, there was an increase of power in the low frequency (LF) band of PI spectrum (12.75 ± 1.04 nu), a decrease in the high frequency (HF) band (87.25 ± 1.04 nu) and an increase of LF/HF ratio (0.16 ± 0.01), when compared to control animals (7.83 ± 1.13 nu LF; 92.16 ± 1.13 nu HF; 0.08 ± 0.01 LF/HF). In addition, there was an increase of power in the LF band of SAP spectrum (7.93 ± 1.39 mmHg2) when compared to control animals (2.97 ± 0.61 mmHg2). BRS was lower in acidic saline injected rats (0.59 ± 0.06 ms/mmHg) when compared to control animals (0.71 ± 0.03 ms/mmHg). Our results showed that induction of CWP in rats shifts cardiac sympathovagal balance towards sympathetic predominance and decreases BRS. These data corroborate findings in humans with FM.
Keywords: Fibromyalgia, chronic muscle pain, blood pressure, cardiovascular variability, baroreflex, sequence method
Introduction
Chronic pain is characterized by an abnormal and non-protective response to a stimulus with duration of more than six months or that outlasts normal tissue healing time (DeSantana and Sluka, 2008). Epidemiological studies show that nearly 14% of the North American population suffers with chronic pain (Clauw and Crofford, 2003), and fibromyalgia alone affects six million of the North American population (2–7% of the whole population) (Wolfe et al., 1990; Clauw and Crofford, 2003).
Fibromyalgia syndrome is characterized by widespread pain, hypersensitivity, stiffness, sleep disorder and pronounced fatigue (Boissevain and McCain, 1991). The underlying mechanisms for fibromyalgia are virtually unknown. However, several mechanisms have been proposed such as ischemia/muscular dysfunction (Bengtsson and Henriksson, 1989; Henriksson and Bengtsson, 1991), central sensitization (Price et al., 2002; Clauw and Crofford, 2003; Staud et al., 2005) or disturbances on endogenous modulatory pain systems (Kosek et al., 1996; Julien et al., 2005). The bulk of the literature supports a disturbance in central pain modulation. To better understand the mechanisms underlying chronic widespread pain, an animal model was developed that involves two intramuscular injections of acidic saline (AS pH 4.0). This model produces long-lasting diffuse mechanical hyperalgesia without motor disturbances or significant tissue damage. The hyperalgesia produced in this model is dependent on the pH and the time (days) between AS administration (Sluka et al., 2001). The hyperalgesia occurs in muscle, skin and viscera (Sluka et al., 2001; Miranda et al, 2004; Yokoyama et al., 2007). This model shows enhanced excitability in the central nervou system. Specifically, there is also an increase in the excitability in spinal neurons characterized by a bilateral increase of the receptive field, phosphorylation of cAMP response element-binding (CREB) transcription factor (Sluka et al., 2003; Hoeger Bement et al., 2003), and an increase in release of glutamate in spinal cord and brainstem (Skyba et al., 2005; Radhakrishnan and Sluka, 2009). Further the hyperalgesia is prevented and reversed by blockade of neuronal activity in the spinal cord and brainstem (Skyba et al., 2002; Tillu et al., 2008; DaSilva et al., 2010). In addition, this model is sensitive to pharmacological and non-pharmacological treatments (exercise) similar to that observed in people with fibromyalgia (Sluka et al., 2002; Nielsen et al., 2004; Yokoyama et al., 2007). Thus, this model is a useful tool to understand the underlying mechanisms of chronic widespread pain with a strong component of central sensitization, like fibromyalgia.
Subjects with fibromyalgia have a higher cardiovascular risk associated with changes in cardiac sympathetic modulation (Figueroa et al., 2008). Further, people with fibromyalgia subjected to tilt maneuvers show postural orthostatic tachycardia syndrome (Staud, 2008a), suggesting autonomic nervous system dysfunction. The brainstem site involved in this model, the rostral ventromedial medulla, in addition to mediating nociception, also plays a role in modulating cardiovascular and autonomic responses (Machado et al., 2000). Therefore, the present study aimed to evaluate the cardiac autonomic modulation in rats after induction of non-inflammatory chronic widespread pain, in an attempt to elucidate the mechanisms involved in the pathophysiology of fibromyalgia. Furthermore, the understanding of the cardiac autonomic modulation in this model could help develop appropriate treatment for fibromyalgia, decreasing the cardiovascular risk of this disease.
Methods
The experimental protocols performed in the current study were in accordance with the Guidelines for Ethical Care of Experimental Animals and were approved by the Animal Research Ethics Committee of the Federal University of Sergipe (Aracaju, SE, Brazil; Protocol #001/2010). Research trials were conducted on male Wistar rats weighing 250–300g, obtained from the animal care facility of the Federal University of Sergipe. Rats were individually housed with unrestricted access to food and tap water and were maintained at an ambient temperature of 22±1°C, on a 12h light-dark cycle. For the study, the animals were allocated in two groups: acidic saline group (pH 4.0; n=8) and normal saline group (pH 7.2; n=6).
Measurement of mechanical sensitivity
Rats were tested for response to mechanical stimulation with von Frey filaments. Animals were placed in clear cubicles on an elevated glass plate and allowed to acclimate to the new environment for at least 30 min before testing. Von Frey filaments of varying bending forces (14.7 to 240.1 mN) were applied to the plantar surface of the posterior paw until the animal withdrew its paw (Gopalkrishnan and Sluka, 2000). The lowest force at which the animal withdrew the paw on one of the two applications was recorded as paw withdrawal threshold. A decrease in withdrawal thresholds was interpreted as secondary mechanical hyperalgesia. We have previously established the test-retest reliability of this method (Sluka, 1999).
Induction of chronic muscle pain
Before the first injection of acidic or normal saline into the gastrocnemius muscle, the animals had their paw withdrawal threshold evaluated in order to record the baseline value. Immediately after establishing the paw withdrawal threshold, animals of both groups were anesthetized with isoflurane (2–5%) and injected with acidic saline (100 µL of sterile saline at pH 4.0) or normal saline (100 µL of sterile saline at pH 7.2) in the left gastrocnemius muscle. This procedure was performed again 5 days after the first injection. This procedure produces bilateral mechanical hyperalgesia lasting for 4 weeks after the second injection (Sluka et al., 2001).
Measurement of cardiovascular responses
One day after the second injection of acidic or normal saline into the gastrocnemius muscle, animals were anesthetized with thiopental sodium (50 mg/kg, i.p.) and were implanted with a polyethylene catheter (PE-10/PE-50, Intramedic, Becton Dickinson and Company, Sparks, MD, USA) into the femoral artery. The catheter was tunneled to the back of the rats and exteriorized on the back neck in the nape, and surgical incision sites were closed by sutures.
Twenty-four hours later, the arterial catheter was connected to a pressure transducer (Edwards Lifescience, Irvine, CA, USA) coupled to a preamplifier (BioData, Model BD-01, PB, Brazil). Pulsatile arterial pressure (BP) was recorded for 30 minutes using an IBM/PC equipped with an analog-to-digital interface (2 kHz; BioData, BD, Brazil). The pulsatile arterial pressure recordings were processed using a computer software (Advanced CODAS / Windaq, Dataq Instruments Inc., Akron, OH, USA) that identifies inflection points on signals and generates beat-by-beat time series with systolic arterial pressure (SAP), diastolic arterial pressure (DAP), mean arterial pressure (MAP), heart rate (HR) and pulse interval (PI) values.
Data analysis
The PI and SAP variability analysis was performed using a custom computer software program (CardioSeries v1.2 - http://sites.google.com/site/cardioseries). Beat-by-beat series obtained from pulsatile arterial pressure recordings were converted to data points every 100 ms using cubic spline interpolation (10 Hz). The interpolated series was divided into half-overlapping sequential sets of 512 data points (51.2 s). Before calculation of the spectral power density, segments were visually inspected and nonstationary data were not taken into consideration. A Hanning window was used to attenuate side effects and the spectrum was computed using a direct FFT algorithm for discrete time series. The spectra were integrated in the low frequency band (LF; 0.2–0.75 Hz) and the high frequency band (HF; 0.75-3 Hz), and results are presented in absolute and normalized form, by dividing LF and HF power by the total power minus very low frequency (VLF; <0.2 Hz) power.
The baroreflex sensitivity (BRS) was assessed in the time-domain by means of the Sequence technique, as described by Di Rienzo and colleagues (1985). A custom computer software (Analyzer v4.4 – http://www.haraldstauss.com) scanned beat-by-beat time series of SAP and PI searching for sequences of at least 4 consecutive beats in which increases in SAP were followed by PI lengthening (up sequence) and decreases in SAP were followed by PI shortening (down sequence), with a linear correlation higher than 0.85. The slope of the linear regression lines between SPB and PI was taken as a measure of BRS.
Statistical analysis
In order to evaluate the cardiac autonomic modulation in rats after induction of non-inflammatory chronic widespread pain, paired and unpaired t-test were carried out to analyze data. Differences were considered statistically significant if p<0.05. The results are presented as mean ± standard error of mean (SEM).
Results
Table 1 shows data of paw withdrawal threshold on day 0, before the first intramuscular injection of normal saline (NSG; n=6) or acidic saline (ASG; n=8), as well as on day 6 (24 hours after the second injection) in both groups. There was a significant decrease in paw withdrawal threshold, bilaterally, in the ASG rats as previously shown (Sluka et al., 2001) and thus establishes the development of enhanced mechanical sensitivity.
Table 1.
Mean values of paw withdrawal threshold on day 0 (first injection) and day 6 (after second injection), mean arterial pressure (MAP) and heart rate (HR) in rats injected with acidic saline or neutral saline into the gastrocnemius muscle.
| Right paw withdrawal threshold (mN) |
Left paw withdrawal threshold (mN) |
MAP (mmHg) |
HR (bpm) |
|||
|---|---|---|---|---|---|---|
| Day 0 | Day 6 | Day 0 | Day 6 | |||
| Acidic saline group | 92±22.40 | 28±4.04* | 132±26.90 | 27±1.82* | 131±2.22 | 409±11.64† |
| Neutral saline group | 108±31.19 | 113±20.83 | 105±16.96 | 105±16.93 | 134±4.05 | 355±11.93 |
p<0.05 compared to Day 0 in the same paw.
p<0.05 compared to Neutral saline group.
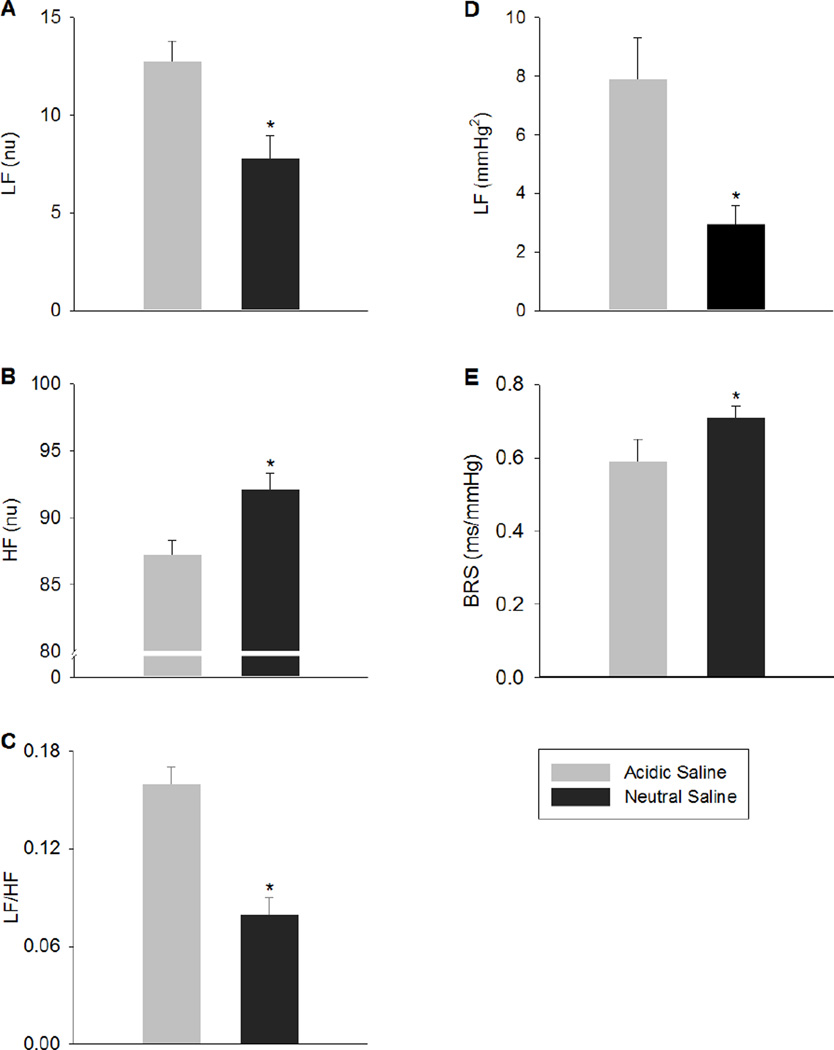
We next determined if there were differences in cardiovascular responses after the development of mechanical hypersensitivity. In the group injected with acidic saline there was an increase in the basal HR (P=0.01) (Table 1). However, the MAP was similar between the group injected with acidic saline and the group injected with normal saline (p=0.4) (Table 1). In the group injected with acidic saline, there was greater power in the LF band of PI spectrum (12.75 ± 1.04 vs. 7.83 ± 1.13 nu) and smaller power in the HF band of the PI spectrum (87.25 ± 1.04 vs. 92.16 ± 1.13 nu) when compared to the group injected with normal saline. This resulted in a greater LF/HF ratio (0.16 ± 0.01 vs. 0.08 ± 0.01; Figure 1), an indication of enhanced sympathetic activation. To further determine alterations in sympathetic activity, we analyzed the LF and HF bands of the SAP spectrum. The group injected with acidic saline showed greater power in LF band of SAP spectrum (7.93 ± 1.39 vs. 2.97 ± 0.61 mmHg2; Figure 1), further supported enhanced sympathetic activation. Lastly, we examined the baroreceptor reflex sensitivity to determine the differences in cardiac baroreflex function after the development of mechanical hypersensitivity.
Fig. 1.
Power of PI spectra at low frequency band (LF; panel A), high frequency band (HF; panel B) and LF/HF ratio (panel C), power of SAP spectra at LF (panel D) and baroreflex sensitivity (BRS; panel E) of rats injected with acidic saline and neutral saline into the gastrocnemius muscle. * p< 0.05 compared to acidic saline.
In the group injected with acidic saline, there was a lower BRS when compared to the group injected with normal saline (0.59 ± 0.06 vs. 0.71 ± 0.03 ms/mmHg; Figure 1).
Discussion
The current study shows, for the first time, that induction of chronic muscle pain in rats results in modulation of the cardiac autonomic system. Specifically, we show that after development of mechanical hypersensitivity, induced by two intramuscular injections of acidic saline (pH 4.0), shifts the cardiac autonomic balance towards a sympathetic predominance and reduction in baroreceptor reflex sensitivity.
Supporting our findings, previous studies in humans show a relationship between cardiovascular disorders (e.g. dysautonomia) and fibromyalgia (FM) (Martínez-Lavín et al., 1997; Staud, 2008a; Solano et al., 2009; Reyes Del Paso et al., 2010). This cardiac autonomic dysfunction (i.e. dysautonomia) may be accompanied by sympathetic hyperactivity at rest and sudden sympathetic changes during orthostatic tests (i.e. increase in HR and decrease in heart rate variability and BRS) (Cohen et al., 2001; Friederich et al., 2005; Furlan et al., 2005). The literature shows that chronic muscle pain pathogenic mechanism is closely associated with sympathetic modulation dysfunction and baroreflex dysfunction (Cohen et al., 2000; Raj et al., 2000; Martínez-Lavín, 2004; Figueroa, 2008). These findings are symptoms of fibromyalgia (Clauw and Crofford, 2003) and other syndromes also characterized by chronic pain, such as chronic fatigue syndrome (Tak et al., 2009). Thus, the current study is the first to document a relationship between cardiac autonomic dysfunction and chronic widespread pain in rats that mimics that found in people with chronic widespread pain (Staud, 2008b; Di Franco et al., 2009).
Chronic widespread pain induced in this animal model by repeated injections of acidic saline is initiated by activation of acid-sensing ion channels in muscle (Sluka et al., 2003) but once developed is maintained by changes in the central nervous system (Sluka et al., 2001 and 2003; Skyba et al., 2005;). While changes in excitability occur in the spinal cord as measured by neuronal hyperexcitability, increased glutamate release, and phospohorylation of transcription factors (Skyba et al., 2002 and 2005; Radharkishnan and Sluka, 2009), supraspinal sites likely mediate the widespread nature of the hyperalgesia.
The rostral ventromedial medulla (RVM) is a brain site involved in pain modulation and can both inhibit and facilitate nociceptive responses. In the acidic saline model, blockade of neuronal activity with a local anesthetic during the second injection of acidic saline prevents the development of hyperalgesia 24h later; and reverses the mechanical hypersensitivity once developed (Tillu et al., 2008), suggesting an enhanced facilitation. The RVM is not only involved in nociception but also in autonomic responses, and thus has multiple effector functions (Foo and Mason, 2005; Mason, 2005). The RVM projects to the spinal cord where it targets the dorsal horn and sympathetic preganglionic neurons (Allen and Cechetto, 1994; Mason, 2005). With respect to sympathetic control, inhibition of the RVM reduces the tachycardia evoked by air-jet stress, and autonomic responses in fear conditioning (Zaretsky et al., 2003; Vianna et al., 2008).
Glutamate in the RVM plays a critical role in the development and maintenance of the hypersensitivity in this model of acidic saline (Radharkishnan and Sluka, 2009; Da Silva et al., 2010a). Glutamate in the RVM can also modulate the cardiac autonomic system (de Toledo Bergamaschi et al., 2006). In response to the second acidic saline injection there is an increase in glutamate release in the RVM and blockade of NMDA glutamate receptors (N-methyl-D-aspartate) in the RVM reversed the mechanical hyperalgesia (Radharkishnan and Sluka, 2009; Da Silva et al., 2010b). With respect to autonomic responses, injection of glutamate into the RVM increases MAP and HR (Bazil and Gordon, 2001; Zaretsky et al, 2003) and glutamate receptors mediate sympathoexcitatory responses in rostral ventrolateral medulla (RLVM) (Bardgett et al., 2010) showing their role in mediating pain facilitation and autonomic nervous system control. These findings show interplay between central pathways, namely the RVM, for both chronic widespread muscle pain and cardiac autonomic modulation. The increases in glutamate and activation of NMDA receptors in the RVM, could explain or result in long term development of permanent and potentially lethal cardiovascular disturbances in people with fibromyalgia.
Conclusions
There are studies in the literature showing that cardiac autonomic dysfunction may be used to diagnose fibromyalgia. To our knowledge, this is the first study to demonstrate the presence of autonomic cardiovascular dysfunction in an animal model of chronic widespread muscle pain, showed by changes in baroreflex control and cardiac autonomic modulation. Additional studies are needed to better understand the relationship between cardiac autonomic control and fibromyalgia pathophysiology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen GV, Cechetto DF. Serotoninergic and nonserotoninergic neurons in the medullary raphe system have axon collateral projections to autonomic and somatic cell groups in the medulla and spinal cord. J Comp Neurol. 1994;15(3):357–366. doi: 10.1002/cne.903500303. 350. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, McCarthy JJ, Stocker SD. Glutamatergic Receptor Activation in the Rostral Ventrolateral Medulla Mediates the Sympathoexcitatory Response to Hyperinsulinemia. Hipertension. 2010;55:284–289. doi: 10.1161/HYPERTENSIONAHA.109.146605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazil MK, Gordon FJ. Spinal NMDA receptors mediate pressor responses evoked from the rostral ventrolateral medulla. Am J Physiol. 1991;260:H267–H275. doi: 10.1152/ajpheart.1991.260.1.H267. [DOI] [PubMed] [Google Scholar]
- Bengtsson A, Henriksson KG. The muscle in fibromyalgia-- a review of Swedish studies. J Rheumatol Suppl. 1989;19:144–149. [PubMed] [Google Scholar]
- Boissevain MD, Mccain GA. Toward an integrated understanding of fibromyalgia syndrome. I. Medical and pathophysiological aspects. Pain. 1991;45:227–238. doi: 10.1016/0304-3959(91)90047-2. [DOI] [PubMed] [Google Scholar]
- Clauw DJ, Crofford LJ. Chronic widespread pain and fibromyalgia: what we know, and what we need to know? Best Pract Res Clin Rheumatol. 2003;17:685–701. doi: 10.1016/s1521-6942(03)00035-4. [DOI] [PubMed] [Google Scholar]
- Cohen H, Neumann L, Shore M, Amir M, Cassuto Y, Buskila D. Autonomic dysfunction in patients with fibromyalgia: application of power spectral analysis of heart rate variability. Semin Arthritis Rheum. 2000;29(4) doi: 10.1016/s0049-0172(00)80010-4. 217-27. [DOI] [PubMed] [Google Scholar]
- Cohen H, Neumann L, Kotler M, Buskila D. Autonomic nervous system derangement in fibromyalgia syndrome and related disorders. Isr Med Assoc J. 2001;(10):755–760. [PubMed] [Google Scholar]
- Da Silva LF, DeSantana JM, Sluka KA. Activation of NMDA Receptors in the Brainstem, Rostral Ventromedial Medulla, and Nucleus Reticularis Gigantocellularis Mediates Mechanical Hyperalgesia Produced by Repeated Intramuscular Injections of Acidic Saline in Rats. The Journal of Pain. 2010a;11(4):378–387. doi: 10.1016/j.jpain.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva LF, Walder RY, Davidson BL, Wilson SP, Sluka KA. Changes in expression of NMDA-NR1 receptor subunits in the rostral ventromedial medulla modulate pain behaviors. Pain. 2010b;151:155–161. doi: 10.1016/j.pain.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Toledo Bergamaschi C, de Arruda Carillo B, Futuro Neto HA, Campos RR. Differential baroreceptor modulation mediated by the ventrolateral medulla. Auton Neurosci. 2006;30:126–127. 156–162. doi: 10.1016/j.autneu.2006.02.012. [DOI] [PubMed] [Google Scholar]
- DeSantana JM, Sluka KA. Central mechanisms in the maintenance of chronic widespread noninflammatory muscle pain. Curr Pain Headache Rep. 2008;12(5):338–343. doi: 10.1007/s11916-008-0057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Franco M, Iannuccelli C, Alessandri C, Paradiso M, Riccieri V, Libri F, Valesini G. Autonomic dysfunction and neuropeptide Y in fibromyalgia. Clin Exp Rheumatology. 2009;27(5 Suppl 56):75–78. [PubMed] [Google Scholar]
- Di Rienzo M, Bertinieri G, Mancia G, Pedotti A. A new method for evaluating the baroreflex role by a joint pattern analysis of pulse interval and systolic blood pressure series. Med Biol Eng Comput. 1985;23(suppl I):313–314. [Google Scholar]
- Figueroa A, Kingsley JD, McMillan V, Panton LB. Resistance exercise training improves heart rate variability in women with fibromyalgia. Clin Physiol Funct Imaging. 2008;28(1):49–54. doi: 10.1111/j.1475-097X.2007.00776.x. [DOI] [PubMed] [Google Scholar]
- Foo H, Mason P. Movement-related discharge of ventromedial medullary neurons. J Neurophysiol. 2005;93:873–883. doi: 10.1152/jn.00750.2004. [DOI] [PubMed] [Google Scholar]
- Friederich HC, Schellberg D, Mueller K, et al. Stress and autonomic dysregulation in patients with fibromyalgia syndrome. Schmerz. 2005;19:185–188. doi: 10.1007/s00482-004-0335-1. [DOI] [PubMed] [Google Scholar]
- Furlan R, Colombo S, Perego F, Atzeni F, Diana A, Barbic F, et al. Abnormalities of cardiovascular neural control and reduced orthostatic tolerance in patients with primary fibromyalgia. J Rheumatol. 2005;32:1787–1793. [PubMed] [Google Scholar]
- Gopalkrishnan P, Sluka KA. Effect of varying frequency, intensity, and pulse duration of transcutaneous electrical nerve stimulation on primary hyperalgesia in inflamed rats. Arch Phys Med Rehabil. 2000;81(7):984–990. doi: 10.1053/apmr.2000.5576. [DOI] [PubMed] [Google Scholar]
- Henriksson KG, Bengtosson A. Fibromyalgia--a clinical entity? Can J Physiol Pharmacol. 1991;69:672–677. doi: 10.1139/y91-100. [DOI] [PubMed] [Google Scholar]
- Hoeger-Bement MK, Sluka KA. Phosphorylation of CREB and mechanical hyperalgesia is reversed by blockade of the cAMP pathway in a time-dependent manner after repeated intramuscular acid injections. J Neurosci. 2003;23:5437–5445. doi: 10.1523/JNEUROSCI.23-13-05437.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien N, Goffaux P, Arsenault P, et al. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain. 2005;114:295–302. doi: 10.1016/j.pain.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Machado BH, Castania JA, Bonagamba LG, Salgado HC. Neurotransmission of autonomic components of aortic baroreceptor afferents in the NTS of awake rats. Am J Physiol Heart Circ Physiol. 2000;279(1):H67–H75. doi: 10.1152/ajpheart.2000.279.1.H67. [DOI] [PubMed] [Google Scholar]
- Martínez-Lavín M, Hermosillo AG, Mendoza C, Ortiz R, Cajigas JC, Pineda C, Nava A, Vallejo M. Orthostatic sympathetic derangement in subjects with fibromyalgia. J Rheumatol. 1997;24(4):714–718. [PubMed] [Google Scholar]
- Martínez-Lavin M. Fibromyalgia as a sympathetically maintained pain syndrome. Curr Pain Headache Rep. 2004;8(5):385–389. doi: 10.1007/s11916-996-0012-4. [DOI] [PubMed] [Google Scholar]
- Mason P. Deconstructing endogenous pain modulations. J Neurophysiol. 2005;94(3):1659–1663. doi: 10.1152/jn.00249.2005. [DOI] [PubMed] [Google Scholar]
- Miranda A, Peles S, Rudolph C, Shaker R, Sengupta JN. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126(4):1082–1089. doi: 10.1053/j.gastro.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Nielsen AN, Mathiesen C, Blackburn-Munro G. Pharmacological characterisation of acid-induced muscle allodynia in rats. Eur J Pharmacol. 2004;8(1–3):93–103. doi: 10.1016/j.ejphar.2004.01.017. 487. [DOI] [PubMed] [Google Scholar]
- Price DD, Staud R, Robinson ME, et al. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain. 2002;99:49–59. doi: 10.1016/s0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R, Sluka KA. Increased glutamate and decreased glycine release in the rostral ventromedial medulla during induction of a pre-clinical model of chronic widespread muscle pain. Neuroscience Letters. 2009;457:141–145. doi: 10.1016/j.neulet.2009.03.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj SR, Brouillard D, Simpson CS, Hopman WM, Abdollah H. Dysautonomia among patients with fibromyalgia: a noninvasive assessment. J Rheumatol. 2000;27(11):2660–2665. [PubMed] [Google Scholar]
- Reyes Del Paso GA, Garrido S, Pulgar A, Martín-Vázquez M, Duschek S. Aberrances in autonomic cardiovascular regulation in fibromyalgia syndrome and their relevance for clinical pain reports. Psychosomatic Medicine. 2010;72(5):462–470. doi: 10.1097/PSY.0b013e3181da91f1. [DOI] [PubMed] [Google Scholar]
- Skyba DA, King EW, Sluka KA. Effects of NMDA and non-NMDA ionotropic glutamate receptor antagonists on the development and maintenance of hyperalgesia induced by repeated intramuscular injection of acidic saline. Pain. 2002;98:69–78. doi: 10.1016/s0304-3959(01)00471-7. [DOI] [PubMed] [Google Scholar]
- Skyba DA, Lisi TL, Sluka KA. Excitatory amino acid concentrations increase in the spinal cord dorsal horn after repeated intramuscular injection of acidic saline. Pain. 2005;119:142–149. doi: 10.1016/j.pain.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Deacon M, Stibal A, Strissel S, Terpstra A. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. J Pharmacol Exp Ther. 1999;289(2):840–846. [PubMed] [Google Scholar]
- Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Rohlwing JJ, Bussey RA, Eikenberry SA, Wilken JM. Chronic muscle pain induced by repeated acid Injection is reversed by spinally administered muand delta-, but not kappa-, opioid receptor agonists. J Pharmacol Exp Ther. 2002;302(3):1146–1150. doi: 10.1124/jpet.102.033167. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Price MP, Breese NM, et al. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–239. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- Staud R, Vierck CJ, Robinson ME, et al. Effects of the Nmethyl- D-aspartate receptor antagonist dextromethorphan on temporal summation of pain are similar in fibromyalgia patients and normal control subjects. J Pain. 2005;6:323–332. doi: 10.1016/j.jpain.2005.01.357. [DOI] [PubMed] [Google Scholar]
- Staud R. Autonomic dysfunction in fibromyalgia syndrome: postural orthostatic tachycardia. Curr Rheumatol Rep. 2008a;10(6):463–466. doi: 10.1007/s11926-008-0076-8. [DOI] [PubMed] [Google Scholar]
- Staud R. Heart rate variability as a biomarker of fibromyalgia syndrome. Future Rheumatology. 2008b;1(5):475–483. doi: 10.2217/17460816.3.5.475. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano C, Martinez A, Becerril L, Vargas A, Figueroa J, Navarro C, Ramos-Remus C, Martinez-Lavin M. Autonomic dysfunction in fibromyalgia assessed by the Composite Autonomic Symptons Scale (COMPASS) J Clin Rheumatol. 2009;15(4):172–176. doi: 10.1097/RHU.0b013e3181a1083d. [DOI] [PubMed] [Google Scholar]
- Tak LM, Riese H, Bock GH, Manoharan A, Kok IC, Rosmalen JGM. As good as it gets? A meta-analysis and systematic review of methodological quality of heart rate variability studies in functional somatic disorders. Biological Psychology. 2009;82:101–110. doi: 10.1016/j.biopsycho.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Vianna DM, Allen C, Carrive P. Cardiovascular and behavioral responses to conditioned fear after medullary raphe neuronal blockade. 2008;153:1344–1353. doi: 10.1016/j.neuroscience.2008.03.033. [DOI] [PubMed] [Google Scholar]
- Walder RY, Rasmussen LA, Rainier JD, Light AR, Wemmie JA, Sluka KA. ASIC1 and ASIC3 Play Different Roles in the Development of Hyperalgesia After Inflammatory Muscle Injury. The Journal of Pain. 2010;11(3):210–218. doi: 10.1016/j.jpain.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- Yokoyama T, Maeda Y, Audette KM, Sluka KA. Pregabalin reduces muscle and cutaneous hyperalgesia in two models of chronic muscle pain in rats. J Pain. 2007;8:422–429. doi: 10.1016/j.jpain.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Zaretsky DV, Zaretskaia MV, Samuels BC, Cluxton LK, DiMicco JA. Microinjection of muscimol into raphe pallidus suppresses tachycardia associated with air stress in conscious rats. J Physiol. 2003;546:243–250. doi: 10.1113/jphysiol.2002.032201. [DOI] [PMC free article] [PubMed] [Google Scholar]