Abstract
Understanding disease dynamics during the breeding season of declining amphibian species will improve our understanding of how remnant populations persist with endemic infection, and will assist the development of management techniques to protect disease-threatened species from extinction. We monitored the endangered Litoria verreauxii alpina (alpine treefrog) during the breeding season through capture-mark-recapture (CMR) studies in which we investigated the dynamics of chytridiomycosis in relation to population size in two populations. We found that infection prevalence and intensity increased throughout the breeding season in both populations, but infection prevalence and intensity was higher (3.49 and 2.02 times higher prevalence and intensity, respectively) at the site that had a 90-fold higher population density. This suggests that Bd transmission is density-dependent. Weekly survival probability was related to disease state, with heavily infected animals having the lowest survival. There was low recovery from infection, especially when animals were heavily infected with Bd. Sympatric amphibian species are likely to be reservoir hosts for the disease and can play an important role in the disease ecology of Bd. Although we found 0% prevalence in crayfish (Cherax destructor), we found that a sympatric amphibian (Crinia signifera) maintained 100% infection prevalence at a high intensity throughout the season. Our results demonstrate the importance of including infection intensity into CMR disease analysis in order to fully understand the implications of disease on the amphibian community. We recommend a combined management approach to promote lower population densities and ensure consistent progeny survival. The most effective management strategy to safeguard the persistence of this susceptible species might be to increase habitat area while maintaining a similar sized suitable breeding zone and to increase water flow and area to reduce drought.
Introduction
The amphibian disease chytridiomycosis (caused by the fungal pathogen Batrachochytrium dendrobatidis, Bd) is a major cause of amphibian declines globally and has been called the most devastating threat from disease to biodiversity [1]. In-depth ecological studies are important for determining disease impact in the wild because many factors can affect disease dynamics. Breeding habitat plays an important role in the prevalence of Bd infection, with higher prevalence more often associated with permanent water bodies [2,3]. Bd is known to exhibit density-dependent disease transmission [4–6]. Bd infection tends to peak seasonally [7–11], and the peak is often attributed to optimal temperature conditions for Bd. However, other factors might play a role [12]. Aggregate breeders often experience dramatic increases in infection prevalence during their short breeding season [13], likely due to both increased density of animals and increased frequency of contact due to breeding behaviour. Intensive population monitoring throughout the breeding season can shed light on the ecological impact of Bd on declining species and can inform management decisions.
Capture-mark-recapture (CMR) studies are an effective ecological tool to determine effects of disease on populations and individuals in the wild and guide management decisions [14]. The in-depth analysis of CMR data allows for greater understanding of how survival and recapture probability are directly affected by disease and the probability of an animal gaining and recovering from infection [15]. CMR studies in amphibian populations with prevalent Bd infection have uncovered factors that influence disease dynamics including a dependence on disease prevalence, infection intensity, temperature, population density, and reservoir hosts [5,14,15]. Infection intensity is known to play an important role in disease dynamics, yet CMR studies often do not separate animals with heavy versus light infection [5,16]. It is important, particularly for pathogens with a strong relationship between infection burden and disease impact, to conduct CMR analyses that include infection load in order to obtain a true understanding of the disease dynamics [5].
Here, we conducted a CMR study to monitor the effects of prevalence and intensity of Bd infection in the endangered alpine treefrog, Litoria verreauxii alpina, with the purpose of better understanding the ecology of chytridiomycosis during the breeding season. Our most important aim was to identify opportunities for intervention that would promote the recovery of this and similar species. Litoria v. alpina is an aggregate breeder and native to the upland regions of the Australian Alps above 1200 m. Once widespread, L. v. alpina’s distribution has declined by over 80% of its former range since the 1980s [17] due primarily to chytridiomycosis [18]. Despite major declines, some populations persist in the presence of the pathogen [17,18]. While adults are known to have high mortality due to the disease, tadpoles and juveniles do not; therefore, high recruitment plays an important role in population persistence [19]. Although this species is endangered, populations have not been intensively monitored for chytridiomycosis during the breeding season when animals are thought to gain infection.
A second aim to this study was to screen for potential Bd reservoir species. Disease reservoirs can play an important role in the disease dynamics of co-occurring susceptible species [5]. A possible amphibian reservoir species present in the Australian Alps is the widespread and abundant common eastern froglet, Crinia signifera [18,19]. Recent research of non-amphibian hosts of Bd identified the North American crayfish, Procambarus clarkii [20,21]. While P. clarkii is not present in Australia, the commercially farmed and invasive crayfish species Cherax destructor is widespread throughout Australia [22] and might be involved in the dynamics of chytridiomycosis.
Materials and Methods
Study site
We conducted an intensive 10-week CMR study of two populations of L. v. alpina during their 12-week breeding season (see S1 File) [23]. Study Site 1: Oglivies Dam is a 0.17 hectares low elevation (1382m) site (S1 File) [23]. Sampling occurred between 1-Sept-2013 through 6-Nov-2013 (weeks 2–11 of breeding). Study Site 2: Sponar’s Creek (S1 File) [23] is a 1.8 hectares high elevation (1515m) site. Sampling occurred between 25-Sept-2013 and 25-Nov-2013 (weeks 1–10 of breeding) (S1 Fig). Air and water temperatures were recorded every two hours at each site using iButtons (S2 Fig).
Field survey
Animals were captured during one to three nights each week over 10 weeks, resulting in the capture of up to 50 new animals on each survey night (S1 Table). Animals were captured with a new, clean, gloved hand and kept individually in a new plastic zip bag. Animals were set aside while we completed our collection and then processed and returned to the site of capture each night. Animals found in amplexus were held together in one bag. Animals were photographed (for individual identification, see S1 File), swabbed for Bd (see below), weighed to the nearest 0.01g and snout to venter length (SVL) was measured to the nearest 0.02mm. Waders and boots were dried between sites to prevent the spread of Bd.
Testing for Bd
We tested for Bd infection by using skin swabs and a qPCR assay [24]. The swabbing protocol was standardized by performing 45 strokes on the venter and limbs with a sterile rayon-tipped swab (MW-113, Medical Wire & Equipment, Wiltshire, United Kingdom). Genomic DNA was extracted from the swabs using the Prepman Ultra (Applied Biosystems®, Life Technologies Pty Ltd, Carlsbad, California, USA) and a bead beater to break the fungal cell walls for two minutes, and then the extract was diluted 3:47 in PCR water. Extracted DNA was then analysed using quantitative real time PCR following Boyle et al. [24]. We conducted the analysis in singlicate to maximise both cost efficiency and test accuracy [25,26] including a positive and negative control and a series of dilution standards (to estimate infection load in zoospore equivalents, ZE).
To test for inhibition of the swab DNA, a subset of 20 samples was haphazardly selected and an internal positive control (VICTM IPC, Applied Biosystems®, Life Technologies Pty Ltd, Carlsbad, California, USA) was added to the qPCR reaction. No inhibition was detected in those samples. Because many samples returned high (>1000ZE) infection loads and prevalence was high, we concluded that inhibition due to high zoospore loads was unimportant. We prioritized resources to increase sample size rather than including IPC’s in every reaction.
Reservoir hosts
Common eastern froglets (Crinia signifera) were collected at both sites (n = 93) between 21-Sept-2013 and 21-Nov-2013 (S2 Table). Animals were collected and individually stored in a clean plastic bag, and swabbed for Bd using the same protocol as for L. v. alpina and then released.
The western blue claw yabbie, Cherax destructor, was present at both sites where L. v. alpina were sampled, but it proved difficult to capture. Instead, crayfish were collected from a nearby site that supported populations of L. v. alpina and C. signifera: Kiandra (S1 Fig; S2 Table) (Lat. -35.867, Long. 148.498: Elevation 1358m). Frogs at this site have similarly high Bd prevalence [19]. Animals were collected with baited (Fancy FeastTM, Nestlé Purina PetCare, St, Louis, Missouri, USA) minnow traps between 1-Oct-2013 and 25-Nov-2013. Traps were left open for 2–5 days before collection. Animals were collected individually from the traps with inverted plastic zip bags, and euthanized by freezing for at least two hours (n = 94).
The gastrointestinal (GI) tract was tested for Bd presence following Brannelly et al. [20]. Because faecal matter inside of the GI tract causes PCR inhibition, it was removed with a sterile swab. The inside of the GI tract was then swabbed using 30 strokes with a new sterile MW113 swab, and the GI tract was swabbed again with a second swab as a backup. We followed the same extraction and qPCR protocol as for the frogs except that we diluted DNA extraction samples 1:10. We included an IPC in each sample to test for inhibition. Samples were analysed in singlicate, but inhibited samples were reanalyzed in triplicate. When all samples returned negative results for Bd, we selected a subset of 24 backup swabs and re-extracted the DNA using Qiagen DNeasy Blood and Tissue Kit using a final elution volume of 200μl. The samples were then analyzed following the above stated qPCR protocol, but the DNA sample was undiluted. No evidence of inhibition was observed.
Statistical analysis
Survival, recapture probability, and disease state were examined using the statistical software M-SURGE [27]. The Conditional Arnason–Schwarz model was used. M-SURGE is a program designed specifically for multi-state CMR studies and can be used to analyse low recapture rates. We determined survival (S), recapture (r) and state change (Ψ) probabilities. The independent variables tested were site (s: Oglivies Dam and Sponar’s Creek), time in weeks (t), current Bd state (f), and Bd state of previous capture (to). The two Bd states were Bd positive and Bd negative. Only data from week 3 through week 10 of the breeding season were included, because both sites were sampled for those eight weeks. We measured goodness of fit as “ĉ” with the program U-CARE, and ĉ = 0.707. Below a value of “1”, ĉ represents under distribution of the data; therefore, ĉ not adjusted, and ĉ = 1 was used in analysis. Because females were never recaptured, they were excluded from the analyses in M-SURGE. While typically only two disease states are investigated [15,28–30] (Bd negative and Bd positive) as above, we decided to further explore the effect of infection intensity. To explore infection intensity, we analysed the data using a three-disease-state model in which the three states were Bd negative, low infection (<350 zoospore equivalents, ZE) and high infection (>350ZE). The goodness of fit was ĉ = 0.661, and it was not adjusted.
Population size for each population was analysed using a Program MARK POPAN model. Variables explored were (t) time in weeks, (Φ) survival probability, (p) recapture probability, and (Pent) probability of entry into the population. Because males were more conspicuous than females and tend to be present at the breeding site for an extended period, the “super-population size” calculated through POPAN refers to all the males that came to breed within the 2013 breeding season. Population density was estimated as the super-population size of males divided by site area.
Model selection was based on Akaike’s information criterion (AIC), with the best fitting model indicated by the lowest AIC value. The seven best fitting models were chosen for analysis. We did not perform model averaging.
Infection intensity over the breeding season was assessed using ANCOVA in SPSS (v21), and individuals are covariates. The data were transformed Log10(N+1) for a normal distribution, and only positive animals were included in the analysis. Infection prevalence was assessed using a logistic regression in which change in infection over time was compared between populations. Prevalence between the two sites within specific weeks was compared using Pearson’s Chi-Squared test in SPSS (v21), and odds ratios were calculated in Microsoft Excel. Because female capture rates were low, infection intensity and prevalence analyses only included males. To compare overall infection status of males and females between the two sites, ANCOVAs in SPSS (v21) were used, in which sex, site, week, site*sex and week*sex were the factors analysed, and individual was a covariate.
Body condition defined as mass/SVL was analysed using ANCOVAs in SPSS (v21). Mass/SVL is an appropriate measure for body condition in this species because it is highly correlative (Female: Pearson’s correlation = 0.817, p < 0.01; Male: Pearson’s correlation = 0.669, p < 0.01) and linear (Linear Regression ANOVA: Female: Mass = -5.819 + 0.272(SVL) p < 0.01; Male: Mass = -1.598 + 0.121(SVL), p < 0.01) [31]. We compared site and time, and individuals were covariates. Effect size was determined using the averaged Cohen’s d statistic across time and was calculated in Microsoft Excel. The effect of infection intensity on body condition was correlated using linear regression analysis in SPSS (v21), and time was not included.
Animal ethics and permits
This study was carried out in strict accordance with the recommendations of Animal Ethics. The protocol was approved by the Animal Ethics Committee at James Cook University (Application A1880). All necessary permits obtained for the described study complied with all relevant regulations. Amphibian and crayfish collection permits were issued by NPWS Wildlife Licencing and Management.
Results
Population density
The best supported model to estimate population abundance of males over the course of the breeding season was Φ(.)p(t)pent(t) for both sites (Oglivies Dam; AIC = 439.318, parameters = 16: Sponar’s Creek; AIC = 816.459, parameters = 17) (Table 1). The outcomes explored were Φ = survival probability, p = recapture probability and pent = probability of entry into the population. The variable that could affect each outcome was t = time in weeks, or (.) indicating that time was not an important variable in predicting the outcome.
Table 1. AIC ranking of best-fit POPAN models.
Model results are for the two sites: Sponar’s Creek and Oglivies Dam.
| Model Variables* | Parameters | Deviance | AIC | Δ AIC |
|---|---|---|---|---|
| Sponar’s Creek | ||||
| Φ(.)p(t)Pent(t) | 17 | 0 | 816.459 | 0 |
| Φ(t)p(.)Pent(t) | 16 | 0 | 826.993 | 10.534 |
| Φ(t)p(t)Pent(t) | 24 | 0 | 829.007 | 12.548 |
| Φ(.)p(.)Pent(t) | 8 | 0 | 830.658 | 14.199 |
| Φ(.)p(.)Pent(.) | 3 | 21818.006 | 23201.514 | 22385.055 |
| Oglivies Dam | ||||
| Φ(.)p(t)Pent(t) | 16 | 0 | 439.318 | 0 |
| Φ(t)p(t)Pent(t) | 23 | 0 | 449.208 | 9.89 |
| Φ(t)p(.)Pent(t) | 18 | 0 | 449.832 | 10.514 |
| Φ(.)p(.)Pent(t) | 9 | 0 | 456.482 | 17.164 |
| Φ(.)p(.)Pent(.) | 3 | 67125.025 | 70049.5921 | 69610.2741 |
* Outcome probabilities determined are (Φ) survival probability, (p) recapture probability, and (Pent) probability of entry into the population. These outcome probabilities are determined by the variable (t) time in weeks, or no variable (.).
The population estimate for Oglivies Dam (0.17 hectares) was 2725 males (95% CI, 1712–4505); and the estimated population density was 16,031 males per hectare (95% CI, 10,070–25,498) (Table 2). Sponar’s Creek (1.8 hectares) population size was smaller with 319 males (95% CI, 277–381); and the estimated population density was 177 males per hectare (95% CI, 154–212) (Table 2). The population density of L. v. alpina at Oglivies Dam was on average 90.53 times higher than at Sponar’s Creek.
Table 2. Recaptured animals are each site.
Number of Litoria verreauxii alpina that were recaptured at the different sites (Sponar’s Creek, 1.8 hectares in area: Oglivies Dam, 0.17 hectares in area) and how many times the individual was recaptured over the course of the breeding season. Population size is the super-population estimate for males.
| Number of recaptures | Oglivies Dam | Sponar's Creek |
|---|---|---|
| 0 | 201 | 139 |
| 1 | 40 | 50 |
| 2 | 17 | |
| 3 | 11 | |
| 5 | 1 | |
| Total captured | 241 | 218 |
| Population size | 2725 | 319 |
Recaptures
A total of 459 animals were captured at both sites over the course of the breeding season. At Oglivies Dam, 241 animals were captured, 18 were female, and 16.60% of males were recaptured (Table 2). At Sponar’s Creek, 218 animals were captured, 21 were female, and 36.24% of males were recaptured (Table 2).
Disease dynamics
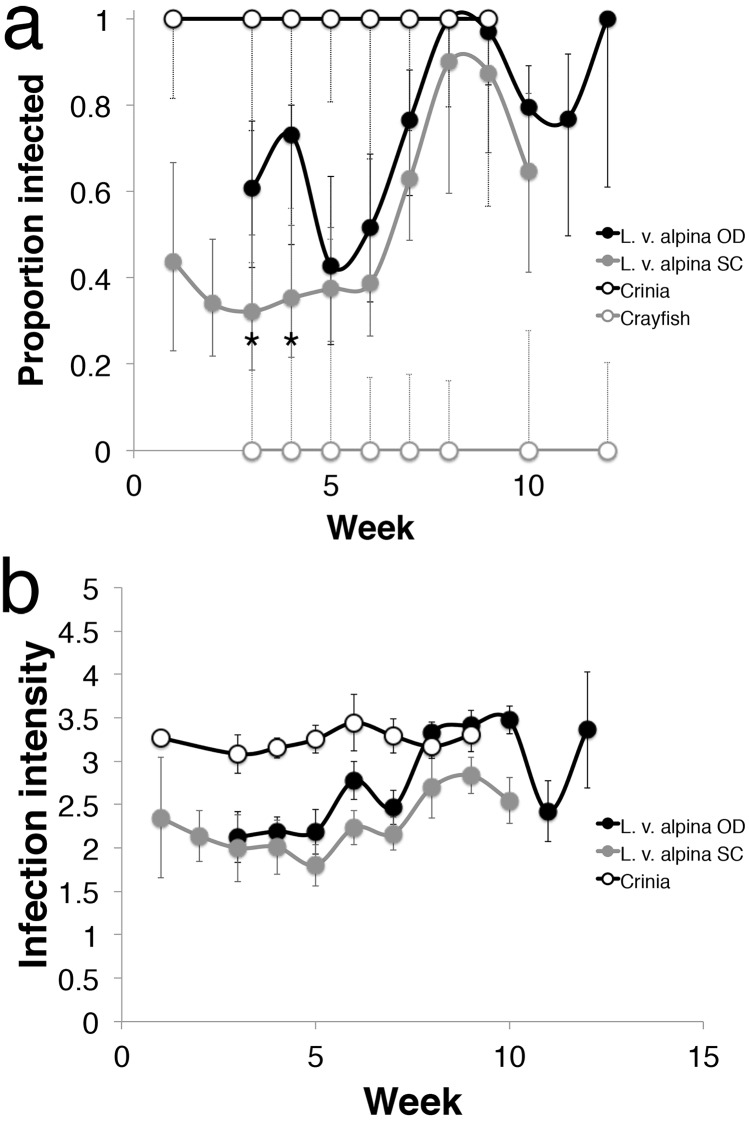
Between recaptures, the disease state detected for some animals often changed (Table 3). The most common disease state change was from Bd negative to low infection intensity at Sponar’s Creek, and from low infection intensity to high infection intensity at Oglivies Dam. It was less common for an animal at either site to reduce or clear infection (Table 3). Therefore, infection prevalence increased each week throughout the course of the breeding season at both sites (Logistic Regression: Exp(B) = 1.313, p<0.001) and also differed significantly between sites (Logistic Regression: Exp(B) = 0.319, p<0.001). Oglivies Dam had higher infection prevalence than Sponar’s Creek throughout the breeding season (Odds ratio = 3.50). Oglivies Dam infection prevalence was greatest at weeks 3 (Chi-Squared: χ2 = 4.800, p = 0.028; Odds ratio = 3.25) and 4 (Chi-Squared: χ2 = 8.422, p = 0.004; Odds ratio = 4.98) (Fig 1A).
Table 3. Disease state change over the course of the study.
The proportion of recaptured individuals that changed disease state over the course of multiple recaptures between the two sites: Oglivies Dam and Sponar’s Creek. This table represents the data collected from the CMR study.
| Recaptures | Oglivies Dam | Sponar’s Creek |
|---|---|---|
| Stay Zero | 0.025 | 0.266 |
| Stay Low | 0.075 | 0.063 |
| Stay High | 0.250 | 0.025 |
| Zero to Low | 0.125 | 0.266 |
| Zero to High | 0.150 | 0.089 |
| Low to High | 0.275 | 0.127 |
| Low to Zero | 0.050 | 0.063 |
| High to Low | 0.025 | 0.013 |
| High to Zero | 0.025 | 0.013 |
| Low to Zero to High | 0.013 | |
| Zero to Low to High | 0.051 | |
| Zero to Low to Zero to Low | 0.013 | |
Fig 1. Infection prevalence and intensity across sites for Litoria verreauxii alpina, Crinia signifera and Cherax destructor.
(a) Prevalence of infection. Error bars indicate 95% confidence intervals. (*) indicates time-points where prevalence is significantly different at each site for Litoria verreauxii alpina, using Pearson’s Chi-Squared test. (b) Infection intensity transformed to log10 scale. Error bars indicate standard error. Sites are Oglives Dam (OD) and Sponar’s Creek (SC).
Similarly, infection intensity increased throughout the course of the breeding season (ANCOVA: F11, 346 = 6.216, p < 0.001) and differed between the two sites (ANCOVA: F1,346 = 6.002, p = 0.015), with Oglivies Dam having 2.02 times greater average infection intensity (d = 0.48) (6,698±16,522ZE) than Sponar’s Creek (3,315±10,034ZE) (Fig 2B).
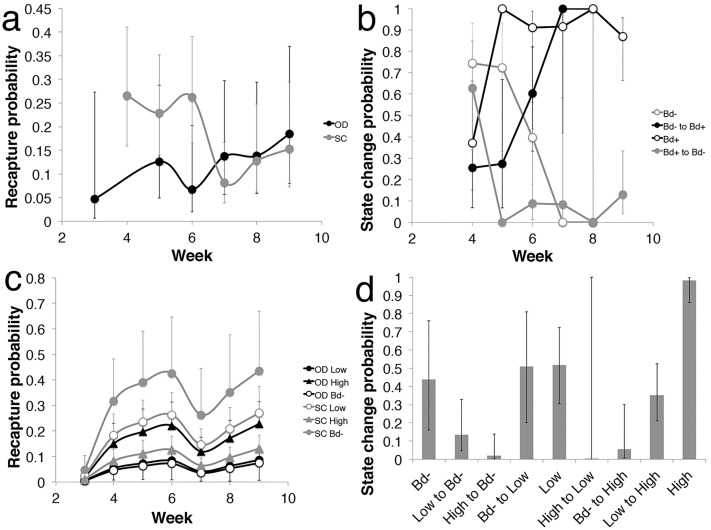
Fig 2. Recapture probability and State change probability.
Conditional Arnason-Schwarz model in which outcome probabilities are (S) survival, (p) recapture, (Ψ) state change, and the variables that can influence the outcomes are (g) site, (t) time in weeks, (to) state at previous capture, (f) state at capture. Panels (a) and (b) represent the two-disease-state model, Bd positive (Bd+) and Bd negative (Bd-), in which the best model was S(g)p(g*t)Ψ(to*f*t). Panels (c) and (d) represent the three-disease-state model, Bd negative (Bd-), low infection intensity of >350ZE (Low) and high infection intensity of >350ZE (High), in which the best model was S(g*f)p(g*to+t)Ψ(to*f). (a) Recapture probability per week in a two-disease-state model. Factors included in the best model for recapture probability were site and week, Error bars indicate 95% confidence interval. (b) Probability of changing state per week in a two-disease-state model. Factors included in the best model for state change probability were week, infection state at current capture, and infection state at previous capture, and error bars indicate 95% confidence interval. (c) Recapture probability per week in a three-disease-state model. Factors included in the best model for recapture probability were site, state of infection and week. Error bars indicate standard error, and only one error bar included for figure clarity. (d) Probability of changing state in a three-disease-state model, error bars indicate 95% confidence interval. Sites are Oglives Dam (OD) and Sponar’s Creek (SC).
Males had higher overall infection intensities than females (averaged across sites: males 5,497±736ZE; females, 2,730±2,513ZE) (ANCOVA: by sex, F1,597 = 612.369, p<0.01; by sex*week F10,597 = 2.335, p = 0.01), but infection intensity did not vary by site for each sex (ANCOVA: by sex*site, F1,597 = 1.289, p = 0.257).
Body condition was not affected by infection intensity (Linear regression ANOVA: F1, 580 = 0.62, p = 0.432). Male body condition did not vary between sites (ANCOVA: F1,580 = 0.812, p = 0.368), but did decrease by an average of 10.51% each week throughout the course of the breeding season (ANCOVA: F11, 580 = 4.984, p < 0.001; d = 0.1051). Body condition of the females did not vary between sites (ANCOVA: F1, 36 = 0.245, p = 0.626) or through time (ANCOVA: F11, 36 = 1.37, p = 0.26).
CMR analysis
Two-disease-state model
Using a two state analysis (Bd positive and Bd negative) the two best fit models were Model 1) S(g)p(g*t)Ψ(to*f*t) (AIC = 927.706, deviance = 867.706, parameters = 30), and Model 2) S(.)p(g*t)Ψ(to*f*t) (AIC = 927.927, deviance = 869.972, parameters = 29) (Table 4). The outcomes explored were (S) survival probability, (p) recapture probability, and (Ψ) state change probability. The variables affecting each probability were (g) site, (t) time in weeks, (to) state of previous capture, (f) state of capture, and (.) indicates that no variable affected the probability. Neither model suggested that disease state influenced survival. In Model 1, survival probability per week differed between sites: animals at Oglivies Dam had a lower estimated survival probability per week (0.721, 95% CI, 0.555–0.843) than Sponar’s Creek (0.861, 95% CI, 0.726–0.935). In Model 2 survival probability was the same for all animals (0.821, 95% CI 0.722–0.890). Recapture and disease state change probability were equivalent in models 1 and 2. Recapture probability differed between sites and weeks but not for different disease states (Fig 2A). Recapture rate was lower earlier in the season for Oglivies Dam, while it was higher early in the season for Sponar’s Creek. Towards the end of the sampling period, both sites had similar weekly recapture rates. The probability of an animal changing infection state was dependent on time and disease status but not site (Fig 2B). Early in the season, there was a higher probability of staying Bd negative or clearing infection, but after week 7 the chance of staying Bd negative or clearing infection dropped to a very low probability. Early in the season animals were not likely to gain Bd infection or remain Bd positive. But after week 7 the likelihood of maintaining infection or becoming infected was high.
Table 4. AIC ranking for Conditional Arnason–Schwarz models.
Model results for both the two-disease-state (Bd negative and Bd positive) and three-disease-state analysis (Bd negative, low Bd infection under 350ZE, and high Bd infection over 350ZE).
| Model Variables* | Parameter | Deviance | AIC | Δ AIC |
|---|---|---|---|---|
| 2-State | ||||
| S(g)p(g*t)Ψ(to*t) | 30 | 867.706 | 927.706 | 0 |
| S(.)p(g*t)Ψ(to*f*t) | 29 | 869.972 | 927.972 | 0.266 |
| S(g+f)p(g*t)Ψ(to*f*t) | 31 | 867.697 | 929.697 | 1.991 |
| S(f)p(g*t)Ψ(to*f*t) | 30 | 869.971 | 929.971 | 2.265 |
| S(g*f)p(g*t)Ψ(to*f*t) | 32 | 867.377 | 931.377 | 3.671 |
| S(f*g)p(g*t)Ψ(to*f*t) | 32 | 867.377 | 931.377 | 3.671 |
| S(g)p(g*t)Ψ(to*f) | 18 | 900.469 | 936.469 | 8.763 |
| S(g*f*to)p(g*to*t)Ψ(g*to*f*t) | 67 | 835.799 | 969.799 | 42.093 |
| S(.)p(.)Ψ(.) | 3 | 987.247 | 993.247 | 65.541 |
| 3-State | ||||
| S(g*f)p(g*to+t)Ψ(to*f) | 24 | 979.337 | 1027.337 | 0 |
| S(g*f)p(g*to+t)Ψ(f) | 21 | 987.779 | 1029.776 | 2.439 |
| S(g*f)p(g*to+t)Ψ(to) | 21 | 996.219 | 1038.219 | 10.882 |
| S(g*f)p(g*to+t)Ψ(to*f*t) | 60 | 921.865 | 1041.865 | 14.528 |
| S(g)p(g*to+t)Ψ(to*f*t) | 56 | 935.674 | 1047.674 | 20.337 |
| S(g*f)p(g*t)Ψ(to*f*t) | 62 | 925.296 | 1049.296 | 21.959 |
| S(f)p(g*to+t)Ψ(to*f*t) | 57 | 935.729 | 1049.729 | 22.392 |
| S(g*f*t)p(g*to*t)Ψ(g*to*f*t) | 113 | 859.464 | 1085.464 | 58.127 |
| S(.)p(.)Ψ(.) | 3 | 1079.595 | 1085.595 | 58.258 |
*Probabilities determined are (S) survival probability, (p) recapture probability, (Ψ) state change probability; and the variables that influence S, p and Ψ are (g) site, (t) time in weeks, (to) state of previous capture, (f) state of capture and no variable (.)
Three-disease-state model
When including three-disease-states in the CMR analysis (Bd negative, “low infection” with intensity of infection under 350ZE, and “high infection” with intensity of infection above 350ZE), the best fit model was S(g*f)p(g*to+t)Ψ(to*f) (AIC = 1027.424, deviance = 979.424, parameters = 26) (Table 4). Disease status and site were important factors influencing weekly survival. Uninfected animals from both sites had similar survival probabilities per week (Oglivies Dam: 0.766, 95% CI, 0.273–0.966; Sponar’s Creek: 0.760, 95% CI 0.552–0.891), and when infected with low Bd loads (Oglivies Dam: 0.994, 95% CI, 0.003–1.0; Sponar’s Creek: 0.997, 95% CI, 0.001–1.0). In contrast, when carrying a high Bd infection, weekly survival at Oglivies Dam was lower (0.460, 95% CI, 0.232–0.706) than survival at Sponar’s Creek (0.771, 95% CI, 0.423–0.939). Recapture probability was dependent on week, site, and state at previous capture (Fig 2C). Recapture probability was higher overall at Sponar’s Creek than at Oglivies Dam, but infection intensity impacted recapture rates differently at the two different sites. At Oglivies Dam, animals were more likely to be captured if they had high Bd infection intensity; but at Sponar’s Creek, Bd negative animals had the highest recapture probability. Infection state change probability was dependent on state of infection at current capture, and state of infection at previous capture, but not time or site (Fig 2D). While some animals remained Bd negative over the breeding season, most became infected. Once lightly infected, some animals appeared to clear the infection; however, if the animals showed a high intensity of infection, they were less likely to become Bd negative at the next capture.
Reservoir hosts
A total of 94 C. destructor crayfish were sampled and all tested negative for Bd (Fig 1A). A total of 93 C. signifera individuals were tested for Bd. Infection prevalence was 100% (Fig 1A), and infection intensity was consistently high (3875±6691ZE) throughout the breeding season (Fig 1B).
Discussion
Density-dependence
Our study supports the concept of density-dependence of Bd infection because the 90–fold higher density site, Oglivies Dam, had 3.49 and 2.02 times higher prevalence and intensity, respectively, throughout the breeding season than the Sponar’s Creek site. This pattern has also been shown in studies of Rana muscosa, in which Bd infection also increased with increased population density [5,6,32]. The transmission and spread of Bd is known to have density-dependent disease transmission characteristics in other studies [4–6]. At higher population densities, uninfected animals are more likely to come in contact with infected animals and the pathogen in the environment, leading to an increase in direct and indirect transmission [33].
Disease dynamics
Infection intensity and prevalence at the beginning of the season were lower at both sites, and then it increased until the end of the breeding season (Fig 1). The increase in infection intensity and prevalence is consistent with what has been observed for other aggregate breeders [13,34]. However, some L. v. alpina animals entered the breeding season with an infection suggesting that they are becoming infected outside the breeding season. These infected animals might have overwintered with infection after exposure to infection in the previous season; or, more likely, they gained infection before the breeding season while they were dispersed in terrestrial habitats. The abundance of terrestrial reservoirs could be a key factor determining exposure in the non-breeding season (see Reservoir Hosts section below). However, overwintering ecology and infection susceptibility are unknown in this species [23].
CMR analysis
Recapture rates differed between the two sites. This was expected because they differed significantly in population density [30,35,36]. Animals in the smaller population, Sponar’s Creek, were more likely to be recaptured as recapture effort was relatively greater per individual (Table 2). Uninfected animals were more likely to be recaptured than infected animals at Sponar’s Creek (Fig 2C), which is a pattern observed in other frog species with Bd [14,15]. However, recapture probabilities were different at Oglivies Dam, where heavily infected animals were more likely to be recaptured. This is consistent with CMR analysis of other wildlife diseases [28]. While the heavily infected category for this study was >350ZE, animals likely do not become ill until a much higher infection load develops [37]. Oglivies Dam animals had higher infection intensities, and more animals were likely experiencing clinical signs of chytridiomycosis in the heavily infected category compared with animals at Sponar’s Creek. Heavily infected animals might be more easily recaptured because they are more lethargic. Therefore, if there is large heterogeneity in infection burden, it is important to include infection intensity in analyses to help tease apart its effects on recapture rates.
The results from the infection state change analysis suggest that the Bd transmission and recovery dynamics were similar in both populations. The two-disease-state analysis suggested that early in the season, some animals were able to recover from infection, but as the breeding season progressed, animals were less likely to recover and unlikely to remain Bd negative. The higher potential for recovery early on might occur because there were fewer heavily infected animals early in the season, and therefore, lower transmission rates and exposure and re-exposure to the pathogen would occur. When the recapture analysis was expanded to include three disease states, it showed that animals were very unlikely to recover from high infection intensity as seen by the high proportion of animals remaining at high infection intensity (Fig 2D). In contrast, recovery from low infection is more likely.
Results of analysis of factors influencing survival depended on whether infection intensity was included. In the two-disease-state model without considering infection intensity, the best-fit model suggested that disease state does not influence survival; however, the lower weekly survival probability for Oglivies Dam is confounded by the higher proportion of infected individuals with higher infection intensities as demonstrated by the three-disease-state model. In the three-disease-state model, survival of the highly infected animals differed between the sites, with Oglivies Dam animals having lower survival (0.46 weekly survival) than the highly infected animals at Sponar’s Creek, and the low infected and uninfected animals at both sites had similar weekly survival rates (0.76–0.99). It is possible that the site differences in survival suggested by the model were not due to site characteristics, but rather they represented differences in infection intensity between the sites that our model was unable to discern using the high intensity threshold of 350ZE. The lower survival of heavily infected animals at Oglivies Dam might be because Oglivies Dam animals were more heavily infected than Sponar’s Creek animals. These results support previous reports of the progression of Bd infection in susceptible species. Infection intensity builds over several weeks, and animals succumb to chytridiomycosis and die when heavy infection burdens are reached [6,13,38]. Analysing field data using multistate models is important to characterize disease dynamics in affected populations [39,40].
Differences in the sexes
A limitation of this study is that females were never recaptured and so we were unable to determine the effect of disease on their behaviour and survival. However, for L. v. alpina, males are likely to be the drivers of Bd within the system due to their breeding behaviour. Males tend to be present in the breeding ponds for weeks at a time, whereas females arrive to mate and deposit their eggs and then return to their non-breeding habitat. Males have higher infection intensity throughout the breeding season because they spend more time in the breeding habitat increasing their chance of pathogen exposure, either through direct contact with other animals, or through indirect transmission in the aquatic environment. While these results suggest that females have lower levels of infection in the breeding pond during capture than males, female L. v. alpina have similar low year-to-year survivorship similar to males [19]. This suggests that total infection rates and disease outcomes are similar in both sexes.
Reservoir hosts
Reservoir species likely play an important role in infection dynamics of L. v. alpina. Infected L. v. alpina die after the breeding season resulting in almost complete population turnover every year [19]. Tadpoles and juveniles leave the pond uninfected [19] and most first time breeders enter a pond uninfected. Under such circumstances, reservoir hosts are the likely cause of pathogen persistence. Two potential reservoir species were analysed in this study: an invasive crayfish species, C. destructor, and a sympatric frog species, C. signifera. We did not find crayfish carrying Bd infection, suggesting that C. destructor is not a reservoir species in the Australian Alps. However, we found 100% prevalence and high intensity of Bd infection in C. signifera. Intensity of infection in C. signifera is higher than L. v. alpina throughout the breeding season (Fig 1B). Additionally C. signifera do not vary in infection prevalence or intensity throughout the season suggesting that this species is tolerant of infection and is likely to be contributing to persistence and spread of this deadly pathogen similar to other reservoir hosts of this pathogen [41]. Litoria v. alpina can be found in close contact with C. signifera during the breeding season and in shared hibernation places during the non-breeding season. It is likely that infection in L. v. alpina is perpetuated by direct and indirect transmission of Bd from C. signifera.
Management implications
Recent laboratory studies suggest that L. v. alpina might be evolving an immune response to fight infection [37], and selection for resistance is a possibility. However, artificial selection for disease resistance to chytridiomycosis has not been successfully attempted, and it is costly in terms of the research required [42,43]. In addition, at higher density site the lower survivorship when heavily infected might reduce the opportunity for the evolution of resistance mechanisms that could permeate the population. Therefore, alternative management strategies might be more efficient in the short term to help secure the conservation of species [42]. Our study supports the density-dependence of Bd. Our site with higher population density of L. v. alpina had higher infection prevalence and intensity and lower survival for heavily infected frogs. In order to promote survivorship and decrease effects of disease, management strategies might aim to decrease population densities. Such management techniques might include increasing the area of the water body while maintaining the same size of suitable breeding habitat within the pond or decreasing environmental disease transmission by increasing water replacement using increased flow into and out of the ponds that L. v. alpina inhabit.
Litoria. v. alpina are highly susceptible to Bd infection, rarely recover from infection, and have near complete population turnover each year in populations with endemic Bd infections [19]. Therefore, high recruitment enables some populations to persist despite the impact of chytridiomycosis, and is crucial to the persistence of L. v. alpina [15,19,44]. While reproductive output in persisting populations is sufficient to maintain populations, it is a risky mechanism for population persistence. Drought is a high risk factor for decreased progeny survival as evidenced by L. v. alpina having been extirpated from all ephemeral water bodies [23]. Management strategies to ensure consistent annual survival of progeny by reducing the effects of drought by increasing water body size and water input would promote population persistence [23]. Increasing water body size and water input (while maintaining breeding habitat size) might serve to both promote lower population densities and enable consistent recruitment. This strategy might be effective in sustaining some reintroduced populations. Because C. signifera appears to be maintaining infection within the system, reducing their abundance or reintroducing L. v. alpina into sites where they are absent could promote L. v. alpina conservation.
Supporting Information
The following supporting information is available for this article online.
Oglivies Dam 35° 57' 29" S, 148° 24' 4" E: Elevation 1382m. Sponar’s Creek, 36° 21' 32.4" S, 148° 30' 0" E: Elevation 1515m. Kiandra, 35° 52' 1" S, 148° 29' 53" E: Elevation 1358m, where the crayfish were collected. The white lines indicate state lines, Victoria to the West and Australian Capital Territory to the Northeast. The grey lines indicate major roadways. Scale bar = 10km. Map data reprinted from Google Imagery under CC BY license, with permission from TerraMetrics, original copyright 2015.
(TIF)
Sites are Oglivies Dam and Sponar’s Creek. Error bars are standard error. Temperatures were collected with iButtons places at the sites.
(TIF)
Further information on the study species, study sites, weather data collection, and individual marking methods.
(DOCX)
Data includes site, sex, date of collection, SVL, mass, and infection load or each animal throughout the study.
(XLSX)
Species, date of collection, site and infection status and intensity.
(XLSX)
Acknowledgments
We would like to thank M. McFadden and P. Harlow for field guidance, and L. Grogan and G. Martin for guidance in modelling the data. We would like to thank C. Scheele, A. Fletcher, T. Fletcher, L. Skerratt, R. Skerratt, T. Skerratt and D. Newell for helping us in the field, and D. Featherstone and P. Virpara for help analysing the crayfish. We thank V. Eldridge, T. Swain, R. O’Neill, R. Mouat, SnowyHydro and National Parks and Wildlife Services, who provided accommodation during the field season.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The project was funded by the Australian Research Council Grants FT100100375, LP110200240 and DP120100811 and Taronga Zoo, received by LFS, LB and DAH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Skerratt LF, Berger L, Speare R, Cashins S, McDonald KR, Phillott AD, et al. (2007) Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. Ecohealth 4: 125–134. [Google Scholar]
- 2. Kriger KM, Hero J-M (2007) The chytrid fungus Batrachochytrium dendrobatidis is non-randomly distributed across amphibian breeding habitats. Divers Distrib 13: 781–788. [Google Scholar]
- 3. Murray KA, Retallick RWR, Puschendorf R, Skerratt LF, Rosauer D, McCallum HI, et al. (2011) Assessing spatial patterns of disease risk to biodiversity: implications for the management of the amphibian pathogen, Batrachochytrium dendrobatidis . J Appl Ecol 48: 163–173. [Google Scholar]
- 4. Briggs CJ, Vredenburg VT, Knapp RA, Rachowicz LJ (2005) Investigating the population-level effects of chytridiomycosis: An emerging infectious disease of amphibians. Ecology 86: 3149–3159. [Google Scholar]
- 5. Briggs CJ, Knapp RA, Vredenburg VT (2010) Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. PNAS 107: 9695–9700. 10.1073/pnas.0912886107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vredenburg VT, Knapp RA, Tunstall TS, Briggs CJ (2010) Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc Natl Acad Sci U S A 107: 9689–9694. 10.1073/pnas.0914111107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berger L, Speare R, Hines HB, Marantelli G, Hyatt AD, McDonald KR, et al. (2004) Effect of season and temperature on mortality in amphibians due to chytridiomycosis. Aust Vet J 82: 434–439. [DOI] [PubMed] [Google Scholar]
- 8. Brannelly LA, Chatfield MWH, Richards-Zawacki CL (2012) Field and laboratory studies of the susceptibility of the green treefrog (Hyla cinerea) to Batrachochytrium dendrobatidis infection. PLoS One 7: e38473 10.1371/journal.pone.0038473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kriger KM, Hero J-M (2007) Large-scale seasonal variation in the prevalence and severity of chytridiomycosis. J Zool 271: 352–359. [Google Scholar]
- 10. Rothermel BB, Walls SC, Mitchell JC, Dodd CK, Irwin LK, Green DE, et al. (2008) Widespread occurrence of the amphibian chytrid fungus Batrachochytrium dendrobatidis in the southeastern USA. Dis Aquat Organ 82: 3–18. 10.3354/dao01974 [DOI] [PubMed] [Google Scholar]
- 11. Sapsford SJ, Alford RA, Schwarzkopf L (2013) Elevation, temperature, and aquatic connectivity all influence the infection dynamics of the amphibian chytrid fungus in adult frogs. PLoS One 8: e82425 10.1371/journal.pone.0082425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Korfel CA, Hetherington TE (2014) Temperature alone does not explain patterns of Batrachochytrium dendrobatidis infections in the green frog Lithobates clamitans . Dis Aquat Organ 109: 177–185. 10.3354/dao02749 [DOI] [PubMed] [Google Scholar]
- 13. Kinney VC, Heemeyer JL, Pessier AP, Lannoo MJ (2011) Seasonal pattern of Batrachochytrium dendrobatidis infection and mortality in Lithobates areolatus: affirmation of Vredenburg’s “10,000 zoospore rule”. PLoS One 6: e16708 10.1371/journal.pone.0016708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Murray KA, Skerratt LF, Speare R, McCallum H (2009) Impact and dynamics of disease in species threatened by the amphibian chytrid Fungus, Batrachochytrium dendrobatidis . Conserv Biol 23: 1242–1252. [DOI] [PubMed] [Google Scholar]
- 15. Phillott AD, Grogan LF, Cashins SD, Mcdonald KR, Berger L, Skerratt LF (2013) Chytridiomycosis and seasonal mortality of tropical stream-associated frogs 15 years after introduction of Batrachochytrium dendrobatidis . Conserv Biol 27: 1058–1068. 10.1111/cobi.12073 [DOI] [PubMed] [Google Scholar]
- 16. Conn PB, Cooch EG (2009) Multistate capture-recapture analysis under imperfect state observation: An application to disease models. J Appl Ecol 46: 486–492. [Google Scholar]
- 17. Osborne W, Hunter D, Hollis G (1999) Population declines and range contraction in Australian alpine frogs In: Campbell A, editor. Declines and Disappearances of Australian Frogs. Canberra, ACT: Biodiversity Group Environmental Australia, Vol. 1 pp. 145–159. [Google Scholar]
- 18.Hunter D, Pietsch R, Clemann N, Scroggie M, Hollis G, Marantelli G (2009) Prevalence of the amphibian chytrid fungus (Batrachochytrium dendrobatidis) in populations of two frog species in the Australian Alps. Report to the Murray Catchment Management Authority.
- 19. Scheele BC, Hunter DA, Skerratt LF, Brannelly LA, Driscoll DA (2015) Low impact of chytridiomycosis on frog recruitment enables persistence in refuges despite high adult mortality. Biol Conserv 182: 36–43. [Google Scholar]
- 20. Brannelly LA, McMahon TA, Hinton M, Lenger D, Richards-Zawacki CL (2015) Batrachochytrium dendrobatidis in natural and farmed Louisiana crayfish populations: prevalence and implications. Dis Aquat Organ 112: 229–235. 10.3354/dao02817 [DOI] [PubMed] [Google Scholar]
- 21. McMahon TA, Brannelly LA, Chatfield MWH, Johnson PTJ, Joseph MB, McKenzie VJ, et al. (2013) Chytrid fungus Batrachochytrium dendrobatidis has nonamphibian hosts and releases chemicals that cause pathology in the absence of infection. PNAS 110: 210–215. 10.1073/pnas.1200592110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beatty S, Morgan D, Gill H (2005) Role of life history strategy in the colonisation of western Australian aquatic systems by the introduced crayfish Cherax destructor Clark, 1936. Hydrobiologia 549: 219–237. [Google Scholar]
- 23. Brannelly LA, Hunter DA, Skerratt LF, Scheele BC, Lenger D, McFadden MS, et al. (2015) Chytrid infection and post-release fitness in the reintroduction of an endangered alpine tree frog. Anim Conserv. 10.1111/acv.12230 [DOI] [Google Scholar]
- 24. Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD (2004) Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Organ 60: 141–148. [DOI] [PubMed] [Google Scholar]
- 25. Kriger KM, Hero J-M, Ashton KJ (2006) Cost efficiency in the detection of chytridiomycosis using PCR assay. Dis Aquat Organ 71: 149–154. [DOI] [PubMed] [Google Scholar]
- 26. Skerratt LF, Mendez D, Mcdonald KR, Garland S, Livingstone J, Berger L, Speare R (2011) Validation of diagnostic tests in wildlife: The case of chytridiomycosis in wild amphibians. J Herpetol 45: 444–450. [Google Scholar]
- 27. Choquet R, Reboulet A- M, Pradel R, Gimenez O, Lebreton J- D (2004) M–SURGE: new software specifically designed for multistate capture–recapture models. Anim Biodivers Conserv 21: 207–215. [Google Scholar]
- 28. Senar JC, Conroy MJ (2004) Multi-state analysis of the impacts of avian pox on a population of Serins (Serinus serinus): The importance of estimating recapture rates. Anim Biodivers Conserv 27: 133–146. [Google Scholar]
- 29. Lachish S, Jones M, McCallum H (2007) The impact of disease on the survival and population growth rate of the Tasmanian devil. J Anim Ecol 76: 926–936. [DOI] [PubMed] [Google Scholar]
- 30. Pilliod DS, Muths E, Scherer RD, Bartelt PE, Corn PS, Hossack BR, et al. (2010) Effects of amphibian chytrid fungus on individual survival probability in wild boreal toads. Conserv Biol 24: 1259–1267. 10.1111/j.1523-1739.2010.01506.x [DOI] [PubMed] [Google Scholar]
- 31. Green AJ (2001) Mass/length residuals: measures of body condition or generators of spurious results? Ecology 82: 1473–1483. [Google Scholar]
- 32. Rachowicz LJ, Briggs CJ (2007) Quantifying the disease transmission function: effects of density on Batrachochytrium dendrobatidis transmission in the mountain yellow-legged frog Rana muscosa. J Anim Ecol 76: 711–721. [DOI] [PubMed] [Google Scholar]
- 33. Ryder JJ, Miller MR, White A, Knell RJ, Boots M (2007) Host-parasite population dynamics under combined frequency- and density-dependent transmission. Oikos 116: 2017–2026. [Google Scholar]
- 34. Terrell VCK, Engbrecht NJ, Pessier AP, Lannoo MJ (2014) Drought reduces chytrid fungus (Batrachochytrium dendrobatidis) infection and mortality but not prevalence in adult crawfish frogs (Lithobates areolatus). J Wildl Dis 50: 56–62. 10.7589/2013-01-016 [DOI] [PubMed] [Google Scholar]
- 35. Longo A V., Burrowes PA (2010) Persistence with chytridiomycosis does not assure survival of direct-developing frogs. Ecohealth 7: 185–195. 10.1007/s10393-010-0327-9 [DOI] [PubMed] [Google Scholar]
- 36. Newell DA, Goldingay RL, Brooks LO (2013) Population recovery following decline in an endangered stream-breeding frog (Mixophyes fleayi) from subtropical Australia. PLoS One 8: e58559 10.1371/journal.pone.0058559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bataille A, Cashins SD, Grogan L, Skerratt LF, Hunter D, McFadden MS, et al. (2015) Susceptibility of amphibians to chytridiomycosis is associated with MHC class II conformation. Proceeding R Soc B 282: 20143127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Voyles J, Young S, Berger L, Campbell C, Voyles WF, Dinudom A, et al. (2009) Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science 326: 582–585. 10.1126/science.1176765 [DOI] [PubMed] [Google Scholar]
- 39. Grogan LF, Berger L, Rose K, Grillo V, Cashins SD, Skerratt LF (2014) Surveillance for emerging biodiversity diseases of wildlife. PLoS Pathog 10: e1004015 10.1371/journal.ppat.1004015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grogan L (2015) Understanding host and environmental factors in immunology and epidemiology of chytridiomycosis in anuran populations in Australia. Doctoral Thesis. James Cook University, Townsville, Australia.
- 41. Reeder NMM, Pessier AP, Vredenburg VT (2012) A reservoir species for the emerging Amphibian pathogen Batrachochytrium dendrobatidis thrives in a landscape decimated by disease. PLoS One 7: e33567 10.1371/journal.pone.0033567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Scheele BC, Hunter DA, Grogan LF, Berger L, Kolby JE, McFadden MS, et al. (2014) Interventions for reducing extinction risk in chytridiomycosis-threatened amphibians. Conserv Biol 28: 1195–1205. 10.1111/cobi.12322 [DOI] [PubMed] [Google Scholar]
- 43. Woodhams DC, Bosch J, Briggs CJ, Cashins S, Davis LR, Lauer A, et al. (2011) Mitigating amphibian disease: strategies to maintain wild populations and control chytridiomycosis. Front Zool 8: 8 10.1186/1742-9994-8-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Muths E, Scherer RD, Pilliod DS (2011) Compensatory effects of recruitment and survival when amphibian populations are perturbed by disease. J Appl Ecol 48: 873–879. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Oglivies Dam 35° 57' 29" S, 148° 24' 4" E: Elevation 1382m. Sponar’s Creek, 36° 21' 32.4" S, 148° 30' 0" E: Elevation 1515m. Kiandra, 35° 52' 1" S, 148° 29' 53" E: Elevation 1358m, where the crayfish were collected. The white lines indicate state lines, Victoria to the West and Australian Capital Territory to the Northeast. The grey lines indicate major roadways. Scale bar = 10km. Map data reprinted from Google Imagery under CC BY license, with permission from TerraMetrics, original copyright 2015.
(TIF)
Sites are Oglivies Dam and Sponar’s Creek. Error bars are standard error. Temperatures were collected with iButtons places at the sites.
(TIF)
Further information on the study species, study sites, weather data collection, and individual marking methods.
(DOCX)
Data includes site, sex, date of collection, SVL, mass, and infection load or each animal throughout the study.
(XLSX)
Species, date of collection, site and infection status and intensity.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.