Abstract
Alcohol and drug use contribute to the pathogenesis of diabetes and are associated with adverse health outcomes, but little research exists on treatments for substance use disorders (SUDs) in patients with diabetes. The aim of this study was to evaluate contingency management (CM) treatments targeting substance use in patients with diabetes. A secondary analysis evaluated the main and interactive effects of diabetes status and treatment condition on outcomes of 681 substance abusers. All participants were enrolled in randomized clinical trials comparing CM to standard care (SC). As in the main trials, CM treatment improved outcomes. However, there was a significant treatment condition X diabetes status interaction effect in terms of durations of abstinence achieved and proportion of negative samples submitted; patients with diabetes responded even more favorably than their counterparts without diabetes when receiving CM. Analyses of post-treatment effects revealed that patients with diabetes, regardless of the type of SUD treatment to which they were earlier assigned, were more likely than those without diabetes to be abstinent at the 9-month follow-up. The findings suggest CM may be an effective treatment for this vulnerable subgroup of substance abusing patients.
Keywords: substance abuse treatment, contingency management, diabetes, alcohol, cocaine
Diabetes is projected to be the seventh leading cause of death by 2030 [World Health Organization (WHO), 2011]. Current treatment guidelines recommend using pharmacotherapies, addressing psychosocial and behavioral factors, and treating comorbid conditions, including substance use disorders (SUDs) [American Diabetes Association (ADA), 2012; Piette & Kerr, 2006]. There is paucity of research on treatments for co-occurring SUDs in patients with diabetes, even though illicit drug use and drinking contribute to its pathogenesis (Ghitza, Wu, & Tai, 2013). Heavy drinking is associated with increased risk of type 2 diabetes (Babor et al., 2012), and substance use can directly and indirectly (e.g., influencing self-management behaviors) contribute to disease progression by disrupting glucose regulation (Ahmed, Karter, & Liu, 2006; Leung, Zhang, Lin, & Clark, 2011a).
Despite the adverse health effects associated with SUDs in patients with diabetes, no SUD interventions have been evaluated explicitly in patients with diabetes. CM is an evidence-based treatment for SUDs based on principles of behavioral analysis. It is included in the National Institute for Health and Clinical Excellence (NICE; 2007) guidelines in the United Kingdom and is being implemented nationwide throughout the Veterans Administration in the United States (Petry, DePhilippis, Rash, Drapkin, & McKay, 2014). CM involves delivering tangible positive reinforcers following objective evidence of a targeted behavior (e.g., negative urine sample, attending treatment) (Petry, 2012), and it is the most effective psychosocial treatment for SUDs (Dutra et al., 2008). Reinforcers typically are chances to win prizes of varying sizes (Petry et al., 2005) or vouchers that can be exchanged for retail goods or services (Higgins, Badger, & Budney, 2000). Numerous studies demonstrate that CM is efficacious in reducing substance use (Lussier, Heil, Mongeon, Badger, & Higgins, 2006; Prendergast, Podus, Finney, Greenwell, & Roll, 2006). CM is also efficacious in subgroups of SUD patients including those with or at high risk for HIV and those with concurrent psychiatric disorders (McDonell et al., 2013; Petry, Alessi, & Rash, 2013; Reback et al., 2010). In these populations, CM appears to have particularly large effect sizes, suggesting its potential for treating other vulnerable populations, such as substance abuse patients with diabetes.
Given increased risks associated with substance use among patients with diabetes, there is a critical need to identify effective SUD treatments in this population. We conducted a retrospective analysis of patients with and without diabetes who participated in randomized trials of CM. Because past research demonstrates very strong effects of CM in other SUD populations with chronic conditions, we hypothesized that patients with diabetes would respond particularly well to CM.
Methods
Participants
Participants were 681 patients enrolled in randomized trials of CM (Petry, Barry, Alessi, Rounsaville, & Carroll, 2012; Petry, Weinstock, & Alessi, 2011). Inclusion criteria were 18 years or older, initiating SUD treatment at a community-based clinic, and ability to understand study procedures. Exclusion criteria were significant uncontrolled psychiatric disorders (e.g., active suicidal ideation, psychosis), or being in recovery for gambling disorder (see Petry et al., 2006, Petry & Alessi, 2010). Patients provided written informed consent, and the University Institutional Review Board approved procedures.
Procedures
Following informed consent, participants completed demographic questionnaires and structured interviews. The Clinical Interview for the DSM-IV (First, Spitzer, Gibbon, & Williams, 1996) evaluated substance use diagnoses and the Addiction Severity Index (ASI; McLellan et al., 1985) assessed medical, drug, alcohol, employment, legal, family/social, and psychiatric problems. ASI composite scores range from 0.00-1.00 on each domain, with higher scores indicating greater severity of symptoms. The ASI-medical section asks if patients have a chronic medical condition, and if so what it is. The Service Utilization Form (SUF; Rosenheck, Neale, Leaf, Milstein, & Frisman, 1995) evaluated medical, psychiatric, and substance use services received and reasons for services received (e.g., diabetes prescription, check-up, or A1c) in the past year at baseline, and since the last evaluation at subsequent assessment periods. The ASI and SUF were re-administered one, three, six, and nine months after treatment initiation. Patients received $35-40 for completion of each assessment, and over 80% participated in each assessment, with no differences between completers and non-completers (Petry et al., 2012; Petry, Weinstock, et al., 2011).
Treatments
A computerized randomization procedure assigned patients to conditions. The Petry et al. (2011) “group” study involved two conditions: group-based CM or standard care (SC). The Petry et al. (2012) study was comprised of two related studies, one for patients initiating treatment with a cocaine positive sample (the “positive” study) and the other for patients initiating treatment with a cocaine negative sample (the “negative” study). Both studies assigned patients to one of three conditions; two involved SC+CM and the third SC only. As treatments are detailed in original reports, they are only briefly described below.
SC treatment
All study participants received intensive outpatient treatment at the clinics; group sessions were held 3-5 days per week for up to four weeks. Care was gradually tapered to a minimum of one group per week. Patients submitted up to 24 study breath and urine samples during the first 12 weeks of treatment. Breath samples were tested for alcohol using Alcosensor-IV Alcometers (Intoximeters, St Louis, MO, USA), and urine samples for opioids and cocaine using Ontrak TesTstiks (Roche, Somersville, NJ, USA).
CM treatment
Patients assigned to CM received SC as above, and submitted breath and urine samples at the same schedule. They also earned reinforcers for providing substance negative samples and/or attending treatment. In all studies (Petry et al., 2011; 2012) reinforcement for abstinence was contingent upon samples testing negative for alcohol, cocaine, and opioids concurrently. Attendance at group therapy was reinforced either alone (in one CM condition of the “negative” study) or in addition to abstinence (in the “group” study). The “positive” study evaluated two magnitudes of reinforcement for abstinence, while the “negative” study compared abstinence versus attendance CM, both providing the same magnitude of reinforcement. Both studies also had a SC condition. The CM condition in the “group” study reinforced both attendance and abstinence, such that minimal reinforcement was provided for attending groups alone, with more reinforcement when samples also tested negative. All studies found benefits of CM relative to SC. Further, all provided comparable treatments (e.g., intensity, duration) and applied identical assessment instruments. These similarities allowed for cross-study analyses. Nevertheless, all analyses controlled for study (see below).
Data analysis
Patients who reported having diabetes, or receiving any diabetes-related services, were classified as having diabetes, while those who had no indication of diabetes or diabetes-related services in their study records were coded as not having diabetes. In total, 28 patients had evidence of diabetes, 96.4% of whom (n=27) reported the condition at the baseline evaluation.
Demographics and baseline characteristics were compared between patients with and without evidence of diabetes using χ2 and independent t-tests. Baseline variables that differed significantly between groups were included as covariates in subsequent analyses.
Multivariate general linear models (GLM) evaluated relationships between diabetes status, age, study (positive, negative or group study), treatment condition (CM or SC), baseline ASI-medical scores, and the interaction between treatment condition and diabetes status on primary study outcomes. Primary outcomes were: retention, longest duration of abstinence (LDA), and percentage of negative samples submitted. Retention, ascertained from clinic records, was weeks engaged in group therapy sessions (0-12 weeks). LDA was operationalized as the longest period of consecutive abstinence from alcohol, cocaine, and opioids during treatment (range 0–12 weeks). Submission of a positive sample for one or more substances or a failure to provide a sample on a testing day broke the string of abstinence. Percentage of samples negative for cocaine, alcohol, and opioids were calculated with the number of samples submitted in the denominator, so missing samples (and study retention) did not impact this variable. These three measures were available from 100% of randomized patients.
Logistic regressions assessed post-treatment predictors of abstinence. The same variables above (diabetes status, study, age, and baseline ASI-medical scores) were entered as independent variables in step one of a logistic regression. In step two, treatment condition (CM or SC) and the interaction between treatment condition and diabetes status were added to the model. The dependent variable was submission of a sample testing negative for alcohol, cocaine and opioids at Month 9. The first regression included only participants with data available at Month 9 (n=543), 80% of the full sample; the second conservatively presumed patients who did not complete the follow-up were positive. All analyses were conducted on SPSS for Windows (v 21), and 2-tailed alphas of < 0.05 were considered significant.
Results
Baseline characteristics
As shown in Table 1, patients with and without diabetes differed on only one baseline demographic and substance use variable. On average, patients with diabetes were older by about seven years, p < 0.001. Consistent with a diagnosis of diabetes, patients with diabetes also had higher ASI-medical scores.
Table 1. Demographic and Baseline Characteristics.
| Variable | No diabetes | Diabetes | Statistical test (df), p |
|---|---|---|---|
| N | 653 | 28 | |
| Study, n (%) | X2(2) = 2.45, .29 | ||
| Petry et al. (2012) Positive study | 104 (15.9) | 5 (17.9) | |
| Petry et al. (2012) Negative study | 316 (48.4) | 17 (60.7) | |
| Petry et al. (2011) Group study | 233 (35.7) | 6 (21.4) | |
| Treatment group, n (%) | X2(1) = .00, .95 | ||
| Contingency management | 400 (61.3) | 17 (60.7) | |
| Standard care | 253 (38.7) | 11 (39.3) | |
| Race, n (%) | X2(2) = .28, .87 | ||
| African American | 280 (42.9) | 13 (36.4) | |
| Caucasian | 282 (43.2) | 12 (42.9) | |
| Other | 91 (13.9) | 3 (10.7) | |
| Male gender, n (%) | 318 (48.7) | 15 (53.6) | X2(1) = .26, .61 |
| Age* | 36.8 (9.6) | 43.8 (11.4) | t (679) = -3.70, <.001 |
| Years of education | 12.2 (2.1) | 11.5 (2.3) | t (678) = 1.53, .13 |
| Employment status, n (%) | X2(2) = 4.80, .09 | ||
| Full Time | 255 (39.1) | 6 (21.4) | |
| Part Time | 151 (23.1) | 6 (21.4) | |
| Unemployed/other | 247 (37.8) | 16 (57.1) | |
| Income | $9,140 (15,703) | $6,714 (12,269) | t (676) = .81, .42 |
| Baseline sample positive for alcohol, cocaine, or opiods, n (%) | 153 (23.4) | 8 (28.6) | X2(1) = .39, .53 |
| DSM-IV Cocaine use disorder, n (%) | 579 (88.7) | 27 (96.4) | X2(1) = 1.65, .20 |
| DSM-IV Opioid use disorder, n (%) | 235 (36.0) | 8 (28.6) | X2(1) = .64, .42 |
| DSM-IV Alcohol use disorder, n (%) | 432 (66.2) | 14 (50.0) | X2(1) = 3.10, .08 |
| Addiction Severity Index scores | |||
| Medical* | .26 (.36) | .42 (.44) | t (679) = -2.26, .02 |
| Employment | .73 (.27) | .81 (.26) | t (679) = -1.51, .13 |
| Alcohol | .22 (.25) | .18 (.26) | t (679) = .81, .42 |
| Drug use | .16 (.10) | .19 (.10) | t (678) = -1.33, .19 |
| Legal | .13 (.21) | .07 (.18) | t (677) = 1.40, .16 |
| Family/social | .17 (.21) | .15 (.20) | t (676) = .65, .53 |
| Psychological | .29 (.24) | .31 (.23) | t (675) = -.50, .62 |
Note. Values represent means and standard deviations unless otherwise indicated; DSM-IV = Diagnostic and Statistical Manual for Mental Disorders, revision IV;
Significant between group difference, p < .05.
During treatment effects
In predicting treatment outcomes, study and treatment condition were significant. Specifically, study was related to all three treatment outcomes, F(2, 678)= 22.22, 104.71, and 25.06, ps< .001, for retention, percent negative samples, LDA, respectively. Patients in the “positive” study from Petry et al. (2012) had poorer outcomes than their counterparts in the Petry et al. (2012) “negative” study, with adjusted means and standard errors (SE) of 5.47 (.50) versus 6.44 (.39) for weeks retained, 2.43 (.50) versus 5.39 (.39) weeks for LDA, and 46.55 % (3.46) versus 89.74 % (2.68) for percent negative samples. Patients in the Petry et al. (2011) “group” study had similar or better outcomes as those in the Petry et al. (2012) “negative” study, with means (SE) of 8.24 (.42), 5.24 (.43), and 82.08% (2.94), for the respective outcomes.
Treatment condition was significantly associated with LDA and percentage of negative samples submitted, F(1, 679) = 18.93 and 8.49, ps < .01, but treatment condition was not significantly related to retention, F(1, 679) = 2.05, p = .15. Overall, patients randomized to CM maintained abstinence for longer durations than those randomized to SC, with means (SE) of 5.93 (.46) and 2.78 (.57) weeks in the respective conditions. Patients randomized to CM also submitted a higher percentage of negative samples than their counterparts in SC, with means (SE) of 80.05% (3.18) and 65.52% (3.92), respectively.
Although the multivariate analysis did not reveal a main effect of diabetes on outcomes (ps > .09), there was a significant diabetes status by treatment condition interaction effect for LDA and percent negative samples, F(1, 677) = 5.47, p<.05 and F(1, 677) = 5.52, p<.05, respectively. Figure 1 shows the weighted means (SE) of the primary outcomes based on diabetes status and treatment condition. Patients with diabetes demonstrated greater improvements in LDA and percentage of negative samples submitted when they were assigned to CM compared to SC. The interaction was not significant for retention, p > .26.
Figure 1.
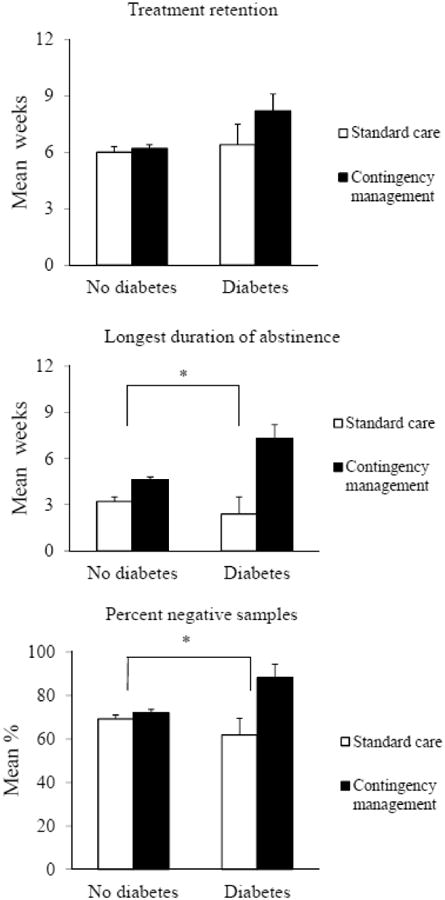
Weighted means (SE) of the primary outcomes by diabetes status and treatment condition.
Follow-up effects
Overall, 82.6% of patients with diabetes submitted samples negative for cocaine, opioids, and alcohol at the Month 9 follow-up versus 61.7% of patients without diabetes. Step 1, including diabetes status, age, baseline ASI-medical scores, and study significantly predicted submission of a negative sample at Month 9, χ2(5) = 14.15, p < .05. Older age was inversely related to submission of a negative sample at follow-up, Beta (SE) = -0.02 (.01), Wald = 5.01, p <.05; odds ratio (OR) = 0.978, 95% confidence interval (CI) =0.960 - 0.997, and having diabetes was associated with a 3.5-fold increase in the likelihood of submitting a negative sample relative to not having diabetes, Beta (SE) = 1.27 (.57), Wald = 4.99, p <.05; OR (95% CI) = 3.57 (1.17 - 10.90). Step 2, including treatment condition and the interaction of treatment condition and diabetes status, was not significant, p > .55.
Results were similar when follow-up non-completers were included in the analyses and coded as having a positive sample. Again, patients with diabetes were more likely than their counterparts without diabetes to submit a negative sample at follow-up, Beta (SE) = .91 (0.42), Wald = 4.63, p <.05; OR (95% CI) = 2.47 (1.08–5.63). Age was not associated with abstinence at follow-up in this analysis, and Step 2 was again not significant.
Discussion
Overall, 4.1% of these SUD patients had diabetes. This rate is consistent with prevalence rates of diabetes in this age group; an estimated 4.1% of adults aged 20-44 years, the age group most represented in this sample, have diabetes [Centers for Disease Control and Prevention (CDC), 2014]. As expected, older age was related to increased likelihood of having diabetes, and scores on the ASI-medical domain were higher in those with diabetes. A limitation of this study is that diabetes status was not explicitly queried or biologically assessed by a hemoglobin A1c or glucose tolerance test. Some patients may have had diabetes but not reported it or received treatment for it during the study assessment periods. Nevertheless, the analyses focused on patients with known diabetes, which they considered a chronic medical condition or for which they reported receiving care.
Analyses of treatment outcomes revealed that CM may be especially beneficial for substance abusers with known diabetes. CM was efficacious overall, but patients with diabetes responded notably well increasing LDA and proportions of negative samples even more so than patients without diabetes. Although preliminary due to the small sample of patients with diabetes, these findings suggest that CM may be a particularly well-suited treatment for patients with diabetes. Our findings are in line with those from a prior study showing that in response to CM, SUD patients with HIV engaged in more health-promoting behaviors than those without HIV (Reback et al., 2010).
Although studies reveal that prize-based CM is cost-effective in improving substance abuse treatment outcomes relative to SC (e.g., Olmstead & Petry, 2009; Sindelar, Elbel, & Petry, 2007), application of this approach to all patients seeking SUD treatment would substantially increase treatment costs. Having an objective index, such as diabetes, to determine who should receive this treatment could be helpful in its expansion to clinical care settings. Because patients with diabetes are a high-risk, high-resource utilizing group (ADA, 2013; Egede et al., 2014; Leung et al., 2011b), reducing substance use in these patients via CM could result in net healthcare savings. Future larger scale studies should estimate diabetes-related healthcare savings associated with CM treatments for SUDs by assessing health outcomes such as glycemic control, complications, and hospitalizations.
Analyses of follow-up data did not reveal a significant treatment condition by diabetes status interaction effect after treatment ended. However, patients with diabetes were more likely to be abstinent at the month 9 follow-up. These findings of improved outcomes are consistent with those from a behavioral weight loss program that found obese participants with diabetes had better response than those without diabetes (Pascale, Wing, Butler, Mullen, & Bononi, 1995). Having diabetes may motivate individuals to change their negative health behaviors, like substance abuse. Relatedly, the healthcare providers of patients with diabetes in this study may have discussed their substance abuse and linked it with potential or existing adverse outcomes, thereby perhaps enhancing their motivation to abstain. Patients with HIV, for example, are more likely to have been advised by a healthcare provider to quit smoking than patients without HIV (Berg et al., 2014). Although this initial study is unable to identify reasons for differences in outcomes between patients with and without diabetes, these results do provide support for further evaluating CM in SUD patients with diabetes.
Although this study is preliminary, it has several novel and important features. It is the first known evaluation of SUD outcomes in patients with diabetes, and it was conducted in community-based settings, making results generalizable. It employed random assignment to conditions, evaluated objective indices of substance use, and assessed both short- and long-term outcomes. Despite these strengths, this study had limitations. First, the number of patients with diabetes was small, and future studies are needed to confirm and extend these results in larger samples. Second, the retrospective nature of the study design precluded a comprehensive and detailed assessment of diabetes and its treatment (e.g., date of diagnosis, type of diabetes, medications, diabetes-related complications). Third, some patients likely were unaware of their diabetes, given that an estimated 28% of adults have undiagnosed diabetes (CDC, 2014). Those with undiagnosed diabetes may represent a subgroup of SUD patients who have different treatment outcomes. Future studies should employ objective diabetes screening tests to identify cases of undiagnosed diabetes (ADA, 2012). Earlier diagnosis is likely to improve diabetes treatment outcomes, and may interact synergistically to improve SUD outcomes as well.
In summary, this study is the first to evaluate outcomes in SUD patients with diabetes. Patients with co-occurring SUDs and diabetes were even more responsive to CM than their counterparts without diabetes. These data suggest that CM may be particularly beneficial for treating this vulnerable, and ever-increasing, subgroup of persons with SUDs. Further, these results bode well for the expansion of this treatment toward addressing other aspects of diabetes prevention and treatment (Petry et al., 2013), including enhancing weight loss (Petry, Barry, Pescatello, & White, 2011; Volpp et al., 2008), increasing ambulatory activities (Petry, Andrade, Barry, & Byrne, 2013), and improving diabetes care activities such as blood glucose self-monitoring (Raiff & Dallery, 2010; Stanger et al., 2013). As CM interventions are gaining traction throughout the world (Garcia-Rodriguez et al., 2009; Hser et al., 2011; Weaver et al., 2014), studies evaluating their application toward diabetes are timely and potentially important for reducing the global health burden of this disease.
Acknowledgments
Funding: This study and preparation of this report were supported in part by National Institutes of Health grants P30-DA023918, R01-DA027615, P50-DA09241, P60-AA03510, R01-HD075630, T32-AA07290, DP3-DK097705, and M01-RR06192.
References
- Ahmed AT, Karter AJ, Liu J. Alcohol consumption is inversely associated with adherence to diabetes self-care behaviours. Diabetic Medicine: A Journal of the British Diabetic Association. 2006;23:795–802. doi: 10.1111/j.1464-5491.2006.01878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes--2013. Diabetes Care. 2012;36:S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Chavez JY, Lee CS, Houser RF, Falcon LM, Tucker KL. Factors associated with alcohol consumption patterns in a Puerto Rican urban cohort. Public Health Nutrition, FirstView. 2014 doi: 10.1017/S1368980014000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor T, Rehm J, Jernigan D, Vaeth P, Monteiro M, Lehman H. Alcohol, diabetes, and public health in the Americas. Revista Panamericana de Salud Pública = Pan American Journal of Public Health. 2012;32:151–155. doi: 10.1590/s1020-49892012000800010. [DOI] [PubMed] [Google Scholar]
- Berg CJ, Nehl EJ, Wang X, Ding Y, He N, Johnson BA, Wong FY. Healthcare provider intervention on smoking and quit attempts among HIV-positive versus HIV-negative MSM smokers in Chengdu, China. AIDS Care. 2014;26:1201–1207. doi: 10.1080/09540121.2014.892565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National diabetes statistics report: estimates of diabetes and its burden in the United States. Atlanta, GA: US Department of Health and Human Services; 2014. [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. The American Journal of Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Egede LE, Gebregziabher M, Zhao Y, Dismuke CE, Walker RJ, Hunt KJ, Axon RN. Impact of mental health visits on healthcare cost in patients with diabetes and comorbid mental health disorders. PloS One. 2014;9:e103804. doi: 10.1371/journal.pone.0103804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for the DSM-IV –TR axis I disorders, clinician version (SCID-CV) Washington, DC: American Psychiatric Press, Inc; 1996. [Google Scholar]
- Garcia-Rodriguez O, Secades-Villa R, Higgins ST, Fernandez-Hermida JR, Carballo JL, Errasti Perez JM, Al-halabi Diaz S. Effects of voucher-based intervention on abstinence and retention in an outpatient treatment for cocaine addiction: a randomized controlled trial. Experimental and Clinical Psychopharmacology. 2009;17:131–138. doi: 10.1037/a0015963. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Wu LT, Tai B. Integrating substance abuse care with community diabetes care: implications for research and clinical practice. Substance Abuse and Rehabilitation. 2013;4:3–10. doi: 10.2147/SAR.S39982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Badger GJ, Budney AJ. Initial abstinence and success in achieving longer term cocaine abstinence. Experimental and Clinical Psychopharmacology. 2000;8:377–386. doi: 10.1037//1064-1297.8.3.377. [DOI] [PubMed] [Google Scholar]
- Hser YI, Li J, Jiang H, Zhang R, Du J, Zhang C, et al. Zhao M. Effects of a randomized contingency management intervention on opiate abstinence and retention in methadone maintenance treatment in China. Addiction (Abingdon, England) 2011;106:1801–1809. doi: 10.1111/j.1360-0443.2011.03490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung GY, Zhang J, Lin WC, Clark RE. Behavioral health disorders and adherence to measures of diabetes care quality. The American Journal of Managed Care. 2011a;17:144–150. [PubMed] [Google Scholar]
- Leung G, Zhang J, Lin WC, Clark RE. Behavioral disorders and diabetes-related outcomes among Massachusetts Medicare and Medicaid beneficiaries. Psychiatric Services (Washington, D C) 2011b;62:659–665. doi: 10.1176/appi.ps.62.6.659. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- McDonell MG, Srebnik D, Angelo F, McPherson S, Lowe JM, Sugar A, et al. Ries RK. Randomized controlled trial of contingency management for stimulant use in community mental health patients with serious mental illness. The American Journal of Psychiatry. 2013;170:94–101. doi: 10.1176/appi.ajp.2012.11121831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, O'Brien CP. New data from the Addiction Severity Index. Reliability and validity in three centers. The Journal of Nervous and Mental Disease. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence. NICE clinical guideline 51: Drug misuse: psychosocial interventions. 2007 http://www.nice.org.uk/nicemedia/live/11812/35973/35973.pdf. [PubMed]
- Olmstead TA, Petry NM. The cost-effectiveness of prize-based and voucher-based contingency management in a population of cocaine- or opioid-dependent outpatients. Drug and Alcohol Dependence. 2009;102:108–115. doi: 10.1016/j.drugalcdep.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascale RW, Wing RR, Butler BA, Mullen M, Bononi P. Effects of a Behavioral Weight Loss Program Stressing Calorie Restriction Versus Calorie Plus Fat Restriction in Obese Individuals With NIDDM or a Family History of Diabetes. Diabetes Care. 1995;18:1241–1248. doi: 10.2337/diacare.18.9.1241. [DOI] [PubMed] [Google Scholar]
- Petry NM. Contingency management for substance abuse treatment: a guide to implementing this evidence-based practice. New York: Routledge; 2012. [Google Scholar]
- Petry NM, Alessi SM. Prize-based contingency management is efficacious in cocaine-abusing patients with and without recent gambling participation. Journal of Substance Abuse Treatment. 2010;39:282–288. doi: 10.1016/j.jsat.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Rash CJ. A randomized study of contingency management in cocaine-dependent patients with severe and persistent mental health disorders. Drug and Alcohol Dependence. 2013;130:234–237. doi: 10.1016/j.drugalcdep.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Andrade LF, Barry D, Byrne S. A randomized study of reinforcing ambulatory exercise in older adults. Psychology and Aging. 2013;28:1164–1173. doi: 10.1037/a0032563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Barry D, Alessi SM, Rounsaville BJ, Carroll KM. A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients. Journal of Consulting and Clinical Psychology. 2012;80:276–285. doi: 10.1037/a0026883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Barry D, Pescatello L, White WB. A low-cost reinforcement procedure improves short-term weight loss outcomes. The American Journal of Medicine. 2011;124:1082–1085. doi: 10.1016/j.amjmed.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Cengiz E, Wagner JA, Hood KK, Carria L, Tamborlane WV. Incentivizing behaviour change to improve diabetes care. Diabetes, Obesity & Metabolism. 2013;15:1071–1076. doi: 10.1111/dom.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, DePhilippis D, Rash CJ, Drapkin M, McKay JR. Nationwide dissemination of contingency management: the Veterans Administration initiative. The American Journal on Addictions/American Academy of Psychiatrists in Alcoholism and Addictions. 2014;23:205–210. doi: 10.1111/j.1521-0391.2014.12092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29:725–731. doi: 10.2337/diacare.29.03.06.dc05-2078. [DOI] [PubMed] [Google Scholar]
- Petry NM, Kolodner KB, Li R, Peirce JM, Roll JM, Stitzer ML, Hamilton JA. Prize-based contingency management does not increase gambling. Drug and Alcohol Dependence. 2006;83:269–273. doi: 10.1016/j.drugalcdep.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Petry NM, Peirce JM, Stitzer ML, Blaine J, Roll JM, Cohen A, et al. Li R. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: a national drug abuse treatment clinical trials network study. Archives of General Psychiatry. 2005;62:1148–1156. doi: 10.1001/archpsyc.62.10.1148. [DOI] [PubMed] [Google Scholar]
- Petry NM, Weinstock J, Alessi SM. A randomized trial of contingency management delivered in the context of group counseling. Journal of Consulting and Clinical Psychology. 2011;79:686–696. doi: 10.1037/a0024813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Raiff BR, Dallery J. Internet-based contingency management to improve adherence with blood glucose testing recommendations for teens with type 1 diabetes. Journal of Applied Behavior Analysis. 2010;43:487–491. doi: 10.1901/jaba.2010.43-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reback CJ, Peck JA, Dierst-Davies R, Nuno M, Kamien JB, Amass L. Contingency management among homeless, out-of-treatment men who have sex with men. Journal of Substance Abuse Treatment. 2010;39:255–263. doi: 10.1016/j.jsat.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenheck R, Neale M, Leaf P, Milstein R, Frisman L. Multisite experimental cost study of intensive psychiatric community care. Schizophrenia Bulletin. 1995;21:129–140. doi: 10.1093/schbul/21.1.129. [DOI] [PubMed] [Google Scholar]
- Sindelar J, Elbel B, Petry NM. What do we get for our money? Cost-effectiveness of adding contingency management. Addiction. 2007;102:309–316. doi: 10.1111/j.1360-0443.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- Stanger C, Ryan SR, Delhey LM, Thrailkill K, Li Z, Li Z, Budney AJ. A multicomponent motivational intervention to improve adherence among adolescents with poorly controlled type 1 diabetes: a pilot study. Journal of Pediatric Psychology. 2013;38:629–637. doi: 10.1093/jpepsy/jst032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpp KG, John LK, Troxel AB, Norton L, Fassbender J, Loewenstein G. Financial incentive-based approaches for weight loss: a randomized trial. JAMA: The Journal of the American Medical Association. 2008;300:2631–2637. doi: 10.1001/jama.2008.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver T, Metrebian N, Hellier J, Pilling S, Charles V, Little N, et al. Strang J. Use of contingency management incentives to improve completion of hepatitis B vaccination in people undergoing treatment for heroin dependence: a cluster randomised trial. Lancet. 2014;384:153–163. doi: 10.1016/S0140-6736(14)60196-3. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Global status report on noncommunicable diseases 2010. Geneva: World Health Organization; 2011. [Google Scholar]


