Abstract
Porphyromonas gingivalis is an etiologic agent of periodontal disease in humans. The disease is associated with the formation of a mixed oral biofilm which is exposed to oxygen and environmental stress, such as oxidative stress. To investigate possible roles for cytochrome bd oxidase in the growth and persistence of this anaerobic bacterium inside the oral biofilm, mutant strains deficient in cytochrome bd oxidase activity were characterized. This study demonstrated that the cytochrome bd oxidase of Porphyromonas gingivalis, encoded by cydAB, was able to catalyse O2 consumption and was involved in peroxide and superoxide resistance, and dioxygen tolerance.
Introduction
Porphyromonas gingivalis is a gram negative anaerobe populating the oral cavity. P. gingivalis resides in the dental plaque and it is a main contributor to periodontal diseases. The oral biofilm is a model of microbial multicellularity and multicellular behaviour ranging from commensal microbiome to virulent infection. The accumulated body of studies of bacterial pathogens in periodontal acute and chronic infections have designated P. gingivalis, Tannerella forsythia and Treponema denticola (also called “the red complex”) as the tripartite cornerstone of the community in its pathogenic state, as recently confirmed by metagenomic microbiome analysis [1]. Not only do they produce proteases, toxins and inflammatory compounds that attack host tissue, but they can shape the whole behaviour of the community [2]. Nevertheless, the physiological properties of each individual of the red complex are not fully understood. In this respect, P. gingivalis remains a puzzling organism. Although it is non-motile, and possesses a rather undersized armament for adaptive responses compared to most ubiquitous organisms, P. gingivalis sustains the infection disease by surviving in the unfavourable environment of the oral cavity and periodontal pockets, and by invading host tissues. For example, the P. gingivalis ATCC 33277 genome encodes only four two-component systems, one orphan histidine kinase, two orphan response regulators, one histidine kinase/response regulator hybrid protein [3] and six extracytoplasmic sigma factors, including SigH (PGN_1740) which is required for resistance to oxygen and oxidative stress [4–6]. As reviewed by Henry et al. [7], the apparent robustness of P. gingivalis is largely promoted by its ability to circumvent oxidative stress via intracellular enzymes that detoxify the ROS originating from many host-dependent and environment-dependent processes in the oral cavity. Surprisingly, this anaerobic organism possesses an O2-dependent respiratory enzyme. CydAB is a prokaryote-specific membrane-bound oxidase, with two heme b cofactors and one heme d, which is considered as the site for oxygen reduction [8]. Thanks to its high affinity for oxygen, CydAB is used for energy generation in oxygen-limiting conditions by facultative anaerobes, and by nanaerobes. This term, ‘nanaerobe’, was implemented to designate bacteria whose cell population grows optimally in an anaerobic environment but which are able to multiply in the presence of oxygen, such as Bacteroides fragilis [9]. In addition to promoting O2-dependent growth, CydAB is involved in the detoxification of ROS and RNS, and promotes survival during the stationary phase of growth, in some bacteria such as Escherichia coli, Azotobacter vinelandii and Shewanella oneidensis [10–12]. In E. coli, the cytochrome bd displayed catalase activity: the purified enzyme catalyzed efficiently the decomposition of H2O2 with formation of O2. Moreover, cyanide, which binds ferric heme d, was found to inhibit the catalase activity. The authors also demonstrated that overexpressing cydAB in E. coli resulted in transformation of H2O2 in O2 in catalase-minus E. coli mutants [13]. The involvement of CydAB in maintaining the redox metabolism of bacterial cells was also demonstrated in E. coli, for which CydAB was described as an electron sink during periplasmic disulphite oxidation, or as a supply of oxidative power for haem synthesis [14, 15]. CydAB also decreases electrons availability for the fumarate reductase Frd. In E. coli, the fumarate reductase Frd is an important source of H2O2 in aerobic conditions; CydAB diminishes the rate of H2O2 formation by Frd when anaerobic cultures are exposed to O2 [16].
In P. gingivalis the main respiratory enzyme is Frd, which supports anaerobic energy generation. Chemical inhibition of Frd has been shown to diminish growth rate and biofilm formation [17]. No P. gingivalis mutant with altered frdAB genes has been reported nor studied, but in Bacteroides fragilis such a mutant grew very poorly [18]. The role of CydAB in P. gingivalis remains unexplored, except as regards the unaltered biofilm forming ability of the cydAB mutant [19]. Transcriptomic data have only revealed that cydAB gene expression was upregulated by oxygen [20] and negatively controlled by a LuxS homolog [21]. Focusing on P. gingivalis, this work examines the implications of CydAB for stress response and adaptation, and for interaction with host cells. These data lead us to rethink the classification of P. gingivalis, which has long been considered as a strict anaerobe in regard to its O2 metabolism. Indeed a new classification was proposed by Morris and Schmidt for bacteria that can harvest O2 present in nanomolar concentrations [22].
Methods
Bacterial strains and growth conditions
Porphyromonas gingivalis ATCC 33277 was cultured in enriched BHI broth containing, per liter, 37 g of BHI powder (AES Chemunex, France), 5 g of yeast extract (Conda, Dutscher) 25 mg of hemin (Sigma), and 10 mg of menadione (Sigma). The strains were maintained on Columbia 3 agar plates supplemented with 5% (v/v) defibrinated horse blood (AES Chemunex, Combourg, France), 25 mg/l of hemin, and 10 mg/l of menadione. The cultures were incubated at 37°C in an anaerobic chamber Macs-VA500 (Don Whitley) flooded with 80% N2, 10% H2 and 10% CO2. However, brief exposures to oxygen outside the chamber cannot be avoided for some experiments. The media were supplemented with erythromycin 5 μg/ml, and tetracycline 1 μg/ml when required. The P. gingivalis cydAB deletion mutant was constructed and is described by Leclerc et al. [19]. This mutant is resistant to erythromycin. The exact role of the cydW gene that is part of the cydWAB operon is unknown. Therefore we have not deleted the gene to study the phenotype of a cytochrome bd oxidase mutant.
Complementation of the cydAB mutant with the native genes in trans
In trans complementation often leads to an overexpression of the genes. Overexpressing cydAB without overexpressing cydW may confer pleiotropic phenotypes, if CydW is involved for example in the correct folding or stabilisation of the cytochrome bd oxidase inside the inner membrane. Therefore we used a 3 425 bp DNA fragment containing the 3 ORF of the cydWAB operon (PGN_1040 to PGN_1042) and the 179 bp 5’ intergenic region between PGN_1039 and PGN_1040, that has been amplified by PCR using the primers CydAB1 and CydAB2 (Table 1). The fragment digested by SphI (NEB England) was then ligated into the SphI linearised vector pYKP028 [23], and the insert sequence was verified by nucleotide sequencing and in silico comparison with the published genome sequence [3]. The resulting vector pYKP028_cydWAB, conferring tetracycline resistance, was introduced into a P. gingivalis cydAB mutant by electroporation as previously described [24]. Complemented mutants were selected by their combined resistance to erythromycin and tetracycline. The expression of cydWAB genes from the recombinant plasmid was verified by RT-PCR. The nucleotide sequence of the DNA insert was verified compared to the native genomic operon.
Table 1. Primers for RT-PCR and qRT-PCR.
| Name | Sequence |
|---|---|
| CydAB1 | GCATGCGAGGGACATTGCTGGTATTG |
| CydAB2 | GCATGCATTGCTATCGTTTGGTCAGG |
| PGN_0564-L (sodB) | AATTCCACCACGGTAAGCAC |
| PGN_0564-R (sodB) | GAGCCGAATTGTTTGTCGAT |
| PGN_0381-L (glk) | ATGAATCCGATCCGCCACCAC |
| PGN_0381-R (glk) | GCCTCCCATCCCAAAGCACT |
| PGN_1040-L (cydW) | GGGAAAGACGCTATGGGCTA |
| PGN_1041-L (cydA) | ACACTGGGATTGGGTGTCAT |
| PGN_1041-R (cydA) | CACCGATCGCAAAGTTGA |
| PGN_1042-R (cydB) | TCCTCCCCCATACATTACCA |
ROS (Reactive Oxygen Species) resistance assay
For H2O2 survival assays, P. gingivalis strains (wild-type, cydAB mutant and cydAB complemented mutant) were grown overnight in enriched BHI at OD600 nm of 0.5 (exponential phase), and 1.8 (stationary phase). For paraquat survival assays, the three P. gingivalis strains were grown overnight in enriched BHI at OD600 nm of 0.5 (exponential phase). Samples of these cultures were used to inoculate an enriched BHI medium to an OD600 nm of 0.1. Each culture was split in two, and one half was treated with 500 μM of H2O2 (Sigma) or 480 μM of paraquat (Sigma). The other half of each culture was left untreated to serve as a control. 10 μl of each sample was removed at 0, 1, 2 and 3 h 30 (for H2O2 treatment) or at 0, 1, 2, 4 and 6 h (for paraquat treatment) of incubation at 37°C in anaerobic conditions (incubation in the anaerobic chamber, but addition of paraquat and sampling for CFU-counting outside the chamber i.e. exposed to atmospheric oxygen) or in aerobic conditions. Serial dilutions were made in duplicate with enriched BHI broth. An aliquot of each dilution was spread on Colombia agar plates supplemented with blood. Colonies were enumerated after five days of anaerobic incubation at 37°C. At least three independent experiments, each in duplicate or triplicate, were conducted.
Dioxygen tolerance
Bacterial cells from overnight cultures in enriched BHI of P. gingivalis (wild-type, cydAB mutant and cydAB complemented mutant) at OD600nm 0.5 were washed and suspended in phosphate buffered saline (PBS) supplemented with 5 g/l of yeast extract to an OD600 nm of 0.1.The CFU/ml of each culture was determined after incubation for six hours at 37°C in aerobic conditions, with shaking at 150 rpm, or in anaerobic conditions. Serial dilutions were made and were spread on supplemented Colombia agar plates. Colonies were enumerated after five days of anaerobic incubation at 37°C.
RT-PCR and qRT-PCR
Overnight cultures of P. gingivalis (wild-type and cydAB mutant) at OD600 nm 0.5 (exponential phase) grown in enriched BHI were used to inoculate enriched BHI cultures to OD600 nm 0.1. Each culture was split in two, and one half was treated with 320 μM of Paraquat (Sigma). After one hour at 37°C in an anaerobic atmosphere, samples were harvested by centrifugation (4000 rpm for 15 min at room temperature). Bacterial lysis and RNA extraction were performed with mirVanaTM miRNA isolation kit (Ambion, Life technologies) according to the manufacturer’s instructions. Residual DNA was removed by DNase treatment using the Turbo DNA-free (Ambion, Life technologies) and extraction was purified with the RNA Clean & ConcentratorTM-5 (Zymo Research, Proteigen) according to the manufacturer’s instructions. The quantity of the extracted RNA was confirmed with a NanoDrop spectrophotometer (Thermo Scientific). The amount of RNA was estimated by determining the absorbance at 260 nm.
Real-Time quantitative Polymerase Chain Reaction (qRT-PCR) was used to assess the level of expression of selected P. gingivalis genes. A reverse transcription reaction was performed with the M-MLV reverse transcriptase (Promega) using random hexamer primers, according to the manufacturer’s recommendations.
Conventional PCR was performed using the OneTaq HS enzyme (New Englands BioLabs) according to the manufacturer’s protocol, using a BioRad C1000 thermal cycler.
qRT-PCR assays were performed using SYBR green/ROX chemistry (Eurogentec) with the ABI Prism 7000 Sequence Detection System and software (Applied Biosystem). The differences in messenger RNA expression, Ct (Cycle threshold) value, were calculated by the 2-ΔΔCt method in which the amount of target RNA was adjusted to the amount of a reference internal RNA, the glk transcript (PGN_0381). Real-time quantitative PCR was carried out for each gene in triplicate from two independent experiments. The primers used in this experiment are described in Table 1.
Analysis of O2 consumption by high resolution respirometry
Oxygen concentration was measured by high-resolution respirometry with an Oroboros Oxygraph-2k (Oroboros Instruments), in a standard configuration, with 2 ml volume of both chambers, at 37°C, and 500 rpm stirrer speed. P. gingivalis cells grown at 37°C in anaerobic conditions (Anaerogen, Oxoid) were harvested in the exponential growth phase (OD600 nm 0.5), washed or not in PBS according to the experimental conditions tested, and transferred into oxygraph chambers. Acquisition was started after 2–3 minutes of equilibration at 37°C in oxic conditions and after closing of the chambers. Measurements were performed on two independent cultures, and the data shown represent typical results. Data were recorded at 1 s intervals using the Datlab 4 Acquisition software (Oroboros, Innsbruck, Austria). Standardized calibration procedures of the oxygen signal were carried out using the enriched BHI medium or PBS. Respiration was automatically corrected for contributions of the polarographic oxygen sensor and of oxygen diffusion to total apparent respiration, as a continuous function of oxygen concentration.
Adhesion and Invasion into epithelial cells Ca9-22
Epithelial cells Ca9-22 (Japanese Collection of Research Bioresources Cell Bank, JCRB) were cultivated at 37°C in aerobiosis with 5% CO2 in humidified atmosphere in Eagles’ Modified Essential Medium (EMEM) enriched with 10% (v/v) fetal calf serum, 1% (v/v) of 200 mM L-glutamine and supplemented or not with 1% (v/v) of an antibiotic mixture containing 10 U/μl penicillin and 10 U/μl streptomycin. Epithelial cells Ca9-22 were seeded into 24-well culture plates at a density of approximately 1,5 105 cells per well. Cells were grown to confluent monolayers over 48 hours and washed twice with 500 μl of PBS. P. gingivalis strains (wild-type, cydAB mutant and complemented cydAB mutant) were incubated with Ca 9–22 cells with a multiplicity of infection of about 1:1000, for 30 minutes at 37°C in aerobiosis with 5% CO2 in humidified atmosphere in enriched -EMEM without antibiotic. After incubation, unattached bacteria were removed by three washes with PBS.
To evaluate the percentage (compared to inoculum) of bacteria that are associated to Ca9-22 (both adherent and internalized bacterial cells), samples were plated on Colombia agar medium supplemented with blood.
To evaluate the percentage of bacteria which have been internalized into Ca9-22 cells, samples were incubated for 1 hour in enriched EMEM (i.e. with antibiotics to kill external bacteria) at 37°C with 5% CO2 in humidified atmosphere. External adherent bacteria were removed by three PBS washes and the samples were plated on Colombia agar medium supplemented with blood.
Colonies were enumerated after 5 days of incubation at 37°C, in anaerobic conditions. At least three independent experiments, each in duplicate, were conducted.
Results and Discussion
The cydWAB genes are transcribed as one polycistronic mRNA
In E. coli, the cytochrome bd oxidase complex is encoded by three genes, from which the last one, cydX, was recently found to be essential for the heme-bound active site stability [25, 26]. Over 300 homologues of cydX were identified in bacteria, including 80 unannotated genes [27]. The sequence analysis of these proteins shows a great degree of variability, with only a few highly-conserved residues. Sequenced genomes of P. gingivalis (http://www.ncbi.nlm.nih.gov/genome/) do not encode the cydX homologue. By further investigation of cydAB operons in bacterial genomes, Allen et al., (2014) identified two additional conserved hypothetical small proteins encoded at the 3’end of some operons: CydY and CydZ [27]. We have not found any cydY or cydZ homologues immediately downstream of cydAB in P. gingivalis genomes.
In the P. gingivalis ATCC 33277 genome, the cydAB genes are located downstream of an unannotated ORF, that we called cydW (PGN_1040) (Fig 1A). It encodes a hypothetical membrane protein of 82 amino acids, and a search was performed using the Blastp program to compare the proteins of the NR database to the CydW sequence. CydW-homologues are widely distributed amongst the members of bacteroidales family (S1 Fig). The function of this putative membrane protein is unknown.
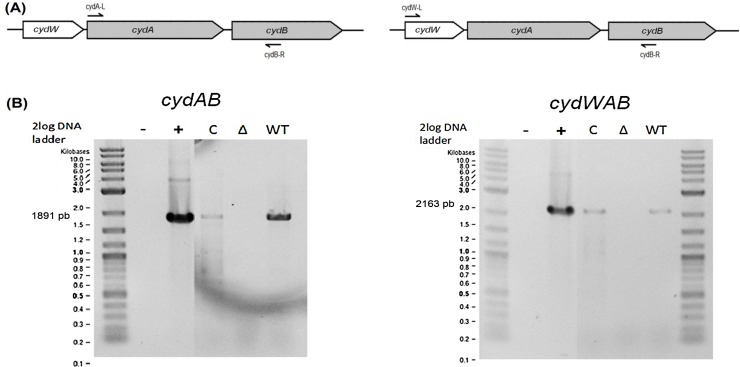
Fig 1. Organisation of the cydWAB genes.
(A) Map of cydWAB operon and the positions of primers used for PCR. To construct the cydAB mutant used in this study, a DNA fragment containing part of both cydA and cydB (in grey) was deleted. (B) PCR performed on cDNA obtained by reverse transcription on total RNA extract from P. gingivalis ATCC 33277 (WT), cydAB mutant (Δ) and complemented mutant (C). H2O and genomic DNA of P. gingivalis ATCC 33277 were used as controls.
By RT-PCR analyses, we demonstrated that cydWAB genes are part of the same operon (Fig 1B) in the wild type strain. Additional controls are displayed in the supporting information (S2 Fig). In the cydAB mutant we verified that the transcription of the cydAB-downstream gene (PGN_1043) and cydW was not defective due to the mutation (data not shown). Therefore, we proved that the cydAB mutant contained a non-polar deletion of the central part of the cydWAB operon (Fig 1B). Moreover, we found that the cydWAB operon was transcribed at both exponential and stationary phases of growth in the wild-type strain, in anaerobic condition, although LuxS was reported as a repressor of cydAB in anaerobiosis [21].
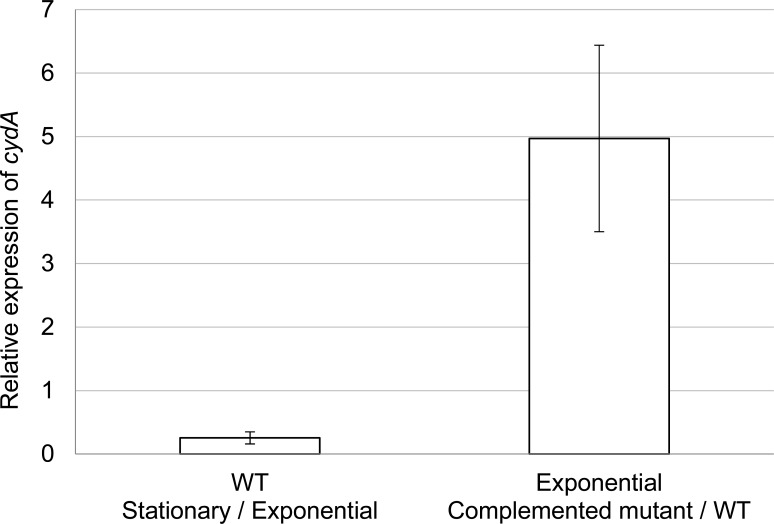
By quantitative RT-PCR, we saw that the expression of cydA was four times higher in the exponential phase of growth than in the stationary phase of growth (Fig 2) in P. gingivalis ATCC 33277. Therefore, survival assays and respirometry assays were carried out with exponentially growing cultures.
Fig 2. Relative expression of cydA.
The relative expression was quantified by qRT-PCR with the 2∆∆Ct method. The normalization was done with the glucokinase house-keeping gene (PGN_0381). The expression of cydA in the wild-type strain was compared between stationary and exponential phases of growth. The white bar represents the ratio of expression in the stationary phase vs. the exponential phase.The relative expression of cydA in the exponential phase was compared between the complemented cydAB mutant and the wild type strains. The grey bar represents the ratio of expression of complemented mutant vs. wild-type. These data are the mean and standard deviations of two biological replicates containing three technical replicates.
A trans complementation restores the cydWAB transcription
We verified that the cydWAB operon was transcribed in the cydAB mutant carrying the cydWAB genes encoded by the pYKP028_cydWAB vector. However, the complemented mutant not only expressed but overexpressed the cydA gene: during the exponential growth phase, expression was over five times the expression of cydA in a wild type strain (Fig 2), and over twelve times at the stationary phase of growth (data not shown), as monitored by qRT-PCR analyses. In the complemented mutant the expression of cydA was constant throughout the growth phases (data not shown). This result may explain the inability of the complemented mutant to behave exactly as the wild type in stress survival assays and respirometry experiments, as described in the following chapters.
The cydAB mutant is more sensitive to oxidative stress
Superoxide donor
Paraquat was used to generate superoxide anion radicals. Paraquat undergoes redox cycling in vivo, being reduced by electron donors such as NADPH, and it needs to be oxidized by an electron receptor such as dioxygen to produce superoxide. In E. coli, paraquat can also alter the intracellular NADPH/NADP+ ratio by diverting electron flow [28] and can induce the SoxRS stress response [29]. Therefore, susceptibility to paraquat depends either on detoxification efficiency or on the intracellular concentration of the electron receptor that is required to generate superoxide. O2°- is unable to cross the cell envelope; therefore, as previously demonstrated in E. coli, the only O2°- which is effective is that generated within the cell by paraquat [30]. Detection of intracellular ROS, using redox probes or agents, may be a source of artefacts. Such detection was discarded in the present work because of the possibilities of interference with paraquat and menadione (from the enriched BHI medium that is used for growth) and the difficulty controlling O2 levels at very low concentrations.
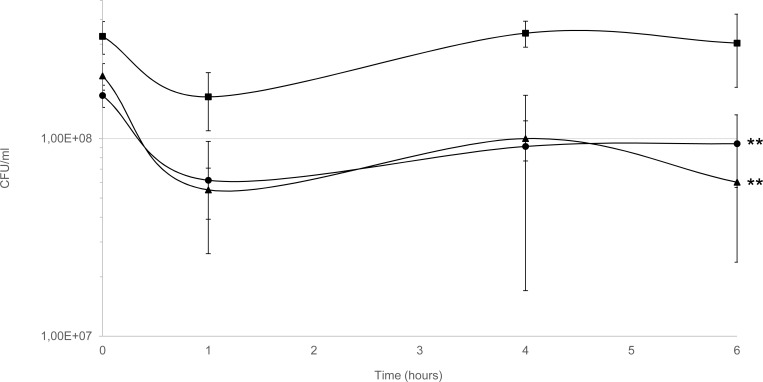
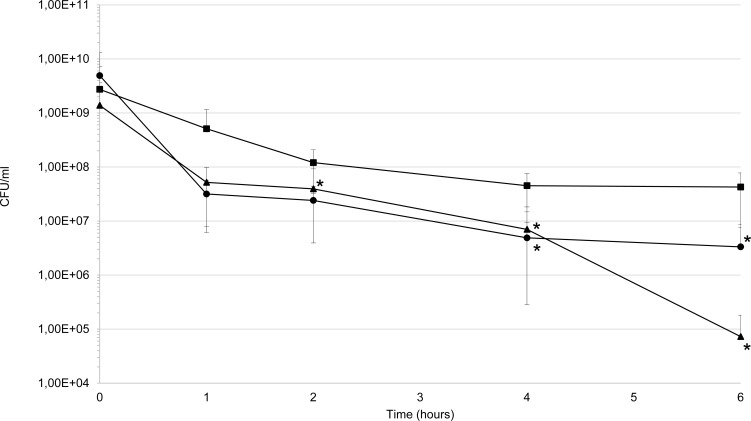
The P. gingivalis wild-type, cydAB mutant and complemented mutant were exposed to an inhibitory (i.e. causing no visible growth after 24 h of incubation) concentration of paraquat (480 μM) in anaerobic conditions following a short exposure to oxygen during paraquat addition and the surviving cells were quantified. The percentages of surviving cells of wild-type, mutant and complemented mutant were respectively 49, 26 and 39%, relative to the untreated samples, after one hour of treatment (Fig 3). The differences in susceptibility between wild-type and mutant suggest that cydAB is either involved in superoxide detoxification or in removing the residual electron acceptor O2 required for the production of ROS from paraquat. When the experiment was performed in aerobic conditions with the same concentration of paraquat, the survival of the cydAB mutant was not affected compared to WT (data not shown). In anaerobiosis, restoring cydAB expression in trans partially restores the wild-type phenotype in the cydAB mutant (Fig 3). However, the reason why overexpressing the cyd operon by plasmid-based complementation did not totally restore the resistance of the mutant to paraquat is not understood.
Fig 3. Survival assay with paraquat, a superoxide generator.
The effect of paraquat on the viability of P. gingivalis wild-type, cydAB mutant and complemented mutant was tested by exposing the strains to 480 μM of paraquat in enriched BHI medium at 37°C in anaerobic condition, and determining the CFU/ml at different time points for six hours. Wild-type (■), cydAB mutant (▲) and complemented cydAB mutant (●). Bars represent standard errors and asterisks indicate statistically significant differences between values of wild-type strain and mutant or complemented mutant (** p<0.01). Statistical analysis were performed with GraphPad Prism software (California, USA). The Mann-Whitney test was used and p-values<0.05 were considered significant.
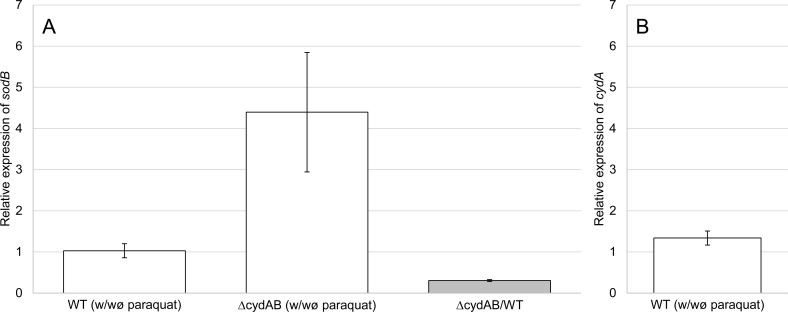
The expression of sodB (PGN_0564) encoding the superoxide dismutase was monitored in P. gingivalis wild type and cydAB mutant. In E. coli redox-cycling drugs were able to activate the SoxR (sodB activator) in anaerobic cells, thus without superoxide production, as long as alternative respiratory acceptors were provided [31]. In P. gingivalis, there is no SoxRS system. sodB expression is activated by exposure to oxygen [20] and by paraquat in aerobiosis [32]. qRT-PCR analyses showed that the expression of sodB was not induced by a sub-inhibitory concentration of paraquat (320 μM) in the wild-type strain, while the expression was four times higher in the cydAB mutant (Fig 4A). Without paraquat, and in anaerobic conditions, the expression level of sodB was three times lower in the cydAB mutant than in the wild-type (Fig 4A). One hypothesis is that CydAB decreased paraquat toxicity in anaerobiosis by consuming the trace of O2 resulting from the short exposure to air during addition of paraquat, oxygen which is needed to generate ROS from paraquat. We demonstrated in this study that CydAB is effectively involved in O2 consumption (see further down). Moreover, the expression of cydA was not affected by paraquat, in the conditions that we used to monitor sodB expression (Fig 4B).
Fig 4. Relative expression of sodB.
The relative expression of sodB was quantified by qRT-PCR with the 2∆∆Ct method. The normalization was done with the glucokinase gene (PGN_0381). The white histograms represent, for wild-type or cydAB mutant strains, the relative expression of sodB (A) or cydA (B) between both conditions: with paraquat (320 μM) and without paraquat (control condition). The grey histogram represents the relative expression of sodB between cydAB mutant and wild-type (control condition) in absence of paraquat. Bars represent standard errors.
Peroxide stress
The absence of cydAB increased the susceptibility of exponentially growing P. gingivalis to 500 μM of H2O2 (Fig 5) while no difference was observed between wild-type and cydAB mutant when H2O2 was added to cultures in the stationary phase of growth (data not shown). This result is consistent with our previous data demonstrating that cydAB genes were expressed in the exponential phase of growth, displayed in Fig 2. However, the complementation of cydAB mutant with the native cydAB genes in trans only partially restored the resistance to H2O2 in the exponential phase: after 2 h exposure to 500 μM H2O2, the survival rate was 44.5% for wild-type, 21% for the complemented mutant, and 10% for the mutant (Fig 5). The susceptibility of P. gingivalis to H2O2 was significantly increased in the stationary phase compared to the exponential phase, with the survival rate dropping to 0.05% after two hours of treatment (data not shown).
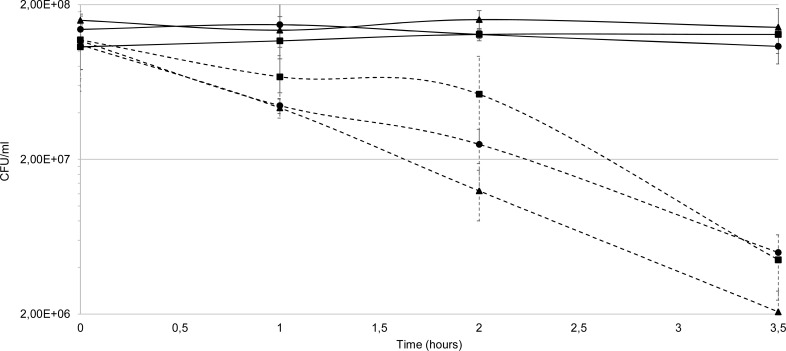
Fig 5. Survival assay with hydrogen peroxide.
The effect of H2O2 on bacterial viability was assayed by counting the colony-forming units after exposing the cultures to 500 μM of H2O2 for 3.5 hours, in enriched BHI broth at 37°C in anaerobic conditions. Broken lines represent the numeration of viable cells after H2O2 addition (500 μM) and solid lines represent the control condition without H2O2. Wild-type (■), cydAB mutant (▲) and complemented cydAB mutant (●). Bars represent standard errors.
CydAB is involved in O2 consumption
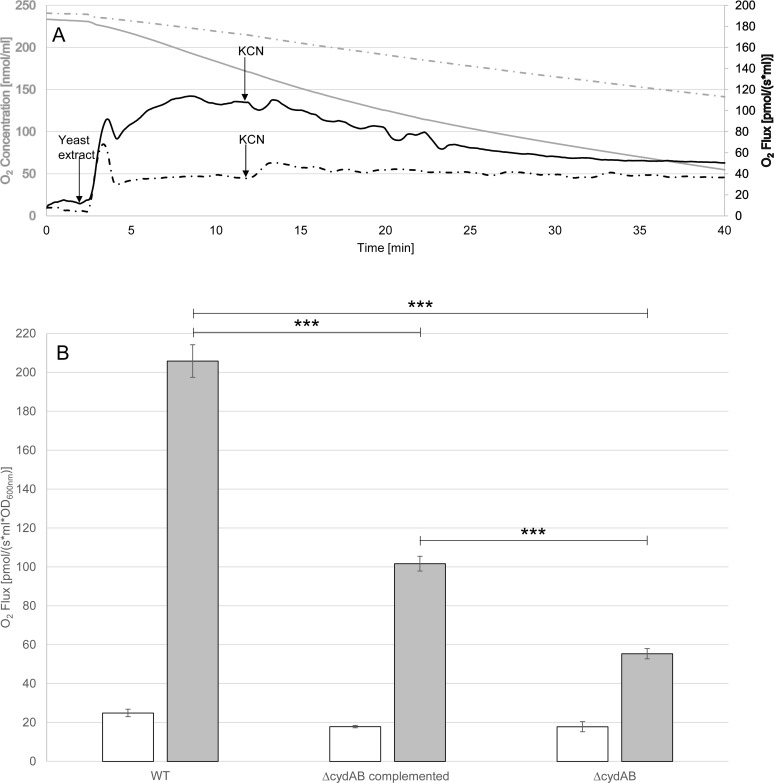
Despite no significant growth in microaerobic (6% O2 generated by campygen microaerophilic gas generator from Oxoid) and aerobic atmospheres in enriched BHI (data not shown), P. gingivalis ATCC 33277 can survive in oxygenated environments. We wanted to analyse the role of CydAB in O2 consumption in P. gingivalis. We were unable to detect any effect of the cydAB mutation on the instant respiration of enriched BHI-grown cells by high resolution respirometry (data not shown). However, O2 consumption at a rate of 18 pmol/(s*ml) was observed with non-inoculated enriched BHI medium (S3A Fig). To discard the effect of enriched BHI on O2 consumption, the cells were harvested by centrifugation and then washed twice in phosphate buffered saline (non O2-consuming S3A Fig). Neither the wild-type, nor the cydAB mutant or complemented mutant displayed a significant O2 consumption in PBS, with basal O2 consumption rates of respectively 17.8, 11.7, 11.4 pmol/(s*ml). This reduced O2 consumption is probably due to the lack of endogenous electron donors in washed cells (PBS). We found that respiration was reinstated in the wild-type by adding yeast extract to the PBS, while no effect was detected in the non-inoculated medium (S3B Fig). In this condition, the medium rate of O2 consumption was significantly lower in the cydAB mutant [35 pmol/(s*ml)] compared to WT [147 pmol/(s*ml)] and complemented mutant [67 pmol/(s*ml)] (Fig 6A and 6B). Interestingly, the addition of KCN (1 mM) decreased, but did not totally end, O2 consumption by wild-type but had no effect on the cydAB mutant (Fig 6A), confirming that the O2 consumption by the wild-type was mainly mediated by a cyanide-sensitive oxidase such as the cytochrome bd oxidase.
Fig 6. O2 consumption measured by high resolution respirometry.
(A) The graphs represent the speed of consumption of O2 (O2 Flux per Volume) (black lines) and remaining exogenous O2 concentrations in the oxygraph chamber (grey lines) for both strains in PBS: wild-type (solid lines) and cydAB mutant (broken lines). OD600 nm for wild-type and cydAB mutant were respectively of 0.588 and 0.544. Yeast extract (5 g/l) and potassium cyanide (KCN; 1 mM) addition were added at 2.5 min and 12 min respectively. (B) The effect of addition of yeast extract in PBS for O2 consumption is represented as the average and standard deviation of speed consumption of O2 (O2 Flux per Volume per OD600 nm) for each strain. The white histogram corresponds to the condition without yeast extract, and the grey histogram to the condition with yeast extract (5 g/l). Bars represent standard errors. Bars represent standard errors and asterisks indicate statistically significant results (*** p<0.0001). Statistical analysis were performed with GraphPad Prism software (California, USA). The Mann-Whitney test was used and p-values<0.05 were considered significant.
We have measured the intracellular concentrations of ATP in the media (enriched BHI or PBS plus yeast extract) that we used to monitor the O2 consumption, both in presence and absence of oxygen. No difference was observed with or without the cydAB genes, as depicted in S4 Fig.
Cytochrome bd oxidase contributes to the dioxygen tolerance of P. gingivalis
To determine whether cydAB genes are required for dioxygen tolerance, wild-type, cydAB mutant and complemented cydAB mutant were exposed for six hours to atmospheric oxygen, in the PBS-yeast extract conditions for which CydAB dependent O2-consumption was detected. Unlike the control assays in anaerobic conditions, the cydAB mutant displayed a lower survival rate than the wild-type and complemented mutant in aerobic conditions, as depicted in Fig 7. However, 0.01% of cydAB mutant cells survived after six hours exposure to an aerobic atmosphere, indicating that CydAB is not the only factor contributing to the aerotolerance of P. gingivalis (Fig 7).
Fig 7. Dioxygen tolerance.
The resistance to dioxygen was assayed by counting colony-forming units of bacterial cells suspended in PBS with yeast extract (5 g/l) and maintained in an aerobic atmosphere for six hours. Wild type (■), cydAB mutant (▲) and complemented cydAB mutant (●). Bars represent standard errors and asterisks indicate statistically significant differences between values of wild-type strain and mutant or complemented mutant strains (* p<0.05). Statistical analysis were performed with GraphPad Prism software (California, USA). The Mann-Whitney test was used and p-values<0.05 were considered significant.
Adhesion to and invasion of epithelial cells by P. gingivalis are not affected by cydAB mutation
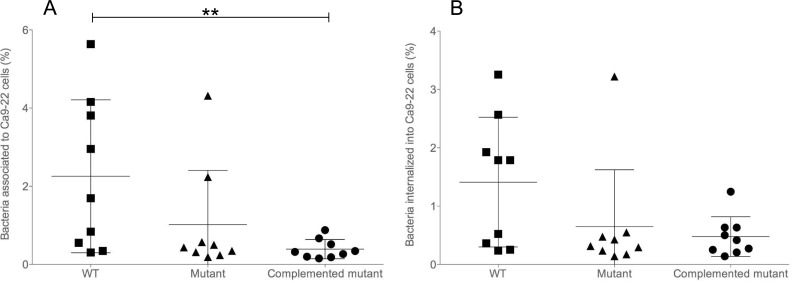
The P. gingivalis cydAB mutant and wild type displayed similar efficiency to adhere and/or invade the Ca9-22 gingival epithelial cells in aerobic atmosphere plus 5% CO2, as shown in Fig 8A which displayed all the Ca9-22-associated (inside and outside) bacteria. It is noteworthy that the efficiency of the complemented cydAB mutant, which overexpressed the cydWAB operon (Fig 2), was significantly altered (Fig 8A). When only the number of invasive bacterial cells was monitored, no difference was observed between the strains (Fig 8B). Survival assays with THP1 macrophages were also performed and neither the cydAB mutant nor the complemented mutant behaved differently than the WT (S5 Fig).
Fig 8. Adhesion and invasion to Ca9-22 epithelial cells.
The percentage (vs. inoculum) of (A) adherent external and internal bacteria (“associated”), or (B) internal bacteria, which are remaining after incubation with Ca9-22 was assayed by counting colony forming units of bacterial cells per millilitre. Wild-type (■), cydAB mutant (▲) and complemented cydAB mutant (●). Bars represent mean with standard errors and asterisks indicate statistically significant differences between values of wild-type strain, mutant and complemented mutant strains (** p<0.01).
The presence of the cytochrome bd oxidase does not seem to be crucial for the interaction of P. gingivalis with host cells although the behavior of the overexpressing complemented strain requires further investigations.
Conclusion
The cydA gene was identified in genomes of prokaryotes that have been classified as strict anaerobes, such as members of the Archaeoglobus, Methanosarcina, Geobacter, Desulfovibrio genera and members of the Bacteroides class [22]. P. gingivalis, a bacteroidetes, is considered as an obligatory anaerobic bacterium, although possessing an oxygen-dependent enzyme CydAB and an oxygen-generating superoxide dismutase SodB. The role of CydAB in O2 consumption and growth at nanomolar concentrations of oxygen was first demonstrated in the anaerobic bacterium Bacteroides fragilis, which was therefore classified as a nanaerobe [9]. This study is the first to evaluate the physiological role of CydAB in relation to oxygen species in the oral pathogen P. gingivalis.
This study shows that the cytochrome bd oxidase of P. gingivalis is necessary to confer an optimal resistance to hydrogen peroxide and superoxide. P. gingivalis is an obligate anaerobe but it is also known as an oxygen-tolerant organism. P. gingivalis possesses several systems that work against oxidative stress damage, including antioxidant enzymes [7], DNA binding proteins [33] and a heme layer that may act as an oxidative sink [34, 35], which altogether were suspected to account for its tolerance to dioxygen. We demonstrated in this study that CydAB of P. gingivalis was also involved in O2 consumption, and in dioxygen resistance. Lewis et al. [20] and Diaz et al. [36] showed that P. gingivalis strains W83 and W50 were able to growth in a microaerobic atmosphere in the presence of excess hemin in medium (1 to 5 mg/l). However, we repeatedly failed to obtain visible growth of P. gingivalis ATCC 33277 in a microaerobic atmosphere (data not shown). Therefore, it is still unknown whether this P. gingivalis strain can benefit from low concentration of O2 for growth, and whether or not CydAB is essential for O2-mediated development. Moreover, in Bacteroides fragilis, a mutation inactivating one single gene, the oxe gene, allowed the growth up to 2% O2 [37] Additional experiments are needed to further investigate the growth of P. gingivalis ATCC 33277 in tiddly-controlled nanomolar concentrations of O2 and to search for possible oxe-like mutations in O2 growing P. gingivalis strains.
In conclusion, CydAB, which promotes ROS resistance, may confer a competitive advantage to P. gingivalis. Our data suggest that CydAB is a key element in the survival of P. gingivalis inside its ecological niche and to shape the oral community. Oral biofilms are constantly exposed to oxygen. Amazingly enough, most identified oral pathogens are classified as anaerobic bacteria. The survival of these oral bacterial species therefore depends on their specific tolerance to oxygen and the local oxygen tension inside the biofilm community. Cooperation also plays a role in shaping the community. For example, Fusobacterium nucleatum supports P. gingivalis growth by providing a capnophilic environment when growing in an oxygenated and CO2 depleted environment [38]. Competition between species is also relevant in oral biofilms: Streptococcus sanguinis or Streptococcus gordonii excrete H2O2 to compete with other species [39, 40]. P. gingivalis is often detected together with Treponema denticola in subgingival plaque. It is hypothesized that T. denticola can produce succinic acid, which is utilised by P. gingivalis in the cell envelope, while P. gingivalis produces isobutyric acid which increases T. denticola growth [41, 42]. Decreasing the local O2 concentration via high affinity cytochrome bd oxidase may favour the co-culture of associated anaerobic pathogens.
Supporting Information
Taxa tree showing conserved operon architecture and co-occurrence of cydWAB genes. The analysis was performed with the String online software (Jensen et al. Nucleic Acids Res. 2009,37:D412-6) and with P. gingivalis ATCC 33277 cydA sequence as anchor. This neighborhood view shows runs of genes that occur repeatedly in close neighborhood in prokaryotic genomes, correlated with taxa tree. + indicated when sub-level species have been collapsed to reduce the taxa tree. For collapsed levels, the organism names are in green. Genes located together in a run are linked with a black line. The maximum allowed intergenic distance for genes to be considered as neighbor is 300 base pairs. Same color represents homologues based on amino-acid sequence similarity. Only predicted functional partners are displayed. PGN_1039 (light blue), PGN_1043 (purple) and PGN_1044 (dark blue) products appeared for Porphyromonas genus because the co-occurrence on the same DNA region of their coding genes in this genus is considered by the software as a parameter for a potential functional partnership.
(PDF)
PCR was performed on cDNA obtained by reverse transcription of total RNA extracted from P. gingivalis ATCC 33277 (wild-type) with the following primers: (A) PGN_1039-L, TTCAGCCATACGCATCTGAG / PGN_1039-R, GTTGAATGCCACAATGTTCG for PGN_1039 gene; (B) PGN_1040-L, TCATGCGTATAGCTCGCTTTT / PGN_1040-R, TACCGACCTGTTGCTTCAGA for cydW gene; (C) PGN_1041-L, CCGGTAGGAATGACCTTCAA / PGN_1041-R, ATCCTTTCGCAGCAGGTAGA for cydA gene; (D) PGN_1042-L, TGGTAATGTATGGGGGAGGA / PGN_1042-R, GAGAACCACGTTCCACAGGT for cydB gene; (E) PGN_1043-L, GTCCCGACATCATAGCAGGT / PGN_1043-L, CAAGGTCCGTTGCCACTATT for PGN_1043 gene. RNA extract was used as negative control (-).The ladder (L) is the DNA Molecular Weight Marker VIII (Roche).
(PDF)
(A) O2 Flux per Volume is represented by black lines and O2 concentration is represented by grey lines for both media: PBS (solid lines) and enriched BHI (dashed lines). (B) The graphs represent the speed of consumption of O2 (O2 Flux per Volume) (black lines) by PBS and exogenous remaining O2 concentrations in the oxygraph chamber (grey lines). Yeast extract (5 g/l) was added at 9 min.
(PDF)
ATP content in P. gingivalis (wild-type, cydAB mutant and cydAB complemented mutant) was quantified by the BacTiter-Glo™ Microbial Cell Viability Assay kit (Promega). Histograms represent the concentration of ATP for 107 bacteria and bars represent standard errors. P. gingivalis strains were grown overnight in enriched BHI at OD600 nm of 0.5 (exponential phase). Samples were used to inoculate an enriched BHI medium to an OD600 nm of 0.1 (for A and B experiments) or were washed and suspended in PBS supplemented with 5 g/l of yeast extract to an OD600 nm of 0.1 (for C and D experiments). Each culture was split in two, one half was incubated for 7 hours in anaerobic conditions (for A and C experiments) and the other half was incubated for 5 hours in anaerobic conditions and shifted to micro-aerobic conditions for 2 hours (for B and D experiments).
(PDF)
Survival of P. gingivalis ATCC 33277 was assayed by counting colony forming units of bacterial cells per millilitre after 1 and 2 hours in presence of THP1 macrophages (JCRB cell bank, Japan). Wild-type (■), cydAB mutant (▲) and complemented cydAB mutant (●). Bars represent standard errors. THP1 monocyte were seeded into 24-well culture plates at a density of approximately 1,5.105 cells per well and were differentiate to macrophages after 72 hours in aerobic conditions at 37°C with 5% CO2 in humidified atmosphere in Roswell Park Memorial Institute-1640 medium (RPMI-1640) enriched with 10% (v/v) fetal calf serum, 1% (v/v) of 200 mM L-glutamine, 1% (v/v) of 100 nM pyruvate sodium, 1% (v/v) of antibiotic mixture (10 U/μl penicillin and 10 U/μl streptomycin), 2% (v/v) of 1 M HEPES and 10 ng/ml of phorbol 12-myristate 13-acetate (PMA). Cells were washed with 500 μl of PBS and P. gingivalis strains (wild-type, cydAB mutant and cydAB complemented mutant) were added to THP1 macrophages with a multiplicity of infection of about 1:4000, in enriched RPMI-1640 without antibiotic mixture nor PMA. Plates were centrifuged for 5 minutes at 1000 g to promote bacteria-cell contact. Plates were incubated for 1 or 2 hours in aerobic conditions at 37°C with 5% CO2 in humidified atmosphere. Unattached bacteria were removed by three PBS washes. Samples were plated on Colombia agar supplemented with blood. Colonies were enumerated after 5 days of anaerobic incubation at 37°C. At least three independent experiments, each in duplicate, were conducted.
(PDF)
Acknowledgments
We thank Ludivine Malherbe for her experimental help.
Abbreviations
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1. Hong BY, Furtado Araujo MV, Strausbaugh LD, Terzi E, Ioannidou E, Diaz PI. Microbiome profiles in periodontitis in relation to host and disease characteristics. PLoS One 2015;10(5):e0127077 10.1371/journal.pone.0127077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Duran-Pinedo AE, Chen T, Teles R, Starr JR, Wang X, Krishnan K, et al. Community-wide transcriptome of the oral microbiome in subjects with and without periodontitis. ISME J 2014. August;8(8):1659–72. 10.1038/ismej.2014.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Naito M, Hirakawa H, Yamashita A, Ohara N, Shoji M, Yukitake H, et al. Determination of the genome sequence of Porphyromonas gingivalis strain ATCC 33277 and genomic comparison with strain W83 revealed extensive genome rearrangements in P. gingivalis . DNA Res 2008. August;15(4):215–25. 10.1093/dnares/dsn013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Onozawa S, Kikuchi Y, Shibayama K, Kokubu E, Nakayama M, Inoue T, et al. Role of extracytoplasmic function sigma factors in biofilm formation of Porphyromonas gingivalis . BMC Oral Health 2015;15:4 10.1186/1472-6831-15-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dou Y, Osbourne D, McKenzie R, Fletcher HM. Involvement of extracytoplasmic function sigma factors in virulence regulation in Porphyromonas gingivalis W83. FEMS Microbiol Lett 2010. November;312(1):24–32. 10.1111/j.1574-6968.2010.02093.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yanamandra SS, Sarrafee SS, Anaya-Bergman C, Jones K, Lewis JP. Role of the Porphyromonas gingivalis extracytoplasmic function sigma factor, SigH. Mol Oral Microbiol 2012. June;27(3):202–19. 10.1111/j.2041-1014.2012.00643.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Henry LG, McKenzie RM, Robles A, Fletcher HM. Oxidative stress resistance in Porphyromonas gingivalis . Future Microbiol 2012. April;7(4):497–512. 10.2217/fmb.12.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borisov VB, Gennis RB, Hemp J, Verkhovsky MI. The cytochrome bd respiratory oxygen reductases. Biochim Biophys Acta 2011. November;1807(11):1398–413. 10.1016/j.bbabio.2011.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baughn AD, Malamy MH. The strict anaerobe Bacteroides fragilis grows in and benefits from nanomolar concentrations of oxygen. Nature 2004. January 29;427(6973):441–4. [DOI] [PubMed] [Google Scholar]
- 10. Giuffre A, Borisov VB, Mastronicola D, Sarti P, Forte E. Cytochrome bd oxidase and nitric oxide: from reaction mechanisms to bacterial physiology. FEBS Lett 2012. March 9;586(5):622–9. 10.1016/j.febslet.2011.07.035 [DOI] [PubMed] [Google Scholar]
- 11. Edwards SE, Loder CS, Wu G, Corker H, Bainbridge BW, Hill S, et al. Mutation of cytochrome bd quinol oxidase results in reduced stationary phase survival, iron deprivation, metal toxicity and oxidative stress in Azotobacter vinelandii . FEMS Microbiol Lett 2000. April 1;185(1):71–7. [DOI] [PubMed] [Google Scholar]
- 12. Fu H, Chen H, Wang J, Zhou G, Zhang H, Zhang L, et al. Crp-dependent cytochrome bd oxidase confers nitrite resistance to Shewanella oneidensis . Environ Microbiol 2013. August;15(8):2198–212. 10.1111/1462-2920.12091 [DOI] [PubMed] [Google Scholar]
- 13. Borisov VB, Forte E, Davletshin A, Mastronicola D, Sarti P, Giuffre A. Cytochrome bd oxidase from Escherichia coli displays high catalase activity: an additional defense against oxidative stress. FEBS Lett 2013. July 11;587(14):2214–8. 10.1016/j.febslet.2013.05.047 [DOI] [PubMed] [Google Scholar]
- 14. Mobius K, Arias-Cartin R, Breckau D, Hannig AL, Riedmann K, Biedendieck R, et al. Heme biosynthesis is coupled to electron transport chains for energy generation. Proc Natl Acad Sci U S A 2010. June 8;107(23):10436–41. 10.1073/pnas.1000956107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bader M, Muse W, Ballou DP, Gassner C, Bardwell JC. Oxidative protein folding is driven by the electron transport system. Cell 1999. July 23;98(2):217–27. [DOI] [PubMed] [Google Scholar]
- 16. Korshunov S, Imlay JA. Two sources of endogenous hydrogen peroxide in Escherichia coli . Mol Microbiol 2010. March;75(6):1389–401. 10.1111/j.1365-2958.2010.07059.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dashper S, Ang CS, Liu SW, Paolini R, Veith P, Reynolds E. Inhibition of Porphyromonas gingivalis biofilm by oxantel. Antimicrob Agents Chemother 2009. March;54(3):1311–4. 10.1128/AAC.00946-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baughn AD, Malamy MH. The essential role of fumarate reductase in haem-dependent growth stimulation of Bacteroides fragilis . Microbiology 2003. June;149(Pt 6):1551–8. [DOI] [PubMed] [Google Scholar]
- 19. Leclerc J, Martin B, Tamanai-Shacoori Z, Le Pottier L, Guyodo H, Meuric V, et al. Involvement of respiratory chain in biofilm formation in Porphyromonas gingivalis . Bull Group Int Rech Sci Stomatol Odontol 2011;50(2):5–6. [PubMed] [Google Scholar]
- 20. Lewis JP, Iyer D, Anaya-Bergman C. Adaptation of Porphyromonas gingivalis to microaerophilic conditions involves increased consumption of formate and reduced utilization of lactate. Microbiology 2009. November;155(Pt 11):3758–74. 10.1099/mic.0.027953-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hirano T, Beck DA, Demuth DR, Hackett M, Lamont RJ. Deep sequencing of Porphyromonas gingivalis and comparative transcriptome analysis of a LuxS mutant. Front Cell Infect Microbiol 2012;2:79 10.3389/fcimb.2012.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morris RL, Schmidt TM. Shallow breathing: bacterial life at low O2 . Nat Rev Microbiol 2014. March;11(3):205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kumagai Y, Yajima A, Konishi K. Peptidase activity of dipeptidyl aminopeptidase IV produced by Porphyromonas gingivalis is important but not sufficient for virulence. Microbiol Immunol 2003;47(10):735–43. [DOI] [PubMed] [Google Scholar]
- 24. Yoshimoto H, Takahashi Y, Hamada N, Umemoto T. Genetic transformation of Porphyromonas gingivalis by electroporation. Oral Microbiol Immunol 1993. August;8(4):208–12. [DOI] [PubMed] [Google Scholar]
- 25. VanOrsdel CE, Bhatt S, Allen RJ, Brenner EP, Hobson JJ, Jamil A, et al. The Escherichia coli CydX protein is a member of the CydAB cytochrome bd oxidase complex and is required for cytochrome bd oxidase activity. J Bacteriol 2013. August;195(16):3640–50. 10.1128/JB.00324-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoeser J, Hong S, Gehmann G, Gennis RB, Friedrich T. Subunit CydX of Escherichia coli cytochrome bd ubiquinol oxidase is essential for assembly and stability of the di-heme active site. FEBS Lett 2014. May 2;588(9):1537–41. 10.1016/j.febslet.2014.03.036 [DOI] [PubMed] [Google Scholar]
- 27. Allen RJ, Brenner EP, VanOrsdel CE, Hobson JJ, Hearn DJ, Hemm MR. Conservation analysis of the CydX protein yields insights into small protein identification and evolution. BMC Genomics 2014;15:946 10.1186/1471-2164-15-946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liochev SI, Hausladen A, Beyer WF Jr., Fridovich I. NADPH: ferredoxin oxidoreductase acts as a paraquat diaphorase and is a member of the soxRS regulon. Proc Natl Acad Sci U S A 1994. February 15;91(4):1328–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pomposiello PJ, Bennik MH, Demple B. Genome-wide transcriptional profiling of the Escherichia coli responses to superoxide stress and sodium salicylate. J Bacteriol 2001. July;183(13):3890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hassan HM, Fridovich I. Paraquat and Escherichia coli. Mechanism of production of extracellular superoxide radical. J Biol Chem 1979. November 10;254(21):10846–52. [PubMed] [Google Scholar]
- 31. Gu M, Imlay JA. The SoxRS response of Escherichia coli is directly activated by redox-cycling drugs rather than by superoxide. Mol Microbiol 2013. March;79(5):1136–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ohara N, Kikuchi Y, Shoji M, Naito M, Nakayama K. Superoxide dismutase-encoding gene of the obligate anaerobe Porphyromonas gingivalis is regulated by the redox-sensing transcription activator OxyR. Microbiology 2006. April;152(Pt 4):955–66. [DOI] [PubMed] [Google Scholar]
- 33. Ueshima J, Shoji M, Ratnayake DB, Abe K, Yoshida S, Yamamoto K, et al. Purification, gene cloning, gene expression, and mutants of Dps from the obligate anaerobe Porphyromonas gingivalis . Infect Immun 2003. March;71(3):1170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smalley JW, Birss AJ, Silver J. The periodontal pathogen Porphyromonas gingivalis harnesses the chemistry of the mu-oxo bishaem of iron protoporphyrin IX to protect against hydrogen peroxide. FEMS Microbiol Lett 2000. February 1;183(1):159–64. [DOI] [PubMed] [Google Scholar]
- 35. Smalley JW, Silver J, Marsh PJ, Birss AJ. The periodontopathogen Porphyromonas gingivalis binds iron protoporphyrin IX in the mu-oxo dimeric form: an oxidative buffer and possible pathogenic mechanism. Biochem J 1998. May 1;331 (Pt 3):681–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Diaz PI, Slakeski N, Reynolds EC, Morona R, Rogers AH, Kolenbrander PE. Role of oxyR in the oral anaerobe Porphyromonas gingivalis . J Bacteriol 2006. April;188(7):2454–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meehan BM, Baughn AD, Gallegos R, Malamy MH. Inactivation of a single gene enables microaerobic growth of the obligate anaerobe Bacteroides fragilis . Proc Natl Acad Sci U S A 2012. July 24;109(30):12153–8. 10.1073/pnas.1203796109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huang GT, Kim D, Lee JK, Kuramitsu HK, Haake SK. Interleukin-8 and intercellular adhesion molecule 1 regulation in oral epithelial cells by selected periodontal bacteria: multiple effects of Porphyromonas gingivalis via antagonistic mechanisms. Infect Immun 2001. March;69(3):1364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zheng L, Itzek A, Chen Z, Kreth J. Environmental influences on competitive hydrogen peroxide production in Streptococcus gordonii . Appl Environ Microbiol 2011. July;77(13):4318–28. 10.1128/AEM.00309-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhu L, Kreth J. The role of hydrogen peroxide in environmental adaptation of oral microbial communities. Oxid Med Cell Longev 2012;2012:717843 10.1155/2012/717843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grenier D. Nutritional interactions between two suspected periodontopathogens, Treponema denticola and Porphyromonas gingivalis . Infect Immun 1992. December;60(12):5298–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhu Y, Dashper SG, Chen YY, Crawford S, Slakeski N, Reynolds EC. Porphyromonas gingivalis and Treponema denticola synergistic polymicrobial biofilm development. PLoS One 2013;8(8):e71727 10.1371/journal.pone.0071727 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Taxa tree showing conserved operon architecture and co-occurrence of cydWAB genes. The analysis was performed with the String online software (Jensen et al. Nucleic Acids Res. 2009,37:D412-6) and with P. gingivalis ATCC 33277 cydA sequence as anchor. This neighborhood view shows runs of genes that occur repeatedly in close neighborhood in prokaryotic genomes, correlated with taxa tree. + indicated when sub-level species have been collapsed to reduce the taxa tree. For collapsed levels, the organism names are in green. Genes located together in a run are linked with a black line. The maximum allowed intergenic distance for genes to be considered as neighbor is 300 base pairs. Same color represents homologues based on amino-acid sequence similarity. Only predicted functional partners are displayed. PGN_1039 (light blue), PGN_1043 (purple) and PGN_1044 (dark blue) products appeared for Porphyromonas genus because the co-occurrence on the same DNA region of their coding genes in this genus is considered by the software as a parameter for a potential functional partnership.
(PDF)
PCR was performed on cDNA obtained by reverse transcription of total RNA extracted from P. gingivalis ATCC 33277 (wild-type) with the following primers: (A) PGN_1039-L, TTCAGCCATACGCATCTGAG / PGN_1039-R, GTTGAATGCCACAATGTTCG for PGN_1039 gene; (B) PGN_1040-L, TCATGCGTATAGCTCGCTTTT / PGN_1040-R, TACCGACCTGTTGCTTCAGA for cydW gene; (C) PGN_1041-L, CCGGTAGGAATGACCTTCAA / PGN_1041-R, ATCCTTTCGCAGCAGGTAGA for cydA gene; (D) PGN_1042-L, TGGTAATGTATGGGGGAGGA / PGN_1042-R, GAGAACCACGTTCCACAGGT for cydB gene; (E) PGN_1043-L, GTCCCGACATCATAGCAGGT / PGN_1043-L, CAAGGTCCGTTGCCACTATT for PGN_1043 gene. RNA extract was used as negative control (-).The ladder (L) is the DNA Molecular Weight Marker VIII (Roche).
(PDF)
(A) O2 Flux per Volume is represented by black lines and O2 concentration is represented by grey lines for both media: PBS (solid lines) and enriched BHI (dashed lines). (B) The graphs represent the speed of consumption of O2 (O2 Flux per Volume) (black lines) by PBS and exogenous remaining O2 concentrations in the oxygraph chamber (grey lines). Yeast extract (5 g/l) was added at 9 min.
(PDF)
ATP content in P. gingivalis (wild-type, cydAB mutant and cydAB complemented mutant) was quantified by the BacTiter-Glo™ Microbial Cell Viability Assay kit (Promega). Histograms represent the concentration of ATP for 107 bacteria and bars represent standard errors. P. gingivalis strains were grown overnight in enriched BHI at OD600 nm of 0.5 (exponential phase). Samples were used to inoculate an enriched BHI medium to an OD600 nm of 0.1 (for A and B experiments) or were washed and suspended in PBS supplemented with 5 g/l of yeast extract to an OD600 nm of 0.1 (for C and D experiments). Each culture was split in two, one half was incubated for 7 hours in anaerobic conditions (for A and C experiments) and the other half was incubated for 5 hours in anaerobic conditions and shifted to micro-aerobic conditions for 2 hours (for B and D experiments).
(PDF)
Survival of P. gingivalis ATCC 33277 was assayed by counting colony forming units of bacterial cells per millilitre after 1 and 2 hours in presence of THP1 macrophages (JCRB cell bank, Japan). Wild-type (■), cydAB mutant (▲) and complemented cydAB mutant (●). Bars represent standard errors. THP1 monocyte were seeded into 24-well culture plates at a density of approximately 1,5.105 cells per well and were differentiate to macrophages after 72 hours in aerobic conditions at 37°C with 5% CO2 in humidified atmosphere in Roswell Park Memorial Institute-1640 medium (RPMI-1640) enriched with 10% (v/v) fetal calf serum, 1% (v/v) of 200 mM L-glutamine, 1% (v/v) of 100 nM pyruvate sodium, 1% (v/v) of antibiotic mixture (10 U/μl penicillin and 10 U/μl streptomycin), 2% (v/v) of 1 M HEPES and 10 ng/ml of phorbol 12-myristate 13-acetate (PMA). Cells were washed with 500 μl of PBS and P. gingivalis strains (wild-type, cydAB mutant and cydAB complemented mutant) were added to THP1 macrophages with a multiplicity of infection of about 1:4000, in enriched RPMI-1640 without antibiotic mixture nor PMA. Plates were centrifuged for 5 minutes at 1000 g to promote bacteria-cell contact. Plates were incubated for 1 or 2 hours in aerobic conditions at 37°C with 5% CO2 in humidified atmosphere. Unattached bacteria were removed by three PBS washes. Samples were plated on Colombia agar supplemented with blood. Colonies were enumerated after 5 days of anaerobic incubation at 37°C. At least three independent experiments, each in duplicate, were conducted.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.