Forward
More than 25 years ago, the author was asked to provide a retrospective analysis of the global status of what were then called “red tides”, but are now known as “harmful algal blooms” or HABs. The occasion was an international conference in Takamatsu, Japan (Okaichi et al. 1989), convened at a time when algal blooms seemed to be affecting more and more countries and causing increased economic losses through a growing number of impacts, but long-term trends were anecdotal and speculative. The challenge was a significant one: to evaluate blooms of many different types of algae – some producing toxins that could affect humans, fish, shellfish, and many different marine organisms and ecosystems, some causing harm in a multitude of other ways – and then assess whether the problem was indeed “growing worse” on a global basis. The resulting paper (Anderson 1989), though highly qualitative in its approach and content, helped to ignite a scientific discussion that motivated many studies and publications, some arguing that indeed the HAB problem was growing worse as a result of pollution (e.g., Smayda 1989) or other factors such as expanded aquaculture operations or ballast water transfer of species (e.g., Hallegraeff 1993), while others contended that the “global expansion of HABs” was not accepted by all or was being exaggerated by scientists and the press. Skeptics counseled caution and argued that the increased number of toxins and impacted resources had other causes, including the simple discovery of toxins and toxic species that had always existed. It is now clear that the “global expansion” of HAB phenomena is real, due in part to our ability to better define the boundaries of the problem. However, those boundaries are not static, but continue to expand due to natural dispersal via storms or currents, as well as to effects from human influences, such as pollution, aquaculture expansion, and ballast water transport. The fact that part of this expansion is simply because of increased scientific awareness and detection capabilities should not temper our concern. The global problem of HABs is serious and much larger than we thought. Now, at the 15th International Conference on Harmful Algae in Korea in 2012, a new challenge has been posed – this time to look forward and envision the nature of HABs, the field of HAB science, and the nature of HAB impacts and management in an era where so many features are changing rapidly due to population pressures, climatic shifts, and many other global, regional, and local forcings. Motivation for this request reflects a desire to anticipate changes that can guide research priorities, technology development, and social and commercial policies in areas that are either affected by, or that affect, HABs. The following thoughts are offered in the same manner as those written in the 1989 retrospective – as personal views offered in hopes that others will expand on these ideas and ultimately create a scholarly and comprehensive perspective on the future of HABs in a changing world.
Introduction
Over the last several decades, capabilities for research and management of HABs have grown at a rapid rate. Scientific advances have been significant in many areas, and now a large and capable research and management community exists where formerly there were only scattered individuals and programs, often working independently. Powerful new technological developments have altered the way HABs can be monitored and managed (e.g., Anderson et al. 2012; Scholin et al. 2009; Campbell et al. 2010). HAB problems are serious, and in some areas of the world are growing worse, but capabilities and knowledge exist that help to minimize impacts and protect public health and marine resources as never before. This scientific and management community, and the HABs they respond to and investigate, now face a world that is changing in many ways due to population growth, pollution, and climate change, to name but a few stressors. Even the current global economic crisis can be viewed as a factor that will affect the future of HAB science and management due to reductions in funding or diversion of scientific teams to other topics, for example. Of equal importance, perhaps, perceptions of HABs are changing, affecting the behavior, needs, and priorities of the public, funding agencies, and those charged with managing these diverse phenomena. Here I explore some aspects of HABs in a changing world, looking several decades into the future in an attempt to envision the manner in which these bloom phenomena will be affected globally, and the associated challenges that the HAB research and management community will need to meet. Given space limitations and the many issues that could be addressed in this context, each can only be covered in a brief or cursory manner, but where possible, more detailed analyses or reviews of individual subject areas will be cited. Taken together, the view that emerges is one of new and exciting opportunities for research and management of HABs in our changing world, but with significant challenges as well.
Population Growth and Food Production
Population growth
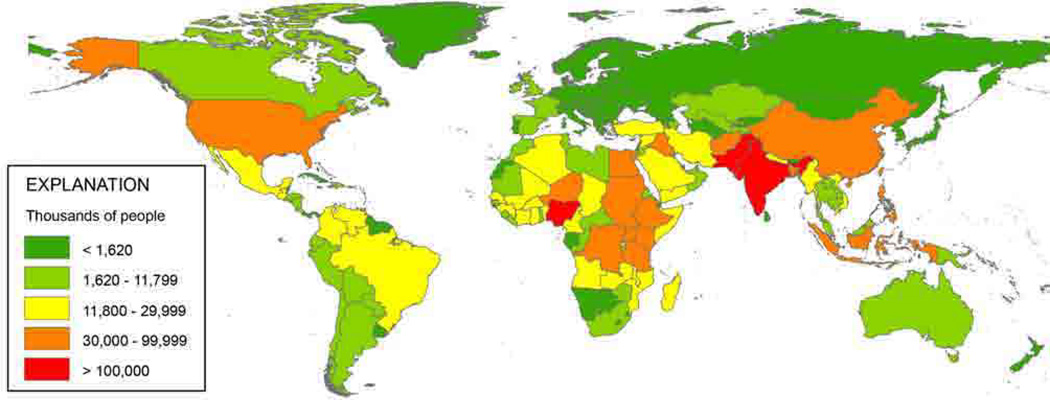
The current world population of close to 7 billion is projected to reach 9.3 billion by 2050 and 10.1 billion by 2100 (United Nations, Department of Economic and Social Affairs, Population Division, 2009). This growth will not be uniform geographically, with much of the increase projected to come from high-fertility countries, 39 of which are in Africa, nine in Asia, six in Oceania and four in Latin America (Fig 1). Nearly 40% of this growing population will inhabit the coastal zone. It is well established that coastal development can lead to changes that affect some HABs – from increased pollution of the coastal zone that can stimulate some HAB species through nutrient enrichment, to alteration of coastal hydrography, nutrient dynamics, and ecosystem health and structure through the destruction of wetlands or the creation of marinas and harbors (GEOHAB 2006).
Fig. 1.
Increases in the number of people by country projected for the period 2010–2050. (Rice and Herman 2012)
Food production and agriculture
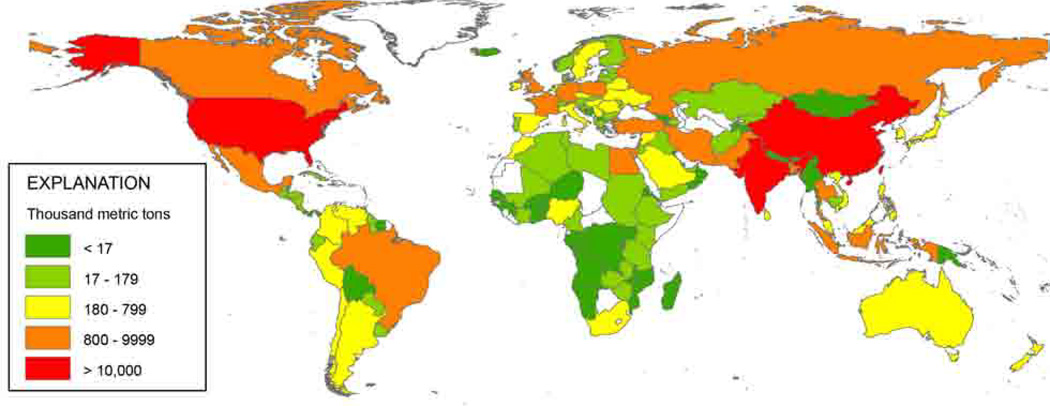
Concurrent with this population growth will be a need to increase global food supplies by 30% by 2050. This is a massive increase, and it will have to be accomplished through expanded agriculture and aquaculture, as well as with technological advances to increase productivity in each. Agriculture will presumably account for the majority of the necessary increase in food capacity, which in turn, will undoubtedly be associated with increased fertilizer usage and thus increased loadings of nitrogen, phosphorous, and other algal nutrients to the coastal zone. Fertilizer application on land remains a major contributor to non-point nutrient pollution, and is expected to increase at an alarming rate in many regions (Seitzinger et al. 2010). Both industrial and developing nations are using significantly higher loadings of fertilizer in agriculture, with global N fertilizer usage increasing more than 10-fold since 1960 (Smil 2001). Looking at where fertilizers have been applied globally as a possible guide to the future, the expected pattern is non-uniform (Fig. 2; Rice and Herman 2012). The heaviest applications will once again be associated with China and India, but the United States and a number of other countries or regions such as Brazil, Canada, Southeast Asia, Russia, Africa, and Europe are expected to be major users as well.
Fig. 2.
Nitrogen fertilizer application by country in 2008 expressed as mass of N in thousands of metric tons. Countries with no reported data are shown as white. (Rice and Herman 2012)
One of the most rapidly increasing sources of nutrients to both freshwaters and the coastal zone is the atmosphere, and here again, we can expect inputs from this source to continue to grow as cities and industrial and commercial centers grow, and as more houses, cars, animal feedlots, and other sources of airborne pollution are created. In estuarine and coastal waters, it has been estimated that 20–40% of N inputs are ultimately of atmospheric origin, derived from industrial, agricultural, and urban sources (Paerl 1995).
Linkages between HABs and land-derived nutrient over enrichment have been noted within the past several decades by many workers (e.g., Smayda 1989, Anderson et al. 2002, Glibert et al. 2006). Whether enhanced nutrient inputs specifically stimulate HABs is a subject of continuing debate (reviewed in Gowen et al. 2012), but few would contest that increased nutrient loadings can lead to increased phytoplankton growth and biomass, and in some cases, in more HABs. Going forward, coastal waters of countries with heavy fertilizer usage (Fig. 2) can thus expect higher nutrient loadings, more algal productivity, and potentially more HABs, but other countries will be affected as well, even those with modest increases in nutrient loadings from agriculture. Note also that the algal proliferations resulting from these nutrient inputs are not always immediately evident as toxic or destructive HABs, but can instead cumulatively lead to major downstream effects known as ‘dead zones’ - areas where the dissolved oxygen levels are so low that marine life cannot be sustained (other than micro-organisms). Since the 1960s, the number of dead zones in coastal waters has doubled every decade (Diaz and Rosenberg 2008), and given the expanding population and food production, it seems inevitable that this aspect of HAB impacts will continue to grow through time.
Capture fisheries and aquaculture
One might hope that a significant portion of the world's growing need for food could derive from wild fisheries, but this is decidedly not the case. Overall global capture fisheries production continues to remain stable at about 90 million tons (FAO 2012). Furthermore, most stocks remain fully exploited or over-exploited. As of 2006, FAO reports that 52% of the world’s commercial fish stocks are fully exploited, 19% are over-exploited, with 8% “significantly depleted” and 1% slowly recovering (FAO 2012). Only 20% of stocks are considered under-exploited or moderately exploited. Of the world’s 15 major fishing regions, productivity has fallen over the past few years in all but four. The alarming state of capture fisheries prompted FAO (2012) to warn: “the maximum wild capture fishery potential from the world's oceans has probably been reached.”
While capture fisheries production remains stable, aquaculture production has been expanding rapidly. Aquaculture remains one of the fastest-growing animal food-producing sectors, and in the next decade, total production from both capture and aquaculture will exceed that of beef, pork or poultry. In the last three decades, for example, world food fish production from aquaculture has expanded almost 12 times, at an average annual rate of 8.8% (FAO 2012). China continues to account for the largest share of farmed marine and freshwater species, with 70% of the total volume and over 50% of the total value. Other major producers in Asia are India, Vietnam, Indonesia, Bangladesh, Thailand, Myanmar, the Philippines, and Japan.
Aquaculture can thus contribute substantially to the global need for increased food production, but it also represents another source of HAB nutrients, provided as feed or fertilizer, and modulated by the biological transformations occurring in these high biomass systems. Cultured fish retain only a fraction of their food – the rest decomposes in the water column or settles to the bottom and decomposes; either way, the nutrients released from this decomposition can stimulate phytoplankton growth. Even molluscan mariculture can be a cause for concern, as the long-held view that such production is always environmentally favorable because the natural water-clearing effects of filter feeding shellfish is now being questioned in certain situations, particularly those with heavy stocking densities (Bouwman et al. 2013). The concern is that, because of their low assimilation efficiencies, molluscs can become point sources of regenerated nutrients, acting as pumps that transform the nutrients in algal biomass into dissolved and particulate forms. Benthic regeneration of the accumulated feces and decomposing feed can thus be a significant and sustained source of nutrients in such systems. In their review, Bouwman et al. (2013) examined several scenarios of future aquaculture development, and estimated that nutrients from aquaculture operations of all types will increase up to six-fold by 2050, and more importantly, that this increase may exceed the nutrient assimilative capacity in those parts of the world where aquaculture is rapidly growing.
To reduce nutrient effects from these expanding operations, production may have to be moved to areas that are well flushed or that are located in deeper, more dynamic offshore waters. This is happening to some extent in the U.S. and some other countries, where fish farming operations have shifted from easily accessible but poorly flushed bays and coves to areas with much stronger currents – resulting in a significant reduction in particulate and dissolved nutrient buildup, and consequently, reduced planktonic and benthic impacts. In contrast, most fish farms in developing countries are located in shallow, easily accessible bays where nutrients can accumulate and stimulate algal blooms. The effect can be worsened when aquaculture sites are located in wetlands (e.g., salt marshes or mangrove swamps) that otherwise would serve as a sink rather than a source of nutrients to the system.
The takeaway messages from these predictions of population growth and the need for expanded food production are several: 1) eutrophication-related HABs are not going to decrease in the near future, but are likely to increase on a global basis; 2) the distribution of nutrient-enhanced HABs will not be uniform, as many countries with both rapid population growth and increased agriculture and aquaculture production (e.g., China, India, Africa) will continue to be “hot spots” for such outbreaks, unless major policy changes are instituted that lead to more efficient application of fertilizers or to the optimal location and operation of aquaculture facilities; and 3) aquaculture operations will continue to increase dramatically going forward, providing high quality food for a growing population, but also contributing to the HAB problem, through nutrient enrichment in poorly flushed areas, as well as the placement of susceptible resources in areas where HABs can cause problems, through both toxicity and high biomass effects.
These scenarios are not speculative – there is already ample evidence that these impacts are occurring. For example, China has experienced rapid increases in eutrophication, mariculture, and other population-related pressures in the coastal zone, and this has been accompanied by an equally dramatic increase in the frequency and diversity of HABs, as well as changes in the species composition of harmful algae (Zhou and Zhu 2006). Virtually all of the main HAB species that we are familiar with worldwide have been observed over the last decade or two in Chinese waters, some detected for the first time (Lu et al. 2014). As might be expected given the heavy nutrient inputs to coastal waters from anthropogenic sources, large-scale, high-biomass blooms (those covering more than 1,000 km2) have been increasing dramatically along the coast. Prorocentrum donghaiense has been a recurrent bloom species in the East China Sea for more than ten years, and other high biomass bloom formers include Phaeocystis globosa, Cochlodinium geminatum, and Karlodinium veneficum. Brown tides caused by Aureococcus anophagefferens have recently occurred in the Bohai Sea. Just 20 years ago, there were only a handful of HAB scientists in China, and only a few recognized species of concern. Clearly times have changed, as China has developed one of the largest national communities of HAB researchers and managers anywhere, and given the trends noted above in population growth, fertilizer usage, and mariculture expansion, this country and others that are developing in similar ways may need to expand their HAB research and management capabilities even further.
Species dispersal, range expansion, and new toxins
From the foregoing, it is apparent that for some parts of the world at least, the HAB problem will remain the same, or more likely, worsen with the combined pressures of a growing world population, food production, aquaculture development, and coastal development. Superimposed on this type of HAB expansion will be the emergence of new species or impacts. Although many HAB species are already widespread, some continue to appear in new locations, as has been the case with Cochlodinium polykrikoides in recent years (Kudela and Gobler 2012). Aureococcus anophagefferens, the tiny brown tide organism that was formerly restricted to the northeastern US and South Africa, is now causing massive blooms along the coast of China (Zhang et al. 2012), and another brown tide species – Aureoumbra lagunensis – has expanded its range from a single area in the Gulf of Mexico to Florida (Gobler et al. 2013), and more recently to Cuba (Koch et al. 2014). Ostreopsis is another emerging problem – formerly of concern as a benthic dinoflagellate potentially linked to ciguatera fish poisoning, but more recently shown to be the source of toxic aerosols that cause respiratory problems and illness among beach goers. As stated by Rhodes (2011), the distribution of Ostreopsis has expanded markedly in the last decade, associated illnesses have also increased, and these trends are likely to continue.
These are but a few examples of the continuing expansion of HAB species globally, and there seems little doubt that these types of reports will continue for many years. One could argue, however, that we will frequently be documenting the dispersal of HAB species that we already know about, with fewer and fewer new species or toxins being described. This reflects the maturity of the HAB field, as well as the global distribution of many HAB species. However, as discussed below, species that are not traditionally considered to be harmful are being identified as HAB species as new societal activities or resources are affected in negative ways by algal blooms.
With respect to toxins, the pace of discovery of new toxins has slowed from the days when saxitoxin and brevetoxin were known, but dinophysistoxins, pectenotoxins, domoic acid, yessotoxins, spirolides, azaspiracids, palytoxins, and others were yet to be identified or described. It has been more than a decade since the characterization of the azaspiracids (James et al. 2003), the last major toxin associated with a new and previously unknown acute human toxicity syndrome (azaspiracid shellfish poisoning – AZP), and about five years since euglenophycins were described (Zimba et al. 2010). This trend suggests that the ‘golden age of discovery’ of new classes of phycotoxins causing human toxin syndromes may be behind us (Anderson et al. 2012). There will, however, still be new toxin discoveries going forward, but these will presumably occur less frequently than in the past. Meanwhile, HAB chemists will remain busy with the development of new analytical technologies and approaches to measure known toxins, identification of derivatives and metabolites of the known toxins, and characterization of mechanisms of biotransformation and bioaccumulation. One compilation (P. Hess, pers. comm.) lists 444 toxins in the major HAB toxin families, with 322 known chemical structures. These include 10 compounds in the domoic acid family, 18 saxitoxins, 60 okadaic acids, 18 azaspiracids, and 7 microcystins, to name but a few. In addition to these, there are numerous metabolites – nearly 40 additional compounds in the saxitoxin family alone (Weiss et al. 2010). Many HAB toxin metabolites are not fully characterized, so it will take many years to document their potency, structure, and public health significance. The latter is of particular importance, as managers are already struggling with the need to monitor multiple toxins in seafood or drinking water, and adding more compounds to that list will be problematic for many. It is important for resource managers to know that there are numerous derivatives and metabolites potentially present, but the realities of measuring so many closely related compounds argues for simplification of the monitoring process (through functional rather than analytical assays, or LC/MS methods that allow concurrent detection of many toxins, for example). ELISA assays are currently available for most of the major HAB toxins, and this brings toxin detection capabilities to many who lack expensive and complex instrumentation for direct chemical analysis. Fishermen can now test shellfish and other commercial products themselves, opening up access to resources that are otherwise restricted. A prime example of the value of these kits is the reopening of the offshore surf clam and ocean quahog industry on Georges Bank, which had been closed to harvest for over 20 years due to the threat of PSP toxins. Development of an Onboard Screening, Dockside Testing Protocol based on saxitoxin ELISA kits has resulted in the sustained harvest of millions of dollars of offshore shellfish in that region (DeGrasse et al. 2014), and it is likely that the approach will be used to maximize the harvest of offshore shellfish in other areas as well.
One major gap in our current toolbox for HAB toxin detection is the lack of a reliable kit-based assay for ciguatoxins. Ciguatera fish poisoning (CFP) remains the most serious of all HAB human poisoning syndromes globally, with estimates of 50,000 or more poisonings annually. There is a clear need for inexpensive and widely-deployable ciguatoxin detection capabilities that can protect populations from poisoning, and allow consumption of reef fish that are presently avoided due to the risk of CFP. Although the technical challenges to developing an antibody with sufficient specificity to the large ciguatoxin molecule are significant, new approaches such as single-domain antibodies may provide this much-needed capability going forward.
Changing perceptions of HABs
One expectation for the future is that some countries or regions may begin to reevaluate the way that algal blooms are viewed, and in particular, which species are considered harmful. The term HAB has always been broad, as it was intended to include toxic blooms as well as those that cause harm in other, diverse ways. Despite the long list of HAB impacts that are well known and recurrent throughout the world, new impacts will emerge going forward, and with that will come the designation of new harmful species. One current example is with desalination plants. The expansion of HABs due to pollution, coastal development, and other factors, is occurring at a time when there is also an increase in the construction of desalination plants to produce drinking water. There are currently more than 14,000 desalination plants in more than 150 countries worldwide, and the desalination market is forecast to grow by 12% per year. The intersection of these plants with nearshore HABs is inevitable. Recent research suggests that algal toxins are effectively removed by desalination processes and pretreatments (e.g., Laycock et al. 2012, Dixon et al. 2011), so the larger concerns relate to algal biomass. It is now clear that algal species can produce organics that pass through ultrafilters and other pretreatment processes, forming gels or polymers (e.g., transparent exocellular compounds or TEPS; Berman 2012.) or extracellular polymeric substances (EPS; Flemming and Wingender, 2001) that are either the direct cause of fouling or that serve as a nutrient source for the microbial communities that foul the membranes. These compounds can be seriously disruptive, particularly to those plants that use reverse osmosis (RO) to produce fresh water. One example is the bloom of Cochlodinium polykrikoides in the Arabian Gulf and Sea of Oman in 2008/2009 that affected a large number of RO desalination plants, closing some for as long as two months (Richlen et al. 2010). Since economic considerations are leading to a huge expansion in RO desalination plants compared to those that use flash evaporation or other thermal processes, we can expect many more impacts of HABs on desalination facilities than have been recorded thus far. It is also very likely that species that are not generally considered harmful to other sectors of society will be harmful to these plants because they produce disproportionally large amounts of dissolved organic materials. Eventually, a list of species that are prolific producers of harmful organic compounds (that are not toxins) will be generated and used by desalination plant operators to plan mitigation strategies.
Another aspect of the changing perception of HABs stems from the fact that many are very well understood and managed in certain countries. This in turn can lead to a shift in research funding to other areas – either to other HAB problems or syndromes, or to unrelated areas. One example is in Japan, where the management of PSP and DSP is so effective that human illnesses are rare. As a result, funding agencies do not feel it is worth investing in research on the fundamental science of those topics; in effect, they are not “problems” any more. Now many HAB scientists in Japan are shifting their research to topic areas where major unknowns remain – to ciguatera fish poisoning, for example (Y. Fukuyo, pers. comm.).
A related shift in some countries is from fundamental research on physiology, ecology, and genetics to topics directly applicable to management, such as bloom control or impact mitigation. In one sense, this is a reflection of the maturity of the field, and the progress made in our efforts to understand the fundamental mechanisms underlying HABs and their impacts. However, this trend towards more practical and pragmatic HAB research is not necessarilly a wise approach. In the U.S., for example, the HAB community has long argued that the most effective national HAB program is one that sustains the full spectrum of research, including the Ecology and Oceanography of HABs (the ECOHAB program; Anderson 1995), Monitoring and Event Response for HABs (http://www.cop.noaa.gov/stressors/extremeevents/hab/current/fact-merhab.aspx) and Prevention, Control, and Mitigation of HABs (PCMHAB; Dortch et al. 2008). However, in a sign of what many other countries are already experiencing or will experience, the funding emphasis within these national programs has changed in recent years due to pressures from mission-oriented agencies with clear mandates for practical outcomes such as improved forecasts or bloom control. The challenge in the U.S. and elsewhere is thus to maintain an emphasis on fundamental HAB science and resist the temptation to shift resources to solely address more practical aspects.
Yet another interesting change in the perception of what is harmful comes from countries that are heavily dependent on their coastal waters for aquaculture and capture fisheries, particularly those with very dense operations such as in China, Japan, Korea, and other Asian countries. Here we are seeing a distinction being made between HABs and FABs or “favorable algal blooms” as countries are recognizing that phytoplankton biomass needs to be at a relatively high level to support such operations. In this sense, algal blooms, even dense, high biomass ones, can be considered beneficial, and thus efforts to reduce pollution or other nutrient inputs as a general bloom mitigation strategy may not be supported by certain sectors of society, such as fishermen. An interesting example comes from the Inland Sea of Japan, an area frequently used as an example of the manner in which reductions in pollution can lead to corresponding reductions in algal blooms, including those that are destructive to fisheries and aquaculture. Between 1965 and 1976, the number of red tide outbreaks (high biomass blooms) increased seven-fold in the Inland Sea, in parallel with the increase in industrial production and chemical oxygen demand (COD) from domestic and industrial wastes (Okaichi 1997). In 1973, Japanese authorities instituted the Seto Inland Sea Law to reduce COD loadings to half of the 1974 levels over a three-year period. The number of red tides began to decrease in 1977, eventually falling to less than 30% of the peak frequency, which had been in excess of 300 blooms per year. However, in the years since that legislation, fisheries productivity has also decreased in the region, leading to requests by the fishing industry for pollution controls to be relaxed, in the hopes that this will lead to more algal productivity and blooms, and foster enhanced fisheries production (Y. Fukuyo, pers. comm.). Implicit in this type of request is the acceptance of an occasional destructive HAB event in return for generally enhanced productivity and higher fishery yields the remainder of the time. One wonders if this view of favorable, high-biomass algal blooms will become more prevalent as countries and agencies worldwide are under increased pressure to maximize coastal fisheries productivity to feed their growing populations.
HAB prevention, control, and mitigation
The subdiscipline of HAB prevention, control, and mitigation (PCM) is diverse, as it covers a wide array of strategies that can reduce the impacts of HABs (Boesch et al. 1997). It is obviously preferable to prevent HABs in the first place rather than combating their impacts, so an array of strategies have been formulated through time, including shellfish toxin monitoring programs, controls on nutrient inputs to water bodies, and early detection and forecasting of blooms. Many believe that some of the global expansion in HAB incidence is linked to increased pollution of the coastal ocean, particularly by plant nutrients (e.g., Smayda 1989, Anderson et al. 2002, GEOHAB 2006). Indeed, there are few other causes that could be responsible for the scale and timing of the observed increases in HABs over the last several decades. As a result, conscientious pursuit of goals for pollution reductions, including excess nutrients, could well prevent HABs in some locations. Such strategies are typically longer-term in nature, however, due to the buildup of a reservoir of nutrients in bottom sediments and adjacent soils in many areas. Careful assessment and precaution against species introductions via ballast water and aquaculture-related activities also can be effective preventative strategies.
Mitigation strategies are based on the acceptance that HABs are going to occur, and therefore strategies for reducing impacts are needed. Example strategies include depuration of toxic shellfish, towing of fish pens to clear waters, harvesting restrictions on toxic shellfish, or early harvesting of threatened fisheries products. The procedures used to keep contaminated fisheries resources from the market have been largely successful when appropriately applied (Anderson et al. 2001). However, in order to contend with more frequent and diverse risks from HABs in an era of declining governmental resources to support labor-intensive monitoring, more sophisticated and reliable detection methods are required, in addition to the immediate expansion of simple methods, such as ELISA toxin kits, or networks of volunteer phytoplankton observers (Hall 1999), for example. These new technologies are rapidly appearing, as discussed below, and will continue to facilitate efforts to mitigate HAB impacts, even in areas with recurrent and serious HABs. Individuals consuming seafood and medical professionals seeing patients also need to be better informed about the risks, and responsible public education and communication should therefore receive increased attention. Progress has been excellent in this important element of HAB impact mitigation, but education and outreach efforts need to be sustained and further strengthened.
The one PCM area where development has been particularly slow and uneven on a global basis is in bloom control or suppression. In 1997, the author wrote a commentary in Nature (Anderson 1997) that attempted to explain why HAB science had such a striking lack of research emphasis and thus progress in this important area. At the time, an international conference on HABs in Vigo, Spain (1997) had one scientific presentation on direct bloom control, out of 400 papers or posters from 58 countries. Multiple explanations were offered, including the lack of targeted funding opportunities specifically for bloom control and impact mitigation, the complexity of the dynamic, three-dimensional ocean and the ecosystems in which marine HABs occur, and the relative difficulty of getting favorable peer reviews for proposals on controversial bloom control strategies in competition with fundamental studies of bloom dynamics or ecophysiology. Now, nearly 20 years later, the situation has improved, but progress is still much slower than in other areas of HAB science. At the recent International Conference on Harmful Algae (ICHA) in Korea in 2012, 30 papers on bloom control were presented, out of 380 total. This may seem like major progress, but most of these studies originate from only a few countries (Korea, China, and Japan for marine HABs), and the total also includes freshwater HAB control studies, which were not included in the 1997 Vigo conference. The challenges to controlling HABs in freshwater lakes and rivers are certainly significant, but are less complex than in the ocean, and there is wider public and management acceptance of such environmental manipulations and mitigation efforts given the long history of water-body treatment for drinking water supplies. It thus seems likely that major HAB control efforts will advance faster in freshwater systems than in the marine environment.
It also seems inevitable that there will be a stronger and stronger push for more progress on HAB control, as this is what the general public and most stakeholders feel should be a major target and outcome of HAB research. In the U.S., legislation has been passed that includes authorization of a funding program that focuses specifically on prevention, control, and mitigation, as many politicians believe that this should be the top priority for the funding they provide. As discussed above, it has sometimes been a battle to convince these legislators to sustain funding for fundamental HAB science, given their desire for practical approaches to control and management. Given the trends noted above, the recognition that politicians and agencies are becoming increasingly pragmatic about HAB research priorities, and the widespread human manipulation of the coastal zone for food and commerce, we can expect that bloom control research efforts will expand in the future. In the U.S., for example, the PCMHAB program was established as a targeted program in which all funding applications fall within the same topic area of practical PCM strategies, such that unfair comparisons between practical versus fundamental science are not made during the peer review process.
Practical control strategies tend to be controversial, and often speculative or unproven. Perhaps even more important is the requirement that all funded PCMHAB projects include a Transition Advisory Committee consisting of stakeholders, managers, and others who will benefit from, or be affected by, the proposed mitigation strategy. By including these individuals in project meetings from the outset, environmental and social objections can be addressed at an early stage, and the transition to full application made less confrontational and controversial. Despite this positive perspective, it seems likely that bloom control efforts will continue to focus on specific, high-value resources in countries that are best able to appease environmental interests, leaving other countries with only prevention and mitigation in their PCM arsenals.
Molecular biology and genetics
Recent development and application of advanced technologies in genomics, transcriptomics, and proteomics has already provided deep insights into the ecology, physiology, taxonomy, and toxicology of HABs, and this trend will surely continue. Noteworthy contributions to HAB science have been made in many areas, one of which is the taxonomic and phylogenetic reclassification of a number of HAB species. For example, the dinoflagellate genera Gymnodinium and Gyrodinium were traditionally separated on the basis of strict morphology (girdle displacement), but molecular phylogenetic approaches led to the creation of the genera Karenia (10+ spp. now known.), Karlodinium (8 spp.) and Takayama (6 spp.) (Daugbjerg et al. 2000, de Salas et al. 2008). Similar reclassifications have now been made for Gambierdiscus (Litaker et al. 2009) and more recently, the tamarense complex of Alexandrium (John et al. in press).
Another major area of progress has been in the elucidation of biosynthetic pathways and identification of genes involved in processes such as toxin production (Kellmann et al. 2008, Stucken et al. 2010) through transcriptomics and gene expression profiling. As the genomes and transcriptomes of more HAB species are described, discoveries of key physiological processes like these will continue, deepening our understanding of the mechanisms underlying bloom dynamics, toxicity, and general impacts. Likewise, molecular tools have revealed genetic heterogeneity at multiple levels during HABs, including within individual bloom populations.
Indeed, the demonstration that HABs are assemblages of multiple strains or genotypes that will differentially adapt to a changing environment is critical to our understanding of bloom dynamics, including responses to climate change and other anticipated forcings. Transcriptome analysis only applies to expression at the gene transcriptional level, but many key genes are post-translationally modified, particularly in dinoflagellates. One way around this constraint is through analysis of the proteome, a cell’s complement of proteins. This has only been applied to HAB taxa to a limited extent (e.g., Chan et al. 2005, Nosenko et al. 2006, Young et al. 2009, Wang et al. 2012) but there is great potential in this technology, and as more protein data are made available in databases, this should be an area of significant growth.
A related field of endeavor – metabolomics – also has the potential for great advancement in the field, but as with proteomics, the study of the composition of low-molecular weight metabolites within cells has yet to be applied to HAB research in a concerted fashion. There are no apparent major technological impediments, and the metabolomics approach has already been successfully applied to the dinoflagellate endosymbiont Symbiodinium (reviewed by Gordon and Leggatt 2010). Among HAB species, metabolic fingerprinting of K. brevis extracts by mass spectrometry was not successful in identifying specific compound(s) responsible for allelopathy (Prince et al. 2008). This frontier field is likely to be more fruitful when additional comprehensive spectral libraries become available, and as structural-functional relationships of the metabolites are better defined. Looking forward once again, it is clear that significant research advances will be achieved through the individual use of the “omic” technologies, but that major advances will also benefit from their combined use as datasets and analytical capabilities expand in each.
New technologies for in situ HAB cell and toxin detection
Anderson et al. (2001) reviewed the different approaches adopted by countries and commercial enterprises worldwide to monitor and manage HABs in coastal waters. There are, however, many challenges associated with these activities due to the complexity and diversity of HAB phenomena. Resource managers and regulatory officials must deal with multiple toxins and multiple toxic algal species, multiple toxic fisheries resources, and large- and small-scale HAB events that occur intermittently. Many new approaches to HAB cell detection and bloom monitoring have been developed, and many more are anticipated in the future.
Among the new approaches to species-specific detection and enumeration are optical instruments, such as the autonomous underwater vehicle (AUV) called the Brevebuster, which enumerates cells using optical features unique to K. brevis, the Florida red tide organism (Richardson and Pinckney 2004). The Imaging FlowCytobot (IFCB) is another powerful new optical tool – it is essentially an underwater flow cytometer that uses a range of optical features in an automated classification approach to photograph, enumerate, and identify cells at the genus, and sometimes species level (Campbell et al. 2010).
Yet another approach to improved cell detection involves the development of species- or strain-specific molecular “probes” that can label HAB cells of interest so they can be detected visually, electronically, or chemically. This line of research has been a hallmark of the HAB field because of the need for species-specific measurements. Progress has been rapid, and probes and assays of multiple types are available for many HAB species, with more being added every year. These developments have reached the stage where the new molecular counting methods are routinely employed in major research and monitoring programs.
These probe methods also opened the door to remote, subsurface, near real-time detection of specific HAB taxa. One instrument that provides these capabilities for in situ HAB cell and toxin detection is the Environmental Sample Processor (ESP; Scholin et al. 2009). The ESP autonomously collects discrete water samples from the ocean subsurface, concentrates microorganisms (particulates), and automates application of molecular probes to identify specific microorganisms and their gene products. Capabilities have also been developed for antibody-based detection of two HAB toxin families – domoic acid (Doucette et al. 2009) and saxitoxin (G. Doucette unpub. data). There are now 18 ESPs in use around the globe, some devoted to HAB studies, but others used for investigations of microbial communities, larvae, and other components of planktonic ecosystems (Scholin et al. 2009). Looking forward, we can anticipate a time when the ESP, IFCB, Brevebuster, and other in situ autonomous instruments are included as operational assets in ocean observing systems – arrays of moored and mobile instruments that can collect and transmit data continuously from remote locations to shore-based scientists and managers. We are still in the early stages of a shift from at-sea research to the use of robots and observing infrastructure to collect data, and this trend will surely continue to shape the nature of HAB monitoring and management in the future. Just as networks of meteorological stations and numerical models of atmospheric dynamics greatly improved our ability to provide accurate forecasts of weather events, ocean observatories and their associated numerical models of ocean dynamics have the potential to document long-term patterns and changes in the sea, to detect infrequent HAB events that previously went unobserved, and to make predictions or forecasts about these and other phenomena that directly affect human populations and marine ecosystems (Anderson et al. 2008).
HAB modeling and forecasts
Technological advances have expanded our capabilities for research and monitoring of HABs, but the blooms will always be undersampled because of the large space and time scales over which they occur (McGillicuddy et al. 2010b). This has led to the development of models of various types, and more recently to forecasts. There are those in the HAB field who feel that it is not possible to model or forecast complex HABs in the dynamic environment of open coastal waters (or large freshwater systems for that matter), yet progress has been significant and the results compelling, with much more progress expected going forward, and many research teams pursuing this important goal.
One example of an innovative and useful empirical model is that of Raine et al. (2010) who described a chain of observable events that lead to blooms of Dinophysis acuminata in Bantry Bay, Ireland. The authors defined a single index that quantifies these patterns and used that to evaluate past outbreaks, and to predict new ones.
Numerical models with varying levels of sophistication have also been developed. Some are three-dimensional physical models that resolve hydrography, into which HAB cells are introduced as passive particles (e.g., Velo-Suarez et al. 2010). A similar approach is used in a HAB forecasting system developed for K. brevis blooms in the Gulf of Mexico (Stumpf et al. 2009). Blooms are detected and defined using ocean color satellite images, and bloom transport is predicted using hydrographic modeling with the HAB cells treated as passive particles. This approach is also being taken for cyanobacterial bloom forecasts in Lake Erie (Wynne et al. 2011). The next step in sophistication and complexity has been to couple a detailed biological submodel to a hydrographic model, as has been done for Alexandrium blooms in the Gulf of Maine region in the U.S. (McGillicuddy et al. 2005). This model has demonstrated good skill at reproducing observations (He et al. 2008) and has been heavily used for hindcasts (i.e., looking at past events to understand underlying mechanisms; He et al. 2008, Li et al. 2009). It is also being used to issue weekly nowcasts and forecasts (looking forward three or four days), and even seasonal or annual forecasts (McGillicuddy et al. 2011).
Despite significant advances in recent years, there are many aspects of the HAB modeling effort that need to be improved. Regional models need to be developed for many parts of the world, but this can be facilitated by the use of computer code and algorithms developed for other areas or other HABs. A major area for advancement will benefit from the collection of in situ HAB data on a real-time basis that can be assimilated into the models to improve accuracy, much as is done with meteorological sensor networks and weather forecasts.
A realistic vision for the future is therefore that of arrays of moored or mobile instruments with sensors for HAB cells and their toxins generating data for the numerical models that provide forecasts with sufficient accuracy to be of use by managers, fishermen, the general public, and other stakeholders.
Climate change
Over the past several decades, many new or unexpected HAB phenomena have been attributed to eutrophication or ballast water introductions, but now, climate change is increasingly invoked as a potential factor (Hallegraeff 2010). This view reflects undeniable changes in atmospheric CO2 concentrations, rising global temperatures, melting of glaciers and ice caps, and changing storm, rainfall, stratification, and acidification patterns. This aspect of HAB research and policy (reviewed in Hallegraeff 2010) is in its infancy, as publications are few, and those that do exist tend to focus on single environmental factors (e.g., CO2, temperature increase, stratification), single biological properties (photosynthesis, toxicity, nutrient uptake), or individual species or strains. Complex physical and ecosystem interactions are rarely considered, and most time series of HABs are not of sufficient duration to permit realistic extrapolations. Another major constraint is the general lack of regional physical-biological models of HAB systems that can be utilized in forecast mode to explore future climate change scenarios. Relatively few of these exist worldwide (McGillicuddy 2010a), and where they do, they are not yet coupled to larger, ecosystem-level models or to global climate models. Although some regional ecosystem models exist, we need to augment the output to include parameters that give us more detail for HAB projections. In other words, one can simulate HAB growth and population development under different climate scenarios, but this will be relatively meaningless unless one also knows what these changes will do to co-occurring phytoplankton, zooplankton, predators on zooplankton, and other elements of the food chain that can directly affect the HABs being simulated. Compounding the problem is the relative coarse resolution and accuracy of the global climate models into which the higher resolution local or regional HAB modeling must be nested. Prediction of the impact of global climate change on HABs is thus fraught with uncertainties, yet many HAB researchers are being asked by resource managers, the general public, and other stakeholders to make those types of forecasts. There is no question that HAB research will have climate as a major focal area for the foreseeable future, and that there will be range extensions and contractions of key HAB species, yet our ability to make definitive statements about expected changes and impacts will continue to be limited for many years.
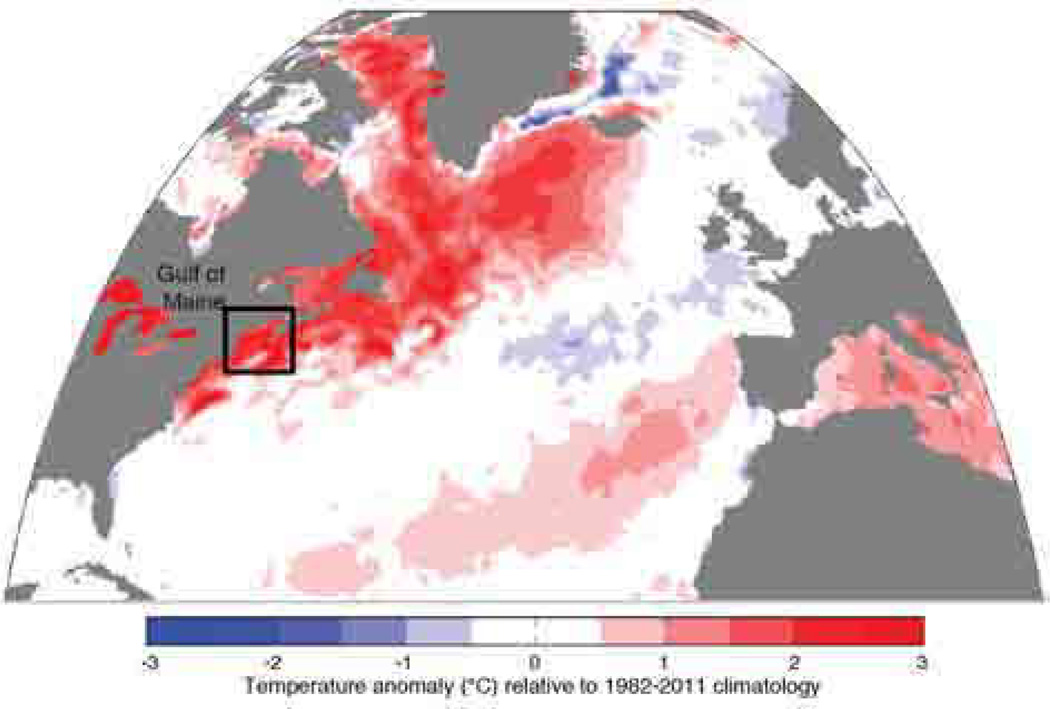
As described above, multi-factorial experiments are necessary, yet are exceedingly time-consuming and difficult to undertake in a meaningful way. In this regard, we need to take maximum advantage of “natural” experiments. Very few long-term records exist of HABs at any single locality, and as a rule we need at least 30 consecutive years before trends can realistically be detected (e.g., Anderson et al. 2014). Much can be learned, however, from long-term data sets for phytoplankton in general, such as those from the Continuous Plankton Recorder (CPR) (e.g., Burkhill and Reid 2010) or from short-term phytoplankton community responses to El Niño-Southern Oscillation (ENSO) and North Atlantic Oscillation (NAO) episodes or other large-scale meteorological and oceanographic events. In the latter context, one of the best ways to study climate impacts may well be to take advantage of weather fluctuations that mimic future climate change scenarios. This might, for example, be a major regional warming event, or an intrusion of a water mass with different properties into an area, as has occurred in a number of areas subject to HABs in recent years (e.g., McGillicuddy et al. 2011). If “rapid response” funding is made available, it might be possible to take advantage of these weather perturbations to study entire eco-and hydrographic systems under a common forcing. Fig. 3 shows the extraordinary scale of a warming event in the North Atlantic in 2012. The anomaly stretched over thousands of kilometers, with temperatures as high as 3 °C above the 30-year mean. Studying a natural event of this type may be more informative than running multiple laboratory experiments on individual organisms and then trying to extrapolate those results to predict the responses of a complex ecosystem. With natural weather fluctuations, entire systems are responding to the change, and if these are well documented, a great deal of information can be obtained, and a more complete story developed.
Fig. 3.
Mean sea surface temperature (SST) anomalies for the Northwest Atlantic and the Gulf of Maine for June-August 2012. The anomoly values are relative to the 1982–2011 climatology. (from Mills et al. 2013)
A re-examination of the phytoplankton fossil record using increasingly sophisticated geochemical tools (e.g., Dale 2001) is also warranted, as climate trends of the magnitude that we will be experiencing in the next 100 years have happened before, albeit at a much slower pace and starting from a cooler baseline than present (IPCC 2008). Past episodes of climate change over long periods of geological and evolutionary history allowed organisms to adapt to their changing environment, and much can be learned from these ancient patterns.
There are clearly a number of challenges facing HAB scientists as they attempt to determine whether the local or regional phenomena that they study are being affected by climate change. This is obviously a long-term endeavor, but without the proper tools and approaches, trends will be difficult or impossible to definitively identify, and mechanisms will be obscure, making it all the more difficult to convince state, federal, and local agencies that changes are indeed occurring and require some level of response or adaptation. Strong efforts should be made to establish long-term datasets of not only HAB cell abundance or toxicity thorough time, but also a range of other parameters that affect HAB abundance. These would include nutrient concentrations, hydrographic features, and regional meteorology, as well as the abundance and distribution of competing phytoplankton species, potential grazers, parasites, or viruses. We need several decades of data of this type to begin to reveal trends that are more than mere fluctuations in a noisy, long-term signal. Realistically, however, establishing and maintaining long-term datasets of this type is extraordinarily expensive and challenging, particularly if efforts are made to obtain a diverse array of data to place the HAB observations in context.
To meet this overarching goal for long-term data, a number of possible programs or approaches might be considered. These would include: a) utilization of data from state or federal monitoring programs; although usually limited to a single parameter (e.g., shellfish toxicity), these data can provide continuity through time and broad spatial coverage, as well as information on the onset, termination, and intensity of outbreaks (e.g., Anderson et al. 2014); b) identification of key locations within individual countries or regions where sufficient data can be collected to provide the necessary perspectives; it will not be possible to determine climate change effects on all HABs, but if a few key representative groups or habitats are characterized, it may be possible to formulate generalizations that apply more broadly to HABs of many types; and c) development and utilization of new sensors and instruments that can collect long-term data in an automated, high-frequency, and sustained fashion. These could be deployed through existing ocean observing networks, but many of those are not in locations where recurrent HABs occur, so HAB-specific deployments will also be needed.
Summary
Clearly, there are many challenges facing HAB scientists and managers as well as the HAB species themselves in this rapidly changing world. A growing world population and the need to provide food for 30% more people in the next 40 years will lead to increased nutrient enrichment of coastal waters. As a result, we can expect more HABs in some areas as well as increased impacts due to the expansion of aquaculture operations in affected regions, and perhaps even the direct stimulation of HABs by these operations. These expanded impacts will be most significant in developing areas of the world that are already struggling with growing HAB problems – areas like China, southeast Asia, India, and Africa. In some areas, pressures to reduce nutrients are going to conflict with the need for enhanced coastal productivity in support of dense aquaculture (the favorable algal bloom or FAB concept). It is also clear that there will be other ramifications of the changing perception of HABs by resource managers and funding agencies, potentially shifting funding away from certain topics (e.g., fundamental ecology and physiology) and into others (e.g., prevention, control, and mitigation). Driven by the need for more accurate yet less-expensive monitoring data, improvements in technologies for cell and toxin detection will continue at a rapid pace, as will the efforts to incorporate some of these technologies into autonomous biosensors that can augment monitoring programs and provide valuable, real-time data to improve detection and forecasting efforts. Molecular biology and ‘omic’ technologies will continue to provide novel insights into HAB taxonomy, evolution, cell physiology, and gene function. With respect to climate change, other than stating that there will be range extensions and contractions for HAB species, little else can be predicted about future changes due to the current lack of long-term data sets and the complexities within the environments and ecosystems where HABs occur. Even the most advanced HAB models are far from the stage where long-term forecasts are possible that can reflect these complexities and incorporate future climate projections in a way that is realistic. Incremental progress in forecasting is needed, however, and should not be discouraged because of its high uncertainty at present. Valuable information about the resilience of HAB species and communities can be gleaned from weather or climate variations that mimic future climate change scenarios, so every effort should be made to take advantage of these as they occur. Overall, there will continue to be many new challenges in HAB research and management, but the tools, technologies, and skilled personnel are in place to minimize impacts and protect public health and marine resources as never before.
Acknowledgements
Funding was provided by the Woods Hole Center for Oceans and Human Health, through National Science Foundation Grants OCE-0430724, OCE-0911031, and OCE-1314642, and National Institute of Environmental Health Sciences Grants 1-P50-ES012742-01 and 1-P01-ES021923-01. Support was also provided by NOAA Grants NA11NOS 4780023, NA10NOS4780137, NA11NOS4780025 and Cooperative Agreement NA09OAR4320129.
References
- Anderson DM. In: Red Tides: Biology, Environmental Science and Toxicology. Okaichi T, Anderson DM, Nemoto T, editors. Elsevier; 1989. pp. 11–16. [Google Scholar]
- Anderson DM. ECOHAB - The Ecology and Oceanography of Harmful Algal Blooms: A National Research Agenda. Woods Hole Oceanographic Institution. Woods Hole, MA: 1995. p. 66. [Google Scholar]
- Anderson DM. Nature. 1997;388:513–514. [Google Scholar]
- Anderson DM, Andersen P, Bricelj VM, et al. Asia Pacific Economic Program, Singapore, and Intergovernmental Oceanographic Commission. Paris: 2001. Monitoring and Management Strategies for Harmful Algal Blooms in Coastal Waters; p. 268. [Google Scholar]
- Anderson DM, Burkholder JM, Cochlan WP, et al. Harmful Algae. 2008;8:39–53. doi: 10.1016/j.hal.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DM, Cembella AD, Hallegraeff GM. Annu. Rev. Mar. Sci. 2012;4:143–176. doi: 10.1146/annurev-marine-120308-081121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DM, Couture DA, Kleindinst JL, et al. Deep-Sea Res. II. 2014;103:264–276. doi: 10.1016/j.dsr2.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DM, Glibert PM, Burkholder JM. Estuaries. 2002;25(4b):704–726. [Google Scholar]
- Berman T. Desal. & Water Treat. 2012 [Google Scholar]
- Boesch DF, Anderson DM, Horner RA, et al. NOAA Coastal Ocean Program, Decision Analysis Series No. 10, Special Joint Report with the National Fish and Wildlife Foundation. 1997 [Google Scholar]
- Bouwman L, Beusen A, Glibert PM, et al. Environ. Res. Lett. 2013;8:5. [Google Scholar]
- Burkhill PH, Reid PC. In: OceanObs ‘09: sustained ocean observations and information for society. Hall J, Harrison DE, Stammer D, editors. Paris, France: European Space Agency; 2010. Publication WPP-306. [Google Scholar]
- Campbell L, Olson RJ, Sosik HM, et al. J. Phycol. 2010;46(1):66–75. [Google Scholar]
- Chan LL, Hodgkiss IJ, Lam PKS, et al. Proteomics. 2005;5:1580–1593. doi: 10.1002/pmic.200401020. [DOI] [PubMed] [Google Scholar]
- Dale B. Sc. Mar. 2001;65:257–272. [Google Scholar]
- Daugbjerg N, Hansen G, Larsen J, et al. Phycologica. 2000;39:302–317. [Google Scholar]
- DeGrasse S, Conrad S, DiStefano P, et al. Deep Sea Res. II. 2014;103:288–300. [Google Scholar]
- de Salas MF, Laza-Martinez A, Hallegraeff GM. J. Phycol. 2008;44(1):241–257. doi: 10.1111/j.1529-8817.2007.00458.x. [DOI] [PubMed] [Google Scholar]
- Diaz RJ, Rosenberg R. Science. 2008;321:926–929. doi: 10.1126/science.1156401. [DOI] [PubMed] [Google Scholar]
- Dixon M, Falconet C, Ho L, et al. J. Hazard. Mat. 2011;188(1–3):288–295. doi: 10.1016/j.jhazmat.2011.01.111. [DOI] [PubMed] [Google Scholar]
- Dortch Q, Anderson DM, Ayres DL, et al., editors. HAB RDDTT Workshop Report. Woods Hole, MA: 2008. [Google Scholar]
- Doucette GJ, Mikulski CM, Jones KL, et al. Harmful Algae. 2009;8:880–888. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2012. 2012 Rome. 209 pp. I2727E/1/ [Google Scholar]
- Flemming H-C, Wingender J. Water Sci. & Tech. 2001;43:1–8. [PubMed] [Google Scholar]
- GEOHAB, Global Ecology and Oceanography of Harmful Algal Blooms Programme. HABs in Eutrophic Systems. Paris and Baltimore: IOC and SCOR; 2006. p. 74. [Google Scholar]
- Glibert PM, Harrison J, Heil C, et al. Biogeochem. 2006;77:441–463. [Google Scholar]
- Gobler CJ, Koch F, Kang J, et al. Harmful Algae. 2013;27:29–41. [Google Scholar]
- Gordon BR, Leggat W. Marine Drugs. 2010;8:2546–2568. doi: 10.3390/md8102546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen RJ, Tett P, Bresnan E, et al. Oceanogr. & Mar. Biol: An Ann. Rev. 2012;50:65–126. [Google Scholar]
- Hall S. Proc. 6th Canadian Workshop on Harmful Marine Algae. In: Martin JL, Haya K, editors. Can. Tech. Rep. Fish. Aquat. Sci. Vol. 2261. 1999. pp. 1–30. [Google Scholar]
- Hallegraeff GM. Phycologia. 1993;32:79–99. [Google Scholar]
- Hallegraeff GM. J. Phycol. 2010;46:220–235. [Google Scholar]
- He R, McGillicuddy DJ, Keafer BA, et al. J. Geophys. Res.-Oceans. 2008;113:C07040. [Google Scholar]
- IPCC. Working Group II contribution to the Fourth Assessment Report of the IPCC. 2008 Intergovernmental Panel on Climate Change. [Google Scholar]
- James KJ, Moroney C, Roden C, et al. Toxicon. 2003;41(2):145–151. doi: 10.1016/s0041-0101(02)00244-1. [DOI] [PubMed] [Google Scholar]
- John U, Medlin LK, Groben R. J. Phycol. in press. [Google Scholar]
- Kellmann R, Mihali TK, Jeon YJ, et al. App. Environ. Microbiol. 2008;74:4044–4053. doi: 10.1128/AEM.00353-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch F, Kang Y, Villareal TA, et al. Appl. & Env. Microbiol. 2014;80(16):4947. doi: 10.1128/AEM.00888-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudela RM, Gobler CJ. Harmful Algae. 2012;14:71–86. doi: 10.1016/j.hal.2022.102236. [DOI] [PubMed] [Google Scholar]
- Laycock MV, Anderson DM, Naar J, et al. Desalin. 2012;293:1–6. [Google Scholar]
- Li Y, He R, McGillicuddy DJ, Jr, et al. Cont. Shelf Res. 2009;29(17):2069–2082. doi: 10.1016/j.csr.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litaker RW, Vandersea MW, Faust MA, et al. Phycologia. 2009;48:344–390. [Google Scholar]
- Lu D, Qi Y, Gu H, et al. Algological Studies. 2014 DOI: http://dx.doi.org/10.1127/1864-1318/2014/0161.
- McGillicuddy Jr, editor. J. Mar. Sys. 2010a;83(3–4):297. doi: 10.1016/j.jmarsys.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillicuddy DJ., Jr J. Mar. Sys. 2010b;83:105–107. doi: 10.1016/j.jmarsys.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillicuddy DJ, Jr, Anderson DM, Lynch DR, et al. Deep-Sea Res. II. 2005;52(19–21):2698–2714. [Google Scholar]
- McGillicuddy DJ, Jr, Townsend DW, He R, et al. Limnol. & Oceanogr. 2011;56(6):2411–2426. doi: 10.4319/lo.2011.56.6.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KE, Pershing AJ, Brown CJ, et al. Oceanogr. 2013;26(2):191–195. [Google Scholar]
- Nosenko T, Lidie KL, Van Dolah FM, et al. Mol. Biol. Evol. 2006;23:2026–2038. doi: 10.1093/molbev/msl074. [DOI] [PubMed] [Google Scholar]
- Okaichi T. In: Sustainable Development in the Seto Inland Sea, Japan - From the Viewpoint of Fisheries. Okaichi T, Yanagi Y, editors. Tokyo, Japan: Terra Scientific Publishing Company; 1997. pp. 251–304. [Google Scholar]
- Okaichi T, Anderson DM, Nemoto T, editors. Red Tides: Biology, Environmental Science, and Toxicology. New York: Elsevier; 1989. p. 439. [Google Scholar]
- Paerl HW. Ophelia. 1995;41:237–259. [Google Scholar]
- Quilliam MA, Lewis NJ, Aasen J, et al. In: Proc. 12th Intern. Conf. on Harmful Algae. Moestrup Ø, editor. Copenhagen; 2008. pp. 377–380. [Google Scholar]
- Prince EK, Myers TL, Kubanek J. Limnol. Oceanogr. 2008;53(2):531–541. [Google Scholar]
- Raine R, McDermott G, Silke J, et al. J. Mar. Sys. 2010;83:150–157. [Google Scholar]
- Rhodes L. Toxicon. 2011;57(3):400–407. doi: 10.1016/j.toxicon.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Rice KC, Herman JS. Appl. Geochem. 2012;27:1–14. [Google Scholar]
- Richardson TL, Pinckney JL. J. Appl. Phycol. 2004;16:315–328. [Google Scholar]
- Richlen ML, Morton SL, Jamali EA, et al. Harmful Algae. 2010;9:163–172. [Google Scholar]
- Scholin C, Doucette G, Jensen S, et al. Oceanogr. 2009;22:158–167. [Google Scholar]
- Seitzinger SP, Mayorga E, Bouwman AF, et al. Global Biogeochem. Cycles. 2010;24:1–16. [Google Scholar]
- Smayda TJ. In: Novel Phytoplankton Blooms: Causes and Impacts of Recurrent Brown Tide and Other Unusual Blooms. Cosper EM, Carpenter EJ, Bricelj M, editors. New York: Springer-Verlag; 1989. pp. 213–228. [Google Scholar]
- Smil V. Cambridge, MA: The MIT Press; 2001. [Google Scholar]
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2008 Revision, Highlights. 2009 [Google Scholar]
- Working Paper No. ESA/P/WP.210. [Google Scholar]
- Stucken K, John U, Cembella A, et al. PLoS ONE. 2010;5(2):e9235. doi: 10.1371/journal.pone.0009235. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf RP, Tomlinson MC, Calkins JA, et al. J. Mar. Sys. 2009;76:151–161. doi: 10.1016/j.jmarsys.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velo-Suárez L, Reguera B, González-Gil S, et al. J. Mar. Sys. 2010;83:242–252. [Google Scholar]
- Wang D-Z, Li C, Zhang Y, et al. J. Proteomics. 2012;75(18):5564–5577. doi: 10.1016/j.jprot.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Weiss M, D’Agostino PM, Mihali TK, et al. Mar. Drugs. 2010;8(7):2185–2211. doi: 10.3390/md8072185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne TT, Stumpf RP, Tomlinson MC, et al. Ecol. Appl. 2011;21(7):2709–2721. doi: 10.1890/10-1454.1. [DOI] [PubMed] [Google Scholar]
- Young C, Truman P, Boucher M, et al. Proteomics. 2009;9:2529–2542. doi: 10.1002/pmic.200800725. [DOI] [PubMed] [Google Scholar]
- Zhang QC, Qiu LM, Yu RC, et al. Harmful Algae. 2012;19:117–124. [Google Scholar]
- Zhou MJ, Zhu MY. Progr. in Earth Sci. 2006;21:673–679. [Google Scholar]
- Zimba PV, Moeller PD, Beauchesne K, et al. Toxicon. 2010;55(1):100–104. doi: 10.1016/j.toxicon.2009.07.004. [DOI] [PubMed] [Google Scholar]